Abstract
Background
Previous epidemiological studies have found positive associations between maternal infections and childhood leukaemia; however, evidence from prospective cohort studies is scarce. We aimed to examine the associations using large-scale prospective data.
Methods
Data were pooled from six population-based birth cohorts in Australia, Denmark, Israel, Norway, the UK and the USA (recruitment 1950s-2000s). Primary outcomes were any childhood leukaemia and acute lymphoblastic leukaemia (ALL); secondary outcomes were acute myeloid leukaemia (AML) and any childhood cancer. Exposures included maternal self-reported infections [influenza-like illness, common cold, any respiratory tract infection, vaginal thrush, vaginal infections and urinary tract infection (including cystitis)] and infection-associated symptoms (fever and diarrhoea) during pregnancy. Covariate-adjusted hazard ratio (HR) and 95% confidence interval (CI) were estimated using multilevel Cox models.
Results
Among 312 879 children with a median follow-up of 13.6 years, 167 leukaemias, including 129 ALL and 33 AML, were identified. Maternal urinary tract infection was associated with increased risk of any leukaemia [HR (95% CI) 1.68 (1.10–2.58)] and subtypes ALL [1.49 (0.87–2.56)] and AML [2.70 ([0.93–7.86)], but not with any cancer [1.13 (0.85–1.51)]. Respiratory tract infection was associated with increased risk of any leukaemia [1.57 (1.06–2.34)], ALL [1.43 (0.94–2.19)], AML [2.37 (1.10–5.12)] and any cancer [1.33 (1.09–1.63)]; influenza-like illness showed a similar pattern but with less precise estimates. There was no evidence of a link between other infections and any outcomes.
Conclusions
Urinary tract and respiratory tract infections during pregnancy may be associated with childhood leukaemia, but the absolute risk is small given the rarity of the outcome.
Keywords: Maternal infection, childhood leukaemia, cohort study, prenatal
Key Messages.
This is the first large-scale, prospective cohort study to examine associations between maternal infections or infection- associated symptoms during pregnancy and childhood leukaemia.
Using data on 312 879 mother-child pairs from six international birth cohorts, we found that maternal urinary tract infection was associated with an increased risk of childhood leukaemia but was not associated with overall cancer risk.
Respiratory tract infection was associated with increased risk of both childhood leukaemia and any cancer. Influenza- like illness showed a similar pattern to respiratory tract infection, but with less precision in the estimates.
Introduction
Leukaemia is the most common cancer in children.1 Although the aetiology remains poorly understood, previous studies have suggested that a significant fraction of childhood leukaemia may originate in utero, based on the evidence that pre-leukaemic clones with acquired genetic lesions have been present at birth.2 Hypotheses for an infectious aetiology have been further developed, proposing that an abnormal immune response to common infections in childhood may trigger the transformation of pre-leukaemic cells into clinical leukaemia.3 Associations between dysregulated immune function at birth and subsequent childhood leukaemia have also been reported,4,5 indicating that prenatal factors that influence the development of the foetal immune system may be related to leukaemia risk.
Maternal infection during pregnancy could be a cause of these in utero chromosomal or immunological changes and potentially lead to leukaemia in children.6,7 Some infections in pregnancy could result in chromosomal aberrations,8,9 which is considered the ‘first hit’ (in utero genetic lesion) in the development of childhood leukaemia. In addition, maternal infection can cause birth defects, some of which have been associated with childhood leukaemia.10 Maternal infection can also alter the development of the foetal immune system.11,12 Previous studies have shown that infection during pregnancy was associated with immune-related diseases in the children, such as asthma,13 type I diabetes14 and infectious diseases.15
Although many studies have investigated the association between maternal infection and childhood leukaemia, results have been inconsistent. In our previous systematic review and meta-analysis, maternal influenza, rubella and varicella during pregnancy were associated with increased risk of childhood leukaemia.16 However, this evidence was derived predominantly from case-control studies which may be prone to recall and selection bias.16 Cohort studies may provide more compelling evidence; however, only two small cohort studies (with six and 11 leukaemia cases, respectively) have been reported.17,18 Due to the rarity of childhood leukaemia (cumulative risk, ∼0.06% by the age of 15 years19), individual cohort studies have insufficient power to test the associations.
We pooled prospectively collected data from six international, well-characterized birth cohorts to examine associations between specific types of maternal infection during pregnancy and the risk of childhood leukaemia in the offspring.
Methods
Ethics approval for each cohort in the International Childhood Cancer Cohorts Consortium (I4C) was obtained from all local research ethics committees. The current study was approved by the I4C Steering Committee.
