Abstract
Introduction
The epidemiology of Clostridium difficile infection (CDI) has changed in the last two decades. There is a lack of information regarding incidence and severity of CDI, especially in the developing world.
Methods
This was a retrospective and observational study from four hospitals of three Mexican cities. Patients were diagnosed with CDI when presented with loose stools and had at least one of the following tests positive: toxins assay, real-time PCR, or an endoscopic image compatible with pseudomembranous colitis. CDI was classified according to international guidelines. Demographic and clinical data as well as information regarding total hospital admissions, total length-of-hospital stay, and other variables related to hospitalization were gathered from the epidemiology and administration departments of each hospital.
Results
A total of 2050 hospital beds were analyzed with 288,171 patients hospitalized accumulating 1,576,446 days of hospitalization during the study period. The average rate of CDI per 1000 hospital-days was lower than the rates reported in the US and Europe, although in 2015 CDI rates were almost persistently above the mean rate for the study period. More than half of PCR positive patients were ribotype 027.
Conclusion
Hospital rates of CDI are increasing in Mexican hospitals with a predominance of infections caused by ribotype 027.
Keywords: Clostridium difficile, Hospital acquired infections, Hospital diarrhea, Infection rates
Introduction
The epidemiology of Clostridium difficile infection (CDI) has changed in the last two decades, with an increasing incidence and severity of the disease.1, 2 In the United States, the burden of CDI has been estimated to be over 450,000 cases per year3 and hospitalizations have increased by >200% in recent years.4
Incidence and infection rates have been mainly described in European countries,5 Australia,6 and in the United States.7 There is a lack of information about incidence and severity of CDI, especially in low and middle income countries around the globe.8, 9 Furthermore, some hypervirulent strains have been documented in patients from Latin America9 including Mexico,8, 10 although their clinical relevance is uncertain. The aim of this study was to describe CDI rates and patient's characteristics from four Mexican hospitals.
Methods
Design and participating healthcare facilities
This study was retrospective and observational. A total of four hospitals participated in the study: Hospital Univiersitario “Dr. José Eleuterio González” (HU) a public teaching hospital with 500 beds, located in Monterrey, Nuevo León (Northeastern Mexico); Hospital Civil de Guadalajara “Fray Antonio Alcalde” (HCG), which is a public teaching hospital of 1000 beds in Guadalajara, Jalisco (Western Mexico); Hospital General “Manuel Gea Gonzalez” (GEA) a public university hospital of 250 beds in Mexico City (Central Mexico). These hospitals provide medical care to a wide range of population, with maternity, pediatric, medical, and surgical wards. The fourth hospital was Instituto Nacional de Cancerología (INCAN) (Central Mexico), a referral cancer hospital for adult patients with 170 beds.
Diagnosis and classification of CDI
Patients included in the study were adults with a suspected diagnosis of CDI who reported ≥3 bowel movements with loose stools (Bristol 5–7) in the preceding 24 h. For CDI confirmation at least one of the following tests had to be positive: detection of toxins using the ImmunoCard toxins A&B assay (Meridian Bioscience, Cincinnati, OH, USA) in stools, real-time PCR (Cepheid Xpert C. difficile/Epi, Cepheid, Sunnyvale CA) in stools, or an endoscopic image consistent with pseudomembranous colitis. All hospitals used the same diagnostic methods with the exception of GEA and INCAN where no PCR-testing was performed. At the time of the study, none of the hospitals used glutamate deshidrogenase test (GDH).
CDI was classified as hospital-onset healthcare facility-associated CDI (HO-HCFA) when patients had been in-hospital for at least 48 h and were CDI free at admission, or as community-onset health care facility-associated (CO-HCFA) when patients were hospitalized for at least 48 h during the previous 12 weeks at CDI onset.11 Data on community-acquired CDI was very limited, with no cases included. Treatment failure was defined as persistence of diarrhea after five days of treatment.12
Patient's characteristics and CDI rates
The medical records of registered cases of CDI from January 1, 2011 to December 30, 2015 were reviewed. Information on demographic and clinical data such as age, gender, comorbidities, predisposing factors, length-of-hospital stay, colectomy, and 30-day hospital mortality among others were collected.
Information regarding hospital admissions at each center, total length-of-hospital stay and other hospital-related variables were collected from epidemiology and administration departments in each hospital.
