Abstract
Mycosis fungoides is a type of cutaneous T-cell lymphoma, which accounts for the majority of cases of cutaneous T-cell lymphoma. Mycosis fungoides can be classified as early-stage (IA–IIA) or late-stage (IIB or greater) disease. In early-stage mycosis fungoides, skin-directed therapies are commonly used to manage the disease. Chlormethine, or mechlorethamine, is a topical chemotherapeutic, which has been in use for over 60 years. In 2013, the US Food and Drug Administration approved chlormethine/mechlorethamine gel (Valchlor®) for treatment of stage IA and IB mycosis fungoides. Chlormethine/mechlorethamine gel is an effective therapy; however, its use may be limited by the development of adverse cutaneous reactions. Off-label dosing modifications, as well as co-administration of topical steroids and an aggressive moisturization regimen, can be used to reduce these side-effects. We report here 4 cases of mycosis fungoides treated with chlormethine/mechlorethamine gel at the Comprehensive Skin Cancer Center at Columbia University Irving Medical Center, which provide insights into the use of this therapy in clinical practice.
Key words: cutaneous T-cell lymphoma, mycosis fungoides, chlormethine, mechlorethamine, prescription drug management, chemotherapeutics
Topical chlormethine, otherwise known as mechlorethamine, was first introduced in the 1950s as an effective skin-directed chemotherapeutic agent for treating early-stage mycosis fungoides (MF), the most common type of cutaneous T-cell lymphoma (CTCL) (1–4). Systemically, mechlorethamine works as an alkylating agent affecting rapidly dividing cells. However, its mechanism of action topically is not fully understood and may also be mediated by immune mechanisms, including interactions with the epidermal cell-Langerhans cell-T-cell axis (5, 6). Various formulations of chlormethine/mechlorethamine were available in the USA before the advent of gel as a compounded product, including aqueous solutions and petrolatum-based ointments. In the randomized, controlled, multicentre trial in 2013 (7), comparing mechlorethamine HCI 0.016% gel (equivalent to 0.02% chlormethine/mechlorethamine HCl ointment) to Aquaphor®-based chlormethine/mechlorethamine HCl 0.02% ointment, 0.02% chlormethine/mechlorethamine gel was proven non-inferior to topical ointment, with an overall response rate of 58.5%, which included complete and partial remissions in patients with early-stage MF. In 2013, based on this pivotal study, the US Food and Drug Administration (FDA) approved the first topical formulation of chlormethine/mechlorethamine 0.016%, in the form of a gel (Valchlor®; Helsinn Therapeutics, Iselin, NJ, USA) for treating patients with stage IA and IB MF who have received prior skin-directed therapies (7, 8). Chlormethine/mechlorethamine gel (CL gel) is an effective and safe therapy for limited or generalized patch and/or plaque (T1–T2) disease (1, 7, 8). Topical CL gel is currently endorsed by international guidelines for use as first-line therapy in adult patients with MF (European Organisation for Research and Treatment of Cancer (EORTC); European Society for Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN)). Subsequent studies of CL gel have reported overall response rates between 57% and 79.2% (1). If complete remission is achieved, CL gel can continue to be used as a maintenance therapy (8). However, the use of CL gel may be limited by the development of adverse cutaneous reactions, including skin irritation, contact dermatitis, delayed hypersensitivity reaction, erythema, pruritus and hyperpigmentation (1, 9, 10). In the pivotal study (7) demonstrating the efficacy of CL gel, skin-related adverse effects, including skin irritation, pruritus, erythema, contact dermatitis, skin hyperpigmentation, and folliculitis, occurred in 62% of participants. Allergic contact dermatitis specifically was observed in 15% of gel-treated participants. Treatment-limiting skin reactions were experienced by 20.3% of patients. Notably, these reactions were found to occur primarily within the first few months of treatment, with 90% of skin-related AEs occurring before 6 months on treatment. In addition, patients in this study were prohibited from using concomitant topical corticosteroids. While FDA prescribing information recommends CL gel be applied daily, off-label modifications to this dosing schedule, as well as co-administration of topical steroids, are commonly employed among expert clinicians, and may improve tolerance by reducing adverse reactions, especially in the first months of therapy (11). However, insights into the clinical decision-making of providers managing patients on CL gel therapy are limited in the literature, posing a challenge for clinicians who are less familiar with topical CL gel. We share our clinical experience here, in order to provide real-life guidance regarding treatment of early-stage MF, as illustrated by 4 cases of patients with MF treated with CL gel at the Comprehensive Skin Cancer Center (CSCC) at Columbia University Irving Medical Center.
