Abstract
The replicator (rep) of the nopaline-type Ti plasmid pTiC58 is located adjacent to the trb operon of this conjugal element. Previous genetic studies of this region (D. R. Gallie, M. Hagiya, and C. I. Kado, J. Bacteriol. 161:1034–1041, 1985) identified functions involved in partitioning, origin of replication and incompatibility, and copy number control. In this study, we determined the nucleotide sequence of a 6,146-bp segment that encompasses the rep locus of pTiC58. The region contained four full open reading frames (ORFs) and one partial ORF. The first three ORFs, oriented divergently from the traI-trb operon, are closely related to the repA, repB, and repC genes of the octopine-type Ti plasmid pTiB6S3 as well as to other repA, -B, and -C genes from the Ri plasmid pRiA4b and three large plasmids from Rhizobium spp. The fourth ORF and the partial ORF are similar to y4CG and y4CF, respectively, of the Sym plasmid pNGR234a. The 363-bp intergenic region between traI and repA contained two copies of the tra box which is the cis promoter recognition site for TraR, the quorum-sensing activator of Ti plasmid conjugal transfer. Expression of the traI-trb operon from the tra box II-associated promoter mediated by TraR and its acyl-homoserine lactone ligand, AAI, was negatively influenced by an intact tra box III. On the other hand, the region containing the two tra boxes was required for maximal expression of repA, and this expression was enhanced slightly by TraR and AAI. Copy number of a minimal rep plasmid increased five- to sevenfold in strains expressing traR but only when AAI also was provided. Consistent with this effect, constitutive expression of the quorum-sensing system resulted in an apparent increase in Ti plasmid copy number. We conclude that Ti plasmid copy number is influenced by the quorum-sensing system, suggesting a connection between conjugal transfer and vegetative replication of these virulence elements.
The Ti and Ri plasmids of Agrobacterium spp. are primary pathogenicity determinants and are responsible for crown gall or hairy root diseases caused by these bacteria. These plasmids are large (≥200 kb), unit copy, and stably maintained in the bacterial cells. Molecular and genetic studies of these plasmids have led to an understanding of functions associated with tumorigenicity, opine catabolism, and conjugal transfer (reviewed in references 12, 14, and 40). However, other functions, most notably the vegetative replication of these plasmids, have been studied in considerably less detail. The rep region of pTiB6S3, a classical octopine-mannityl opine-type Ti plasmid, has been mapped (25, 26), and the DNA sequence has been determined (47). Similarly, the rep region of pRiA4b, the Ri plasmid from Agrobacterium rhizogenes A4, and the rep region of an otherwise undescribed Ti plasmid, pTi-SAKURA, have been sequenced (34, 35, 46).
Recent studies of several plasmids from Rhizobium spp. have spurred interest in the replication regions of these large plasmids. These plasmids include pNGR234a, the Sym plasmid from Rhizobium sp. strain NGR234 (15), pRL8JI, a cryptic plasmid from Rhizobium leguminosarum 3841 (50), and p42d, the Sym plasmid from Rhizobium etli CFN42 (39). Comparative sequence analysis indicates that the rep genes and their products, and the organization of the genes on these plasmids, are similar and are related to those of pTiB6S3, pRiA4b, and pTi-SAKURA. These replication systems all contain three genes, repA, repB, and repC, organized in tandem. Surveys based on the presence of a repC homologue have indicated that this replicator may exist in many other large rhizobial plasmids (6, 41, 49). Furthermore, in addition to these agrobacterial and rhizobial plasmids, the second rep region from pTAV1, a large cryptic plasmid from the unrelated soil bacterium Paracoccus versutus UW1, also belongs to this group (3).
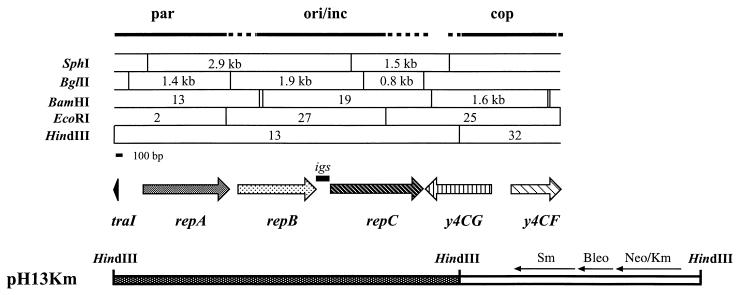
The rep region of pTiC58 is located near the 2 o'clock position on the plasmid and is adjacent to the trb operon, the locus responsible for the mating-pair formation (Mpf) functions of the Ti plasmid conjugal transfer system (17, 27, 28). In our sequence analysis of the Mpf region of pTiC58 we identified the 5′ end of an open reading frame (ORF) that is oriented divergently from traI, the first gene of the trb operon (27). This ORF, which is separated from traI by a 363-bp intergenic region, is almost identical to the repA genes of pTi-SAKURA and the octopine-type Ti plasmid pTiB6S3. Furthermore, the position of this ORF coincides with the par locus of pTiC58 genetically defined by Gallie et al. (17) (Fig. 1). These findings, coupled with the facts that the nopaline- and octopine-type Ti plasmids are incompatible (19) and that the rep loci of these two Ti plasmids belong to the same heteroduplex region (13), led us to predict that the rep region of pTiC58 belongs to the repABC-type replicator family.
FIG. 1.
Physicogenetic map of the rep region of pTiC58 and structure of pH13Km. The phenotype designations par, ori/inc, and cop are according to Gallie et al. (17). The restriction map and gene designations are based on nucleotide sequence analysis as described in the text.
In this study, we determined the nucleotide sequence of the region responsible for vegetative replication of pTiC58. Comparison of the sequence with the genetic map described by Gallie et al. (17) allowed us to assign functions to some of the genes and also to potential cis-acting regions. We also show that the conserved cis-acting tra box elements residing in the intergenic region between traI and repA can affect expression of the traI-trb operon as well as repA. Furthermore, we provide evidence for the coregulation of conjugal transfer and the copy number of the Ti plasmid by the TraR-dependent quorum-sensing regulatory system.
MATERIALS AND METHODS
Bacterial strains, growth conditions, and plasmids.
The Agrobacterium tumefaciens and Escherichia coli strains and the plasmids used in this study are listed in Table 1. A. tumefaciens strains were grown at 28°C in L broth (LB) (42), in ABM minimal medium (9), or on nutrient agar plates (Difco Laboratories, Detroit, Mich.). E. coli strains were grown at 37°C in LB or on L agar plates. Antibiotics were added at the following concentrations when required: for Agrobacterium, carbenicillin, 100 or 200 μg/ml; kanamycin, 100 μg/ml; and tetracycline, 2 μg/ml; for E. coli, ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; and tetracycline, 10 μg/ml. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Gibco-BRL, Gaithersburg, Md.) was included in media at 40 μg/ml to assay for the production of β-galactosidase.
