Key Features.
The Ovarian Cancer Cohort Consortium (OC3) was established to facilitate prospective studies of ovarian cancer risk factors, biomarkers, risk prediction and outcomes while accounting for ovarian cancer subtypes.
The consortium currently includes 1.3 million women, among whom 7314 incident invasive epithelial ovarian cancer cases have been identified, enrolled across 23 prospective studies based in the USA or Canada (16 studies), Europe (five studies), Singapore (one study) and Australia (one study).
The studies' enrolment periods range from 1976 to currently ongoing and the median age at enrolment ranges from 35 to 62 years. Many of the studies enrolled individuals living in a particular region, and others targeted groups with specific shared characteristics, such as religion, occupation, education or participation in a screening programme or randomised trial.
Data were collected using mailed or online questionnaires or in-person or telephone interviews and laboratory analysis. Of the 21 studies that collected updated exposure and outcome information from participants after baseline, most collected data every 2–5 years. Data are available on body size, reproductive and contraceptive history, use of medications (e.g. postmenopausal hormone therapy, non-steroidal anti-inflammatory drugs) and selected disease diagnoses.
Collaboration requests are accepted via a contact form on the OC3 website [theoc3.org].
Why was the consortium set up?
Ovarian cancer is the most common fatal gynaecological malignancy, but its incidence in the general population is low.1 Due to the lack of effective prevention or early detection approaches and the late clinical presentation, most ovarian cancers are detected at advanced stages with poor outcomes. Two large ovarian cancer screening trials have not shown a meaningful survival benefit in women screened with the cancer antigen 125 (CA-125) blood test and transvaginal ultrasound.2,3 Known ovarian cancer risk factors are related to high-penetrance genetic mutations (e.g. in BRCA1 or BRCA2), incessant ovulation, hormone exposures and inflammation. With the exception of BRCA1/2 status, known risk factors poorly predict individual-level risk of developing ovarian cancer, hindering prevention efforts.4,5 Epithelial ovarian cancers are highly heterogeneous, with multiple subtypes that can be distinguished by morphology and molecular pathways, risk factor profile, degree of aggressiveness and prognosis.6,7 The four main histological subtypes (histotypes) of ovarian cancer are serous, endometrioid, clear cell and mucinous tumours; the first two types are further distinguished by level of differentiation (grade). There is increasing evidence that ovarian cancer subtypes have different cells of origin, with a subset of high-grade serous cancers likely originating in the Fallopian tubes and some endometrioid cancers deriving from orthotopic or ectopic endometrial cells, and are clearly characterized by different somatic events (e.g. nearly all high-grade serous tumours have p53 mutations whereas other subtypes do not).8 Whereas precursors in the ovarian surface epithelium have been elusive, there are now several candidate precursors that have been found in the fallopian tubes, such as serous tubal intraepithelial carcinomas.8,9 Currently there is insufficient evidence regarding risk factors and risk of progression of these putative precursors. Since tubal intraepithelial carcinomas are typically incidental findings in clinical series of patients undergoing salpingectomy and salpingo-oophorectomy, these presumptive precursors cannot be studied in epidemiological cohorts with cancer endpoints.
Much of the understanding about risk factors, genetics and molecular pathways of ovarian cancer has been derived from case-control studies. Case-control studies can accrue large numbers of cases and have made important contributions to establishing risk factor associations and genetic susceptibility, but risk factors and clinical measures are ascertained at or after the time of diagnosis. Recall bias has been shown to affect some risk factor associations for ovarian cancer.10 Anthropometric measures obtained at diagnosis, such as weight, can be substantially affected by disease either due to cancer-related weight loss or due to extensive ascites which may increase weight in a subset of cases. Generally, most risk factors established in case-control studies have also been observed in cohort studies. However, case-control studies are unsuitable for most blood- or urine-based biomarker studies of ovarian cancer incidence, which require samples collected before disease development. Prospective cohort studies can provide questionnaire-based exposure data and prospectively collected biospecimens without differential bias by disease status, but due to the rarity of ovarian cancers in the general population, it is challenging to study risk factors and biomarkers for ovarian cancer prospectively in individual cohorts. With the increasing recognition of the substantial heterogeneity of ovarian cancer and the need to study subtypes, it has become virtually impossible to study risk factors in individual prospective studies .
To address the need for well-powered prospective studies with ovarian cancer endpoints, we developed the Ovarian Cancer Cohort Consortium (OC3), a large population-based resource of prospective ovarian cancer cases from a base sample of over 1.3 million women. The OC3 provides a unique resource to conduct studies of ovarian cancer risk factors, biomarkers, risk prediction and outcomes while accounting for ovarian cancer subtypes. The OC3 protocol was approved by the Institutional Review Board of Brigham and Women’s Hospital (Protocol # 2012P000378) and the Advarra Institutional Review Board (Protocol # 00022137).
Who is in the consortium?
The OC3 was initiated through the National Cancer Institute (NCI) Cohort Consortium, a global partnership of more than 55 cohort studies focused on conducting coordinated parallel and pooled analyses to address cancer-related research questions that would be difficult to address in any single cohort.11 NCI Cohort Consortium members were initially approached in 2010 to participate in the OC3. Cohorts that prospectively ascertained ovarian cancer diagnoses and collected basic epidemiological data, including oral contraceptive use and parity, were eligible to join. Availability of biospecimens or information on ovarian histotypes was considered a plus but not a prerequisite and there was no minimum number of participants or ovarian cancer cases required. Currently, there are 23 studies in the OC3, including 21 full cohort studies and two case-cohort studies (CSDLH and NLCS; see Table 1 for full study names), and three additional cohorts (BWHS, CPS3, JSB) are in the process of joining.
Table 1.