SIGNIFICANCE
Mycosis fungoides is a type of cutaneous T-cell lymphoma. In early-stage mycosis fungoides, skin-directed therapies are commonly used to manage the disease. In 2013, the US Food and Drug Administration approved chlormethine/mechlorethamine gel (Valchlor®) for treatment of early- stage mycosis fungoides. Chlormethine/mechlorethamine gel is an effective therapy; however, its use may be limited by development of side-effects. Dosing modifications, co-administration of topical steroids and an aggressive moisturi zation regimen can be used to reduce these side-effects. We report here 4 cases of mycosis fungoides treated with chlormethine/mechlorethamine gel at Columbia University Irving Medical Center, which provide insights into the use of this therapy in clinical practice.
CASE REPORTS
Case 1. A 55-year-old woman, skin type II, had ongoing skin patches for many years which were responsive to clobetasol and fluocinonide ointments. However, she experienced rapid relapse whenever steroids were stopped. Biopsy revealed MF and the patient was then referred to the CSCC for further management. She presented with erythematous patches with fine scaling over the trunk and lower extremities, covering 8% of her total body surface area (TBSA), with no lymphadenopathy and no hepatosplenomegaly. The patient was assigned stage IA MF, T1aN0M0B0. Skin-directed therapies (SDT) including light therapy, and various topical formulations were discussed and the patient elected to initiate CL gel. Because of the potential for contact dermatitis leading to intolerance of CL gel, the decision was made to start treatment at a frequency of 3 times a week. She was instructed to stop clobetasol ointment and use mid-potency topical steroid ointment twice daily as needed. She was also started on an aggressive moisturization regimen (Geskin regimen, see details in the Discussion), which included nightly dilute vinegar soaks for 20 min, followed by application of topical steroid ointment or bland emollient under occlusion. On her 1-month follow-up, she demonstrated approximately 40% improvement from baseline, with less than 5% TBSA of patches (Fig. 1). She did not need to use any topical steroids during this 1-month period because she had no dermatitis or other side-effects. In light of this considerable response with no evidence of skin reaction, she was instructed to increase use of CL gel to 5 times a week. She was also instructed to use topical steroids as needed and to continue the aggressive moisturization regimen. At her 4-month follow up visit, she maintained response with no irritation and the frequency of CL gel application was increased to 6 times per week. After 6 months of CL gel use, there was an 87.5% reduction of TBSA from baseline (from 8% to 1% TBSA). During this period, she also developed inflammation and irritation under bilateral breast folds. She was instructed to hold application of CL gel to irritated areas for few days and apply triamcinolone (TAC) 0.1% ointment, then resume application to these areas 3 times per week and titrate up as tolerated. She was also instructed to continue application of CL gel 6 times per week to non-irritated areas. Over the next 3 months, irritation along the breast folds significantly improved and patient remained stable at 1% TBSA. The frequency of application of CL was reduced to 3-4 times per week for continued management.
Fig. 1.
