Abstract
In the first nine weeks of implementation of a Zika Virus Preparedness Plan in a Mexican Public Hospital, we cared for 221 pregnant women with any signal or symptom suggesting Zika virus infection and 99 (44.8%) patients were found to be positive for Zika virus.
The median age of patients was 25.3 years (range 13–49). Symptoms in PCR-positive patients were rash (91.4%) followed by headache (53.1%), myalgia (46.9%), arthralgia (45.7%), pruritus (35.8%), retroocular pain (29.6%), conjunctivitis (21%), and fever (21%). The women's epidemiologic exposure history indicates local transmission and a community outbreak.
Keywords: Zika virus disease, Congenital Zika virus syndrome, Microcephaly
Zika virus (ZIKV), a vector-borne flavivirus, is responsible for a dengue-like illness. Although many infections are asymptomatic, among those with symptoms, fever, rash, joint pain, are characteristically observed. In 2013, a ZIKV outbreak was reported in French Polynesia with 19,000 cases estimated.1 Two years later, local transmission of ZIKV was documented in Brazil, with a suspected number of cases of ZIKV associated disease of 1,300,000 by December 2015.1, 2
Studies from Brazil have provided growing evidence of an association between ZIKV infection in pregnant women and the development of severe neural developmental disorders such as microcephaly in fetuses and newborns.3 Newborns with microcephaly may develop hearing loss, motor skills defects, intellectual disability, developmental delay, and vision problems.4
ZIKV is primarily transmitted by mosquitoes; however, sexual and perinatal transmission also have been demonstrated.5
In Mexico, the first autochthonous Zika virus disease (ZVD) case was reported in November of 2015 and occurred in a resident of Monterrey.6, 7
From that time and until mid-July 2016, the National Surveillance System count remained at five ZVD cases for our entire state, and then began a slow rise that accelerated at the beginning of September.8
Herein we report the first eight weeks of surveillance of ZIKV infection among pregnant women in a third-level teaching hospital in Monterrey, Mexico.
This is a teaching public hospital in Monterrey, Nuevo Leon that serves the population of Monterrey metropolitan area and surroundings states. The hospital has 500 beds, and on average has 22,000 hospitalization and 9000 deliveries annually. Several intra-institutional meetings between the laboratory and clinical (infectious diseases and gynecology-obstetrics) services lead to a jointly developed ZVD Preparedness Plan.
The ZVD Plan details the clinical and the epidemiologic risk assessment to be used for any pregnant woman presenting for care with any signal or symptom suggesting ZIKV infection (rash, headache, myalgia, arthralgia, pruritus, retroocular pain, conjunctivitis, or/and fever) and directs the clinicians to obtain blood and urine clinical samples for diagnostic testing.9 The ZVD Plan was presented at our hospital-wide grand rounds on September 13, 2016, and the surveillance of ZIKV infection in pregnant women began with no reports of until September 25. From Sunday evening September 25 to September 26, nine pregnant and unrelated women presented to our emergency room (ER) with any signal or symptom suggesting ZIKV infection. During the remainder of that week, and up to Saturday, November 25, an additional 212 women presented for care to the ER with any signal or symptom suggesting ZIKV infection. The median age of patients was 25.33 years (range 13–49).
In the initial 9-week period of simultaneous diagnostic testing for the three arboviruses (dengue virus (DENV), chikungunya virus (CHIKV), and ZIKV), we processed samples belonging to 221 patients (7 single serums, 3 single urines, and 422 paired serum and urine samples). Viral RNA was extracted from the serum samples by using the QIAamp Viral RNA Mini Kit (QIAGEN, Hilden, Germany). RNA was reverse transcribed by using the Superscript III Platinum OneStep Real Time RT-PCR system (Invitrogen, Carlsbad, CA, USA) and subjected to PCRs specific for DENV, CHIKV, and ZIKV using the CDC Trioplex Real-time RT-PCR Assay.10
Of the 221 pregnant patients tested by Trioplex RT-PCR, one patient was positive for CHIKV (and not for DENV or ZIKV); three patients were positive for DENV (and not for CHIKV or ZIKV) and 99 (44.8%) patients were positive for ZIKV. Symptoms in PCR-positive patients were rash (91.4%) followed by headache (53.1%), myalgia (46.9%), arthralgia (45.7%), pruritus (35.8%), retroocular pain (29.6%), conjunctivitis (21%), fever (21%), and diarrhea (2.5%). The gestational ages of the 221 pregnancies ranged from 4.5 to 41.5 weeks (8.3% in the first and 20.4% in the second trimester). All had a high-resolution ultrasonographic (HRUS) evaluation and no cranial abnormalities or other abnormal findings in the fetuses, or the placentas were detected in this baseline assessment. Two women with conditions deemed to be unrelated to ZVD (one at risk of preterm labor, one with a UTI) were hospitalized and resolved satisfactorily; all others were discharged home from the diagnostic ER visit.