Descriptive information on OC3 cohorts
| Study name | Abbreviation | Institution | Country | Enrolment period | OC3 sample sizea | Median baseline age, years | Latest year of follow-up in OC3 dataset | Follow-up questionnaires? (active or passive follow-up) |
|---|---|---|---|---|---|---|---|---|
| Adventist Health Study II | AHS2 | Loma Linda University | USA, Canada | 2001–07 | 39 059 | 53 | 2015 | Yes (active) |
| Black Women's Health Studyb | BWHS | Boston University | USA | 1995 | 59 000 | 38 | NA | Yes (active) |
| Breast Cancer Detection Demonstration Project | BCDDP | National Cancer Institute | USA | 180 | 36 395 | 61 | 1999 | Yes (passive) |
| California Teachers Study | CTS | City of Hope | USA | 1995–96 | 44 176 | 50 | 2012 | Yes (active) |
| Campaign Against Cancer and Stroke | CLUEII | Johns Hopkins | USA | 1989 | 12 395 | 46 | 2012 | Yes (passive) |
| Canadian Study of Diet, Lifestyle, and Healthc | CSDLH | Canadian Study of Diet, Lifestyle, and Health | Canada | 1992–98 | 3019 (39 618) | 58 | 2011 | Yes (passive) |
| Cancer Prevention Study-II | CPSII | American Cancer Society | USA (incl. Puerto Rico) | 1982 | 82 435 | 62 | 2009 | Yes (passive) |
| Cancer Prevention Study-3b | CPS3 | American Cancer Society | USA (incl. Puerto Rico) | 2006–13 | 198 580 | 47 | NA | Yes (active) |
| European Prospective Investigation into Cancer and Nutrition | EPIC | International Agency for Research on Cancer/German Cancer Research Center | France, Italy, Spain, UK, The Netherlands, Germany, Sweden, Denmark, Norway | 1992–2000 | 249 489 | 51 | 2010 | Yes (active) |
| Generations Study (formerly Breakthrough Generations Study) | BGS | Institute of Cancer Research | UK | 2003–13 | 101 894 | 48 | 2014 | Yes (active) |
| Iowa Women's Health Study | IWHS | University of Minnesota/University of Iowa | USA | 1986 | 30 671 | 61 | 2011 | Yes (passive) |
| Janus Serum Bankb | JSB | Cancer Registry of Norway | Norway | 1972–2004 | 152 491 | 42 | NA | No (passive) |
| Melbourne Collaborative Cohort Study | MCCS | Cancer Council Victoria | Australia | 1990–94 | 20 863 | 55 | 2010 | Yes (passive) |
| Multiethnic Cohort Study (White women only) | MEC | University of Hawaii/University of Southern California | USA | 1993–96 | 16 510 | 57 | 2012 | Yes (active) |
| Netherlands Cohort Studyc | NLCS | Maastricht University | The Netherlands | 1986 | 2883 (62 573) | 62 | 2010 | No (passive) |
| NIH-AARP Diet and Health Study | AARP | National Cancer Institute at the National Institutes of Health | USA | 1995–96 | 201 510 | 62 | 2007 | Yes (passive) |
| Nurses’ Health Study | NHS | Brigham and Women's Hospital | USA | 1976 | 98 605 | 47 | 2010 | Yes (active) |
| Nurses’ Health Study II | NHSII | Harvard T.H. Chan School of Public Health | USA | 1989 | 114 033 | 35 | 2011 | Yes (active) |
| NYU Women's Health Study | NYUWHS | New York University | USA | 1985–91 | 12 439 | 49 | 2012 | Yes (active) |
| Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial | PLCO | National Cancer Institute | USA | 1993–2001 | 60 846 | 62 | 2009 | Yes (passive) |
| Singapore Chinese Health Study | SCHS | National University of Singapore | Singapore (Chinese participants) | 1993–98 | 34 021 | 56 | 2012 | Yes (active) |
| Sister Study | SIS | National Institute of Environmental Health Sciences | USA (incl. Puerto Rico) | 2003–09 | 48 163 | 56 | 2012 | Yes (active) |
| Swedish Mammography Cohort | SMC | Uppsala University/Karolinska Institutet | Sweden | 1987–90 | 37 205 | 60 | 2012 | Yes (passive) |
| Vitamins and Lifestyle Study | VITAL | Fred Hutchinson Cancer Research Center | USA | 2000–02 | 28 343 | 60 | 2011 | No (passive) |
| Women’s Health Study | WHS | Brigham and Women’s Hospital | USA | 1992–95 | 39 807 | 53 | 2012 | Yes (active) |
| Swedish Women’s Lifestyle and Health | WLH | Karolinska University | Sweden | 1991–92 | 49 229 | 40 | 2013 | Yes (passive) |
NA, not applicable; OC3, Ovarian Cancer Cohort Consortium.
The OC3 study population is restricted to women with a non-missing age at baseline questionnaire return, a non-missing age at censoring event (i.e. ovarian cancer diagnosis, death or latest follow-up, whichever is earliest), a censoring event that occurred after baseline and no history of cancer before baseline. For the case-cohort studies, the total number of women in the full cohort is shown in parentheses.
Study is currently in the process of joining OC3; sample size and median age at baseline are based on female participants in the whole cohort.
Case-cohort study design.
Of the 26 studies, 18 are based in the USA or Canada, six in Europe, one in Asia and one in Australia (Table 1). The studies' enrolment periods range from 1972 to currently ongoing. Many of the studies sought to enrol individuals in the general population of a particular region and used a variety of registries to identify potential participants [e.g. driver's licence list (IWHS, MEC), electoral roll and phone directory (MCCS), population registry (NLCS, WLH) or commercial mailing list (VITAL)]. However, several studies targeted groups with specific shared characteristics, such as religion (AHS2), occupation or education (CTS, NHS, NHSII, CSDLH), enhanced breast cancer risk (SIS) or screening programme participation (BCDDP, NYUWHS, SMC). Two studies are observational extensions of randomized trials (PLCO, WHS).
When considering only participants that are typically eligible for OC3 analyses (see Supplementary Methods, available as Supplementary data at IJE online) and excluding three studies that have not yet submitted data to the OC3 (BWHS, CPS3 and JSB), the median study size is 39 059 women (interquartile range = 20 863 to 82 435) and the total pooled population in the consortium is 1 363 990 women. After the addition of BWHS, CPS3 and JSB, the total pooled population will be approximately 1.7 million women. The median age at enrolment was 50 years or older for 17 of the 23 studies (Table 1).
The current pooled OC3 dataset (median end of follow-up = 2011; Table 1) includes 7314 incident invasive epithelial ovarian cancer cases, of whom 5109 (69.9%) have data on histotype (Table 2). Of the 7314 cases, 50% are from four studies (AARP, NHS, EPIC, CPS2) with each contributing 8–18% of the total number of cases. The addition of BWHS, CPS3 and JSB is expected to add more than 2000 invasive ovarian cancer cases to the OC3.
Table 2.