Data source: International Childhood Cancer Cohorts Consortium
I4C is a research platform that aims to study the aetiology of childhood cancer through the pooling data from multiple birth cohorts.20 At the time of this analysis, six cohorts have contributed data to I4C, and another seven newer large birth cohorts have been involved in I4C activities but have not contributed data yet. The six cohorts contributing data include the Avon Longitudinal Study of Parents and Children (ALSPAC, UK; n = 14 049), the Collaborative Perinatal Project (CPP, USA; n = 53 738), the Danish National Birth Cohort (DNBC, Denmark; n = 94 690), the Jerusalem Perinatal Study (JPS, Israel; n = 90 079), the Norwegian Mother and Child Cohort Study (MoBa, Norway; n = 111 399) and the Tasmanian Infant Health Study (TIHS, Australia; n = 10 624). These cohorts have recruited 374 579 mother-child pairs over five decades (1950s-2000s). As JPS only collected data on maternal infection in subsets of participants (11 467 from the antenatal cohort and 16 912 from postnatal interview), the total sample of the six cohorts eligible for this analysis was 312 879. Data on all children of ALSPAC, CPP, JPS and TIHS were included in I4C. Due to the data-sharing agreements, a case-cohort design was adopted for DNBC and MoBa, where a random 10% sample of the cohort and all childhood cancer cases (including leukaemias) were included. After excluding non-singleton births and children with Down syndrome, we had an analytical sample of 120 507 children for the current study. The case-cohort design for DNBC and MoBa was taken into account in the analysis. Sharing of de-identified data for pooling in the I4C was approved by the ethics committee for each cohort.
Outcome ascertainment
Primary outcomes were any childhood leukaemia and acute lymphoblastic leukaemia (ALL). Secondary outcomes included acute myeloid leukaemia (AML) and any cancer (including leukaemia). Childhood (<15 years of age) cancer cases were identified through record linkage of the cohorts to national/regional cancer registries (ALSPAC, DNBC, JPS, MoBa and TIHS) or via clinical follow-up (CPP). Children in ALSPAC, JPS and TIHS had been followed to at least 15 years of age, and those in CPP up to 8 years of age. Follow-up in DNBC and MoBa is ongoing, and the latest linkages to cancer registries were performed at the end of 2017, with a median follow-up of 14.3 and 12.5 years, respectively. Tumours were classified according to the International Classification of Diseases for Oncology, Third Edition: C00.0-C80.9 for any cancer, morphology codes 9800–9948 for any leukaemia, 9800–9837 for ALL and 9840–9946 for AML.
Exposure assessment and harmonization
Data on maternal infections during pregnancy were collected using self-reported questionnaires (ALSPAC, JPS, MoBa, TIHS), telephone interviews (DNBC) or medical records and self-reported interviews (CPP). Supplementary Table S1 (available as Supplementary data at IJE online) shows information about when the questionnaires/interviews were completed and what time intervals they covered for each cohort; 29 infection variables were identified from the cohorts’ questionnaires/data dictionaries. Given the relatively small case numbers, we only considered infectious exposures with prevalence >5% and where data were available from at least two cohorts. Based on these criteria, seven infections (influenza-like illness, common cold, any respiratory tract infection, vaginal thrush, vaginal infections, cystitis, any urinary tract infection) and two infection-associated symptoms (fever and diarrhoea) were included for analysis. Similarities of infection variables across cohorts were evaluated before harmonization. Based on infection definitions and exposure timings, we assigned a compatibility category for each harmonized infection variable across cohorts: high—very similar definitions and exposure timings; moderate—similar definitions and exposure timings but with certain variations; low—substantial difference in the definition and exposure timings. Compatibility categories and infection variables in each cohort are shown in Supplementary Table S2 (available as Supplementary data at IJE online).
For each harmonized infection variable, we defined the primary exposure as infection reported at any time during pregnancy. When the primary exposure variable showed an evident association with the outcome, we further examined exposure variables at different stages of pregnancy: infection during 1st trimester (≤12 + 6 weeks or ≤3 months of gestation) and infection during 2nd/3rd trimester (>12 + 6 weeks or >3 months of gestation), if data on exposure timing were available. All infection variables were categorized as binary (yes/no) (Supplementary Table S2).
Covariates
We reviewed the literature to identify potential confounders, and a directed acyclic graph (DAG) was constructed (Supplementary Figure S1, available as Supplementary data at IJE online). Based on the DAG, we included maternal age at time of index child’s birth (continuous), education level (≥12 or <12 years), smoking during pregnancy (yes/no), pre-pregnancy body mass index (BMI; continuous), parity (1 or ≥2) and any diabetes before childbirth (yes/no) in the model for adjustment, except: (i) models for AML, where diabetes was excluded because of its low prevalence (∼1%) and small number of cases; and (ii) models examining ‘any urinary tract infection’, where diabetes was also excluded as one of the cohorts (TIHS) did not collect data on this covariate. Models for common cold, influenza-like illness and respiratory infections were additionally adjusted for birth seasons (spring vs summer, autumn or winter). Details about harmonization for covariates can be found elsewhere.20
Missing data and imputation
Percentages of missing values for covariates and exposure variables ranged 0–33% (Supplementary Table S3, available as Supplementary data at IJE online). We performed multiple imputation using chained equations to impute 30 datasets separately for each cohort. Full details are available in Supplementary Methods (available as Supplementary data at IJE online).