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Relevant genotype, phenotype, or characteristic(s)a | Reference or source |
|---|---|---|
| Escherichia coli | ||
| DH5α | supE44 ΔlacU169(φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 42 |
| S17-1 | pro (r− m+) Mob+ Tpr Smr | 44 |
| Agrobacterium tumefaciens | ||
| C58 | Wild-type pathogenic strain carrying pTiC58 | Our collection |
| NT1 | Ti plasmid-cured C58 | 11 |
| NTL4 | NT1 derivative with tetA tetR mutation | 29 |
| Plasmids | ||
| pBluescript SK(±) | Cloning vector; Apr | Stratagene |
| pCF1 | 3,680-bp BamHI fragment 13 encoding traI, trbB, and repA from pTiC58 cloned in pTZ18U; Apr | 21 |
| pCMA1 | pTiC58traM::nptII; Kmr | 20 |
| pH13Km | 4,906-bp HindIII fragment containing the rep region of pTiC58 ligated with 3,484-bp HindIII fragment from Tn5; Smr Bmr Kmr | This study |
| pJB3 | Broad-host-range IncP cloning vector; Apr | 5 |
| pKP19 | traI::lacZY including 161 bp of the traI promoter region cloned into pLAFR6 | 21 |
| pLKC482 | pUC8-derived vectors for constructing translational fusion with lacZ; Apr Kmr | 48 |
| pPLH13 | 4,906-bp HindIII fragment containing the rep region of pTiC58 cloned in pBluescript SK(+); Apr | This study |
| pPLrep | 6.3-kb HindIII-HindIII fragment containing lacZY genes from pLKC482 cloned into SphI site in pCF1; Kmr Apr | This study |
| pPLrep4 | 6.7-kb PstI-PstI fragment from pPLrep cloned into pJB3; Apr | This study |
| pPLrep5 | 6.7-kb HindIII-HindIII fragment from pPLrep cloned into pJB3; Apr | This study |
| pPLrep6 | 5.6-kb BglII-BglII fragment from pPLrep cloned into pJB3; Apr | This study |
| pRK415 | Broad-host-range IncP cloning vector; Tcr | 24 |
| pRKE33 | 1.8-kb EcoRI fragment encoding traR from pTiC58 cloned into pRK415; Tcr | 36 |
| pSVB33 | 1.8-kb EcoRI fragment encoding traR from pTiC58 cloned into pSa152; Kmr Gmr Smr | 36 |
| pTB12 | traI::lacZY including 167 bp of the traI promoter region cloned in pLAFR6 | This study |
| pTiC58ΔaccR | A Trac mutant of pTiC58 | 4 |
Abbreviations: Smr, streptomycin resistance; Tcr, tetracycline resistance; Cbr, carbenicillin resistance; Kmr, kanamycin resistance; Apr, ampicillin resistance; Gmr, gentamicin resistance; Spr, spectinomycin resistance; Tpr, trimethoprim resistance; Bmr, bleomycin resistance.
DNA manipulation and strain constructions.
Plasmids in E. coli and A. tumefaciens were isolated by an alkaline lysis method (42, 45). Preparations for plasmid copy number assessment were conducted as follows. A. tumefaciens strains containing the plasmid being tested were inoculated into 2 ml of LB without antibiotics and incubated overnight at 28°C with aeration. The cultures were used to reinoculate fresh 2-ml volumes of LB, and the optical density at 600 nm (OD600) of each culture was adjusted to 0.1. The cultures were incubated to late exponential phase (OD600 ∼ 1), and cells from a 1-ml volume were harvested by centrifugation for plasmid preparation. The viable titer of each culture was determined at the time of harvest to ensure that all samples contained roughly the same number of cells (ca. 109 cells). The cells were washed with a 1-ml volume of Agrowash (18), and plasmids were extracted by a modified alkaline lysis method as described previously (18). Following lysis, all extractions, mixings, and other manipulations were performed as gently as possible to minimize shearing.
Standard recombinant DNA techniques were used as described by Sambrook et al. (42). Digestions with restriction endonucleases were conducted according to the manufacturers' instructions. Plasmids were introduced into E. coli by using standard techniques as described previously (42). IncP-based plasmids were introduced into A. tumefaciens strains via E. coli S17-1-mediated biparental mating (10). Other plasmids, including pH13Km, were introduced into A. tumefaciens strains by electroporation (7).
Agarose gel analysis.
Immediately after preparation, closed circular Ti plasmid DNA and HindIII-digested pH13Km were electrophoresed in 0.8% (wt/vol) agarose gels at 4 V/cm for 3 to 6 h, using Tris-borate-EDTA buffer. To compare copy numbers among the Ti plasmids or among samples containing pH13Km, relative intensities of the appropriate bands on the agarose gel were analyzed by densitometry as follows. After electrophoresis, the agarose gel was stained in a solution containing ethidium bromide (5 μg/ml) for 30 min. The stained gel was rinsed with distilled water and exposed to UV light, and the gel image was captured and digitized with a charge-coupled device camera (Fotodyne, Heartland, Wis.). The bands on the digitized gel image were analyzed using the Gel Plot macro of the public domain NIH Image program (version 1.62; National Institutes of Health, Bethesda, Md. [http://rsb.info.nih.gov/nih-image/]) on a Macintosh computer.
DNA sequence analysis and bioinformatics.
DNA fragments were sequenced on both strands by the dideoxy method as described previously (27). Nucleotide sequences were assembled and analyzed, and ORFs were identified and translated by using the DNA Strider program (30) and the Map program of the Genetics Computer Group (GCG) software package (version 10; GCG, Madison, Wis.). Nucleotide and deduced amino acid sequences were compared to those in the databases by using the BLAST2 search protocol (2). PileUp, Gap, and BestFit programs of the GCG package were used to compare sequences and to identify regions conserved among several nucleotide or protein sequences. Unrooted phylogenetic trees of RepC and the repBC intergenic sequence (igs) were constructed by using the maximum parsimony algorithm with bootstrap method by the PAUPSearch program of the GCG package.
β-Galactosidase assay.
β-Galactosidase activity was determined qualitatively on ABM agar medium containing 40 μg of X-Gal per ml. Quantitative assays were conducted as described previously (20). Each sample was assayed in duplicate, and each experiment was repeated at least twice. Levels of activity were expressed as units of β-galactosidase per 108 or 109 CFU.
Nucleotide sequence accession numbers.
The nucleotide sequence of the entire rep region of pTiC58 was deposited in the GenBank database under accession no. AF060155. The accession numbers of rep genes from other plasmids are listed in Table 2.
TABLE 2.
Relatedness among the predicted gene products of the Ti plasmid rep region and those of the other repABC-type replicatorsa
| Gene of pTiC58 | Relatedness (% similarity/% identity) to rep gene product of:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| pTi-SAKURA (AB006875b) | pTiB6S3 (M24529) | pRiA4b (X04833) | pNGR234a (U00090) | p42d (U80928) | pRL8JI (X89447) | pTAV1 (U60522) | pRmeGR4a (X69105) | |
| repA | 100/100 | 97/96 | 65/55 | 63/52 | 64/56 | 67/59 | 64/55 | |
| repB | 99/99 | 84/79 | 48/36 | 49/41 | 46/36 | 48/42 | 41/34 | |
| repC | 99/99 | 80/74 | 57/47 | 57/46 | 53/43 | 55/47 | 48/38 | 37/29 |
| y4CG | 71/63 | |||||||
| y4CFc | 52/44 | |||||||
pRmeGR4a from Sinorhizobium meliloti GR4 contains a repC homologue but not repA or repB (31).
Accession number for the sequence of the rep region.
Incomplete ORF.
RESULTS
DNA sequence analysis of the rep region of pTiC58.