Statistical analysis
Descriptive statistics were used to analyze the variables. For continuous variables with normal distribution, mean and standard deviation were calculated; for those variables with non-normal distribution, median and range were calculated. For all other variables, number and percentage, or range, was calculated as appropriate. Infection rates for 1000 patient-days and per 100 hospital admissions were calculated. Odds ratio was calculated using SPSS (version 20.0).
Ethics approval
The study protocol was reviewed and approved by the Hospital Civil de Guadalajara (number 034/16) and Instituto Nacional de Cancerología Institutional Board Review (number REV/41/16).
Results
Demographic and clinical characteristics of patients
A total of 487 patients who met CDI criteria (HU 168, HCG 267, GEA 30 and INCAN 22) were included, mean age 47.4 ± SD 19.6 years, and a slight predominance of males (Table 1). Almost half of the patients had between 4 and 6 bowel movements per day. Abdominal pain was reported by 52% of patients and 45.1% presented fever. Nearly half of the patients were treated initially with a combination of vancomycin and metronidazole (48.2%) and 35.3% with metronidazole alone. The average length of treatment was 9.7 days, and the mean time between initiation of treatment and diarrhea disappearance was three days; for fever remission the average time was 1.3 days. The overall failure rate was 19.3%, with a 30-day mortality rate of 18.1%. Attributable mortality was estimated at 9.0% (Table 1).
Table 1.
Characteristics of the 487 patients included and their outcome charts.
| Characteristic | Average ± SD (%) |
|---|---|
| Demographic and clinical data | |
| Age | 47.47 ± 19.68 |
| Gender | |
| Male | 292 (60.0) |
| Female | 195 (40.0) |
| Fever | 220 (45.1) |
| Abdominal pain | 253 (52.2) |
| Leucocytes, k/ml | 15.11 ± 12.14 |
| Albumin | 2.17 ± 0.71 |
| Creatinine | 2.10 ± 3.16 |
| Bowel movements per daya | |
| ≤3 | 84 (26.4) |
| 4–6 | 158 (49.7) |
| ≥7 | 76 (23.9) |
| ATLAS score | 4.91 + 1.71 |
| 0–3 points | 41 (18.7) |
| 4–7 points | 160 (73.4) |
| 8–10 points | 17 (7.8) |
| Primary diagnosis | |
| Medical | 70.0% |
| Surgical | 30.0% |
| Comorbidities and predisposing risk factors | |
| Total LOS LOS before diagnosis LOS after diagnosis |
20.19 ± 21.37 13.66 ± 14.96 15.25 ± 15.65 |
| Previous ICU stay | 68 (13.9) |
| Immunosuppression | |
| Invasive mechanical ventilation | 40 (8.2) |
| Proton pump inhibitors | 373 (76.5) |
| H2 blockers | 40 (8.2) |
| Diagnosis of CDI | |
| Immunoassay for toxins A/B | 192 (39.4) |
| PCR | 284 (58.3) |
| C. difficile ribotype 027 | 145/284 (51.1) |
| C. difficile non-027 ribotype | 139/284 (48.9) |
| Endoscopy (pseudomembranous colitis) | 43 (8.8) |
| Initial treatment of CDI | |
| Metronidazole | 77 (35.3) |
| Vancomycin | 34 (15.7) |
| Vancomycin + Metronidazole | 105 (48.2%) |
| Fecal microbiota transplant | 8 (3.7%) |
| Rifaximin | 1 (0.5) |
| Colectomy | 1 (0.5) |
| Treatment duration (days) | 9.76 ± 4.74 |
| Treatment failurea | 42/218 (19.3) |
| 30-day mortality | 88 (18.1) |
| Attributable mortality | 44 (9.0) |
Clinical information was not available for all of the patients.
CDI infection
From the 487 patients included, 200 were tested by immunoassay and 192 patients turned out positive (96%); PCR was performed in 311 and 284 (88.5%) tested positive. Out of those positive by real time PCR, 145/284 (51.1%) were also positive for C. difficile ribotype 027 (detected in the same test) and 139/284 were negative for 027 thus classified as non-027 ribotype (Table 1). There were no statistical differences regarding treatment failure, 30-day mortality, and colectomy between patients with 027 or non-027 ribotype (Table 2).