Statistical analysis
We used Cox proportional hazards models to assess the association between each infection and cancer outcomes in children. Time-to-event was calculated as the interval from birth until a diagnosis of cancer or censoring (last follow-up/record linkage, death or age of 15 years), whichever came first. Overall hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated using multilevel Cox models (one-stage meta-analysis), with random baseline hazard and random coefficient of the exposure to take into account the heterogeneity across cohorts.21 Tau-squared (Tau2, variance of effects across cohorts) was used to assess the heterogeneity. An inverse probability weighting approach was used to account for the case-cohort design for DNBC and MoBa.22 For the non-cancer children of DNBC and MoBa, the weights were set to 10 (which is calculated as one over the sampling fraction of 10%). For the cancer cases of DNBC and MoBa and all children in other cohorts, the weights were set to one, because all these children were included.23 Parameter estimates and corresponding standard errors were obtained based on the penalized partial likelihood.24,25 Models were fit in each imputation dataset. Effect estimates were then pooled across 30 imputations using Rubin’s rules.26 A two-stage meta-analysis was also performed,27 but did not change the conclusions. Therefore, we only report results for the one-stage analysis.
The proportional hazard assumption was tested using log-log survival plots and Schoenfeld residuals and was roughly met across all models. Non-linearity of continuous variables (maternal age and BMI) was tested using fractional polynomials and was not evident.
Several sensitivity analyses were performed. We restricted the analyses of urinary tract infection to the five cohorts with maternal diabetes data and additionally adjusted for this covariate. We additionally included birth seasons in models to account for seasonality. We excluded preterm (<37 weeks of gestation) babies who potentially had a shorter exposure window.
Analyses were performed using Stata version 14 (StataCorp, College Station, TX, USA) and R version 3.6.0 (R Foundation for Statistical Computing, Vienna, Austria). Names of the commands or packages are shown in Supplementary Methods.
Results
Characteristics of the cohorts and participants
Among 312 879 children with a median follow-up of 13.6 years, 556 children were diagnosed with cancer, of which 167 were leukaemia (including 129 ALL and 33 AML). The two Nordic cohorts (DNBC and MoBa) had higher maternal age and education level and heavier babies (Table 1) compared with the other cohorts. The prevalence of maternal smoking was highest in TIHS and CPP. The oldest cohorts (CPP and JPS) had higher proportions of multiparous women and lower prevalence of caesarean section.
Table 1.
Characteristics of the cohorts and their participants
| Characteristics | ALSPAC | CPP | DNBCa | JPS (Israel)b |
MoBaa | TIHS | Pooled | |
|---|---|---|---|---|---|---|---|---|
| (UK) | (USA) | (Denmark) | Prenatal | Postnatal | (Norway) | (Australia) | ||
| Recruitment years | 1991-92 | 1959-65 | 1996-2002 | 1965-68 | 1974-76 | 1999-2009 | 1987-95 | 1959-2009 |
| Sample size | ||||||||
| Original full cohort | 14 049 | 53 738 | 94 690 | 11 467 | 16 912 | 111 399 | 10 624 | 312 879 |
| Analytical sample in current study | 13 664 | 50 342 | 9362a | 10 919 | 16 279 | 10 579a | 9362 | 120 507 |
| Years of follow-up, median (range) | 15.0 (0.01-15.0) | 7.8 (0.01-8.0) | 14.3 (0.01-15.0) | 15.0 (0.2-15.0) | 15.0 (0.3-15.0) | 12.5 (0.04-15.0) | 15.0 (0.02-15.0) | 13.6 (0.01-15.0) |
| Number of cases | ||||||||
| Any cancer | 22 | 49 | 190a | 27 | 27 | 217a | 24 | 556 |
| Any leukaemia | 3 | 15 | 61a | 4 | 9 | 71a | 4 | 167 |
| Acute lymphoblastic leukaemia | 3 | 11 | 44a | 3 | 9 | 56a | 3 | 129 |
| Acute myeloid leukaemia | 0 | 2 | 14a | 1 | 0 | 15a | 1 | 33 |
| Maternal age (years), mean ± SD | 28.0 ± 5.0 | 24.1 ± 5.9 | 30.5 ± 4.3 | 27.5 ± 5.9 | 27.4 ± 5.3 | 30.2 ± 4.6 | 23.6 ± 4.4 | 26.3 ± 5.9 |