The nucleotide sequences of both strands of the entire rep region of pTiC58 as defined by Gallie et al. (17), including BamHI fragment 13 and EcoRI fragments 27 and 25 (Fig. 1), were determined. This yielded 6,146 bp of new sequence, continuing from the BglII site located 155 bp upstream of traI (27) to the EcoRI site at the right end of EcoRI fragment 25 (Fig. 1). The nucleotide sequence is 99.7% identical to that of a corresponding region of pTi-SAKURA (46) and 78% identical to that of the octopine-type Ti plasmid pTiB6S3 (47). The sequenced region contains four significant ORFs and one partial ORF. The first three ORFs, which are oriented opposite to those of the traI-trb operon or clockwise on the Ti plasmid, are very similar at both nucleotide and deduced amino acid sequence levels to the replication genes repA, repB, and repC, respectively, from pTi-SAKURA and pTiB6S3 (Table 2). On this basis, we designated these three ORFs repA, repB, and repC. These three genes also are similar to replicator genes from several other plasmids from the family Rhizobiaceae, including pRiA4b from A. rhizogenes A4 (35), pNGR234a, the Sym plasmid from Rhizobium sp. NGR234 (15), pRL8JI, a cryptic plasmid from R. leguminosarum 3841 (50), and another Sym plasmid, p42d from R. etli CFN42 (39). The three genes also are related to three genes found in the second replicator region from pTAV1, a large cryptic plasmid from P. versutus UW1 (3). The fourth ORF, which is oriented opposite to repABC (Fig. 1), showed similarities to the y4CG gene from pNGR234a and to genes coding for DNA invertases or resolvases from other bacteria, phages, or transposons (Table 2). The partial ORF (Fig. 1) showed similarity to y4CF (Table 2), a gene of unknown function also from pNGR234a, but to no other sequences in the databanks. Whereas repC is separated from y4CG by 8 bp in pTiC58, these two genes are separated by about 3 kb in the corresponding region of pNGR234a (15).
Influence of the traI-repA intergenic region on the expression of traI.
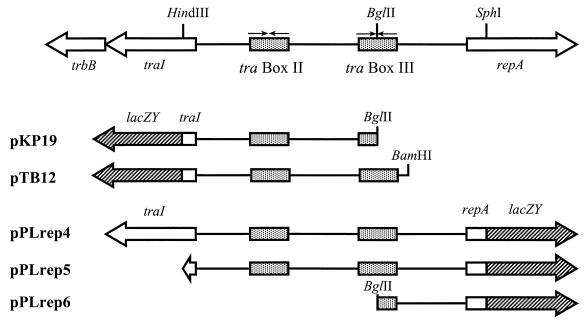
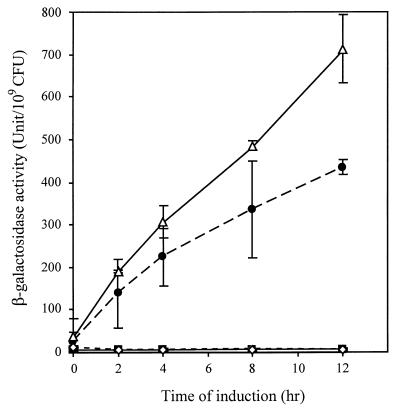
Transcription of the traI-trb operon is activated by TraR in a quorum-dependent manner (21). The 363-bp intergenic sequence between traI and repA, which presumably constitutes a divergent promoter system, contains two copies of an 18-bp inverted repeat sequence called the tra box (Fig. 2). This element constitutes the cis-acting site recognized by TraR and its coinducer, Agrobacterium autoinducer [AAI; N-(3-oxooctanoyl)-l-homoserine lactone (HSL)] (16, 29, 54). Previously we showed that pKP19, which contains the upstream promoter region of traI and a lacZ gene fused to traI (Fig. 2), expresses β-galactosidase activity but only in the presence of TraR and AAI (21). In this construct, the BglII site, which is located upstream of the promoter region, is situated in the middle of the distal tra box (tra box III [Fig. 2]). As a result, pKP19 contains the intact tra box II but only half of tra box III. Using PCR, we modified the 5′ end of this region to reconstitute the intact tra box III. The resulting plasmid, pTB12, is identical to pKP19 except that it contains both copies of the tra box (Fig. 2). Strains harboring each plasmid were tested for expression of the reporter. Similar to the case for pKP19, expression of the traI::lacZ fusion in pTB12 requires both TraR and AAI (Fig. 3 and data not shown). However, the traI::lacZ fusion in pKP19 was induced with significantly faster kinetics than the same reporter in pTB12 (Fig. 3). Thus, the presence of an intact tra box III down-modulates the rate of induction of traI from the tra box II-associated promoter mediated by TraR and AAI.
FIG. 2.
Structure of the traI-repA intergenic region and of the traI and repA expression clones. Shaded boxes indicate the extents and locations of the conserved tra box elements. Hatched arrows represent the lacZY genes from pLKC482. The vector for pKP19 and pTB12 is pLAFR6; the vector for pPLrep4, -5, and -6 is pJB3. The gene sizes of trbB, traI, repA, and lacZY are not drawn to scale.
FIG. 3.
Influence of tra boxes II and III on expression of traI. Overnight cultures of the test strains were diluted into and incubated in fresh ABM medium supplemented with synthetic AAI (40 nM). Samples were removed at the indicated times and assayed for β-galactosidase activity as described in Materials and Methods. The experiment was repeated twice. Bars show the standard deviation for each data point. ▵, NT1(pKP19, pSVB33); ●, NT1(pTB12, pSVB33); ◊, NT1(pKP19); ■, NT1(pTB12).
Influence of TraR-AAI on the expression of rep.
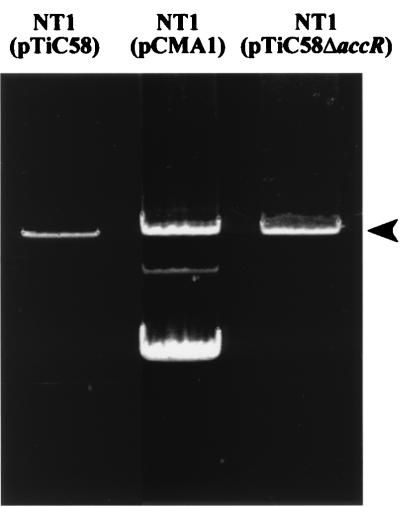
In our studies, we consistently have observed increased amounts of Ti plasmid DNA isolatable from strains harboring the transfer-constitutive (Trac) mutant pTiC58ΔaccR compared to those containing its wild-type transfer-inducible parent, pTiC58. pTiC58ΔaccR contains a deletion in accR which codes for a repressor responsible for the regulation of expression of traR (4, 37, 38). Strains harboring this mutant plasmid express traR constitutively, produce large amounts of AAI, and transfer the Ti plasmid in the absence of induction by the conjugal opine. We examined this anecdotal observation more closely by comparing the amount of Ti plasmid DNA recoverable from strains harboring pTiC58ΔaccR and pTiC58. Following electrophoresis of plasmid DNA prepared from similar numbers of cells as described in Materials and Methods, the band intensity of pTiC58ΔaccR was three- to fivefold greater than that of the wild-type parent plasmid (Fig. 4). While the increase in plasmid yield could be due to other reasons, we suspected that constitutive production of TraR and AAI in some way affects the copy number of this Ti plasmid. We also examined another Trac mutant of pTiC58, pCMA1, which is wild type for accR but contains a deletion in the antiactivator, traM (20). This Ti plasmid expresses traR at its repressed level, but the activator is not inhibited by TraM (20). Like that of pTiC58ΔaccR, the band corresponding to pCMA1 was six- to sevenfold more intense than that of the wild-type parent plasmid (Fig. 4). These observations, along with the negative effect of an intact tra box III on the expression of the divergently expressed traI-trb operon, prompted us to explore the possibility that TraR, together with its signal ligand, influences vegetative replication of the Ti plasmid.