Table 2.
Comparison of outcomes among patients with ribotype 027 and non-027 ribotype.
| Characteristics | Ribotype |
OR (95% CI) | p | |
|---|---|---|---|---|
| 027 (N = 145) n (%) |
Non-027 (N = 139) n (%) |
|||
| Treatment failure | 3 (2.07) | 1 (0.72) | 2.91 (0.29–28.37) | 0.356 |
| 30-Day mortality | 22 (15) | 22 (16) | 0.95 (0.50–1.80) | 0.878 |
| Attributable mortality | 11 (7.59) | 7 (5.04) | 1.54 (0.58–4.11) | 0.381 |
| Colectomy | 2 (1.38) | 0 (0) | 4.86 (0.23–102.15) | 0.308 |
Out of the 487 cases of CDI included, 43 (8.8%) were diagnosed in 2012, 22 (4.5%) in 2013, with a sharp increase in 2014 with 121 (24.8%) cases, and 301 (61.8%) in 2015. The mean hospital length-of-stay was 20.1 ± 21.3 (SD) days, with an average 13.6 ± 14.9 (SD) days prior to CDI diagnosis. After CDI diagnosis patients had an extra hospital stay of 15.2 ± 15.6 (SD) days (Table 1).
Hospital CDI rates
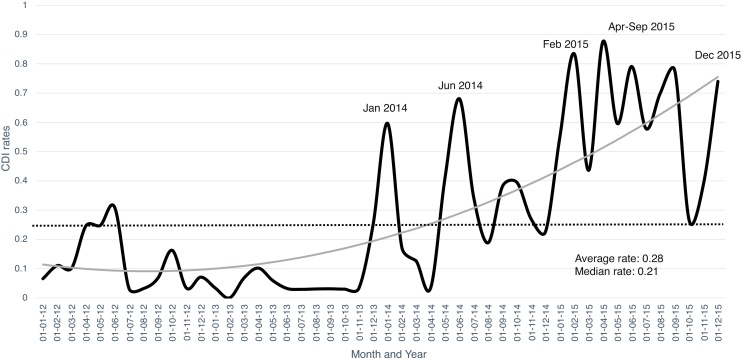
A combined number of 2050 hospital beds were analyzed, with a total of 288,171 patients hospitalized accumulating 1,576,446 days of hospital stay during the study period. The average rate of CDI per 1000 hospital-days was 0.28, and 0.15 cases per 100 hospital admissions. Throughout 2015, CDI rates were almost persistently above the mean rate for the study period (Fig. 1) with a remarkable increasing trend. The lowest rates of CDI were recorded in 2013 with an average of 0.003 cases per 1000 hospital-days compared to 0.34 in 2015. February and April 2015 had the highest CDI rates per 100 admissions, namely 0.83 and 0.87 cases, respectively.
Fig. 1.
Combined CDI rates per 1000 hospital-days during the study period. The dotted line represents the average CDI rate during the study period, the gray line represents the tendency.
Discussion
CDI is an increasing public health problem in many regions of the world.13 Information on CDI hospital rates in middle and low-income countries in Latin-America, Asia, and Eastern Europe is limited. Data on microbiology, infection rates, and clinical characteristicsis are needed in order to better understand local and regional behavior of these infections. Implementation of infection control policies in different settings is also necessary.
In this study, the average rate of CDI was 0.28 per 1000 patient-days. These rates are lower than the 0.6 per 1000 patient-days those reported by Durkins14 et al., in a study that included 29 hospitals, or the 0.6, 0.8, and 0.9 cases per 1000 patient-days reported in Poland from 2011 through 2013, respectively.14, 15 The reported rates in this series are also lower than the rates observed in a large European study in 2012–20135 with an average rate of 6.9 cases per 1000 patient bed-days. It is important to mention that diagnostic methods used in both studies were different from those used in our study.
Considering that the CDI rate in 2015 was 0.6 per 1000 hospital-days, and that the current survey showed an increasing trend in the rate of infections, we can speculate that rates are now similar to the studies mentioned above. The 7-fold increase in 2015 rates compared to the initial period of the study was observed in all four participating hospitals.