| Education ≥12 years, n (%) | 4286 (35.3) | 20 767 (41.5) | 4388 (65.5) | 2607 (24.3) | 8898 (55.7) | 6297 (63.0) | 1690 (18.1) | 48 933 (42.5) |
| Smoking during pregnancy, n (%) | 3561 (29.6) | 23 269 (46.5) | 2365 (25.7) | 1029 (9.5) | 2218 (13.8) | 2370 (23.6) | 5023 (53.7) | 39 835 (33.9) |
| Pre-pregnancy BMI (kg/m2) mean ± SD | 22.9 ± 3.8 | 22.7 ± 4.3 | 23.6 ± 4.4 | – | 22.1 ± 3.1 | 24.0 ± 4.2 | 23.2 ± 4.8 | 22.9 ± 4.1 |
| Maternal diabetes, n (%) | 497 (4.1)c | 391 (0.8) | 172 (1.8) | 30 (0.3) | 130 (0.8) | 145 (1.4) | – | 1365 (1.25) |
| Parity, primipara, n (%) | 5640 (45.3) | 15 329 (30.5) | 4157 (47.1) | 3106 (28.5) | 4916 (30.3) | 4700 (44.4) | 4387 (46.9) | 42 235 (35.6) |
| Sex of child, male, n (%) | 7052 (51.6) | 25 461 (50.7) | 4774 (51.0) | 5590 (51.2) | 8470 (52.0) | 5321 (50.3) | 6673 (71.3)d | 63 341 (52.6) |
| Gestational age at birth (weeks) median (25th, 75th) | 40 (39, 41) | 40 (38, 41) | 40 (39, 41) | 40 (39, 41) | 40 (39, 41) | 40 (39, 41) | 40 (38, 40) | 40 (39, 41) |
| Birthweight (grams), mean ± SD | 3410 ± 551 | 3177 ± 531 | 3587 ± 563 | 3280 ± 493 | 3253 ± 509 | 3604 ± 561 | 3195 ± 751 | 3294 ± 573 |
| Caesarean section, n (%) | 1393 (10.5) | 2440 (4.9) | 1486 (15.9) | 372 (3.4) | 1039 (6.4) | 1477 (14.0) | 1764 (18.9) | 9971 (8.3) |
ALSPAC, Avon Longitudinal Study of Parents and Children; BMI, body mass index; CPP, Collaborative Perinatal Project; DNBC, Danish National Birth Cohort; JPS, Jerusalem Perinatal Study; MoBa, Norwegian Mother and Child Cohort Study; SD, standard deviation; TIHS, Tasmanian Infant Health Study.
DNBC and MoBa provide data on a random 10% sample of the cohort and all cancer cases.
JPS collected data on maternal infection in two subsets (prenatal and postnatal interviews).
Including glycosuria.
High percentage of boys was due to the study design focused on children at highest risk for sudden infant death syndrome; -, data not collected.
The prevalences of influenza-like illness, cystitis and urinary tract infection during pregnancy were just over 10%; fever, vaginal thrush, vaginal infections and diarrhoea ranged from 21% to 25%; common cold and respiratory tract infection were >30% (Supplementary Table S4, available as Supplementary data at IJE online).
Associations of maternal infections with cancer outcomes
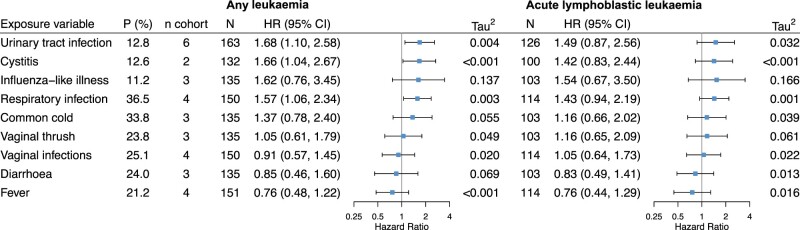
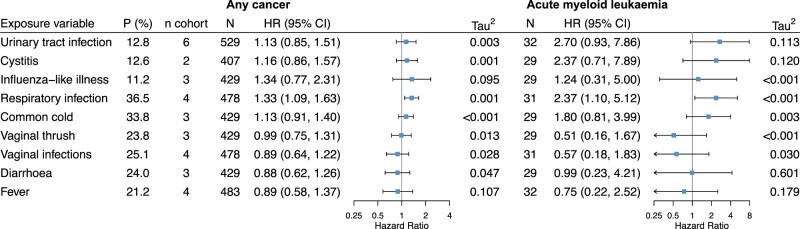
Of the nine infection-related variables tested, three had positive associations with childhood leukaemia: urinary tract infection, cystitis and respiratory infection (Figures 1 and 2). A positive association was also found between respiratory infection and any cancer (Figure 2). No evident association for other infections was observed.
Figure 1.
Associations of each maternal infection during pregnancy with risk of any childhood leukaemia and acute lymphoblastic leukaemia. P (%), prevalence of the infection, weighted by the inverse of the sampling probability in Danish National Birth Cohort and Norwegian Mother and Child Cohort Study; n cohort, number of cohorts with available infection data; N, total number of cases in the pooled dataset; HR (95% CI), hazard ratio and 95% confidence interval; Tau2, variance of effect sizes across cohorts; and its square root (Tau) is the standard deviation of the distribution of effect sizes across cohorts. Hazard ratios were obtained using multilevel Cox modelling with random baseline hazards and random coefficients of the infection and were adjusted for maternal age, education level, smoking during pregnancy, pre-pregnancy body mass index, parity and diabetes. Models for influenza-like illness, common cold and respiratory tract infection were additionally adjusted for birth seasons. Compatibility categories of infection variables across cohorts: high for urinary tract infection, cystitis and vaginal thrush; moderate for influenza-like illness, vaginal infections and diarrhoea; low for respiratory tract infection, common cold and fever
Figure 2.