FIG. 4.
Electrophoretic analysis of wild-type pTiC58 and its Trac mutants. Cells were grown and plasmids were isolated and subjected to electrophoresis in agarose gels as described in Materials and Methods. Lanes contain equal loading volumes of plasmid DNA isolated from 9.0 × 108 CFU of NT1(pTiC58), 8.5 × 108 CFU of NT1(pCMA1), and 1.4 × 109 CFU of NT1(pTiC58ΔaacR). The arrowhead indicates the position of the Ti plasmid. The bands below the Ti plasmid in NT1(pCMA1) correspond to the open circular and closed circular forms of pPH1JI present in this strain (20). The experiment was repeated four times with indistinguishable results; in all repetitions, the intensities of the bands corresponding to pTiC58ΔaccR and pCMA1 always were brighter than those of pTiC58.
We tested the influence of TraR and AAI, as well as the intergenic region between traI and the replication region, on the expression of repA by constructing three clones containing the lacZ gene translationally fused to the 5′ end of repA (Fig. 2). pPLrep4, which contains the entire traI gene and an intact traI-repA intergenic region, confers production of AAI but only in cells expressing TraR. pPLrep5 contains the entire traI-repA intergenic region and the first 17 codons of traI and does not confer production of the acyl-HSL. The shortest clone, pPLrep6, contains the upstream region of repA but extends only to the BglII site at the center of tra box III. Expression of the repA::lacZ fusion in each of these plasmids was assessed in the presence and absence of TraR and AAI. pPLrep6, which contains the shortest intergenic region, consistently expressed the reporter fusion at two- to threefold lower levels compared to the clones with the full intergenic region (Table 3). Expression of the repA::lacZ fusion in each of the three reporter plasmids was not affected by TraR in the absence of AAI. However, when signal molecule was added, expression of the reporter in pPLrep5 was slightly but consistently enhanced (Table 3), and this enhancement was dependent on TraR. Similarly, expression from the reporter in pPLrep4, which itself codes for the production of AAI, was enhanced slightly when TraR was coexpressed in the cells (Table 3). On the other hand, expression of the reporter from pPLrep6, which lacks half of tra box III, as well as upstream untranslated DNA was slightly but consistently inhibited when TraR and AAI were provided (Table 3).
TABLE 3.
Influence of the 5′ upstream region and TraR on expression of a repA::lacZ fusion
| Test plasmid | TraRa | AAI production | β-Galactosidase activity (U/108 CFU)b
|
Ratio of enzyme activitiesc | |
|---|---|---|---|---|---|
| −AAI | +AAId | ||||
| pPLrep4 | − | − | 106 | NTe | |
| + | +++ | 150 | NT | 1.42 (1.42–2.53) | |
| pPLrep5 | − | − | 183 | 211 | 1.15 (0.79–1.15) |
| + | − | 173 | 237 | 1.37 (1.09–2.68) | |
| pPLrep6 | − | − | 78 | 96 | 1.23 (1.23–1.26) |
| + | − | 89 | 74 | 0.83 (0.64–0.83) | |
TraR was provided by introducing pSVB33 in trans to the test plasmid.
Values shown are from a representative experiment in which all strains were tested in parallel.
Activity ratios for pPLrep5 and pPLrep6 were calculated by dividing the β-galactosidase activity in cells incubated with AAI by the activity in cells grown in the absence of AAI, using the data shown. Values for pPLrep4 were calculated by dividing the β-galactosidase activity in cells expressing TraR by the activity in cells lacking traR. Numbers in parentheses show the range in calculated ratios obtained in four or five repetitions of the experiment.
Synthetic AAI was added at a concentration of 40 nM, and the cultures were incubated for 6 h.
NT, not tested.
Construction of a minimal rep plasmid and the effect of TraR and AAI on copy number.
The 4,906-bp HindIII fragment 13, containing the first 17 codons of traI, the traI-repA intergenic region, and all three rep genes as well as 496 bp from the 3′ end of y4CG (Fig. 1), was cloned into pBluescript SK(+) to generate pPLH13. This plasmid replicates in A. tumefaciens as well as in E. coli (data not shown). Thus, all functions essential for replication of the Ti plasmid are contained in this HindIII fragment. To eliminate any influence from the rep region of the vector plasmid, we ligated HindIII fragment 13 with the 3,424-bp HindIII internal fragment from Tn5 to generate a minimal rep plasmid pH13Km (Fig. 1). This construct is identical with respect to pTiC58 DNA to pUCD510 constructed by Gallie et al. (17). pUCD510 replicates in A. tumefaciens with the same copy number and stability as wild-type pTiC58. Although not carefully examined, pH13Km, like pUCD510, replicates at the anticipated low copy number and is very stable in our A. tumefaciens test strain (data not shown).
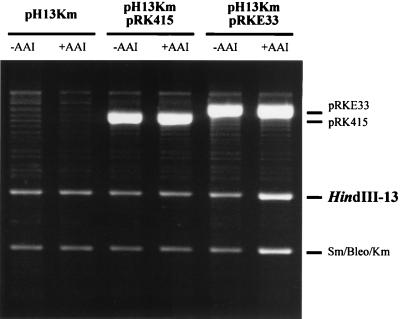
We then assessed the influence of TraR and AAI on the copy number of pH13Km. pRKE33, which codes for traR, or its vector, pRK415, was introduced into NTL4(pH13Km). We then compared the copy numbers of pH13Km in these three strains grown in the presence or absence of AAI by measuring the relative intensities of bands on an agarose gel corresponding to HindIII fragment 13 (Fig. 5). In these comparisons, the intensities of the bands corresponding to pRK415 and the vector portion of pRKE33 served as internal controls. The addition of AAI had virtually no effect on the copy number of pH13Km or pRK415 in strains lacking TraR. However, in strain NTL4(pH13Km, pRKE33), which contains the TraR-producing plasmid, the copy number of the rep plasmid was increased five- to sixfold but only upon addition of AAI (Fig. 5 and Table 4). This increase is similar to the apparent increase in copy number that we observed for the two Trac Ti plasmids (Fig. 4). This was not a generalized effect; the quorum-sensing regulators had no effect on the copy number of pRKE33 or its parent vector, pRK415 (Fig. 5 and data not shown), nor did these factors affect the copy number of pAtC58, the 450-kb catabolic plasmid present in C58 and its derivatives. The HindIII digestion products of this plasmid are visible as faint bands in each lane of Fig. 5.
FIG. 5.
