Abstract
Background
The ideal therapeutic option for ventilator associated pneumonia caused by carbapenem-resistant Enterobacteriaceae is not defined. The aim of this study was to assess mortality-associated risk factors in patients with VAP by CRE and determine the outcome of several treatment options.
Methods
This was a retrospective study performed in two tertiary hospitals involving patients with VAP caused by CRE between January 2010 and August 2014. The outcomes were mortality within 30 days of VAP diagnosis and overall mortality during hospital admission. Risk factors for mortality were assessed by comparing variables of survivors and non-survivors.
Results
One hundred and twelve patients with CRE-VAP were included, 73 (65%) male, median age 56 years. The 30-day mortality was 57.1% and the overall hospital mortality was 67%. In the binary logistic regression analysis, only age >50 years was independently associated to increased mortality. Polymyxin was the most used drug (47.5%), followed by tigecycline (29.2%) and aminoglycosides (2.4%). Combined therapy with two active drugs was used by 17 patients (20.8%). No therapeutic option was independently associated to survival. However, combined therapy with two active drugs was superior to the therapy with a single active drug when inappropriate therapy was the comparator (p = 0.044). The addition of carbapenem was not associated with increased survival.
Conclusion
The best therapeutic option for VAP by CRE is still not completely defined, but the therapy with at least two active drugs was superior in this study.
Keywords: Carbapenemase-producing Klebsiella pneumoniae, Ventilator-associated pneumonia, Risk factors, Mortality, Treatment
Introduction
Emergence of Klebsiella pneumoniae carbapenemase (KPC) among Enterobacteriaceae has severely challenged antimicrobial therapy, as it provides high-level resistance to all beta-lactams and distinct levels of resistance to carbapenems.1 The best therapy for carbapenem-resistant Enterobacteriaceae (CRE) is not well defined in medical literature. Some authors have suggested combined therapy based on few retrospective clinical studies, as well as in vitro and in vivo models.2, 3
Polymyxins, aminoglycosides, and tigecycline have been used as core treatment drugs, considering local susceptibility patterns.4, 5 Other drugs may be suggested according to infection site, such as fosfomycin for urinary tract infection.6 Ventilator-associated pneumonia (VAP) is still one of the main infections in intensive care units. Microbiological etiology of VAP widely varies according to local epidemiology. The incidence of CRE has increased in Southern Brazil, mainly associated with VAP.7, 8 Studies on the treatment of CRE causing VAP (CRE-VAP) are scarce, only case reports and small series are available.9, 10, 11 Risk factors associated with mortality and the optimal treatment of CRE-VAP are not well-established. The main objective of this study was to describe risk factors associated with CRE-VAP mortality. Secondary objective was to determine the outcome with different antibiotic regimens.
Methods
Study design
A cross-sectional study was carried out at two tertiary-care, trauma and surgery reference hospitals, in South of Brazil, with a total number of 870 beds and 58 intensive care unit beds. All patients aged ≥16 years with VAP caused by CRE in the period from January 2010 to August 2014 were included in the study. Only the first episode of each patient was analyzed. Data were collected from medical charts and/or hospital computer system databases. This study was approved by the Ethics Committee of FEPAR (42918014.1.0000.0103).
Microbiological procedures
All included patients had positive quantitative cultures for CRE obtained from the respiratory tract through bronchoalveolar lavage (BAL) (>104 CFU/mL) or tracheal aspiration (>106 CFU/mL). The BAL methodology was previously described by our study-group.12
All identified bacteria were tested for susceptibility by disk diffusion method and/or Gram-negative card for automated method according to the CLSI guidelines.13 Minimal inhibitory concentration (MIC) was determined by automated method in mg/L (Vitek 2®, Biomérieux, Marcy-LÉtoile, France) and/or E-test method, as recommended. Screening for CRE was performed with modified Hodge's test according to CLSI.13 Modified Hodge's test positive isolates were submitted to PCR for blaKPC amplification using Easy Q KPC (Biomérieux, Marcy-LÉtoile, France) with Escherichia coli ATCC 25922 and blaKPC-carrying K. pneumoniae strain ATCC BAA-1705 as negative and positive controls, respectively.14 Briefly, the amplified product was purified using Exonuclease I and Shrimp Alkaline Phosphatase (ExoSAP, USB Corporation) for DNA sequencing. Sequencing reactions were performed using BigDye v1.1 Sequencing Kits (Applied Biosystems, Foster City, CA, USA). Sequence data were acquired on ABI 3100 (Applied Biosystems Inc., CA). The gene sequences were compared to entries in databases queried by NCBI BLAST (nucleotide sequence database; available at http://www.ncbi.nlm.nih.gov/).
