Abstract
Parkinson’s disease (PD) is characterized by the selective death of substantia nigra pars compacta (SNpc) dopaminergic neurons and includes both motor and non-motor symptoms. While numerous models exist for the study of typical PD motor deficits, fewer exist for non-motor symptoms. Previous studies have shown that a Pitx3−/− mouse model (aphakia or ak mouse) has specific developmental failure of the dopaminergic neuron population in the SNpc and that it can be used for the study of PD-related gross motor dysfunction as well as cognitive functional deficits. It remains unclear whether the aphakia mouse, both male and female, might also be used to model fine motor deficits and for additional studies of non-motor deficits associated with PD. Here, using an extensive battery of behavioral tests, we demonstrate that the aphakia mouse shows both gross and fine motor functional deficits compared with control mice. Furthermore, aphakia mice show deficits of olfactory function in buried pellet, odor discrimination and odor habituation/dishabituation tests. We also found that aphakia mice suffer from gastrointestinal dysfunction (e.g., longer whole gut transit time and colon motility deficits), suggesting that the mutation also affects function of the gut-brain axis in this animal model. Moreover, our data demonstrate that in the aphakia mouse, L-DOPA, the gold standard PD medication, can rescue both gross and fine motor function deficits but neither olfactory nor gastrointestinal symptoms, a pattern much like that seen in PD patients. Altogether, this suggests that the aphakia mouse is a suitable model for fine motor, olfactory and gastrointestinal behavioral studies of PD as well as for the development of novel disease-modifying therapeutics. Significance statement: While several animal models are available to study the major motor symptoms of PD, there are fewer that replicate non-motor symptoms, which constitute a major source of morbidity for patients. Moreover, available models often require manipulations resulting in sudden massive cell loss and inflammation, both of which may interfere with understanding of the direct effects of dopaminergic neuronal loss in the SNpc. We describe a model of congenital SNpc cell deficiency in a Pitx3−/− mouse and characterize it with a battery of behavioral tests suggesting that it closely mimics non-motor as well as motor symptoms of PD, providing a useful insight into the effects of the nigrostriatal dopamine deficit. Taken together, these data suggest that the ak mouse represents a useful model to study dopaminergic system function for both motor and non-motor symptoms of PD.
Keywords: Parkinson’s disease, Pitx3, motor function, olfactory function, gastrointestinal function, inflammation
1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease. Loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) is a prominent feature (de Lau and Breteler, 2006) and histopathological hallmark of PD, along with Lewy bodies and Lewy neurites (Balestrino and Schapira, 2020). At present, dopamine (DA) replacement therapy (e.g., L-DOPA and/or DA agonists) is the gold standard treatment and remains the therapeutic mainstay. However, this pharmacological therapy generally only addresses motor symptoms, including bradykinesia, resting tremor, rigidity, and postural instability. Clinically, PD is often undiagnosed until onset of motor symptoms which adversely affect the ability of patients to complete tasks of daily living (Armstrong and Okun, 2020). However, there are many non-motor symptoms associated with PD, some of which can appear years before the onset of the motor phenotype, and that continue through the course of the disease (Katunina and Titova, 2017). These non-motor symptoms are not only difficult to treat medically but also significantly diminish quality of life (Huang et al., 2019). The major non-motor symptoms observed in PD patients include cognitive decline, hyposmia, gastrointestinal dysfunction, sleep disturbances, anxiety, and depression (Chaudhuri et al., 2006; Schapira et al., 2017). Some of these symptoms respond to DA replacement therapies, but many do not (Schaeffer and Berg, 2017), and their underlying pathophysiology remains unclear. The clinical heterogeneity of non-motor symptoms of PD may reflect a wide range of cellular and molecular pathology, as almost the entire range of neurotransmitters present in the brain and the periphery have been implicated (Konno et al., 2017; Schapira et al., 2017) but whether these changes are primary aspects of the disease or secondary effects of dopaminergic dysfunction is unclear. This highlights the need for an effective animal model that exhibits both motor and non-motor symptomatology.
There are two main categories of PD rodent models: neurotoxin-induced (environmental) and genetic (Blandini and Armentero, 2012; Dauer and Przedborski, 2003; Vingill et al., 2018). The former includes treatments with 6-hydroxydopamine (6-OHDA), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), rotenone, and paraquat (Dawson et al., 2010; Tieu, 2011). Genetic models include those overexpressing α-synuclein or LRRK2, as well as DJ-1 knockout, PINK1 knockout, VMAT2−/−, Nurr1+/−, MitoPark and Pitx3−/− (aphakia) mouse models (Chesselet and Richter, 2011; Dawson et al., 2010). These mouse models have been crucial for understanding the basis of motor symptomatology. Moreover, some of these models also display symptoms highly suggestive of non-motor aspects of PD seen in humans (Langley et al., 2021; McDowell and Chesselet, 2012).