Electrophoretic analysis of pH13Km in cells with and without traR. Cells were grown, plasmids were isolated and digested with HindIII, and equal loadings of DNA were subjected to electrophoresis in agarose gels as described in Materials and Methods. The band labeled Sm/Bleo/Km represents the 3.4-kb HindIII fragment from Tn5 cloned in pH13Km. The weakly staining bands in the background come from pAtC58, a 450-kb catabolic plasmid present in C58 and its derivatives (14).
TABLE 4.
Influence of TraR and AAI on copy number of pH13Km
| Test strain | Relative intensity of pH13Kma
|
Ratio of intensities (+AAI/−AAI) | |
|---|---|---|---|
| −AAI | +AAIb | ||
| NTL4(pH13Km) | 50 | 41 | 0.82 |
| NTL4(pH13Km, pRK415) | 39 | 38 | 0.97 |
| NTL4(pH13Km, pRKE33) | 47 | 352 | 7.49 |
Assessed by measuring the intensity of the 4.9-kb HindIII-13 band on the inverted image of an agarose gel, using NIH Image version 1.62. The experiment was repeated two times with indistinguishable results.
Synthetic AAI was added at a concentration of 40 nM, and the cultures were incubated for 6 h.
DISCUSSION
Sequence and function of the rep region of pTiC58.
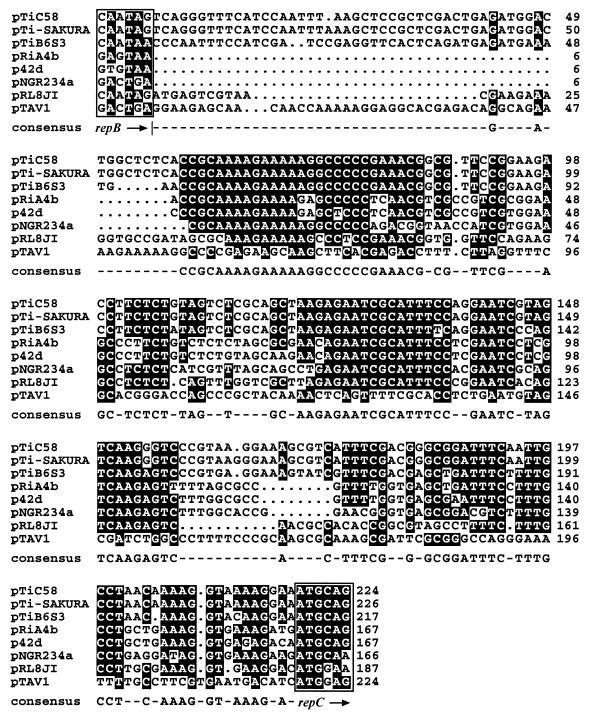
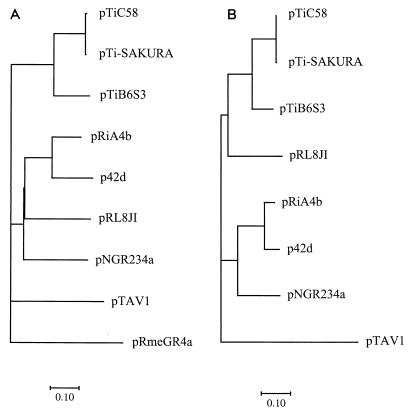
The repABC-type replicators are common among the large, low-copy-number plasmids present in members of the family Rhizobiaceae. These replicators exhibit similarities in individual genes and also in overall organization. In addition to the three rep genes, the igs between repB and repC is believed to serve as a cis-acting site, perhaps as the origin of replication (oriV) or for incompatibility (inc) (47, 50). Alignment of the igs regions indicates that several domains are strongly conserved among the different repABC-type replicators for which complete sequence is available (Fig. 6). On the other hand, a similar alignment of the intergenic sequence between repA and repB does not show such a strong conservation in primary sequence (data not shown). That the rep region of pTiC58 possesses all of these characteristics clearly indicates that this replicator is a member of the repABC family. Furthermore, the rep region of pTiC58 is closely related to the rep regions of other Ti plasmids but more distantly related to those of the Ri plasmid pRiA4b, the two Sym plasmids, and a cryptic plasmid from R. leguminosarum (Table 2). Phylogenetic analysis of the RepC protein, which has been used as an indicator of plasmid diversity (6, 41, 49) (Fig. 7A), and the igs (Fig. 7B) indicate that the rep complexes of the three Ti plasmids form a tight cluster, whereas those of the Ri plasmid and the three Rhizobium plasmids form a second cluster less closely related to that of the Ti plasmid replicators. The rep regions of pRmeGR4a, which contains a RepC homologue, and of pTAV1, present in a host bacterium outside of the family Rhizobiaceae, are more distantly related to the members of this replicator family (Fig. 7). The close relatedness between the replicators of pTiC58 and pTiB6S3 is consistent with the observation that these two plasmids belong to the same incompatibility group, IncRh1 (19, 33). The incompatibility properties of pTi-SAKURA have not been reported, but we anticipate that this plasmid also is a member of the IncRh1 group. In this regard, the divergence between the rep regions of pRiA4b and the Ti plasmids also is consistent with the observation that the Ri plasmid is compatible with the IncRh1 Ti plasmids (52). Turner et al. (49) and Rigottier-Gois et al. (41) reported that the repC sequence is widely distributed among strains of Rhizobium spp. and that these repC sequences cluster into different groups. They suggested that the different repC families correspond to incompatibility groups and that divergence in RepC may be responsible for the compatibility properties exhibited by many members of this family. Our analysis of RepC from the Ti and Ri plasmids supports this hypothesis. However, even though RepC of pTAV1 is distantly related to that of the Ti plasmids, a recombinant clone containing only this replicator is reportedly incompatible with pTiB6S3 (3). On the other hand, Rigottier-Gois et al. (41) also reported that two plasmids classified to the same repC group coexist in a field isolate of R. leguminosarum bv. viciae. Therefore, incompatibility properties of the repABC-type family may involve functions in addition to RepC. Consistent with this conclusion, Tabata et al. reported that a clone containing only the igs region exerted incompatibility against an IncRh1 plasmid (47).
FIG. 6.
Alignment of the igs region from seven repABC-type replication systems. Identical bases are shown as white letters on a black background. Boxed sequences indicate the last codon and the translational stop codon of repB and the first two codons of repC. The consensus sequence is based on identities in at least five of the eight aligned igs regions.
FIG. 7.
Phylogenetic relationships among cis and trans elements of repABC-type replicators. The unrooted phylogenetic trees for RepC amino acid sequences (A) and the igs regions (B) are presented. Note that pRmeGR4a codes for a RepC homologue but does not contain genes related to repA or repB (31).