Variables and definitions
VAP was defined according to CDC criteria.15 Other criteria, such as diagnostic scores/indexes or clinical impression were excluded. Patients with concomitant infections were excluded. Secondary bacteremia was not evaluated. Potential risk factors assessed were: age, gender, Charlson comorbidity index (and all comorbidities were evaluated separately),16 APACHE II,17 burn, HIV infection, chronic corticoid use, trauma on admission, elective or emergency surgery, length of hospitalization and mechanical ventilation duration before VAP, and previous antibiotic use during current hospitalization. Risk was neither adjusted for sepsis nor pneumonia severity scores.
Antimicrobial therapy
Antimicrobial treatment was deemed inappropriate if antibiotics initially prescribed were not active against the identified pathogens based on in vitro susceptibility testing. Appropriate therapy was defined by active drug use with adequate schedule in two situations for additional analysis: antibiotic started within the first 24 h with no switches; and treatment adjustment within five days (arbitrarily defined considering average time of culture results). For analysis of different antibiotics schedules, the term monotherapy was used when a single drug was active against CRE and combined therapy whenever two or more drugs were active considering in vitro susceptibility. The dose of antibiotics was also evaluated. Polymyxin B was used without loading dose and renal adjustment (30,000 IU/kg); meropenem dose ranged from 1 g to 2 g each 8 h with renal adjustment; tigecycline is used with doubled dose (100 mg q12 h) without renal adjustment; amikacin dose was 15 mg/kg without loading dose and renal adjustment.
Statistical analysis
All statistical analyses were carried out using SPSS 18.0. Bivariate analysis was performed separately for each variable. p-Values were calculated using the χ2 test or Fisher's exact test for categorical variables and Student's t-test or Wilcoxon rank-sum test for continuous variables. Variables for which the p-value was ≤0.10 in the univariate analysis were included in a logistic regression model. A p-value of 0.05 was set as the limit for acceptance or removal of the new terms in the model. Goodness-of-fit was assessed by Hosmer–Lemeshow test. Thirty-day mortality curves were constructed and Gehan-Breslow-Wilcoxon test was performed. All tests were two-tailed, and a p-value ≤0.05 was considered significant.
Results
A total of 112 patients with CRE-VAP were included in the study, with 102 cases of K. pneumoniae and 10 cases of Enterobacter aerogenes. Male was the predominant gender (n = 73, 65.2%) and global mean age was 54.9 ± 20.7 years-old (median = 56). The mean hospital stay was 47.7 ± 57.6 days (median = 31 days), mean duration of hospitalization before CRE-VAP 18.6 ± 21.0 days (median = 14 days). The mean ICU stay after CRE-VAP was 14.8 ± 8.3 days (median = 10 days). The Charlson index was low (mean = 1.55 ± 2.36). Data are detailed in Table 1.
Table 1.
Risk factors analysis for ventilator-associated pneumonia caused by Carbapenem-resistant Enterobacteriaceae.