Distribution of the four major ovarian cancer histotypes by study in OC3a
| All invasive epithelial ovarian cancer cases |
All invasive epithelial ovarian cancer cases classified as one of the four major histotypes |
Serous or poorly differentiated |
Endometrioid |
Mucinous |
Clear cell |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study abbreviation | Median age at diagnosis | n (%) | Median age at diagnosis | n (%) | Median age at diagnosis | n (%)b | Median age at diagnosis | n (%)b | Median age at diagnosis | n (%)b | Median age at diagnosis | n (%)b |
| USA and Canada | ||||||||||||
| AARP | 69.9 | 1350 (18.5) | 68.0 | 701 (13.7) | 68.4 | 546 (77.9) | 65.3 | 85 (12.1) | 66.8 | 44 (6.3) | 64.8 | 26 (3.7) |
| AHS2 | 67.7 | 98 (1.3) | 65.4 | 67 (1.3) | 66.4 | 45 (67.2) | 61.6 | 13 (19.4) | 79.8 | 5 (7.5) | 65.2 | 4 (6.0) |
| BCDDP | 68.5 | 175 (2.4) | 68.0 | 96 (1.9) | 69.0 | 67 (69.8) | 66.0 | 21 (21.9) | 74.0 | 5 (5.2) | 58.0 | 3 (3.1) |
| CSDLH | 62.7 | 112 (1.5) | 60.0 | 72 (1.4) | 65.5 | 40 (55.6) | 53.6 | 14 (19.4) | 57.4 | 10 (13.9) | 46.0 | 8 (11.1) |
| CLUEII | 66.5 | 90 (1.2) | 64.0 | 52 (1.0) | 64.0 | 39 (75.0) | 62.0 | 9 (17.3) | 54.0 | 2 (3.8) | 69.5 | 2 (3.8) |
| CPS2 | 70.0 | 578 (7.9) | 70.0 | 455 (8.9) | 70.0 | 358 (78.7) | 69.0 | 51 (11.2) | 68.0 | 26 (5.7) | 69.0 | 20 (4.4) |
| CTS | 66.0 | 216 (3) | 65.0 | 161 (3.2) | 68.0 | 104 (64.6) | 61.0 | 29 (18.0) | 70.0 | 7 (4.3) | 56.0 | 21 (13.0) |
| IWHS | 74.0 | 380 (5.2) | 73.0 | 242 (4.7) | 73.0 | 194 (80.2) | 71.5 | 20 (8.3) | 69.0 | 17 (7.0) | 73.0 | 11 (4.5) |
| MEC | 69.4 | 104 (1.4) | 67.6 | 64 (1.3) | 68.7 | 49 (76.6) | 65.8 | 6 (9.4) | 71.0 | 4 (6.3) | 56.5 | 5 (7.8) |
| NHS | 65.9 | 910 (12.4) | 65.8 | 818 (16.0) | 66.6 | 626 (76.5) | 62.7 | 110 (13.4) | 62.0 | 42 (5.1) | 62.1 | 40 (4.9) |
| NHS2 | 51.2 | 282 (3.9) | 50.8 | 230 (4.5) | 51.3 | 136 (59.1) | 49.6 | 49 (21.3) | 45.7 | 12 (5.2) | 52.3 | 33 (14.3) |
| NYUWHS | 63.9 | 136 (1.9) | 63.9 | 104 (2.0) | 64.8 | 81 (77.9) | 57.1 | 7 (6.7) | 64.5 | 8 (7.7) | 57.8 | 8 (7.7) |
| PLCO | 70.0 | 412 (5.6) | 70.0 | 273 (5.3) | 70.0 | 230 (84.2) | 65.0 | 23 (8.4) | 64.0 | 8 (2.9) | 70.0 | 12 (4.4) |
| SIS | 58.6 | 47 (0.6) | 58.0 | 36 (0.7) | 58.0 | 28 (77.8) | 59.7 | 4 (11.1) | NA | NA | 56.3 | 4 (11.1) |
| VITAL | 67.6 | 142 (1.9) | 67.1 | 106 (2.1) | 68.0 | 89 (84.0) | 62.6 | 8 (7.5) | 71.9 | 3 (2.8) | 62.9 | 6 (5.7) |
| WHS | 63.4 | 207 (2.8) | 63.3 | 158 (3.1) | 63.6 | 131 (82.9) | 60.4 | 20 (12.7) | 65.0 | 7 (4.4) | NA | NA |
| Europe | ||||||||||||
| BGS | 62.1 | 104 (1.4) | 62.1 | 70 (1.4) | 62.6 | 48 (68.6) | 53.2 | 5 (7.1) | 67.6 | 13 (18.6) | 54.7 | 4 (5.7) |
| EPIC | 62.6 | 837 (11.4) | 61.9 | 602 (11.8) | 62.9 | 435 (72.3) | 59.6 | 81 (13.5) | 59.5 | 49 (8.1) | 59.9 | 37 (6.1) |
| NLCS | 71.0 | 471 (6.4) | 70.0 | 333 (6.5) | 71.0 | 240 (72.1) | 70.0 | 37 (11.1) | 68.0 | 41 (12.3) | 68.0 | 15 (4.5) |
| SMC | 67.7 | 213 (2.9) | 67.0 | 161 (3.2) | 66.9 | 116 (72.0) | 67.4 | 30 (18.6) | 76.3 | 10 (6.2) | 63.4 | 5 (3.1) |
| WLHS | 53.8 | 224 (3.1) | 54.2 | 129 (2.5) | 54.3 | 89 (69.0) | 52.8 | 18 (14.0) | 53.3 | 15 (11.6) | 54.3 | 7 (5.4) |
| Other regions | ||||||||||||
| MCCS | 68.7 | 107 (1.5) | 68.7 | 88 (1.7) | 69.5 | 61 (69.3) | 63.6 | 9 (10.2) | 61.5 | 10 (11.4) | 65.0 | 8 (9.1) |
| SCHS | 63.2 | 119 (1.6) | 61.2 | 91 (1.8) | 64.3 | 42 (46.2) | 60.9 | 15 (16.5) | 59.3 | 18 (19.8) | 60.8 | 16 (17.6) |
| All studies | 66.5 | 7314 (100) | 65.4 | 5109 (100) | 67.0 | 3794 (74.2) | 62.9 | 664 (13.0) | 64.1 | 356 (7.0) | 61.2 | 295 (5.8) |
NA, not applicable; OC3, Ovarian Cancer Cohort Consortium.
See Table 1 for full study names corresponding to each abbreviation. BWHS, CPS3 and JSB are not included in this table because they have not yet contributed data to the OC3.
Percentages represent the proportion of cases of each histotype, among cases classified as one of the four major histotypes within each study.
In all the OC3 studies except SCHS, serous cancer was the histotype in the majority of cases, followed by endometrioid and mucinous cancers, which have similar prevalence, and clear cell cancer, the least common of the four major histotypes (Supplementary Figure S1, available as Supplementary data at IJE online). This distribution is consistent with observations in previous studies focused on women of European ancestry.12,13 SCHS, which enrolled Chinese adults living in Singapore, is unique in that the serous histotype is observed in less than half of the cases, and mucinous and clear cell tumours are more common than in the other studies. This distribution is consistent with international registry data showing a greater prevalence of clear cell tumours among Asian versus White women.14 Pooled case numbers are 3794 for serous, 664 for endometrioid, 356 for mucinous and 295 for clear cell. Moreover, case numbers for serous and endometrioid tumours by grade are large enough to allow for exploration of potential differences in risk factor associations by grade (Supplementary Table S1, available as Supplementary data at IJE online).