Murad et al. demonstrated that the use of PCR for detection of CDI is associated to an overestimation of cases, especially during outbreaks.16 We consider that this is not the case in our findings, because PCR test was used only in symptomatic patients with more than three loose stools, and at least in one hospital (INCAN) PCR was not available during the entire study period.
For CDI cases, length-of-stay (LOS) was similar in the four participating Institutions. It was not different from those reported in Madrid, but it was lower than the LOS reported in Barcelona and Rome between 2011 and 2013.16, 17 The LOS is of particular importance in busy, overcrowded, public hospitals.18 The duration of hospital stay was longer after diagnosis than before CDI diagnosis, with no difference in the total LOS in the participating hospitals despite an increase in the number of CDI cases diagnosed during the study. The latter contrasts with the findings in the study by Miller et al.,19 where the total LOS was increased in hospitals with a higher rate of CDI and even patients without CDI had longer LOS in hospitals with higher CDI rates; in our study the average global hospital LOS was 5.7 days at the beginning of the study and 5.4 days in 2015.
It should be pointed out that more than half of the CDI identified by PCR were ribotype 027. This percentage is almost 3-fold higher than the average 18% reported by Davies5 et al. In that study, Germany was the country with the highest rate for ribotype 027 (43%).5 This data may suggest a higher severity of CDI. The relevant clinical outcomes of CDI in our study such as treatment failure or attributable mortality were similar to other reports.17 In addition, in the current study there were no differences between patients infected by ribotype 027 compared to non-027 strains regarding treatment failure, colectomy, and mortality.
The increasing prevalence of ribotype 027 of C. difficile in Mexico has been highlighed in previous reports from two of the participating hospitals.8, 10
Our study has some limitations such as the lack of standardized diagnostic algorithms, with diagnosis based on the positive result of either, one of two laboratory tests, and to a lesser extend to endoscopic findings. The absence of a standardized protocol leaves room for false negative reports, particularly when immunoassay was the only test used. Although the four Institutions included perform regular surveillance and have protocols for identification of CDI patients, variations in reporting between Institutions may also exist. Another limitation is that not all cases could be differentiated by a molecular method lacking information about the possible ribotypes diversity in these hospitals. No information could be gathered regarding treatment with fidaxomicin since at present time its distribution is not approved in Mexico.
Despite the above limitations, we consider that this report demonstrates the increasing trend of CDI in a middle-income country in Latin America, and highlights the emergent CDI problem in different types of public and academic hospitals in our country. Reporting CDI cases and rates occurring in Mexican hospitals is not mandatory, a phenomenon frequently seen in other countries. Therefore, we recommend mandatory notification of CDI cases to be implemented.
To the best of our knowledge, this is the first multi-Institutional report on CDI rates in Mexico and Latin-America.
Conclusion
Hospital rates of CDI are increasing in the Mexican hospitals analyzed in this study. In recent years CDI rates have been similar to those reported in Europe and in the United States with a predominance of infections caused by ribotype 027.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Khanna S., Pardi D.S. The growing incidence and severity of Clostridium difficile infection in inpatient and outpatient settings. Expert Rev Gastroenterol Hepatol. 2010;4:409–416. doi: 10.1586/egh.10.48. [DOI] [PubMed] [Google Scholar]
- 2.Song X., Bartlett J.G., Speck K., Naegeli A., Carroll K., Perl T.M. Rising economic impact of Clostridium difficile-associated disease in adult hospitalized patient population. Infect Control Hosp Epidemiol. 2008;29:823–828. doi: 10.1086/588756. [DOI] [PubMed] [Google Scholar]