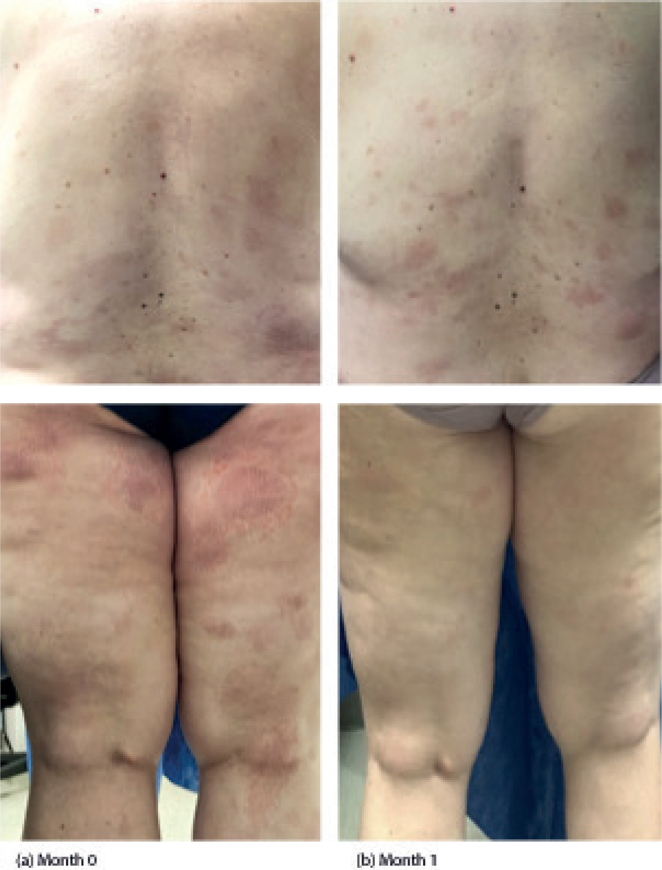
Case 1 clinical images. (a) Baseline photos taken at initial visit (month 0) prior to treatment. (b) Follow-up after 1 month of treatment with chlormethine/mechlorethamine gel.
Case 2. A 26-year-old woman, skin type IV, with a long history of eczema since early childhood that had been treated on and off with topical steroids and who received a renal transplant in 2011, maintained on tacrolimus and mycophenolate mofetil since that time. Six months prior to presenting to CUIMC in January 2020, she had developed non-pruritic, white patches on her thighs that gradually spread to her trunk and arms. Biopsies were obtained and were supportive of a diagnosis of hypo-pigmented MF. At her initial visit, she presented with hypopigmented patches over the trunk, arms and legs, 6% of TBSA, with no lymphadenopathy. The patient was assigned stage IA MF, T1aN0M0B0. Skin-directed therapies (SDT) including light therapy, and various topical formulations were discussed, and the patient elected to initiate CL gel. The Geskin regimen was also initiated (see Fig. 2). After 4 months using CL gel 3 times a week, she saw significant improvement and reduction in hypopigmented patches. She only used topical steroids occasionally initially and does not need to use them at the time of publication. On follow-up after 11 months of use, TBSA had reduced considerably to near complete remission (TBSA of 0.1%). Over the period of use, she denied any skin irritation, redness, itching, increased number of skin lesions, hyperpigmentation, or any other reaction to the gel.
Fig. 2.
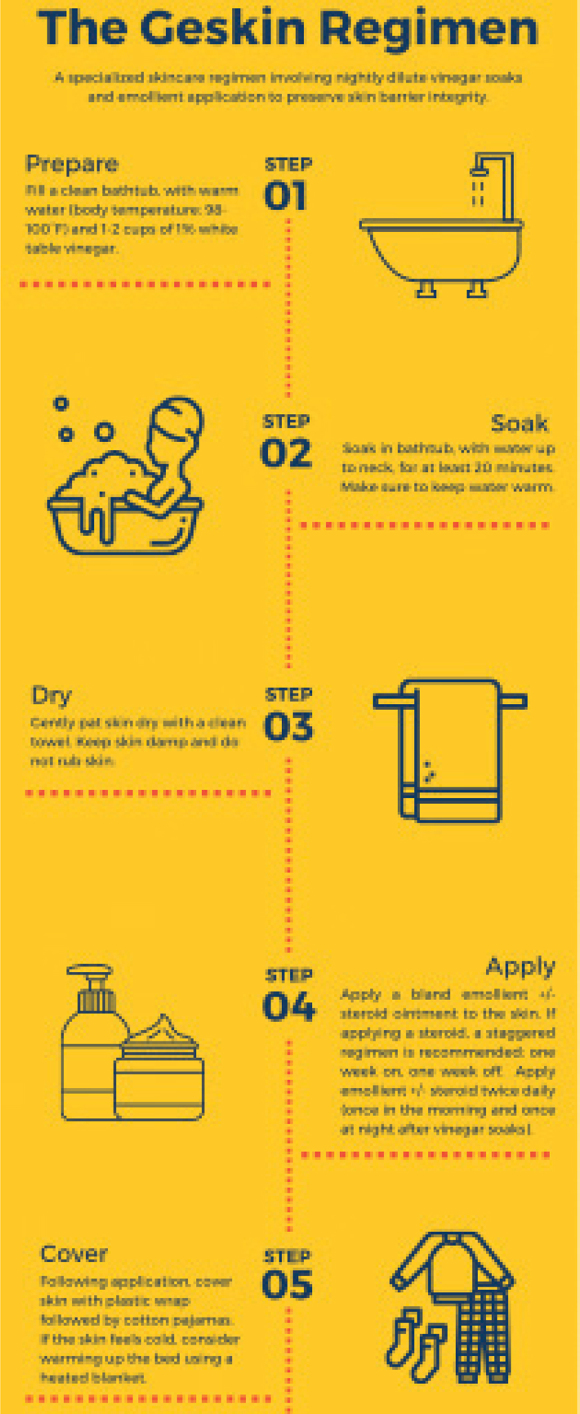
Detailed diagram of the Geskin regimen.