How often have they been followed up?
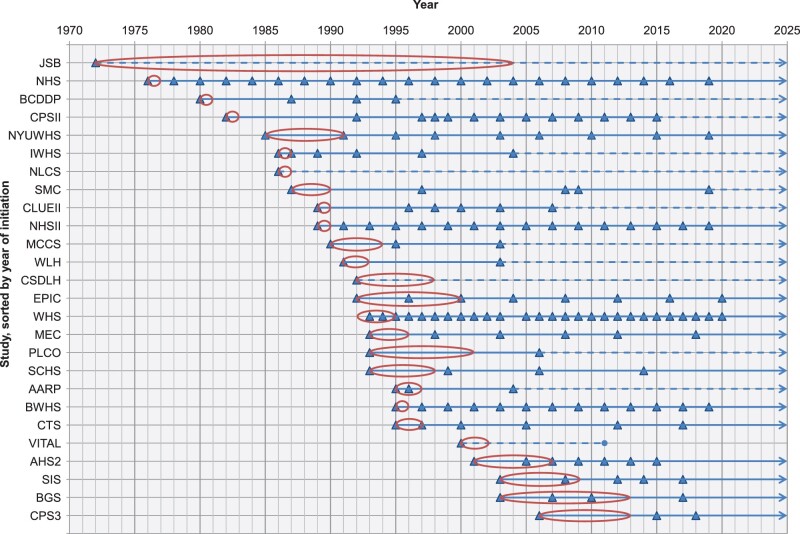
Mailed or online questionnaires were the primary mode of data collection across all the studies, although a few studies incorporated face-to-face or telephone interviews with participants to collect data. Of the 23 studies, 21 sent at least one follow-up questionnaire to participants (or conducted follow-up interviews) to collect updated exposure and outcome information after baseline (Table 1, Figure 1). The interval between questionnaires ranged from annually to 13 years, with most studies collecting data every 2–5 years. Currently, 11 studies are actively following participants through periodic questionnaires or in-person interviews. However, most studies that are no longer actively contacting participants continue to passively follow them for cancer diagnosis and death via linkage with cancer and death registries.
Figure 1.
Timing of questionnaires in the Ovarian Cancer Cohort Consortium, by study.
See Table 1 for full study names corresponding to each abbreviation. Triangles indicate a questionnaire mailing or interview. Ovals indicate the study enrolment period. Solid arrow lines indicate studies that are still actively following participants (i.e., planning to send additional questionnaires or conduct additional interviews to collect updated exposure and/or disease information). Dotted arrow lines indicate studies that no longer actively follow participants but are passively collecting participant endpoints via linkage with cancer and death registries. The countries within the European Prospective Investigation into Cancer and Nutrition (EPIC) collect data at different time intervals; data are shown for the country within EPIC with the shortest interval between questionnaires.
What has been measured?
To initiate the consortium, the OC3 data coordinating centre requested baseline questionnaire data from each study as well as information on ovarian cancer diagnoses and deaths that had been ascertained in the study (see Supplementary Methods for details about procedures for requesting data and harmonizing variables). Information on body mass index, menarche and menopause, parity, oral contraceptive use, hysterectomy and oophorectomy status, postmenopausal hormone therapy use, and several other putative risk factors (e.g. alcohol intake, smoking, diabetes, cardiovascular disease, aspirin use) are consistently available across many of the studies (Table 3). The studies identify cancer cases and case characteristics (e.g. stage, histotype, morphology, grade) either through participant self-reports on questionnaires with subsequent confirmation by review of pathology reports or through linkage with regional or national cancer registries, or a combination of the two methods. Histotype was coded by the individual studies using International Classification of Diseases for Oncology codes,15 and the data coordinating centre created a consensus codebook to classify histotype in alignment with the World Health Organization classification (first revision).16 Almost all the studies confirm deaths through linkage with regional or national death registries. Details about ovarian cancer treatment and cause of death are available in a subset of studies (Table 3).
Table 3.
Availability of data on known and suspected ovarian cancer risk factors at baseline, cause of death and ovarian cancer treatment, and biospecimens collected in each OC3 studya
| Variable | AARP | AHS2 | BCDDP | BGS | CLUEII | CPS2 | CSDLH | CTS | EPIC | IWHS | MCCS | MEC | NHS | NHSII | NLCS | NYUWHS | PLCO | SCHS | SMC | SIS | VITAL | WHS | WLH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Anthropometric variables |
|||||||||||||||||||||||
| Height | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Body mass index in young adulthoodb | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||
| Body mass index at baseline | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Menarche and menopause variables | |||||||||||||||||||||||
| Age at menarche | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Menopausal status | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Age at natural menopause | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Reason for menopause | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Reproductive history | |||||||||||||||||||||||
| Parity | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Age at first birth | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Age at latest birth | X | X | X | X | X | X | X | X | X | ||||||||||||||
| Duration of breastfeeding | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| Contraception history | |||||||||||||||||||||||
| Tubal ligation | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Ever used oral contraceptives | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Oral contraceptive use duration | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Age first used oral contraceptives | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Age last used oral contraceptives | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| Family history of breast or ovarian cancer | |||||||||||||||||||||||
| 1st degree family history of breast cancer | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| 1st degree family history of ovarian cancer | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Hysterectomy/oophorectomy status | |||||||||||||||||||||||
| Hysterectomy status | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Oophorectomy status | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Postmenopausal use of hormone therapy | |||||||||||||||||||||||
| Postmenopausal HT use | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Duration of postmenopausal HT use | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Ever use of oral oestrogen only | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Duration of use of oral oestrogen only | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||
| Ever use of oral oestrogen+progestin only | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||
| Duration of use of oral oestrogen+progestin | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| Ever use of other postmenopausal HT | X | X | X | X | X | X | X | ||||||||||||||||
| Duration of use of other postmenopausal HT | X | X | X | X | X | X | |||||||||||||||||
| Other putative risk factors | |||||||||||||||||||||||
| Endometriosis | X | X | X | X | X | X | X | X | X | ||||||||||||||
| Alcohol intake | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Smoking status | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Pack-years (among ever smokers) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Cardiovascular disease | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Diabetes | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Ever diagnosed with autoimmune disease | X | X | X | X | X | X | X | ||||||||||||||||
| Ever diagnosed with PID | X | X | X | X | X | ||||||||||||||||||
| Regular aspirin or NSAID use | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Race | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Education | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Dietc | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
| Cause of death, ovarian cancer treatment | |||||||||||||||||||||||
| Treatment data from pathology, surgical reports, or tumour registry | X | X | X | X | X | X | X | X | X | ||||||||||||||
| Cause of death | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||
| Biospecimensd | |||||||||||||||||||||||
| Blood | (X) | X | X | X | X | X | X | (X) | (X) | (X) | X | X | X | (X) | X | X | |||||||
| Urine | (X) | (X) | X | (X) | (X) | (X) | X | (X) | X | ||||||||||||||
| Buccal cells | X | (X) | (X) | X | (X) | (X) | X | ||||||||||||||||
| Ovarian tumour tissue | X | X | X | X | X | X | X | X | X |
HT, hormone therapy; NSAID, non-steroidal anti-inflammatory drug; OC3, Ovarian Cancer Cohort Consortium; PID, pelvic inflammatory disease.