Associations of each maternal infection during pregnancy with risk of any childhood cancer and acute myeloid leukaemia. P (%), prevalence of the infection, weighted by the inverse of the sampling probability in Danish National Birth Cohort and Norwegian Mother and Child Cohort Study; n cohort, number of cohorts with available infection data; N, total number of cases in the pooled dataset; HR (95% CI), hazard ratio and 95% confidence interval; Tau2, variance of effect sizes across cohorts; and its square root (Tau) is the standard deviation of the distribution of effect sizes across cohorts. Hazard ratios were obtained using multilevel Cox modelling with random baseline hazards and random coefficients of the infection and were adjusted for maternal age, education level, smoking during pregnancy, pre-pregnancy body mass index, parity and diabetes. Models for influenza-like illness, common cold and respiratory tract infection were additionally adjusted for birth seasons. Compatibility categories of infection variables across cohorts: high for urinary tract infection, cystitis and vaginal thrush; moderate for influenza-like illness, vaginal infections and diarrhoea; low for respiratory tract infection, common cold and fever
In a pooled analysis of all six cohorts, urinary tract infection (compatibility category, high) was associated with an increased risk of any leukaemia [HR (95% CI) 1.68 (1.10–2.58)], Tau2 = 0.004; Figure 1). Results for ALL and AML also show an increased risk, but the 95% CIs ranged from a slightly decreased risk to a considerably increased risk (Figures 1 and 2). When stratified by exposure timing, urinary tract infection in 2nd/3rd trimester, but not 1st trimester, was associated with increased risk of any leukaemia [1.90 (1.00–3.63); Tau2 = 0.055] (Supplementary Figure S2, available as Supplementary data at IJE online). Analysis for cystitis (compatibility category, high) was performed in two Nordic cohorts (DNBC and MoBa) that had data for this variable; results were similar to those for urinary tract infection (Figure 1; and Supplementary Figure S2).
Respiratory tract infection was associated with increased risk of any leukaemia [HR (95% CI) 1.54 (1.05–2.26); Tau2 = 0.002], ALL [1.43 (0.94–2.18); Tau2 <0.001], AML [2.35 (1.08–5.10); Tau2 = 0.003] and any cancer [1.32 (1.08–1.62); Tau2 <0.001] (Figures 1 and 2). It should be noted that the definitions of this exposure were heterogeneous across cohorts (Supplementary Table S2; compatibility category, low), despite the low statistical heterogeneity of effect (Tau2). To address the issue of heterogeneity for this variable, we conducted a post hoc sensitivity analysis by excluding CPP (which had only data on pneumonia and tuberculosis) from the analysis. We found the results were similar to the overall analysis, although the confidence intervals of HRs became wider (data not shown). We also performed a stratified analysis by maternal smoking during pregnancy (yes/no) for respiratory infection. The associations tend to be more pronounced among women who did not smoke than those who smoked (Supplementary Table S5, available as Supplementary data at IJE online). However, a test of heterogeneity shows that HRs between the two groups were not statistically different (P-values >0.1).
In three cohorts (ALSPAC, DNBC and MoBa) with available data, the HRs for influenza-like illness (compatibility category, moderate) were 1.62 (95% CI 0.76–3.42; Tau2 = 0.130) for any leukaemia and 1.51 (95% CI 0.68–3.39; Tau2 = 0.145) for ALL (Figure 1).
Sensitivity analysis
Results were similar to those of the main analysis when we additionally adjusted for maternal diabetes for the analysis on urinary tract infection, additionally adjusted for birth seasons, or excluded babies born preterm (Supplementary Table S6, available as Supplementary data at IJE online).
Discussion
Based on a large pooled dataset from six international cohorts, our study is the first to show a positive association of maternal urinary tract infection during pregnancy with childhood leukaemia in offspring. Our study is also the first cohort study with relatively large numbers of cases to demonstrate associations of maternal respiratory tract infections with higher risk of childhood leukaemia and any cancer.
Urinary tract infection is one of the most common infections in pregnant women,28 and has been associated with maternal and neonatal adverse outcomes such as preeclampsia, preterm delivery and perinatal death.29 Such infections during pregnancy may also have long-term effects on the offspring’s health (increasing risk of infectious morbidity15 and neuropsychiatric disease30). Risks were not elevated for any leukaemia or ALL in our previous meta-analysis of three case-control studies,16 but we observed stronger positive associations for both outcomes in our prospective data which were characterized by a high compatibility for this infection.
Only one case-control study explored the association of maternal respiratory tract infection with childhood leukaemia and reported an odds ratio (OR) of 1.46 (95% CI 0.58–3.67).31 We found that respiratory tract infection was associated with increased risk of any leukaemia, ALL, AML and any cancer in children. Regarding influenza during pregnancy, our previous meta-analysis showed a pooled OR of 1.77 (1.01–3.11) for childhood leukaemia and 3.64 (1.34–9.90) for ALL.16 In the current study, the effect size was similar for leukaemia (HR 1.61) but lower for ALL (HR 1.44); however, both estimates had wide confidence intervals. As the heterogeneity of the definitions for respiratory tract infection and influenza was high across the cohorts (compatibility: low and moderate, respectively), further studies with more accurate measurement (e.g. medical records or laboratory-confirmed cases) are needed to clarify the associations.