By aligning the functional map of the pTiC58 replicator described by Gallie et al. (17) with our sequence, the par region, which is involved in partitioning of the plasmid, corresponds to repA (Fig. 1). Consistent with this conclusion, mutational analysis indicates that RepA and RepB are required for stable maintenance of pTiB6S3 and pRiA4b but are not essential for replication per se (35, 47). repC, which is believed to code for the major replicator protein, corresponds to ori/inc, the origin of replication and the incompatibility region (Fig. 1 and reference 17). The close relatedness between the three rep genes of the two Ti plasmids, coupled with prior genetic analyses (17, 47), prompts us to conclude that the rep gene products of pTiC58 play roles identical to those of pTiB6S3. Gallie et al. (17) proposed a third function, copy number control or cop, associated with this region. The cop locus, which maps distal to repC, overlaps y4CG and perhaps also y4CF (Fig. 1 and reference 17). y4CG, which shows similarities to DNA invertases and resolvases from several bacteria and phage, is not part of the minimum stable, unit-copy replicator as defined by pH13Km. Furthermore, except for pNGR234a, most of the repABC-type replicators lack this gene, although our analysis of the limited available sequence suggests that a similar ORF may be located immediately downstream from repC of pTi-SAKURA and pTiB6S3 (data not shown). However, in pNGR234a the y4CG ORF, while present, is located nearly 3 kb downstream of repC. Thus, it is unlikely that y4CG itself plays a role in replication or copy number control. Furthermore, studies on other repABC-type replicators provide little support for the involvement of accessory genes outside of repABC. However, Cevallos et al. (8) recently reported that a 152-bp region located immediate downstream of repC of p42d exerts incompatibility and is essential for replication of this Sym plasmid. It is possible that a sequence with similar cis-acting functions resides in the 503-bp region downstream of repC from pTiC58 that is contained in pH13Km.
Relationship between tra boxes and the rep region.
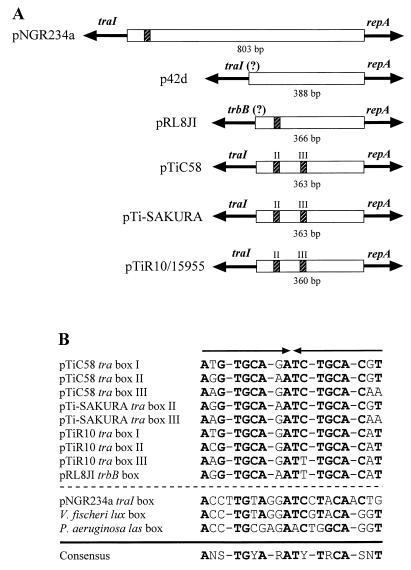
Two tra boxes are located in the intergenic region between traI and repA from both octopine- and nopaline-type Ti plasmids (Fig. 8A) (1, 27). Fuqua and Winans (16) reported that tra box II is required for the expression of the traI-trb operon, whereas tra box III exerts no detectable effect on expression in either direction in the octopine-type Ti plasmid. Our results suggest that an intact tra box III somewhat impairs the tra box II-dependent TraR-mediated expression of traI in pTiC58 (Fig. 3). Moreover, the upstream region which contains tra box III is required for full expression of repA of the nopaline-type Ti plasmid (Table 3). Intriguingly, expression of repA, as assessed by our lacZ reporter fusions, is enhanced slightly by TraR and AAI, but only when the entire upstream region is present (Table 3).
FIG. 8.
Comparison of structures of the traI-repA intergenic regions of six plasmids from the family Rhizobiaceae. (A) Extents of the intergenic regions and distribution of tra box-like sequences. As noted by the (?), the designation of traI in p42d and trbB in pRL8JI is based on similarities from limited available sequence. Roman numerals II and III in the Ti plasmid sequences indicate the two tra boxes located in the intergenic regions between traI and repA. (B) Alignment of the tra boxes, the lux box of V. fischeri, and the las box of Pseudomonas aeruginosa. Arrowheads indicate the symmetry of the sequences; the dashed line separates sequences that are more related to the tra box consensus from those more related to the lux or las box sequences. Symbols in the consensus sequence: R, purine; Y, pyrimidine; S, G or C; N, any nucleotide.
tra boxes are present in the region upstream of repA in all three Ti plasmids examined to date and also in the corresponding regions of other plasmids with a repABC-type replicator (Fig. 8A). The tra box-like sequence in pRL8JI is more closely related to those of the Ti plasmids, whereas the cis element in pNGR234a is more similar to the lux box of Vibrio fischeri (Fig. 8B). Furthermore, two Sym plasmids encode traI-like genes divergently oriented from repA (Fig. 8A). As with the Ti plasmids, traI of pNGR234a is the first gene of the trb operon (15). Apparently, the association of the quorum-sensing regulatory system with Tra occurred on the replicon ancestral to pNGR234a and the Ti plasmids. On the other hand, pRL8JI, which is self-conjugal (23), lacks a traI homologue, and the repABC cluster is linked directly to a gene with relatedness to trbB, the second gene of the Ti plasmid-type trb operon. This finding suggests that quorum sensing is not a de rigueur component of the conjugal transfer systems of these types of plasmids. However, a tra box-like sequence is located in the intergenic region between the trbB homologue and repA of pRL8JI (Fig. 8A), suggesting that the Tra system and perhaps the rep genes of this plasmid were regulated by quorum sensing at one time. It also is possible that conjugal transfer of pRL8JI is controlled by quorum sensing but that the traI homolog is located in some other region of the genome. Interestingly, although there is a traI-like gene present in the region upstream from repA of p42d, there is no identifiable tra box in this region.
A plasmid replicator influenced by quorum sensing.
Our results indicate that copy number of pTiC58 is influenced by TraR and AAI. That both pTiC58ΔaccR and pCMA1 exhibit such an increase indicates that the effect is not due to the influence of some other gene in the arc operon (37). Furthermore, the effect can be seen on the minimal rep plasmid, pH13Km, indicating that TraR influences copy number through repABC and not by some indirect interaction with another component of the Ti plasmid. This is the first report of plasmid replication being influenced by a quorum-sensing system. How TraR and AAI influence Ti plasmid copy number remains to be determined. While transcription of the rep genes does not require the quorum-sensing activator, our analysis suggests that TraR, coupled with AAI, enhances expression of repA (Table 3). This enhancement might be due to weak activation by TraR. Alternatively, TraR bound to tra box III may alter the structure of the DNA around the origin, leading to an increase in the rate of replication initiation. Furthermore, the repA promoter most probably contains cis-acting signals located in the vicinity of tra box III since removing this region resulted in lowered levels of expression and the loss of the enhancing effect associated with TraR and AAI (Table 3).
The connection between DNA replication and quorum sensing is not without precedence. Withers and Nordström (53) reported that an extracellular factor negatively influences chromosomal DNA replication of E. coli in a quorum-dependent manner. Apparently replicon copy number needs in some way to respond to changes in the environment and the population size itself. Studies with RP4 point to a relationship between plasmid replication and conjugal transfer. In this IncP1α plasmid, the replicator gene, trfA, and the conjugal transfer genes in the Tra2 region are coregulated by the product of the trbA gene (22, 32). Sia et al. (43) suggested that conjugal transfer contributes to the maintenance of RK2 by reducing the proportion of plasmidless segregants in a growing population when growth conditions are favorable for conjugation.
Regulating copy number in a quorum-dependent manner could serve several purposes. First, since opine availability regulates quorum sensing (37), increasing the copy number of the Ti plasmid in response to population density results in an increase in components of the opine catabolism systems. Such an increase in opine transporters, for example, may be advantageous to a bacterium under conditions in which availability of these nutritional resources becomes limiting due to increased numbers of opine utilizers. Second, elevated plasmid copy number could augment conjugal transfer by increasing the number of mating pores as well as relaxosome assemblies. This, in turn, could compensate for the occasional loss of the Ti plasmid from some fraction of the agrobacterial population, especially at high population densities. Upregulating plasmid copy number also may explain the observation by Veluthambi et al. (51) that octopine, the conjugal opine of pTiA6, enhances the level of vir gene induction by acetosyringone some 2- to 10-fold. It is conceivable that this stimulation in expression of the vir regulon results from an increase in copy number mediated by the octopine-inducible TraR-AAI quorum-sensing system of this Ti plasmid. These observations suggest that upregulating plasmid copy number in response to conditions that signal an environment favorable for transfer is an important component of the biology of the Ti plasmid.