See Table 1 for full study names corresponding to each abbreviation. BWHS, CPS3, and JSB are not included in this because they have not yet contributed data to the OC3.
For BMI in young adulthood, all studies used recalled weight at age 18 except AHS2, CSDLH and PLCO which used age 20, and CLUEII which used age 21.
Data on diet were collected after baseline in some studies.
(X) indicates that a subset of the study population was targeted for biospecimen collection; X (without parentheses) indicates that the study attempted to collect the biospecimen in the whole study population.
Prospectively collected biospecimens are available in many of the studies (Table 3). Blood was the most commonly collected biospecimen in the studies. Given the prospective blood collection, there is a unique opportunity in the OC3 to examine whether a biomarker-risk association varies by timing of blood collection in relation to ovarian cancer diagnosis. Blood was collected 5 or more years before ovarian cancer diagnosis for 60% of ovarian cancer cases, providing an opportunity to study a range of biomarkers related to aetiology and natural history (Supplementary Table S2, available as Supplementary data at IJE online). Blood was collected within 3 years before diagnosis for 23% of cases, highlighting the potential to conduct studies of biomarkers for early detection. Germline DNA is also available in many studies for all or some participants and several have collected buccal cells and urine. The subset of participants who provided blood or buccal cell samples tended to be younger and more likely to have ever used oral contraceptives, be premenopausal and have shorter duration of postmenopausal hormone therapy use than all participants in the OC3 (Supplementary Table S3, available as Supplementary data at IJE online). This is largely explained by the influence of NHSII, which has the youngest participants of all the OC3 studies (median age at enrolment = 35 years) and collected biospecimens from more than 29 600 women.
Measurement of tissue markers by immunohistochemistry, or newer approaches such as multiplex immunofluorescence, holds promise for a deeper understanding of the mechanisms underlying associations between risk factors and cancer risk. In this regard, some of the OC3 studies have received, or continue to request, consent from participants diagnosed with ovarian cancer to obtain ovarian tumour tissue from the hospitals where participants were treated, or have tissue blocks or tissue microarrays available for research purposes (Table 3).
The consortium has received funding to request additional variables from the member studies, including data from follow-up questionnaires and updated information on cancer incidence and deaths. The consortium will continue to expand the pooled dataset as more projects are funded.
What has been found?
Several pooled analyses of risk factor data and of previously measured biomarker data have been published by the OC3 (see Supplementary Methods for the general approach to statistical analyses in the OC3). Depending on the availability of exposure and biomarker data, the analyses included different subsets of OC3 cohorts.
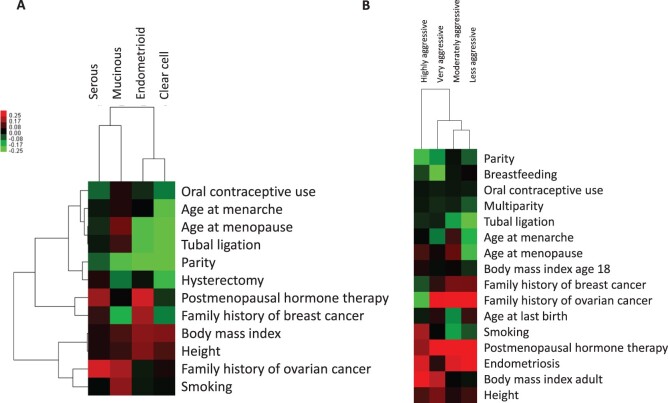
Ovarian cancers can be subdivided by various characteristics, including histotype, tumour aggressiveness (based on survival time) and anatomical location of presentation (ovary, fallopian tube, peritoneum). The first paper from the OC3 found substantial heterogeneity of 12 risk factor associations by histotype, with the weakest associations observed for most risk factors with serous tumours, the most common and aggressive subtype of ovarian cancer.7 Unsupervised clustering of selected risk factors highlighted similar risk factor patterns between endometrioid and clear cell tumours (Figure 2A). When evaluating risk factors by tumour aggressiveness, several risk factors, particularly high body mass index (BMI) and cigarette smoking, were associated with more aggressive, rapidly fatal ovarian cancer, which could reflect a combination of factors such as tumour biology, access to care and/or treatment response (Figure 2B).17 The heterogeneity was not fully explained by histotype, suggesting that both histotype and tumour aggressiveness contribute independently to ovarian cancer heterogeneity. A separate analysis found that most risk factor associations did not vary by anatomical site, except for age at first pregnancy, tubal ligation and early-adult BMI.18 Currently, there is not a single classification that by itself distinguishes aetiologically or clinically meaningful subgroups of ovarian cancers. In the future, molecular classifications based on immunohistochemistry and molecular profiling will be used to further classify ovarian cancers and study risk factor and biomarker associations.19–21
Figure 2.
Unsupervised hierarchical clustering of ovarian cancer subtypes by their associations with risk factors.
Unsupervised hierarchical clustering of (A) the four major ovarian cancer histotypes and (B) four ovarian tumour aggressiveness categories (highly aggressive = time between diagnosis and death <1 year; very aggressive = death in 1 to <3 years; moderately aggressive = death in 3 to <5 years; less aggressive = lived ≥5 years). Unless otherwise noted, the categories used in the cluster analysis were ever versus never parous, ever versus never oral contraceptive use, ever versus never tubal ligation, ever versus never endometriosis, age at menarche >15 vs ≤11 years, age at menopause <40 versus 50 to 55 years, ever versus never menopausal hormone therapy use, ever versus never hysterectomy, family history of breast cancer (yes vs no), family history of ovarian cancer (yes vs no), body mass index >35 versus 20 to 25 kg/m2, height (per 5-cm increase), and ever versus never smoking. The colour scale shows the range of β-values for each exposure.