Case 3. A 47-year-old woman, skin type II, with a month-long history of a pruritic, non-painful dermatitis on the lower legs, which spread to her arms and trunk, was referred to the CSCC for further management of possible MF. She did not report systemic symptoms, such as fever, chills, night sweats or unintentional weight loss. The patient had a medical history significant for hypertension and a 20-year history of discoid lupus. She was started on losartan 1 week prior to rash emergence, on amlodipine 6 months before and hydroxychloroquine 2 years before. She stopped taking amlodipine and hydroxychloroquine when the rash appeared, but continued on losartan. She consulted an outside dermatologist, at which time a biopsy was consistent with early MF. At her initial evaluation, the patient presented with annular erythematous patches with fine scales on the trunk and extremities bilaterally with TBSA of 20%, no lymphadenopathy, and no hepatosplenomegaly. Flow cytometry was negative for peripheral blood involvement. This presentation was consistent with Stage IB disease, T2aN0M0B0. Skin-directed therapies (SDT) including light therapy, and various topical formulations were discussed, and the patient elected to initiate CL gel. The Geskin regimen was also initiated (see Fig. 2). After 10 months, the patient noted significant improvement with 80% reduction in TBSA involvement (from 20% to 4%). She reported using TAC ointment twice per week for pruritus associated with the disease. She noted that she tolerated CL gel application and did not experience any adverse symptoms. Over the following 6 months, the patient’s skin continued to improve, with a 95% reduction (from 20% to 1% TBSA). Sixteen months after starting CL gel therapy, the patient developed new hyperpigmented patches on the bilateral calves, with TBSA 2.5%. She was instructed to increase the frequency of CL gel application daily for affected areas, while continuing vinegar soaks and her moisturizing regimen. She achieved complete remission (Fig. 3) at the last follow-up after 21 months of CL gel therapy, with the patient reporting no adverse symptoms from daily spot application.
Fig. 3.
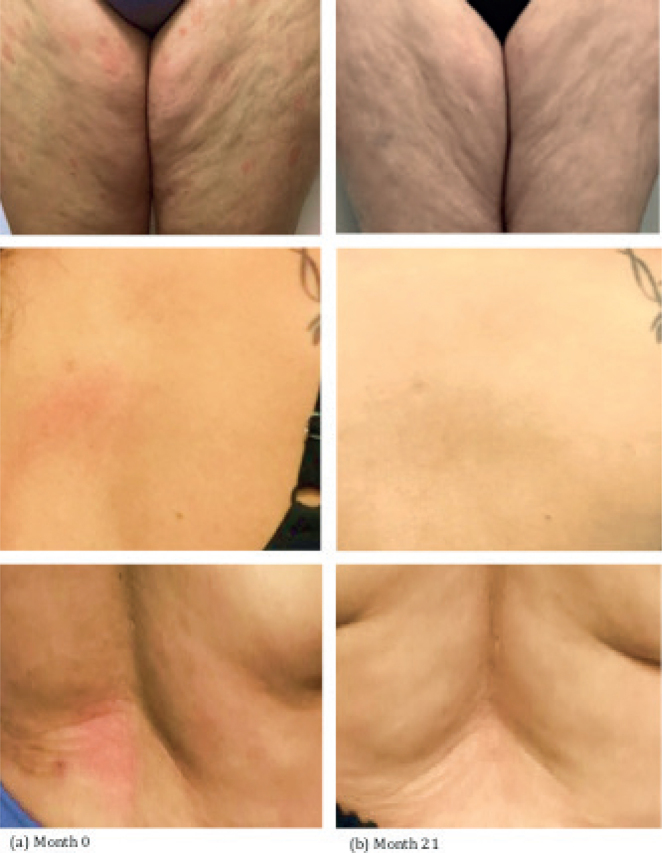
Case 2 clinical images. (a) Baseline at initial visit (month 0) prior to treatment. (b) Follow-up after 21 months of treatment with chlormethine/mechlorethamine gel.