The aphakia (ak) mouse is a Pitx3-deficient genetic PD model that shows selective failure of development of the SNpc dopaminergic neurons due to homozygous disruption of the Pitx3 homeobox gene, which is uniquely expressed in these neurons (Brandt et al., 2017; Hwang et al., 2003; Nunes et al., 2003; Semina et al., 2000; Singh et al., 2007; Smidt et al., 2004; Suarez et al., 2018; van den Munckhof et al., 2003). The ak mouse model has been previously described as a model of moderate PD (Solis et al., 2015) precisely because it presents a clear dorso-ventral gradient of TH expression and DA release (Alberquilla et al., 2020). Previous work by our group and others established that ak mice display PD-like motor deficits that can be rescued by acute 3,4-dihydroxyphenylalanine (L-DOPA) treatment (Ding et al., 2007; Hwang et al., 2005; van den Munckhof et al., 2006) or by dopaminergic cell transplantation (Chung et al., 2011; Moon et al., 2013; Zenchak et al., 2020). Intriguingly, ak mice show deficits in learning and memory function (Ardayfio et al., 2008), and apparent depressive behavior (Kim et al., 2014), indicating that the ak mouse has both motor and non-motor functional deficits. Recent studies have raised the possibility of role for Pitx3 in circadian rhythms and metabolic homeostasis(Del Rio-Martin et al., 2019; Fernandez-Perez et al., 2022; Scarpa et al., 2022). It remains unknown whether the ak mouse also exhibits other non-motor deficits such as olfactory or gastrointestinal dysfunction similar to those seen in PD patients. Since the ak mouse exhibits selective absence of SNpc dopamine neurons without any other cell loss (e.g., noradrenergic neurons in the locus coeruleus), it is an ideal model to address whether these non-motor symptoms are associated with the loss of SNpc neurons specifically. To tackle these issues, we performed an extensive battery of both motor and non-motor behavioral tests and revealed that the ak mouse indeed shows both motor and non-motor symptoms. Further, in addition to gross motor deficits, the ak mouse also shows fine motor deficits suggestive of PD symptoms as demonstrated by adhesive removal test, grip strength test and nest building test. Significant non-motor symptoms include cognitive deficits, olfactory dysfunction and gastrointestinal dysmotility. These findings support the use of the ak mouse as a viable PD mouse model to study mechanisms and novel therapeutics development for both motor and non-motor deficits.
2. Results
2.1. ak mice show both gross and fine motor deficits
Because Pitx3 also controls the development of the cornea, leading to an underdeveloped cornea and blindness in ak mice, visual impairment must be ruled out as a source of functional deficits in behavioral testing. We therefore used functionally blind retinal degeneration 1 (rd1) mice with intact SNpc dopamine neurons as controls. We previously showed that the ak mouse exhibits no impairment in overall spontaneous locomotor activity, but displays motor deficits in nigrostriatal pathway-sensitive behavioral tests, including the beam challenge test and cylinder test (Hwang et al., 2005). These behavior tests are recognized as analog measures of PD gross motor skills or whole-body coordination, but not of fine motor skills. Thus, we examined age- and sex-matched rd1 and ak male mice for both gross motor skills (challenging beam test, cylinder test and rotarod test) and fine motor skills by behavioral tests requiring the use of orofacial and forepaw movements, including adhesive removal tests, grip strength tests and nest-building tests.