| Continuous variables | Death | Survival | p-Value | ||
|---|---|---|---|---|---|
| (n = 64) |
(n = 48) |
||||
| Meana | SDc | Mean | SD | ||
| Age | 61.7 | 19.0 | 45.9 | 19.6 | <0.001f |
| ICU total admission | 14.1 | 11.1 | 15.9 | 28.5 | 0.657 |
| VM days | 14.1 | 11.1 | 15.2 | 28.4 | 0.781 |
| Total admission (days) | 30.3 | 16.6 | 70.9 | 81.1 | <0.001f |
| Days before VAP | 19.0 | 13.0 | 18.1 | 28.6 | 0.826 |
| Days after VAP | 11.3 | 9.4 | 52.8 | 59.5 | <0.001f |
| Charlson index | 2.1 | 2.5 | 0.8 | 1.6 | 0.003f |
| APACHE | 26.1 | 5.5 | 26.5 | 4.7 | 0.702 |
| Categorical variables | nb | % | n | % | p-Value |
|---|---|---|---|---|---|
| Gender (male) | 39 | 60.9% | 35 | 72.9% | 0.109 |
| Burn | 2 | 3.1% | 4 | 8.3% | 0.209 |
| HIV infection | 1 | 1.6% | 1 | 2.1% | 0.671 |
| Diabetes | 10 | 15.6% | 3 | 6.3% | 0.122 |
| Diabetes with complications | 6 | 9.4% | 2 | 4.2% | 0.257 |
| Chronic renal failure | 8 | 12.5% | 1 | 2.1% | 0.046f |
| Previous myocardial infarction | 9 | 14.1% | 6 | 12.5% | 0.533 |
| Congestive heart failure | 7 | 10.9% | 2 | 4.2% | 0.177 |
| Peripheral artery disease | 2 | 3.1% | 0 | 0.0% | 0.329 |
| Previous stroke | 10 | 15.6% | 3 | 6.3% | 0.122 |
| Previous stroke with plegia | 1 | 1.6% | 1 | 2.1% | 0.671 |
| Dementia | 3 | 4.7% | 2 | 4.2% | 0.641 |
| COPD | 4 | 6.3% | 2 | 4.2% | 0.492 |
| Hypertension | 31 | 48.4% | 12 | 25.0% | 0.011f |
| Neoplasm | 9 | 14.1% | 1 | 2.1% | 0.028f |
| Lymphoma | 1 | 1.6% | 0 | 0.0% | 0.575 |
| Leukemia | 0 | 0.0% | 0 | 0.0% | – |
| Metastatic tumor | 5 | 7.8% | 0 | 0.0% | 0.059 |
| Rheumatic disease | 2 | 3.1% | 1 | 2.1% | 0.613 |
| Peptic ulcer | 0 | 0.0% | 0 | 0.0% | – |
| Hepatic disease | 2 | 3.1% | 1 | 2.1% | 0.613 |
| Cirrhosis | 0 | 0.0% | 1 | 2.1% | 0.425 |
| Chronic corticoid use | 3 | 4.7% | 0 | 0.0% | 0.187 |
| Other immunosuppressants | 6 | 9.4% | 1 | 2.1% | 0.121 |
| Trauma on admission | 26 | 40.6% | 28 | 58.3% | 0.041f |
| Elective surgery | 7 | 10.9% | 5 | 10.4% | 0.692 |
| Emergency surgery | 32 | 50.0% | 29 | 60.4% | 0.162 |
| Treatment | nb | % | n | % | p-Value |
|---|---|---|---|---|---|
| Incorrect treatment<24 h | 45 | 70.3% | 36 | 75.0% | 0.390 |
| Correct treatment <24 hd | 19 | 29.7% | 12 | 25.0% | 0.390 |
| Incorrect treatment <5 days | 18 | 28.1% | 11 | 22.9% | 0.298 |
| Correct treatment<5 days | 46 | 71.9% | 37 | 77.1% | 0.298 |
| Monotherapyd | 40 | 62.5% | 26 | 54.2% | 0.276 |
| Combined therapye | 6 | 9.4% | 11 | 22.9% | 0.044f |
Mean of total deaths or survival.
Number of cases.
Standard deviation.
At least one active drug.
At least two active drugs.
p < 0.05.
Thirty-day mortality was 57.1% (64/112) for all CRE-VAP patients and in-hospital global mortality was 67.0% (75/112). Aging, high Charlson index, chronic renal failure, systemic arterial hypertension, neoplasm, and trauma on admission were the clinical findings associated with increased mortality. These data were evaluated in a binary logistic regression and only age > 50 years old was independently associated with increased mortality (Table 2).
Table 2.
Multivariate analysis of risk factors for ventilator-associated pneumonia caused by Carbapenem-resistant Enterobacteriaceae.