ACKNOWLEDGMENTS
We thank Ingyu Hwang and Philippe Oger for helpful discussions.
This work was supported by grants R01 GM52465 from the NIH and AG92-3312-8231 from the USDA to S.K.F. P.-L.L. was supported in part by HATCH project 15-0326 to S.K.F.
REFERENCES
- 1.Alt-Mörbe J, Stryker J L, Fuqua C, Li P-L, Farrand S K, Winans S C. The conjugal transfer system of Agrobacterium tumefaciens octopine-type Ti plasmids is closely related to the transfer system of an IncP plasmid and distantly related to Ti plasmid vir genes. J Bacteriol. 1996;178:4248–4257. doi: 10.1128/jb.178.14.4248-4257.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartosik D, Baj J, Wlodarczyk M. Molecular and functional analysis of pTAV320, a repABC-type replicon of the Paracoccus versutus composite plasmid pTAV1. Microbiology. 1998;144:3149–3157. doi: 10.1099/00221287-144-11-3149. [DOI] [PubMed] [Google Scholar]
- 4.Beck von Bodman S, McCutchan J E, Farrand S K. Characterization of conjugal transfer functions of Agrobacterium tumefaciens Ti plasmid pTiC58. J Bacteriol. 1989;171:5281–5289. doi: 10.1128/jb.171.10.5281-5289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blatny J M, Brautaset T, Winther-Larsen H C, Haugan K, Valla S. Construction and use of a versatile set of broad-host-range cloning and expression vectors based on the RK2 replicon. Appl Environ Microbiol. 1997;63:370–379. doi: 10.1128/aem.63.2.370-379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgos P A, Velázquez E, Toro N. Identification and distribution of plasmid-type A replicator region in Rhizobia. Mol Plant-Microbe Interact. 1996;9:843–849. doi: 10.1094/mpmi-9-0843. [DOI] [PubMed] [Google Scholar]
- 7.Cangelosi G A, Best E A, Martinetti G, Nester E W. Genetic analysis of Agrobacterium. Methods Enzymol. 1991;204:384–397. doi: 10.1016/0076-6879(91)04020-o. [DOI] [PubMed] [Google Scholar]
- 8.Cevallos M A, Ramírez-Romero M A, Soberón N E, Pérez-Oseguera A, Téllez J M, González V. The RepABC plasmid family: a structural analysis. Plasmid. 1999;41:155. [Google Scholar]
- 9.Chilton M D, Currier T C, Farrand S K, Bendich A J, Gordon M P, Nester E W. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci USA. 1974;71:3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook D M, Farrand S K. The oriT region of the Agrobacterium tumefaciens Ti plasmid pTiC58 shares DNA sequence identity with the transfer origins of RSF1010 and RK2/RP4 and with T-region borders. J Bacteriol. 1992;174:6238–6246. doi: 10.1128/jb.174.19.6238-6246.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cook D M, Li P-L, Ruchaud F, Padden S, Farrand S K. Ti plasmid conjugation is independent of vir: reconstitution of the tra functions from pTiC58 as a binary system. J Bacteriol. 1997;179:1291–1297. doi: 10.1128/jb.179.4.1291-1297.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dessaux Y, Petit A, Farrand S K, Murphy P J. Opines and opine-like molecules involved in plant-Rhizobiaceae interactions. In: Spaink H P, Kondorosi A, Hooykaas P J J, editors. The Rhizobiaceae. Dordrecht, The Netherlands: Kluwer Academic Publishing; 1998. pp. 173–197. [Google Scholar]
- 13.Engler G, Depicker R, Maenhaut R, Villarroel R, Van Montagu M, Schell J. Physical mapping of DNA base sequence homologies between an octopine and a nopaline Ti plasmid of Agrobacterium tumefaciens. J Mol Biol. 1981;152:183–208. doi: 10.1016/0022-2836(81)90239-4. [DOI] [PubMed] [Google Scholar]
- 14.Farrand S K. Conjugal plasmids and their transfer. In: Spaink H P, Kondorosi A, Hooykaas P J J, editors. The Rhizobiaceae. Dordrecht, The Netherlands: Kluwer Academic Publishing; 1998. pp. 199–233. [Google Scholar]
- 15.Freiberg C, Fellay R, Bairoch A, Broughton W J, Rosenthal A, Perret X. Molecular basis of symbiosis between Rhizobium and legumes. Nature (London) 1997;387:394–401. doi: 10.1038/387394a0. [DOI] [PubMed] [Google Scholar]
- 16.Fuqua C, Winans S C. Conserved cis-acting promoter elements are required for density-dependent transcription of Agrobacterium tumefaciens conjugal transfer genes. J Bacteriol. 1996;178:435–440. doi: 10.1128/jb.178.2.435-440.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallie D R, Hagiya M, Kado C I. Analysis of Agrobacterium tumefaciens plasmid pTiC58 replication region with a novel high-copy-number derivative. J Bacteriol. 1985;161:1034–1041. doi: 10.1128/jb.161.3.1034-1041.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayman G T, Farrand S K. Agrobacterium plasmids encode structurally and functionally different loci for catabolism of agrocinopine-type opines. Mol Gen Genet. 1990;223:465–473. doi: 10.1007/BF00264455. [DOI] [PubMed] [Google Scholar]
- 19.Hooykaas P J J, Den Dulk-Ras H, Ooms G, Schilperoort R A. Interactions between octopine and nopaline plasmids in Agrobacterium tumefaciens. J Bacteriol. 1980;143:1295–1306. doi: 10.1128/jb.143.3.1295-1306.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hwang I, Cook D M, Farrand S K. A new regulatory element modulates homoserine lactone-mediated autoinduction of Ti plasmid conjugal transfer. J Bacteriol. 1995;177:449–458. doi: 10.1128/jb.177.2.449-458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hwang I, Li P-L, Zhang L, Piper K R, Cook D M, Tate M E, Farrand S K. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc Natl Acad Sci USA. 1994;91:4639–4643. doi: 10.1073/pnas.91.11.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagura-Burdzy G, Khanim F, Smith C A, Thomas C M. Crosstalk between plasmid vegetative replication and conjugative transfer: repression of the trfA operon by trbA of broad host range plasmid RK2. Nucleic Acids Res. 1992;20:3939–3944. doi: 10.1093/nar/20.15.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnston A W B, Homebrecher G, Brewin N J, Cooper M C. Two transmissible plasmids in Rhizobium leguminosarum strain 300. J Gen Microbiol. 1982;128:85–93. [Google Scholar]
- 24.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 25.Koekman B P, Hooykaas P J J, Schilperoort R A. Localization of the replication control region on the physical map of the octopine Ti plasmid. Plasmid. 1980;4:184–195. doi: 10.1016/0147-619x(80)90008-6. [DOI] [PubMed] [Google Scholar]
- 26.Koekman B P, Hooykaas P J J, Schilperoort R A. A functional map of the replicator region of the octopine Ti plasmid. Plasmid. 1982;7:119–132. doi: 10.1016/0147-619x(82)90072-5. [DOI] [PubMed] [Google Scholar]