Several OC3 studies have focused on inflammation-related exposures or biomarkers. Of these, two examined sources of inflammation that might influence cancer risk via direct impact on the ovaries or fallopian tubes [lifetime ovulatory cycles (LOCs)22 and genital powder use23]. The LOC analysis precisely quantified the risk associated with number of ovulatory cycles and showed heterogeneity by ovarian cancer subtype. Further, LOCs were independently associated with ovarian cancer risk after adjusting for the individual contributors to LOC calculations. Regarding genital powder use, previous findings were primarily based on case-control studies that are at risk of recall bias, and the few prospective studies were underpowered to find weaker risk associations. In the largest prospective study so far, the OC3 found a very small, positive association between genital powder use and ovarian cancer risk among all women [hazard ratio (HR) 1.08, 95% confidence interval (CI) 0.99–1.17] as well as among women with intact uterus and fallopian tubes (HR 1.13, 95% CI 1.01–1.26).23 Other potential risk factors examined in the OC3 reflect systemic exposure to inflammation. For example, in a pooled evaluation in a subset of OC3 cohorts with previously measured plasma or serum C-reactive protein (CRP), CRP concentrations >10 versus <1 mg/L were associated with more than 3-fold increased risks of endometrioid and mucinous cancers.24 A study of analgesic use and ovarian cancer risk from the OC3 confirmed findings from previous case-control studies showing an inverse association between frequent use of aspirin, a non-steroidal anti-inflammatory drug, and risk of ovarian cancer. No subtype heterogeneity in this association was observed.25 A complete list of OC3 publications can be found at [theoc3.org/research].
What are the main strengths and weaknesses?
Major strengths of the consortium are the large prospective dataset of more than 1.3 million women, including more than 7300 incident ovarian cancer cases, and extensive data on histotype which permit conduct of well-powered studies of risk factors and biomarkers in ovarian cancer subtypes. The consortium setting enables relatively quick evaluation of the reproducibility of associations across many study populations. The cohort studies have a rich set of risk factor data covering all established ovarian cancer risk factors and many other exposures that could be relevant for specific ovarian cancer subtypes. A subset of OC3 studies has prospective blood collections, and a few have repeated blood samples, allowing conduct of well-powered biomarker studies including research to identify or validate markers of early detection. Furthermore, several studies in the OC3 have tissue specimens available that enable molecular studies designed to understand ovarian cancer aetiology and to evaluate molecular subtypes. The OC3 is closely connected to the Ovarian Cancer Association Consortium (OCAC), the largest consortium of ovarian cancer studies, which includes primarily case-control studies and clinical case series focused on genetic association studies but has also a strong epidemiological component. Several projects are under way which will pool data from both consortia, which are particularly suited to study rare exposures in rare subtypes. Furthermore, the OCAC has completed several genome-wide association studies (GWAS) and some cohorts in OC3 contributed case-control sets for genotyping in OCAC. These data and other GWAS sources in OC3 studies create opportunities for studies of risk factor by gene interactions. The OC3 is ideally situated to validate associations of individual risk factors, genetic susceptibility and integrated risk models developed in OCAC.
However, some weaknesses of the OC3 need to be noted. Although the OC3 includes cohorts from around the world, the racial/ethnic diversity is very limited with only one cohort from Asia and none from Africa, which reflects the few cohort studies conducted in these regions. Most cohorts in the OC3 cover a limited age range at enrolment, which limits the exposure windows that can be studied with biomarkers. Despite the size of the consortium, there is still limited sample size for analyses of rare histotypes. The OC3 database currently only includes exposures measured at baseline, but an ongoing effort is under way to obtain follow-up data in a subset of studies which will enable analyses accounting for time-varying exposures. Biospecimens are only available in a subset of studies, and repeated blood collections are available only in very few studies. The simultaneous evaluation of different exposures and biomarkers may be limited by missing variables and missing data across the studies, but this might be addressed by large-scale imputation approaches.
How can I access the data?
Making OC3 data widely available for epidemiological studies on ovarian cancer is one of the core missions of the OC3. The OC3 welcomes collaboration requests from studies wishing to join the consortium as well as from researchers seeking to use the data for specific research projects. Researchers do not need to be affiliated with an OC3 member study to submit a study proposal. Research proposals from junior scientists are encouraged. Interested researchers should visit the OC3 website [theoc3.org] to fill out a contact form, view an up-to-date list of currently approved projects and read instructions for proposing a new project. Briefly, to propose a project, the researcher must submit an analysis proposal form which is then circulated to the OC3 Steering Committee for review. OC3 studies have agreed to an opt-in approach for analyses. Therefore, the researcher must obtain approval from the main contact of each study for that study’s data to be included in the analysis. Finally, to access data, a data use agreement is set up between the researcher's institution and the data coordinating centre (based at Moffitt Cancer Center) and appropriate institutional review board approvals may need to be obtained. Researchers involved in active analyses participate in bi-weekly analysis calls to provide updates on progress, present preliminary results and receive early feedback. The OC3 website [theoc3.org] contains further details related to OC3 leadership and policies. Enquiries can also be submitted directly to Dr Shelley Tworoger [shelley.tworoger@moffitt.org]. The data underlying this article were provided by each of the member OC3 studies under a Data Use Agreement with Moffitt Cancer Center. Data will be shared following OC3 analysis proposal guidelines which can be found at [theoc3.org/policies]. These guidelines include approval of an analysis proposal by the OC3 Steering Committee, written approval from the main contact of each study, set-up of a data use agreement between the requestor’s institution and Moffitt Cancer Center, and approval by the appropriate institutional review board.
Supplementary Data
Supplementary data are available at IJE online.
Funding
The OC3 received support from the U.S. Department of Defense Ovarian Cancer Research Program (grant numbers W81XWH-12–1-0561, W81XWH-19–1-0346), National Cancer Institute at the National Institutes of Health (grant numbers R01 CA258679, U01 CA164973) and Intramural Research Program of the National Cancer Institute at the National Institutes of Health.