| Variable | p-Value (univariate) | B | OR | CI (95%) | p-Value | |
|---|---|---|---|---|---|---|
| Age > 50 years | 0.003 | 0.11 | 3.39 | 1.27 | 9.17 | 0.016 |
| Charlson index > 1 | 0.013 | 1.22 | 1.13 | 0.32 | 3.96 | 0.853 |
| Chronic renal failure | 0.046 | 0.11 | 0.90 | 0.34 | 2.38 | 0.825 |
| Hypertension | 0.011 | 2.14 | 8.55 | 0.78 | 94.21 | 0.080 |
| Neoplasm | 0.028 | 1.20 | 3.34 | 0.29 | 38.65 | 0.334 |
| Trauma on admission | 0.041 | 0.43 | 1.55 | 0.56 | 4.33 | 0.401 |
Out of 112 patients, only 31 (27.7%) received at least one active drug within 24 h of VAP diagnosis (first clinical signs plus altered chest x-ray). After five days from the diagnosis, 83 patients (74.1%) received at least one active drug; 66 patients received one active drug and 17 (20.8%) received two active drugs for CRE-VAP. Polymyxin B (1,500,000 IU to 3,000,000 IU/day) was the preferred active drug (47.5%), followed by tigecycline (29.2%) and amikacin (1000 mg/day) (2.4%). From 17 patients with two active drugs, polymyxin B with tigecycline was the preferred combination (15/17). Despite of the high MIC for meropenem in all clinical samples from the respiratory tract (MIC ≥16 mg/L), meropenem was combined with at least one active drug in 49 patients (59.8%). One patient was treated with two carbapenems (ertapenem plus meropenem) with good clinical outcome. None of these therapeutic options were independently associated with survival. However, combined therapy (two active drugs) had significantly lower mortality than monotherapy (only one active drug) (p = 0.044). Combination of meropenem was not associated with improved survival.
After culture results, 83 patients received adequate therapy (74%). From 29 patients (26%) who maintained inappropriate treatment after culture results, one third (nine patients) had progressed to death upon disclosure of culture result. Despite having a low Charlson index, the assessed patients had high APACHE II score, ranging from 9 to 33, mean and median of 26 and 27, respectively.
Considering the fact that age was an independent mortality risk factor and combination therapy was a protective factor, age was dichotomized (≥50 years old and <50 years old), according to mortality and antibiotic adequacy. Antibiotic adequacy did not change mortality in patients with ≥50 or <50 years old.
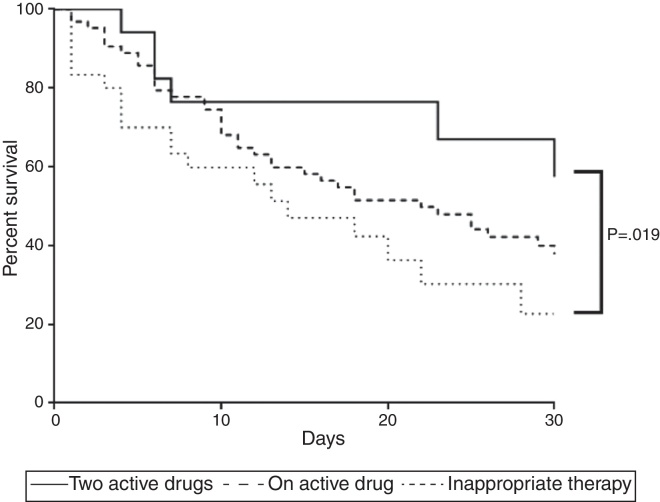
Survival analysis confirmed the aforementioned findings. Combined therapy showed higher survival rate than inappropriate therapy (p = 0.032) whereas survival of those with monotherapy was similar to those who received no active drug (p = 0.129) or combined therapy (p = 0.113) (Fig. 1).
Fig. 1.
Mortality curve of 112 patients with ventilator-associated pneumonia caused by Carbapenem-resistant Enterobacteriaceae. The curve compares mortality of patients receiving adequate therapy as monotherapy or combined as well as inadequate treatment. Two active drugs showed fewer deaths (p < 0.05).
Discussion
KPC-producing K. pneumoniae is endemic in Southern Brazil and it is among the most common pathogens in intensive care units. Similar condition has occurred in Greece.10 This may be associated with poor assistance conditions and low healthcare professional qualification in the intensive units. In developed countries such situation is different and KPC is not as important as Gram-positive cocci (Staphylococcus aureus).18
Some small case series have been published, but not exclusively evaluating CRE-VAP. This is the first study to focus on risk factors for mortality associated with CRE-VAP. From the 112 assessed patients, 81 (72.3%) received inappropriate empirical treatment within 24 h. The most frequent empirical treatment was the use of carbapenem, considered inadequate, even in combined therapy, as none of the tested strains had a MIC < 8 mg/L. Probably due to increased MIC levels, we could not evidence superiority of carbapenem use in combined therapies.