Case 4. A 71-year-old woman, skin type II, was referred to CSCC for evaluation of possible MF. The patient reported a 5-year history of a rash behind her left knee that was intermittently pruritic and had become increasingly erythematous over the last 2 years. The rash was originally diagnosed as post-inflammatory hyperpigmentation. Two weeks before the patient presented at CUIMC, an outside dermatologist performed a biopsy of the rash that revealed severely atypical epidermotropic cerebriform CD4+ lymphocytic infiltrate. During the patient’s initial visit, she presented with a large 10-cm violaceous circular plaque involving the entire left popliteal fossa measuring 1.2% TBSA. Subtle erythematous scaly patches and plaques also covered the buttocks, right upper arm, left upper arm and elbow, and posterior right thigh, with a TBSA of 20%. No lymphadenopathy or splenomegaly was noted, peripheral blood flow cytometry was negative for involvement, and the patient was diagnosed with Stage IB MF, T2N0M0B0. Skin-directed therapies (SDT) including light therapy, and various topical formulations were discussed, and the patient elected to initiate CL gel. The Geskin regimen was also initiated (see Fig. 2). The patient was initiated on CL gel and was instructed to apply it from the neck down, because her patches were very faint and poorly defined, and it would be difficult to focus on the affected areas alone. In the first month of therapy, the patient showed significant improvement with reduction of patches to 7% TBSA. However, she developed superficial erosions in the left popliteal fossa, and was instructed to hold CL gel and apply TAC 0.1% ointment twice daily for 2 weeks. After resolution of the irritation and healing of the erosion, CL gel was restarted at a reduced dose of twice a week to the popliteal fossa only. On 2 months follow-up, there were no problems noted and the frequency increased to 3 times a week again, this time with no irritation. The patient was using TAC 0.1% for occasional itching in the area. No additional adverse symptoms were noted. After 3 months of treatment, skin involvement decreased to 1% TBSA, patch only, and remained stable for 7 months. The patient elected to take a drug holiday at this time. When she returned for follow-up 5 months later, the patient’s CTCL had progressed to 3% TBSA. The patient was instructed to resume CL gel once weekly. Over the next year, the patient remained stable with skin involvement at 1–2% TBSA patches only. After 24 months of treatment with CL gel no more than twice weekly, she remained stable with no adverse effects. Forty months after starting CL gel, the patient is stable at 0.7% TBSA, applying full-body CL gel no more than twice weekly and following the Geskin regimen.
DISCUSSION
Chlormethine/mechlorethamine gel is an effective skin-directed therapy for MF, but its use can be limited by adverse skin reactions, most frequently various types of dermatitis, mainly seen during the first months of treatment (10). In the pivotal study investigating the efficacy of CL gel, time-to-response analyses noted that patients treated with CL gel for a longer interval achieved a greater response (7). Accordingly, a by-time re-analysis of the study data demonstrated that responses to CL gel increased over time, with a peak response at 10 months, based on a 12-month study (12). Early, intermittent and late clinical response patterns to CL gel have been observed, but typical peak response occurs past 24 weeks, emphasizing the importance of continued CL gel treatment (7, 12). To augment its use in the non-research setting, off-label modifications to the application and dosage of CL gel are commonly made by clinicians based on clinical assessments. The use of CL gel in real clinical settings varies by provider and the pattern of usage is often dynamic, including utilization as an adjuvant or salvage therapy (13).