The member studies of the OC3 received support from the: National Cancer Institute at the National Institutes of Health (grant number 1U01CA152939), World Cancer Research Fund (grant number 2009/93) and Ardmore Institute of Health (Adventist Health Study II); American Cancer Society and National Cancer Institute at the National Institutes of Health (Breast Cancer Detection Demonstration Project); National Cancer Institute at the National Institutes of Health (grant numbers P30 CA006973, CA97857, CA86308) (Campaign Against Cancer and Stroke); National Cancer Institute at the National Institutes of Health (grant number R01 CA39742) (Iowa Women’s Health Study); National Cancer Institute at the National Institutes of Health (grant number CA164973) (Multiethnic Cohort Study); Intramural Research Program of the National Cancer Institute at the National Institutes of Health (NIH-AARP Diet and Health Study); National Cancer Institute at the National Institutes of Health (grant numbers UM1 CA186107, P01 CA87969, R01 CA49449, UM1 CA176726, R01 CA67262) (Nurses’ Health Study and Nurses’ Health Study II); National Cancer Institute at the National Institutes of Health (grant numbers UM1 CA182934, P30 CA016087, P30 ES000260) (NYU Women’s Health Study); National Cancer Institute at the National Institutes of Health (grant number UM1 CA182876) (Singapore Chinese Health Study); Intramural Research Program of the National Institute of Environmental Health Sciences at the National Institutes of Health (fund number Z01ES044005) (Sister Study); Swedish Research Council (Swedish Mammography Cohort); National Cancer Institute and Office of Dietary Supplements at the National Institutes of Health (grant number K05 CA154337) (Vitamins and Lifestyle Study); and National Cancer Institute (grant number CA047988) and National Heart, Lung, and Blood Institute at the National Institutes of Health (grant numbers HL043851, HL080467, HL099355) (The Women’s Health Study). The California Teachers Study and the research reported in this publication were supported by National Cancer Institute at the National Institutes of Health (grant numbers U01 CA199277, P30 CA033572, P30 CA023100, UM1 CA164917, R01 CA077398). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. The collection of cancer incidence data used in the California Teachers Study was supported by the: California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California and contract HHSN261201800009I awarded to the Public Health Institute. The opinions, findings and conclusions expressed herein are those of the author(s) and do not necessarily reflect the official views of the State of California, Department of Public Health, the National Cancer Institute, National Institutes of Health, Centers for Disease Control and Prevention or their Contractors and Subcontractors, or the Regents of the University of California or any of its programmes. All aspects of the Cancer Prevention Study II were funded by the Intramural Research Program of the American Cancer Society and by the National Cancer Institute at the National Institutes of Health Intramural Research Program. The coordination of the European Prospective Investigation into Cancer and Nutrition (EPIC) is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by the: Danish Cancer Society (Denmark); Ligue Contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); German Cancer Aid, German Cancer Research Center (DKFZ), Federal Ministry of Education and Research (BMBF) (Germany); Associazione Italiana per la Ricerca sul Cancro-AIRC-Italy and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF), Statistics Netherlands (The Netherlands); Nordforsk (Norway); Health Research Fund (FIS), PI13/00061 to Granada, PI13/01162 to EPIC-Murcia, Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra, ISCIII RETIC (RD06/0020) (Spain); Swedish Cancer Society, Swedish Research Council and County Councils of Skåne and Västerbotten (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C570/A16491 and C8221/A19170 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk, MR/M012190/1 to EPIC-Oxford) (UK). The Generations Study was supported by Breakthrough Breast Cancer and the Institute of Cancer Research. The Institute for Cancer Research is supported by National Health Service funding to the National Institute for Health Research Biomedical Research Centre. Cohort recruitment for the Melbourne Collaborative Cohort Study (MCCS) was funded by VicHealth and Cancer Council Victoria. The MCCS was further augmented by Australian National Health and Medical Research Council (grant numbers 209057, 396414, 1074383) and by infrastructure provided by Cancer Council Victoria. Cases and their vital status were ascertained through the Victorian Cancer Registry and the Australian Institute of Health and Welfare, including the National Death Index and the Australian Cancer Database. The Swedish Women’s Lifestyle and Health Study was supported by the Swedish Cancer Society and Swedish Research Council. Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization .
Supplementary Material
Acknowledgements
The authors would like to thank the California Teachers Study Steering Committee that is responsible for the formation and maintenance of the Study within which this research was conducted. A full list of California Teachers Study team members is available at [https://www.calteachersstudy.org/team]. The authors would like to acknowledge the Channing Division of Network Medicine, Department of Medicine, Brigham and Women's Hospital, as the home of the Nurses' Health Study. We would like to thank the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Author contributions
Author contributions are available as a Supplementary file at IJE online.
Conflict of interest
None declared.
Contributor Information
Mary K Townsend, Department of Cancer Epidemiology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Britton Trabert, Metabolic Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Renée T Fortner, Division of Cancer Epidemiology, German Cancer Research Centre (DKFZ), Heidelberg, Germany.
Alan A Arslan, Division of Epidemiology, Departments of Obstetrics and Gynecology, Population Health, and Environmental Medicine, New York University School of Medicine, New York, NY, USA.
Julie E Buring, Department of Medicine, Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA.
Brian D Carter, Behavioral and Epidemiology Research Group, American Cancer Society, Atlanta, GA, USA.
Graham G Giles, Cancer Epidemiology Division, Cancer Council Victoria, Melbourne, VIC, Australia; Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, VIC, Australia; Precision Medicine, School of Clinical Sciences at Monash Health, Monash University, Clayton, VIC, Australia.
Sarah R Irvin, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Michael E Jones, Division of Genetics and Epidemiology, Institute of Cancer Research, London, UK.
Rudolf Kaaks, Division of Cancer Epidemiology, German Cancer Research Centre (DKFZ), Heidelberg, Germany.
Victoria A Kirsh, Ontario Health Study, Ontario Institute for Cancer Research, Toronto, ON, Canada; Dalla Lana School of Public Health, University of Toronto, Toronto, ON, Canada.
Synnove F Knutsen, School of Public Health, Loma Linda University, Loma Linda, CA, USA.
Woon-Puay Koh, Healthy Longevity Translational Research Programme, Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore; Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore, Singapore.
James V Lacey, Jr, Beckman Research Institute, City of Hope, Duarte, CA, USA.
Hilde Langseth, Department of Research, Cancer Registry of Norway, Oslo, Norway; Department of Epidemiology and Biostatistics, Imperial College London, London, UK.
Susanna C Larsson, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden.
I-Min Lee, Department of Medicine, Harvard Medical School, Boston, MA, USA; Department of Epidemiology, Harvard T.H. Chan School of Public Health, Boston, MA, USA; Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA.
María Elena Martínez, Moores Cancer Center, University of California, San Diego, La Jolla, CA, USA.
Melissa A Merritt, Cancer Epidemiology Program, University of Hawaii Cancer Center, Honolulu, HI, USA.
Roger L Milne, Cancer Epidemiology Division, Cancer Council Victoria, Melbourne, VIC, Australia; Centre for Epidemiology and Biostatistics, Melbourne School of Population and Global Health, University of Melbourne, Melbourne, VIC, Australia; Precision Medicine, School of Clinical Sciences at Monash Health, Monash University, Clayton, VIC, Australia.
Katie M O’Brien, Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA.
Michael J Orlich, School of Public Health, Loma Linda University, Loma Linda, CA, USA.
Julie R Palmer, Slone Epidemiology Center, Boston University School of Medicine, Boston, MA, USA.
Alpa V Patel, Behavioral and Epidemiology Research Group, American Cancer Society, Atlanta, GA, USA.
Ulrike Peters, Division of Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA, USA; Department of Epidemiology, University of Washington School of Public Health, Seattle, WA, USA.
Jenny N Poynter, Division of Pediatric Epidemiology and Clinical Research, University of Minnesota, Minneapolis, MN, USA.
Kim Robien, Department of Exercise and Nutrition Sciences, Milken Institute School of Public Health, George Washington University, Washington, DC, USA.