Combination therapy was previously associated with better survival in a multivariate analysis of 41 patients with KPC bacteremia reported by Qureshi et al. The 28-day mortality was 13.3% in the combination therapy group compared to 57.8% in the monotherapy group (p = 0.01).19 In a review article of case series of KPC infection treatment, Lee and Burgess have analyzed 38 articles with a total of 105 patients, of which 30% had KPC pneumonia. From the total evaluated patients, 47% received monotherapy and 53% received combination therapy, the global treatment failure rate was 36%. Among therapeutic failure cases, the pulmonary site was the most frequent (47%), followed by bacteremia (39%). In our study, more treatment failures were seen in cases treated with monotherapy than in those treated with combination therapy, but the small sample size precluded definitive conclusions. The most common successful regimen for treating pulmonary infections consisted of tigecycline plus colistin (8 patients), followed by aminoglycoside plus colistin (3 patients), but the overall failure rates were not significantly different in the three most commonly used antibiotic combinations: polymyxin plus carbapenem, polymyxin plus tigecycline, polymyxin plus aminoglycoside (30%, 29%, and 25%, respectively, p = 0.06).
Clinical trials are recruiting patients in order to identify the best options, but the answer is still far away. The current concept is to associate antibiotics for CRE, which is based on retrospective studies showing reduced mortality.2, 19, 20
Oliveira et al. analyzed 118 patients with CRE infection and found polymyxin treatment as an independent risk factor associated with higher mortality. Combined therapy in such study was not superior to monotherapy, but monotherapy with carbapenem was protective. The authors discussed that changes in CLSI breakpoints of enterobacteria for carbapenems was based on in vitro data and that for clinical purposes, carbapenems could be active when MIC is between 1 and 4 mg/L. Furthermore, some authors have included a carbapenem as third drug in regimens with two active drugs when the MIC is less than 8 mg/L. However, no CRE showed this pattern in the present cases. This may be one of the explanations for ineffectiveness of carbapenems in terms of CRE-VAP survival.
A recent meta-analysis showed that carbapenem combination should be revised, once it does not decrease mortality and is a potential harm for patients, considering side effects, Clostridium difficile colitis, and resistance induction.21 In our study, none of the therapeutic combinations was independently associated with increased survival; however, combined therapy within five days, with at least two active drugs, was associated to a lower mortality (p = 0.044).
Some limitations of our study included the lack of sepsis severity assessment upon onset of symptoms, evaluation of local complications, such as empyema and lung abscess, and drug dosage. Colistin and tigecycline require a loading dose, even with renal failure. Aminoglycosides serum levels are not routinely evaluated as well as daily dose adjustment of other drugs according to renal clearance. However, even in patients using the recommend dosage, most of them in intensive care units may not achieve adequate levels, despite pharmacodynamic characteristics (Cmax:MIC for aminoglycosides, AUC:MIC for colistin/polymyxin or T%:MIC for carbapenem) in approved FDA dosages.22 Tigecycline requires very low serum levels in order to be considered a safe drug in severe patients. Moreover, lung concentrations of all these drugs are not well known, even with perfect conditions of serum levels. Other limitation comes from the study design including only two hospitals. As it does not have external validity, we encourage other hospitals to compare combined therapy with monotherapy. The better outcome observed among patients receiving combined drugs until five days was interesting. It could mean that adding a second active drug could be recommended. Unfortunately, the small number of patients and the lack of robust data precluded us from performing a multivariate analysis.
Despite such limitations, our findings for VAP are in accordance with the current evidence, which points to better results with combined therapy for CRE severe infections.
In conclusion, patients with CRE-VAP treated with combined therapy with two active drugs within five days have lower mortality. In hospitals with high incidence of CRE, a combined therapy with two active drugs should be initiated until final culture results become available or further publications proving otherwise.
Ethical approval
This study was approved by the Ethics Committee of FEPAR (42918014.1.0000.0103).
Conflicts of interest
Felipe F. Tuon received grants from Pfizer, Novartis, Astellas and Teva in the last year.
Felipe F. Tuon is a CNPQ researcher.
Jaime L. Rocha received grants from Merck, Pfizer, Novartis, Sanofi and AztraZeneca in the last year.
Other authors declare no conflicts of interest.
Acknowledgement
Alexandre Zavascki for the comments and manuscript review.