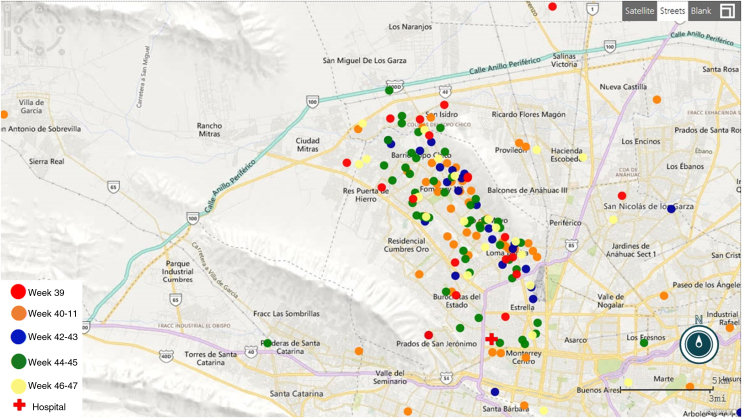
All the women are residents of the Monterrey metropolitan area (Fig. 1). None reported a travel history consistent with ZIKV transmission occurring somewhere besides their place of residency. Seventy RT-PCR positive patients provided information about local exposure: 37 (52.9%) had community contacts with similar febrile rash illness, 18 (25.7%) had both community and intradomiciliary ill contacts, and 4 (5.7%) had in-house contacts. Among the 118 RT-PCR-negative patients with probable ZVD, the most common symptom was rash (81.3%), and 78.4% reported community ill contacts.
Fig. 1.
Distribution of Zika cases through the four weeks in the Metropolitan area of Monterrey Nuevo Leon, Mexico.
We composed the ZVD Plan expecting to activate it gradually; but instead, we quickly faced an epidemic. Our ZVD Preparedness Plan further includes (a) monthly obstetric HRUS for possible congenital findings, (b) ZIKV RNA testing of babies and mothers, and (c) monitoring the children by the neuropediatric specialists. To date, six women have delivered a healthy live infant, with no evidence of neurological damage in newborns. Maternal and infant blood and urines all tested PCR-negative for ZIKV.
Our health facility is a teaching hospital in Monterrey, Nuevo Leon State, Mexico. Nuevo Leon is northern landlocked Mexico State, and our environmental conditions permit the existence of the Aedes vector that transmits Dengue, Chikungunya and Zika. The unpredicted swiftness of ZIKV spread that caused our Action Plan to be put into implementation mode at top speed, to respond to what appears to be a very large outbreak occurring which is still occurring in our city and among the population that uses our hospital for their primary health care needs, including prenatal and obstetric care. We want to document the lessons learned and challenges faced and share them with other institutions facing the same problem.
This report describes the rapid spread of an ongoing outbreak of ZVD affecting the pregnant women cared for at our hospital, and the involvedness of managing an institutional response to a health crisis.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We thank Mr. Gregorio Bustos for his technical assistance and to the U.S. Centers for Disease Control and Prevention for providing our laboratory with the Trioplex Real-time RT-PCR Assay.
This publication was made possible through support provided by the Bureau for Global Health, U.S. Agency for International Development, under the terms of an Interagency Agreement with the U.S. Centers for Disease Control (CDC). The opinions expressed herein are those of the author(s) and do not necessarily reflect the views of the U.S. Agency for International Development.
References
- 1.Cao-Lormeau V.M., Roche C., Teissier A., et al. Zika virus, French Polynesia, South pacific, 2013. Emerg Infect Dis. 2014;20:1085–1086. doi: 10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hennessey M., Fischer M., Staples J.E. Zika virus spreads to new areas – region of the Americas, May 2015–January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:55–58. doi: 10.15585/mmwr.mm6503e1. [DOI] [PubMed] [Google Scholar]
- 3.Driggers R.W., Ho C.Y., Korhonen E.M., et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med. 2016;374:2142–2151. doi: 10.1056/NEJMoa1601824. [DOI] [PubMed] [Google Scholar]
- 4.Nunes M.L., Carlini C.R., Marinowic D., et al. Microcephaly and Zika virus: a clinical and epidemiological analysis of the current outbreak in Brazil. J Pediatr (Rio J) 2016;92:230–240. doi: 10.1016/j.jped.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Besnard M., Lastere S., Teissier A., Cao-Lormeau V., Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014;19 [PubMed] [Google Scholar]
- 6.Jimenez Corona M.E., De la Garza Barroso A.L., Rodriguez Martínez J.C., et al. Clinical and epidemiological characterization of laboratory-confirmed autochthonous cases of Zika virus disease in Mexico. PLoS Curr. 2016:8. doi: 10.1371/currents.outbreaks.a2fe1b3d6d71e24ad2b5afe982824053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dirección General de Epidemiología, Secretaría de Salud. Sistema Único de Información. Boletín Epidemiológico. Número 47, vol. 32. Semana 47. Available from: http://www.epidemiologia.salud.gob.mx/doctos/boletin/2015/sem47.pdf.
- 8.Dirección General de Epidemiología, Secretaría de Salud. Sistema Único de Información. Available from: http://www.epidemiologia.salud.gob.mx/.
- 9.Madad S.S., Masci J., Cagliuso N.V., Allen M. Preparedness for Zika virus disease – New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1161–1165. doi: 10.15585/mmwr.mm6542a2. [DOI] [PubMed] [Google Scholar]
- 10.Trioplex Real-time RT-PCR Assay. Available from: http://www.fda.gov/downloads/MedicalDevices/Safety/EmergencySituations/UCM491592.pdf.