Thomas E Rohan, Department of Epidemiology and Population Health, Albert Einstein College of Medicine, Bronx, NY, USA.
Lynn Rosenberg, Slone Epidemiology Center, Boston University School of Medicine, Boston, MA, USA.
Sven Sandin, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden; Department of Psychiatry, Icahn School of Medicine, Mount Sinai, New York, NY, USA; Seaver Autism Center for Research and Treatment, Icahn School of Medicine, Mount Sinai, New York, NY, USA.
Dale P Sandler, Epidemiology Branch, National Institute of Environmental Health Sciences, Research Triangle Park, NC, USA.
Leo J Schouten, Department of Epidemiology, GROW-School for Oncology and Developmental Biology, Maastricht University, Maastricht, The Netherlands.
V Wendy Setiawan, Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Anthony J Swerdlow, Division of Genetics and Epidemiology and Division of Breast Cancer Research, Institute of Cancer Research, London, UK.
Giske Ursin, Cancer Registry of Norway, Oslo, Norway; Department of Nutrition, Institute of Basic Medical Sciences, University of Oslo, Oslo, Norway; Department of Preventive Medicine, University of Southern California, Los Angeles, CA, USA.
Piet A van den Brandt, Department of Epidemiology, GROW-School for Oncology and Developmental Biology, Maastricht University, Maastricht, The Netherlands.
Kala Visvanathan, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Elisabete Weiderpass, Office of the Director, International Agency for Research on Cancer, World Health Organization, Lyon, France.
Alicja Wolk, Institute of Environmental Medicine, Karolinska Institutet, Stockholm, Sweden; Department of Surgical Sciences, Uppsala University, Uppsala, Sweden.
Jian-Min Yuan, UPMC Hillman Cancer Center, University of Pittsburgh, Pittsburgh, PA, USA; Department of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA.
Anne Zeleniuch-Jacquotte, Department of Population Health and Department of Environmental Medicine, New York University School of Medicine, New York, NY, USA.
Shelley S Tworoger, Department of Cancer Epidemiology, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL, USA.
Nicolas Wentzensen, Clinical Genetics Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Buys SS, Partridge E, Black A. et al. ; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011;305:2295–303. [DOI] [PubMed] [Google Scholar]
- 3. Jacobs IJ, Menon U, Ryan A. et al. Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial. Lancet 2016;387:945–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epidemiology Working Group Steering Committee, Ovarian Cancer Association Consortium Members of the EWG SC in alphabetical order:, Doherty JA, Jensen A, Kelemen LE. et al. Current gaps in ovarian cancer epidemiology: the need for new population-based research. J Natl Cancer Inst 2017;109:djx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pfeiffer RM, Park Y, Kreimer AR. et al. Risk prediction for breast, endometrial, and ovarian cancer in white women aged 50 y or older: derivation and validation from population-based cohort studies. PLoS Med 2013;10:e1001492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peres LC, Sinha S, Townsend MK. et al. Predictors of survival trajectories among women with epithelial ovarian cancer. Gynecol Oncol 2020;156:459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wentzensen N, Poole EM, Trabert B. et al. Ovarian cancer risk factors by histologic subtype: an analysis from the Ovarian Cancer Cohort Consortium. J Clin Oncol 2016;34:2888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kurman RJ, Shih IM.. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol 2016;186:733–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wu RC, Wang P, Lin SF. et al. Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J Pathol 2019;248:41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trabert B. Body powder and ovarian cancer risk-what is the role of recall bias? Cancer Epidemiol Biomarkers Prev 2016;25:1369–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Swerdlow AJ, Harvey CE, Milne RL. et al. The National Cancer Institute Cohort Consortium: an international pooling collaboration of 58 cohorts from 20 countries. Cancer Epidemiol Biomarkers Prev 2018;27:1307–19. [DOI] [PubMed] [Google Scholar]
- 12. Peres LC, Risch H, Terry KL. et al. ; African American Cancer Epidemiology Study and the Ovarian Cancer Association Consortium. Racial/ethnic differences in the epidemiology of ovarian cancer: a pooled analysis of 12 case-control studies. Int J Epidemiol 2018;47:460–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fuh KC, Shin JY, Kapp DS. et al. Survival differences of Asian and Caucasian epithelial ovarian cancer patients in the United States. Gynecol Oncol 2015;136:491–97. [DOI] [PubMed] [Google Scholar]
- 14. Coburn SB, Bray F, Sherman ME, Trabert B.. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int J Cancer 2017;140:2451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization International Agency for Research on Cancer. International Classification of Diseases for Oncology. Geneva: WHO Press, 2013. [Google Scholar]
- 16. Kurman RJ, Carcangiu ML, Herrington CS, Young RH.. WHO Classification of Tumours of Female Reproductive Organs. 4th edn. Lyon: International Agency for Research on Cancer, 2014. [Google Scholar]
- 17. Fortner RT, Poole EM, Wentzensen NA. et al. Ovarian cancer risk factors by tumor aggressiveness: an analysis from the Ovarian Cancer Cohort Consortium. Int J Cancer 2019;145:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fortner RT, Rice MS, Knutsen SF. et al. Ovarian cancer risk factor associations by primary anatomic site: the Ovarian Cancer Cohort Consortium. Cancer Epidemiol Biomarkers Prev 2020;29:2010–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bodelon C, Killian JK, Sampson JN. et al. Molecular classification of epithelial ovarian cancer based on methylation profiling: evidence for survival heterogeneity. Clin Cancer Res 2019;25:5937–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Millstein J, Budden T, Goode EL. et al. Prognostic gene expression signature for high-grade serous ovarian cancer. Ann Oncol 2020;31:1240–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Talhouk A, George J, Wang C. et al. Development and validation of the gene-expression predictor of high-grade-serous ovarian carcinoma molecular subTYPE (PrOTYPE). Clin Cancer Res 2020;26:5411–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trabert B, Tworoger SS, O'Brien KM. et al. The risk of ovarian cancer increases with an increase in the lifetime number of ovulatory cycles: an analysis from the Ovarian Cancer Cohort Consortium (OC3). Cancer Res 2020;80:1210–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Brien KM, Tworoger SS, Harris HR. et al. Association of powder use in the genital area with risk of ovarian cancer. JAMA 2020;323:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peres LC, Mallen AR, Townsend MK. et al. High levels of C-reactive protein are associated with an increased risk of ovarian cancer: results from the Ovarian Cancer Cohort Consortium. Cancer Res 2019;79:5442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trabert B, Poole EM, White E. et al. ; Ovarian Cancer Cohort Consortium (OC3). Analgesic use and ovarian cancer risk: an analysis in the Ovarian Cancer Cohort Consortium. J Natl Cancer Inst 2019;111:137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.