References
- 1.Nordmann P., Naas T., Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791–1798. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tumbarello M., Viale P., Viscoli C., et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 3.Toledo P.V., Tuon F.F., Arend L., Aranha Junior A.A. Efficacy of tigecycline, polymyxin, gentamicin, meropenem and associations in experimental Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae non-lethal sepsis. Braz J Infect Dis. 2014;18:574–583. doi: 10.1016/j.bjid.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lat A., Clock S.A., Wu F., et al. Comparison of polymyxin B, tigecycline, cefepime, and meropenem MICs for KPC-producing Klebsiella pneumoniae by broth microdilution, Vitek 2, and Etest. J Clin Microbiol. 2011;49:1795–1798. doi: 10.1128/JCM.02534-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Castanheira M., Sader H.S., Jones R.N. Antimicrobial susceptibility patterns of KPC-producing or CTX-M-producing Enterobacteriaceae. Microb Drug Resist. 2010;16:61–65. doi: 10.1089/mdr.2009.0031. [DOI] [PubMed] [Google Scholar]
- 6.Tuon F.F., Rocha J.L., Formighieri M.S., et al. Fosfomycin susceptibility of isolates with blaKPC-2 from Brazil. J Infect. 2013;67:247–249. doi: 10.1016/j.jinf.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Toledo P.V., Arend L.N., Pilonetto M., Costa Oliveira J.C., Luhm K.R. Working Group in Healthcare Associated I. Surveillance programme for multidrug-resistant bacteria in healthcare-associated infections: an urban perspective in South Brazil. J Hosp Infect. 2012;80:351–353. doi: 10.1016/j.jhin.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro V.B., Andrade L.N., Linhares A.R., et al. Molecular characterization of Klebsiella pneumoniae carbapenemase-producing isolates in southern Brazil. J Med Microbiol. 2013;62(Pt 11):1721–1727. doi: 10.1099/jmm.0.062141-0. [DOI] [PubMed] [Google Scholar]
- 9.Maltezou H.C., Giakkoupi P., Maragos A., et al. Outbreak of infections due to KPC-2-producing Klebsiella pneumoniae in a hospital in Crete (Greece) J Infect. 2009;58:213–219. doi: 10.1016/j.jinf.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Kontopidou F., Giamarellou H., Katerelos P., et al. Infections caused by carbapenem-resistant Klebsiella pneumoniae among patients in intensive care units in Greece: a multi-centre study on clinical outcome and therapeutic options. Clin Microbiol Infect. 2014;20:O117–O123. doi: 10.1111/1469-0691.12341. [DOI] [PubMed] [Google Scholar]
- 11.Daly M.W., Riddle D.J., Ledeboer N.A., Dunne W.M., Ritchie D.J. Tigecycline for treatment of pneumonia and empyema caused by carbapenemase-producing Klebsiella pneumoniae. Pharmacotherapy. 2007;27:1052–1057. doi: 10.1592/phco.27.7.1052. [DOI] [PubMed] [Google Scholar]
- 12.Tuon F.F., Gortz L.W., Penteado-Filho S.R., Soltoski P.R., Hayashi A.Y., Miguel M.T. Bacteriological study of bronchoalveolar lavage in the antibiotic management of suspected ventilator-associated pneumonia of patients in surgical intensive care units. Rev Col Bras Cir. 2012;39:353–357. doi: 10.1590/s0100-69912012000500002. [DOI] [PubMed] [Google Scholar]
- 13.CLSI (Clinical Laboratory Standard Institute) 2013. Performance standard for antimicrobial susceptibility testing; Twenty-third informational supplement. [Google Scholar]
- 14.Tuon F.F., Rocha J.L., Toledo P., et al. Risk factors for KPC-producing Klebsiella pneumoniae bacteremia. Braz J Infect Dis. 2012;16:416–419. doi: 10.1016/j.bjid.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 15.Horan T.C., Andrus M., Dudeck M.A. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 17.Knaus W.A., Draper E.A., Wagner D.P., Zimmerman J.E. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 18.Jones R.N. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51(Suppl. 1):S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 19.Qureshi Z.A., Paterson D.L., Potoski B.A., et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasink L.B., Edelstein P.H., Lautenbach E., Synnestvedt M., Fishman N.O. Risk factors and clinical impact of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Infect Control Hosp Epidemiol. 2009;30:1180–1185. doi: 10.1086/648451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paul M., Carmeli Y., Durante-Mangoni E., et al. Combination therapy for carbapenem-resistant Gram-negative bacteria. J Antimicrob Chemother. 2014;69:2305–2309. doi: 10.1093/jac/dku168. [DOI] [PubMed] [Google Scholar]
- 22.Sun H.K., Kuti J.L., Nicolau D.P. Pharmacodynamics of antimicrobials for the empirical treatment of nosocomial pneumonia: a report from the OPTAMA Program. Crit Care Med. 2005;33:2222–2227. doi: 10.1097/01.ccm.0000181528.88571.9b. [DOI] [PubMed] [Google Scholar]