Among the plausible mechanisms underlying the observed associations are a direct oncogenic effect of infectious agents and an immune response triggered by the infection which influences the development of the foetal immune system and subsequent immune response to infections in childhood.11,12 Active viral infections or dysregulated cytokines due to maternal infections might also have a direct mutagenic effect on foetal haematopoietic cells via the activation of cytidine deaminase enzymes such as activation-induced deaminase (AID) or apolipoprotein B mRNA-editing catalytic polypeptide-like (APOBEC) (a mutational signature seen in ALL32), which are capable of causing both translocations and point mutations.33 Some infections (e.g. gastrointestinal infections) could be less likely to cause chromosomal or immunological abnormalities and thus not contribute to the risk of childhood leukaemia; this hypothesis is supported by our finding that no association was observed for diarrhoea and childhood leukaemia.
Other childhood cancers (non-leukaemia) may serve as a negative control outcome to assess the possibility that the observed associations between maternal infections and childhood leukaemia were biased by unobserved confounders. Assuming that: (i) other cancers are not affected by maternal infections; and ii) the unobserved confounders of the infections-leukaemia association and the infections-other cancers association are identical, an empirical association between maternal infections and other cancers would suggest that the infections-leukaemia association may be biased by the unobserved confounders; by contrast, no association between maternal infections and other cancers would suggest little unobserved confounding for the infections-leukaemia association. In our dataset, urinary tract infection was associated with childhood leukaemia but not with other types of cancer (Supplementary Table S7, available as Supplementary data at IJE online), suggesting that unobserved confounding does not account for the association of urinary tract infection with leukaemia. On the other hand, the higher risk of both leukaemia and other cancers associated with respiratory infection (Supplementary Table S7) supports the possibility of uncontrolled confounders for this infection. However, this uncontrolled confounding would not fully explain the association with leukaemia, given that the empirical association of respiratory infection with other cancers was weaker than that with leukaemia (HR 1.25 vs 1.54) if the two above-mentioned assumptions hold.
An alternative explanation may be related to antimicrobial treatment for the infection, which was shown to be associated with a higher risk of childhood leukaemia (especially under 5 years of age).34 It is also possible that adverse perinatal outcomes mediate the association between maternal infections and childhood leukaemia. However, there is no evident association between preterm birth or caesarean section and childhood leukaemia in our dataset, suggesting few mediating effects of these two perinatal outcomes. Future studies are needed to test these hypothesized mechanisms.
Strengths of our study included: the large-scale, prospective design thereby reducing the potential for recall or selection bias; assessment of the associations during different stages of pregnancy; adjustment for potential confounders, some of which were usually unavailable in previous studies16; use of self-report which might better capture exposure information for common infections which are usually mild, compared with medical records which reflect more severe cases.
Limitations include: possible misclassification of exposure due to self-report; lack of information on specific pathogens or asymptomatic infections; different definitions of some infections (e.g. influenza) across cohorts; modest numbers of leukaemias and even smaller numbers of leukaemia subtypes; the 10% cohort sample from DNBC and MoBa (since the imputation for missing data in such a subcohort may be less efficient compared with use of the entire cohort)35,36; and potential live-birth bias, as we only used live births as the study population. Although we have adjusted for several potential confounders, we could not rule out the possibility of common residual confounders across all cohorts (e.g. genetic factors) or different residual confounders (e.g. maternal environmental and occupational exposures) that varied between cohorts. Finally, we did not adjust for the multiple comparisons, given the limited number of cases.
In conclusion: using large-scale prospective data from several countries, we found that urinary tract and respiratory tract infections during pregnancy were associated with a higher risk of childhood leukaemia. However, the absolute risk remains small, given the rarity of the outcome. Replication and validation of these findings are needed.
Data availability
The data underlying this article were provided by the original cohorts in I4C by permission. Data will be shared on request to the corresponding author with permission of the original cohorts in I4C.
Supplementary data
Supplementary data are available at IJE online.
Funding
J-R.H. was supported by China Scholarship Council-University of Oxford Joint Scholarship. S.E.H and P.M. are supported by the Research Council of Norway through its Centres of Excellence funding scheme (project number 262700) and by the Norwegian Institute of Public Health.
Supplementary Material
Acknowledgements
We would like to thank all cohorts from the I4C which participated in our study. The Danish cancer cases were ascertained by the Danish Childhood Cancer Registry (Steering Committee: Catherine Rechnitzer, Peter Skov Wehner, Steen Rosthøj and Henrik Schrøder).
Author contributions
J.R.H., J.E.H. and T.D. were responsible for the study concept and design. O.P., A.L.P., M.K., J.O., S.H. and J.G. were responsible for data collection in original cohorts. J.R.H., G.T.. and G.S.P. assembled and harmonized the data. J.R.H. did the statistical analyses and drafted the manuscript. All authors participated in the data interpretation, provided critical review and approved the final manuscript. T.D. will act as guarantor for the paper.
Conflict of interest
None declared.
Contributor Information
Jian-Rong He, Nuffield Department of Women’s and Reproductive Health, University of Oxford, Oxford, UK; Division of Birth Cohort Study, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China; George Institute for Global Health, University of Oxford, Oxford, UK.