In our clinical practice, patient education as well as prevention and management of potential adverse skin reactions are important to optimize the use of CL gel. The appropriate application process is reviewed with the patient, including the use of nitrile gloves to apply the medication, avoidance of sensitive skin areas, such as the face and intertriginous areas, if possible, and taking care to avoid exposure of other persons to the medication, especially children and pregnant women. Patients are educated on how to modify the dosing schedule and how to appropriately use topical steroid ointment (e.g. triamcinolone acetonide ointment) should irritation occur.
Patients are initially started on CL gel 3 times a week with close monitoring. Starting at a reduced dose schedule allows the provider to titrate the dose up or down based on clinical response or the development of adverse effects. Adverse reactions can be effectively managed by reducing the frequency of application and the concomitant use of topical steroids. Upon initiation of CL gel, patients are not only instructed to start topical steroids as needed, but also encouraged to use the Geskin regimen, a specialized skincare protocol to promote normal skin barrier functions (see Fig. 2). The hallmark of the Geskin regimen is long soaks in an acidified medium, followed by the application of a topical ointment under an occlusive dressing. It is therefore distinct from typical “soak and smear” or “wet wrap” procedures in that it combines both extended vinegar soaks with modified, dry occlusive wraps. Soaking for at least 20 min serves to hydrate the stratum corneum and also helps in the removal of scale and crust; short soaks and showers do not provide the same benefit (14). Topical use of acids, such as acetic acid (the main component of vinegar), lowers the pH of the skin, which increases antimicrobial activity, increases the release of oxygen, and promotes tissue healing (15). Application of an emollient and/or topical steroid with occlusion following soaking enhances hydration and allows for increased penetration in the stratum corneum (14).
In line with our experience, A PROspective, Observational, US-based Study Assessing Outcomes, Adverse Events, Treatment Patterns, and Quality of Life in Patients Diagnosed With Mycosis Fungoides Cutaneous T-cell Lymphoma and Treated With Valchlor, NCT02296164 (PROVe Study), which examined the use of CL gel in clinical settings, reported that dermatitis/skin irritation rates were much lower than observed in the pivotal randomized trial demonstrating its efficacy, possibly due to concomitant topical steroid use and/or dosing modifications to CL gel (7, 11). Earlier studies have also demonstrated that concomitant use of CL gel and topical steroids was effective at treating early-stage MF, while decreasing the incidence of severe cutaneous reactions (2, 16). By limiting the development of adverse cutaneous reactions using topical steroids, flexible dosing schedules, and vinegar soaks followed by occlusion, CL gel can be used for a longer time, allowing for increased efficacy.
ACKNOWLEDGEMENTS
This work was supported by Helsinn Healthcare. No writing assistance was utilized in the production of this manuscript. The authors are fully responsible for all content and editorial decisions regarding this manuscript.