- 27.Li P-L, Everhart D M, Farrand S K. Genetic and sequence analysis of the trb locus on pTiC58, a mating-pair formation system related to members of the type IV secretion family. J Bacteriol. 1998;180:6164–6172. doi: 10.1128/jb.180.23.6164-6172.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li P-L, Hwang I, Miyagi H, True H, Farrand S K. Essential component of the Ti plasmid trb system, a type IV macromolecular transporter. J Bacteriol. 1999;181:5033–5041. doi: 10.1128/jb.181.16.5033-5041.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo Z-Q, Farrand S K. Signal-dependent DNA binding and functional domains of the quorum-sensing activator TraR as identified by repressor activity. Proc Natl Acad Sci USA. 1999;96:9009–9014. doi: 10.1073/pnas.96.16.9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mark C. “DNA Strider”: a “C” program for the fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988;16:1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mercado-Blanco J, Olivares J. The large nonsymbiotic plasmid pRmeGR4a of Rhizobium meliloti GR4 encodes a protein involved in replication that has homology with the RepC protein of Agrobacterium plasmids. Plasmid. 1994;32:75–79. doi: 10.1006/plas.1994.1046. [DOI] [PubMed] [Google Scholar]
- 32.Motallebi-Veshareh M, Balzer D, Lanka E, Jagura-Burdzy G, Thomas C M. Conjugative transfer functions of broad-host-range plasmid RK2 are coregulated with vegetative replication. Mol Microbiol. 1992;6:907–920. doi: 10.1111/j.1365-2958.1992.tb01541.x. [DOI] [PubMed] [Google Scholar]
- 33.Nester E W, Kosuge T. Plasmids specifying plant hyperplasias. Annu Rev Microbiol. 1981;35:531–565. doi: 10.1146/annurev.mi.35.100181.002531. [DOI] [PubMed] [Google Scholar]
- 34.Nishiguchi R, Oka A. Structure of the hairy-root-inducing plasmid and identification of its replicator region. Bull Inst Chem Res Kyoto Univ. 1986;64:79–87. [Google Scholar]
- 35.Nishiguchi R, Takanami M, Oka A. Characterization and sequence determination of the replicator region in the hairy-root-inducing plasmid pRiA4b. Mol Gen Genet. 1987;206:1–8. [Google Scholar]
- 36.Piper K R, Beck von Bodman S, Farrand S K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature (London) 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 37.Piper K R, Beck von Bodman S, Hwang I, Farrand S K. Hierarchical gene regulatory systems arising from fortuitous gene associations: controlling quorum sensing by the opine regulon in Agrobacterium. Mol Microbiol. 1999;32:1077–1089. doi: 10.1046/j.1365-2958.1999.01422.x. [DOI] [PubMed] [Google Scholar]
- 38.Piper K R, Farrand S K. Conjugal transfer but not quorum-dependent tra gene induction of pTiC58 requires a solid surface. Appl Environ Microbiol. 1999;65:2798–2801. doi: 10.1128/aem.65.6.2798-2801.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramírez-Romero M A, Bustos P, Girard L, Rodriguez O, Cevallos M A, Dávila G. Sequence, localization and characteristics of the replicator region of the symbiotic plasmid of Rhizobium etli. Microbiology. 1997;143:2825–2831. doi: 10.1099/00221287-143-8-2825. [DOI] [PubMed] [Google Scholar]
- 40.Ream W, Gelvin S B, editors. Crown gall: advances in understanding interkingdom gene transfer. St. Paul, Minn: American Phytopathological Society; 1996. [Google Scholar]
- 41.Rigottier-Gois L, Turner S L, Young J P W, Amarger N. Distribution of repC plasmid-replication sequences among plasmids and isolates of Rhizobium leguminosarum bv. viciae from field populations. Microbiology. 1998;144:771–780. doi: 10.1099/00221287-144-3-771. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 43.Sia E A, Roberts R C, Easter C, Helinski D R, Figurski D H. Different relative importances of the par operons and the effect of conjugal transfer on the maintenance of intact promiscuous plasmid RK2. J Bacteriol. 1995;177:2789–2797. doi: 10.1128/jb.177.10.2789-2797.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simon R, Priefer U, Pühler A. Vector plasmids for in vivo and in vitro manipulations of gram-negative bacteria. In: Pühler A, editor. Molecular genetics of the bacteria-plant interaction. Berlin, Germany: Springer-Verlag KG; 1983. pp. 98–106. [Google Scholar]
- 45.Slota J E, Farrand S K. Genetic isolation and physical characterization of pAgK84, the plasmid responsible for agrocin 84 production. Plasmid. 1982;8:175–186. doi: 10.1016/0147-619x(82)90055-5. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki K, Ohta N, Hattori Y, Uraji M, Kato A, Yoshida K. Novel structural difference between nopaline- and octopine-type trbJ genes: construction of genetic and physical map and sequencing of trb/traI and rep gene clusters of a new Ti plasmid pTi-SAKURA. Biochim Biophys Acta. 1998;1396:1–7. doi: 10.1016/s0167-4781(97)00182-6. [DOI] [PubMed] [Google Scholar]
- 47.Tabata S, Hooykaas P J J, Oka A. Sequence determination and characterization of the replicator region in the tumor-inducing plasmid pTiB6S3. J Bacteriol. 1989;171:1665–1672. doi: 10.1128/jb.171.3.1665-1672.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiedeman A A, Smith J M. lacZY fusion cassettes with KanR resistance. Nucleic Acids Res. 1988;16:3587. doi: 10.1093/nar/16.8.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turner S L, Rigottier-Gois L, Power R S, Amarger N, Young J P W. Diversity of repC plasmid-replication sequences in Rhizobium leguminosarum. Microbiology. 1996;142:1705–1713. doi: 10.1099/13500872-142-7-1705. [DOI] [PubMed] [Google Scholar]
- 50.Turner S L, Young J P W. The replicator region of the Rhizobium leguminosarum cryptic plasmid pRL8JI. FEMS Microbiol Lett. 1995;133:53–58. doi: 10.1111/j.1574-6968.1995.tb07860.x. [DOI] [PubMed] [Google Scholar]
- 51.Veluthambi K, Krishnan M, Gould J H, Smith R H, Gelvin S B. Opines stimulate induction of the vir genes of the Agrobacterium tumefaciens Ti plasmid. J Bacteriol. 1989;171:3696–3703. doi: 10.1128/jb.171.7.3696-3703.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White F F, Nester E W. Relationship of plasmids responsible for hairy root and crown gall tumorigenicity. J Bacteriol. 1980;144:710–720. doi: 10.1128/jb.144.2.710-720.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Withers H L, Nordström K. Quorum-sensing acts at initiation of chromosomal replication in Escherichia coli. Proc Natl Acad Sci USA. 1998;95:15694–15699. doi: 10.1073/pnas.95.26.15694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu J, Winans S C. Autoinducer binding by the quorum-sensing regulator TraR increases affinity for target promoters in vitro and decreases TraR turnover rates in whole cells. Proc Natl Acad Sci USA. 1999;96:4832–4837. doi: 10.1073/pnas.96.9.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]