Jane E Hirst, Nuffield Department of Women’s and Reproductive Health, University of Oxford, Oxford, UK; George Institute for Global Health, University of Oxford, Oxford, UK.
Gabriella Tikellis, Murdoch Children’s Research Institute, Royal Children’s Hospital, University of Melbourne, Melbourne, VIC, Australia.
Gary S Phillips, Retired from Center for Biostatistics, Department of Biomedical Informatics, Ohio State University, Columbus, OH, USA.
Rema Ramakrishnan, Nuffield Department of Women’s and Reproductive Health, University of Oxford, Oxford, UK; George Institute for Global Health, University of Oxford, Oxford, UK; University of New South Wales, Faculty of Medicine, Sydney, NSW, Australia.
Ora Paltiel, Braun School of Public Health, Hadassah-Hebrew University Medical Center, Jerusalem, Israel.
Anne-Louise Ponsonby, Murdoch Children’s Research Institute, Royal Children’s Hospital, University of Melbourne, Melbourne, VIC, Australia.
Mark Klebanoff, Center for Perinatal Research, Abigail Wexner Research Institute at Nationwide Children's Hospital, Columbus, OH, USA.
Jørn Olsen, Department of Clinical Epidemiology, Aarhus University, Aarhus, Denmark.
Michael F G Murphy, Nuffield Department of Women’s and Reproductive Health, University of Oxford, Oxford, UK.
Siri E Håberg, Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway.
Stanley Lemeshow, Division of Biostatistics, College of Public Health, Ohio State University, Columbus, OH, USA.
Sjurdur F Olsen, Centre for Fetal Programming, Department of Epidemiology Research, Statens Serum Institut, Copenhagen, Denmark.
Xiu Qiu, Division of Birth Cohort Study, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, China.
Per Magnus, Centre for Fertility and Health, Norwegian Institute of Public Health, Oslo, Norway.
Jean Golding, Centre for Academic Child Health, Population Health Sciences, Bristol Medical School, University of Bristol, Bristol, UK.
Mary H Ward, Occupational and Environmental Epidemiology Branch, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Rockville, MD, USA.
Joseph L Wiemels, Department of Preventative Medicine, University of Southern California, Los Angeles, CA, USA and.
Kazem Rahimi, Nuffield Department of Women’s and Reproductive Health, University of Oxford, Oxford, UK; George Institute for Global Health, University of Oxford, Oxford, UK.
Martha S Linet, Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, MD, USA.
Terence Dwyer, Nuffield Department of Women’s and Reproductive Health, University of Oxford, Oxford, UK; George Institute for Global Health, University of Oxford, Oxford, UK.
References
- 1. Bhakta N, Force LM, Allemani C. et al. Childhood cancer burden: a review of global estimates. Lancet Oncol 2019;20:e42–53. [DOI] [PubMed] [Google Scholar]
- 2. Greaves MF, Wiemels J.. Origins of chromosome translocations in childhood leukaemia. Nat Rev Cancer 2003;3:639–49. [DOI] [PubMed] [Google Scholar]
- 3. Greaves M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat Rev Cancer 2018;18:471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soegaard SH, Rostgaard K, Skogstrand K, Wiemels JL, Schmiegelow K, Hjalgrim H.. Neonatal inflammatory markers are associated with childhood B-cell precursor acute lymphoblastic leukemia. Cancer Res 2018;78:5458–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chang JS, Zhou M, Buffler PA, Chokkalingam AP, Metayer C, Wiemels JL.. Profound deficit of IL10 at birth in children who develop childhood acute lymphoblastic leukemia. Cancer Epidemiol Biomarkers Prev 2011;20:1736–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Smith M. Considerations on a possible viral etiology for B-precursor acute lymphoblastic leukemia of childhood. J Immunother 1997;20:89–100. [DOI] [PubMed] [Google Scholar]
- 7. Zur Hausen H, de Villiers EM.. Prenatal Infections with Subsequent Immune Tolerance Could Explain the Epidemiology of Common Childhood Cancers. Lyon, France: International Agency for Research on Cancer, 2014. [Google Scholar]
- 8. Cheeran MC, Lokensgard JR, Schleiss MR.. Neuropathogenesis of congenital cytomegalovirus infection: disease mechanisms and prospects for intervention. Clin Microbiol Rev 2009;22:99–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ornoy A, Ergaz Z.. Parvovirus B19 infection during pregnancy and risks to the fetus. Birth Defects Res 2017;109:311–23. [DOI] [PubMed] [Google Scholar]
- 10. Lupo PJ, Schraw JM, Desrosiers TA. et al. Association between birth defects and cancer risk among children and adolescents in a population-based assessment of 10 million live births. JAMA Oncol 2019;5:1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dauby N, Goetghebuer T, Kollmann TR, Levy J, Marchant A.. Uninfected but not unaffected: chronic maternal infections during pregnancy, fetal immunity, and susceptibility to postnatal infections. Lancet Infect Dis 2012;12:330–40. [DOI] [PubMed] [Google Scholar]
- 12. Goldman M, Marchant A.. The impact of maternal infection or immunization on early-onset autoimmune diabetes. Vaccine 2003;21:3422–25. [DOI] [PubMed] [Google Scholar]