Footnotes
Conflicts of interest. TJG-S receives research support from Helsinn Healthcare. LG reports research support/PI for Helsinn Group, J&J, Mallinckrodt, Kyowa Kirin, Soligenix, Innate, miRagen, Galderma, Merck, BMS, and Stratpharma; speakers’ bureau for Helsinn Group and J&J; and scientific advisory board for Helsinn Group, J&J, Mallinckrodt, Sanofi, Regeneron, and Kyowa Kirin. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
REFERENCES
- 1.Denis D, Beneton N, Laribi K, Maillard H. Management of mycosis fungoides-type cutaneous T-cell lymphoma (MF): focus on chlormethine gel. Cancer Manag Res 2019; 11: 2241–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Esteve E, Bagot M, Joly P, Souteyrand P, Beylot-Barry M, Vaillant L, et al. A prospective study of cutaneous intolerance to topical mechlorethamine therapy in patients with cutaneous T-cell lymphomas. French Study Group of Cutaneous Lymphomas. Arch Dermatol 1999; 135: 1349–1353. [DOI] [PubMed] [Google Scholar]
- 3.Ramsay DL, Halperin PS, Zeleniuch-Jacquotte A. Topical mechlorethamine therapy for early stage mycosis fungoides. J Am Acad Dermatol 1988; 19: 684–691. [DOI] [PubMed] [Google Scholar]
- 4.Vonderheid EC, Tan ET, Kantor AF, Shrager L, Micaily B, Van Scott EJ, et al. Long-term efficacy, curative potential, and carcinogenicity of topical mechlorethamine chemotherapy in cutaneous T cell lymphoma. J Am Acad Dermatol 1989; 20: 416–428. [DOI] [PubMed] [Google Scholar]
- 5.Kim YH, Martinez G, Varghese A, Hoppe RT. Topical nitrogen mustard in the management of mycosis fungoides: update of the Stanford experience. Arch Dermatol 2003; 139: 165–173. [DOI] [PubMed] [Google Scholar]
- 6.Tang L, Cao L, Bernardo O, Chen Y, Sundberg JP, Lui H, et al. Topical mechlorethamine restores autoimmune-arrested follicular activity in mice with an alopecia areata-like disease by targeting infiltrated lymphocytes. J Invest Dermatol 2003; 120: 400–406. [DOI] [PubMed] [Google Scholar]
- 7.Lessin SR, Duvic M, Guitart J, Pandya AG, Strober BE, Olsen EA, et al. Topical chemotherapy in cutaneous T-cell lymphoma: positive results of a randomized, controlled, multi-center trial testing the efficacy and safety of a novel mechlorethamine, 0.02%, gel in mycosis fungoides. JAMA Dermatol 2013; 149: 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trautinger F, Knobler R, Willemze R, Peris K, Stadler R, Laroche L, et al. EORTC consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome. Eur J Cancer 2006; 42: 1014–1030. [DOI] [PubMed] [Google Scholar]
- 9.Talpur R, Venkatarajan S, Duvic M. Mechlorethamine gel for the topical treatment of stage IA and IB mycosis fungoides-type cutaneous T-cell lymphoma. Expert Rev Clin Pharmacol 2014; 7: 591–S97. [DOI] [PubMed] [Google Scholar]
- 10.Gilmore ES, Alexander-Savino CV, Chung CG, Poligone B. Evaluation and management of patients with early-stage mycosis fungoides who interrupt or discontinue topical mechlorethamine gel because of dermatitis. JAAD Case Rep 2020; 6: 878–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim EJ, Geskin L, Guitart J, Querfeld C, Girardi M, Musiek A, et al. Real-world experience with mechlorethamine gel in patients with mycosis fungoides-cutaneous lymphoma: preliminary findings from a prospective observational study. J Am Acad Dermatol 2020; 83: 928–930. [DOI] [PubMed] [Google Scholar]
- 12.Geskin LJ, Kim EJ, Angello JT, Kim YH. Evaluating the treatment patterns of chlormethine/mechlorethamine gel in patients with Stage I-IIA mycosis fungoides: by-time reanalysis of a randomized controlled phase 2 study. Clin Lymphoma Myeloma Leuk 2021; 21: 119–124. [DOI] [PubMed] [Google Scholar]
- 13.Duffy R, Jennings T, Sahu J. Mechlorethamine gel usage in patients with mycosis fungoides in a lymphoma clinic. Indian J Dermatol 2020; 65: 237–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutman AB, Kligman AM, Sciacca J, James WD. Soak and smear: a standard technique revisited. Arch Dermatol 2005; 141: 1556–1159. [DOI] [PubMed] [Google Scholar]
- 15.Nagoba BS, Suryawanshi NM, Wadher B, Selkar S. Acidic environment and wound healing: a review. Wounds 2015; 27: 5–11. [Google Scholar]
- 16.de Quatrebarbes J, Esteve E, Bagot M, Bernard P, Beylot-Barry M, Delaunay M, et al. Treatment of early-stage mycosis fungoides with twice-weekly applications of mechlorethamine and topical corticosteroids: a prospective study. Arch Dermatol 2005; 141: 1117–1120. [DOI] [PubMed] [Google Scholar]