- 3.Lessa F.C., Mu Y., Bamberg W.M., et al. Burden of Clostridium difficile infection in the United States. N Engl J Med. 2015;372:825–834. doi: 10.1056/NEJMoa1408913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peery A.F., Dellon E.S., Lund J., et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology. 2012;143:1179–1187. doi: 10.1053/j.gastro.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davies K.A., Longshaw C.M., Davis G.L., et al. Underdiagnosis of Clostridium difficile across Europe: the European, multicentre, prospective, biannual, point-prevalence study of Clostridium difficile infection in hospitalized patients with diarrhoea (EUCLID) Lancet Infect Dis. 2014;14:1208–1219. doi: 10.1016/S1473-3099(14)70991-0. [DOI] [PubMed] [Google Scholar]
- 6.Worth L.J., Spelman T., Bull A.L., Brett J.A., Richards M.J. Epidemiology of Clostridium difficile infections in Australia: enhanced surveillance to evaluate time trends and severity of illness in Victoria, 2010–2014. J Hosp Infect. 2016;93:280–285. doi: 10.1016/j.jhin.2016.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Davis B.M., Yin J., Blomberg D., Fung I.C. Impact of a prevention bundle on Clostridium difficile infection rates in a hospital in the Southeastern United States. Am J Infect Control. 2016 doi: 10.1016/j.ajic.2016.05.014. S0196-6553; 30577-6. [DOI] [PubMed] [Google Scholar]
- 8.Lopardo G., Morfin-Otero R., Moran-Vazquez I.I., et al. Epidemiology of Clostridium difficile: a hospital-based descriptive study in Argentina and Mexico. Braz J Infect Dis. 2015;19:8–14. doi: 10.1016/j.bjid.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López-Ureña D., Quesada-Gómez C., Miranda E., Fonseca M., Rodríguez-Cavallini E. Spread of epidemic Clostridium difficile NAP1/027 in Latin America: case reports in Panama. J Med Microbiol. 2014;63:322–324. doi: 10.1099/jmm.0.066399-0. [DOI] [PubMed] [Google Scholar]
- 10.Camacho-Ortiz A., López-Barrera D., Hernández-García R., et al. First report of Clostridium difficile NAP1/027 in a Mexican hospital. PLoS One. 2015;27:e0122627. doi: 10.1371/journal.pone.0122627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen S.H., Gerding D.N., Johnson S., et al. Society for Healthcare Epidemiology of America; Infectious Diseases Society of America. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA) Infect Control Hosp Epidemiol. 2010;31:431–455. doi: 10.1086/651706. [DOI] [PubMed] [Google Scholar]
- 12.Surawicz C.M., Brandt L.J., Binion D.G., et al. Guidelines for diagnosis, treatment and prevention of Clostridium difficile infections. Am J Gastroenterol. 2013;18:478–498. doi: 10.1038/ajg.2013.4. [DOI] [PubMed] [Google Scholar]
- 13.Freeman J., Bauer M.P., Baines S.D., et al. The changing epidemiology of Clostridium difficile infections. Clin Microbiol Rev. 2010;2:529–549. doi: 10.1128/CMR.00082-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durkin M.J., Baker A.W., Dicks K.V., et al. A comparison between National Healthcare Safety Network laboratory-identified event reporting versus traditional surveillance for Clostridium difficile infection. Infect Control Hosp Epidemiol. 2015;36:125–131. doi: 10.1017/ice.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pituch H., Obuch-Woszczatyński P., Lachowicz D., et al. Hospital-based Clostridium difficile infection surveillance reveals high proportions of PCR ribotypes 027 and 176 in different areas of Poland, 2011 to 2013. Euro Surveill. 2015;20 doi: 10.2807/1560-7917.ES.2015.20.38.30025. [DOI] [PubMed] [Google Scholar]
- 16.Murad Y.M., Perez J., Nokhbeh R., et al. Impact of polymerase chain reaction testing on Clostridium difficile infection rates in an acute health care facility. Am J Infect Control. 2015;43:383–386. doi: 10.1016/j.ajic.2014.12.021. [DOI] [PubMed] [Google Scholar]
- 17.Asensio A., Di Bella S., Lo Vecchio A., et al. The impact of Clostridium difficile infection on resource use and costs in hospitals in Spain and Italy: a matched cohort study. Int J Infect Dis. 2015;36:31–38. doi: 10.1016/j.ijid.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Magee G., Strauss M.E., Thomas S.M., Brown H., Baumer D., Broderick K.C. Impact of Clostridium difficile-associated diarrhea on acute care length of stay, hospital costs, and readmission: a multicenter retrospective study of inpatients, 2009–2011. Am J Infect Control. 2015;43:1148–1153. doi: 10.1016/j.ajic.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Miller A.C., Polgreen L.A., Cavanaugh J.E., Polgreen P.M. Hospital Clostridium difficile infection rates and prediction of length of stay in patients without C. difficile infection. Infect Control Hosp Epidemiol. 2016;37:404–410. doi: 10.1017/ice.2015.340. [DOI] [PMC free article] [PubMed] [Google Scholar]