- 13. Stokholm J, Sevelsted A, Bonnelykke K, Bisgaard H.. Maternal propensity for infections and risk of childhood asthma: a registry-based cohort study. Lancet Respir Med 2014;2:631–37. [DOI] [PubMed] [Google Scholar]
- 14. Yue Y, Tang Y, Tang J. et al. Maternal infection during pregnancy and type 1 diabetes mellitus in offspring: a systematic review and meta-analysis. Epidemiol Infect 2018;146:2131–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cohen R, Gutvirtz G, Wainstock T, Sheiner E.. Maternal urinary tract infection during pregnancy and long-term infectious morbidity of the offspring. Early Hum Dev 2019;136:54–59. [DOI] [PubMed] [Google Scholar]
- 16. He JR, Ramakrishnan R, Hirst JE. et al. Maternal infection in pregnancy and childhood leukemia: a systematic review and meta-analysis. J Pediatr 2020;217:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fine PE, Adelstein AM, Snowman J, Clarkson JA, Evans SM.. Long term effects of exposure to viral infections in utero. Br Med J (Clin Res Ed) 1985;290:509–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fedrick J, Alberman ED.. Reported influenza in pregnancy and subsequent cancer in the child. Br Med J 1972;2:485–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Steliarova-Foucher E, Colombet M, Ries LAG. et al. ; IICC-3 contributors. International incidence of childhood cancer, 2001-10: a population-based registry study. Lancet Oncol 2017;18:719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tikellis G, Dwyer T, Paltiel O. et al. ; the International Childhood Cancer Cohort Consortium. The International Childhood Cancer Cohort Consortium (I4C): a research platform of prospective cohorts for studying the aetiology of childhood cancers. Paediatr Perinat Epidemiol 2018;32:568–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Therneau T. Mixed Effects Cox Models. 2015. https://cran.r-project.org>package=coxme (1 July 2019, date last accessed).
- 22. Kim RS. A new comparison of nested case-control and case-cohort designs and methods. Eur J Epidemiol 2015;30:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Johansson AL, Andersson TM, Hsieh CC, Cnattingius S, Dickman PW, Lambe M.. Family history and risk of pregnancy-associated breast cancer (PABC). Breast Cancer Res Treat 2015;151:209–17. [DOI] [PubMed] [Google Scholar]
- 24. Breslow NE, Clayton DG.. Approximate inference in generalized linear mixed models. J Am Stat Assoc 1993;88:9–25. [Google Scholar]
- 25. Ripatti S, Palmgren J.. Estimation of multivariate frailty models using penalized partial likelihood. Biometrics 2000;56:1016–22. [DOI] [PubMed] [Google Scholar]
- 26. Rubin DB. Multiple Imputation for Survey Nonresponse. New York, NY: Wiley, 1987. [Google Scholar]
- 27. Burke DL, Ensor J, Riley RD.. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat Med 2017;36:855–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gilbert NM, O'Brien VP, Hultgren S, Macones G, Lewis WG, Lewis AL.. Urinary tract infection as a preventable cause of pregnancy complications: opportunities, challenges, and a global call to action. Glob Adv Health Med 2013;2:59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kalinderi K, Delkos D, Kalinderis M, Athanasiadis A, Kalogiannidis I.. Urinary tract infection during pregnancy: current concepts on a common multifaceted problem. J Obstet Gynaecol 2018;38:448–53. [DOI] [PubMed] [Google Scholar]
- 30. Al-Haddad BJS, Jacobsson B, Chabra S. et al. Long-term risk of neuropsychiatric disease after exposure to infection in utero. JAMA Psychiatry 2019;76:594–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McKinney PA, Juszczak E, Findlay E, Smith K, Thomson CS.. Pre- and perinatal risk factors for childhood leukaemia and other malignancies: a Scottish case control study. Br J Cancer 1999;80:1844–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alexandrov LB, Nik-Zainal S, Wedge DC, Campbell PJ, Stratton MR.. Deciphering signatures of mutational processes operative in human cancer. Cell Rep 2013;3:246–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Siriwardena SU, Chen K, Bhagwat AS.. Functions and malfunctions of mammalian DNA-cytosine deaminases. Chem Rev 2016;116:12688–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Momen NC, Olsen J, Gissler M, Kieler H, Haglund B, Li J.. Exposure to systemic antibacterial medications during pregnancy and risk of childhood cancer. Pharmacoepidemiol Drug Saf 2015;24:821–29. [DOI] [PubMed] [Google Scholar]
- 35. Keogh RH, Seaman SR, Bartlett JW, Wood AM.. Multiple imputation of missing data in nested case-control and case-cohort studies. Biometrics 2018;74:1438–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Keogh RH, White IR.. Using full-cohort data in nested case-control and case-cohort studies by multiple imputation. Stat Med 2013;32:4021–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by the original cohorts in I4C by permission. Data will be shared on request to the corresponding author with permission of the original cohorts in I4C.