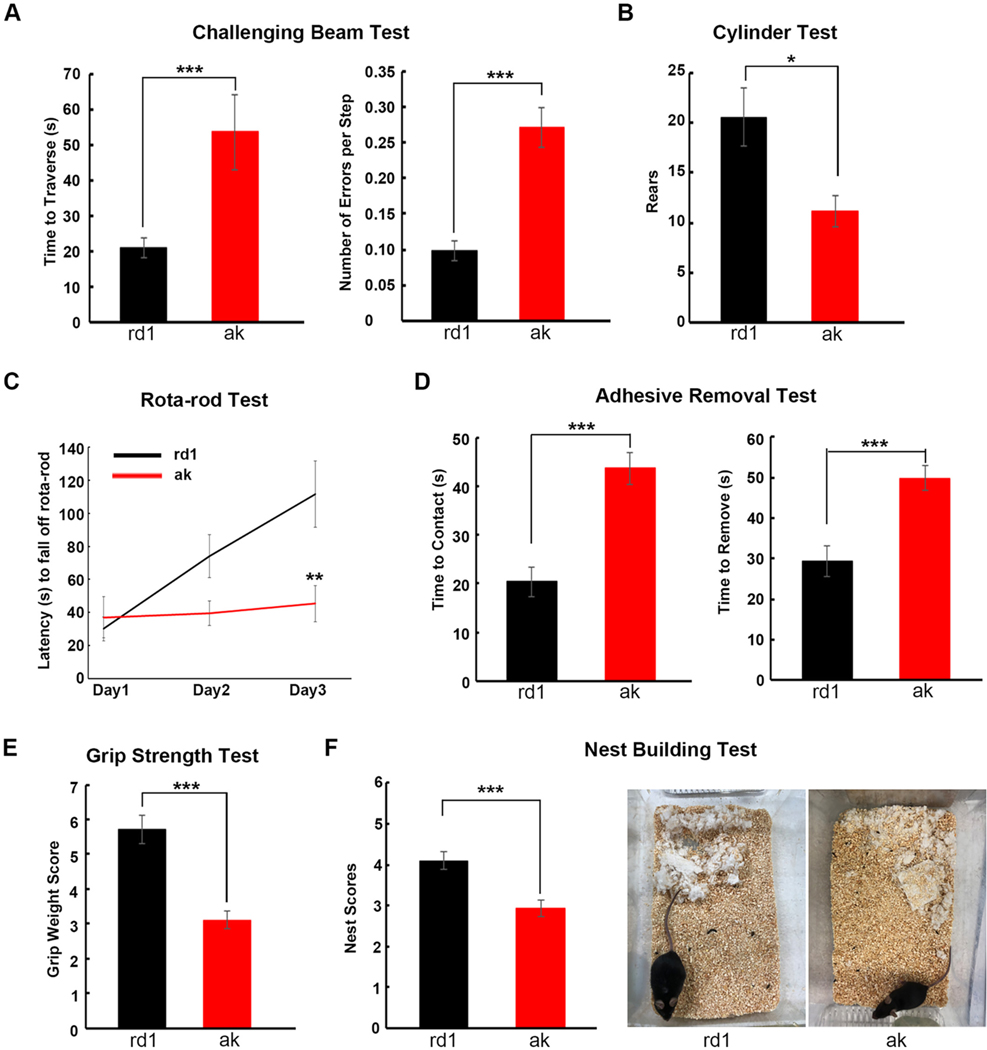
As shown in Fig. 1A, during the challenging beam test, ak mice took significantly longer to traverse the beam (1 m) than rd1 mice (53.7 vs 21 s, p < 0.001). During the traverse, ak mice made more errors per step compared to rd1 mice (average 0.27 vs 0.10 errors per step, p < 0.001, Fig. 1A). On the cylinder test, ak mice showed fewer rearing behaviors than rd1 mice (11.1 vs 20.6 rears, p < 0.01, Fig. 1B). On the rotarod test, on day 1, ak and rd1 mice showed similar latencies. Over the next 2 days, however, rd1 mice improved their performance markedly while ak mouse performance scores remained nearly flat, indicating that ak mice have deficits in motor performance and skill acquisition (Fig. 1C). To examine fine motor function, rd1 and ak mice were subjected to three different tests (adhesive removal test, grip strength test and nest building test). Small adhesive stimuli (quarter inch round stickers) were placed on the snout of each mouse. The mouse would raise both forelimbs towards its face and attempt to wipe off the stimulus with both forepaws (Bouet et al., 2009; Fleming et al., 2004). The time required to make contact and remove the stimulus was recorded. As shown in Fig. 1D, ak mice took longer for both contact and for removal than did rd1 mice (contact time: 43.6 vs 20.4 s, p < 0.001; removal time: 49.8 vs 29.3 s, p < 0.05, Fig. 1D), indicating that ak mice have either forepaw movement deficits, orofacial sensory deficits, or both. The grip strength test was used to detect muscle strength and coordination ability (Deacon, 2013). The testing apparatus consisted of four weighted chains attached to a fine mesh onto which the mice were challenged to grip. The grip score was calculated by the numbers of chains the mouse was able to support and how long it was able to avoid falling. ak mice had lower grip scores than rd1 mice (3.11 vs 5.71, p < 0.001, Fig. 1E). Nest building is a natural rodent behavior that requires coordinated orofacial and forepaw motor skills. It has been used to assess fine motor and sensorimotor skills in PD mouse models (Fleming et al., 2004; Paumier et al., 2013). Typically, a mouse first shreds a tightly packed cotton square, then arranges it into a nest. We applied a five-point nest-rating scale (Deacon, 2006) to measure the nest building ability of ak and rd1 mice. Our data showed that ak mice had lower nest-rating scores than rd1 mice (3.0 vs 4.1, p < 0.001, Fig. 1F) and in addition ak mice left obvious untorn cotton squares in the cage while rd1 mice did not. Taken together, these findings demonstrate that ak mice display both gross and fine motor/sensorimotor functional deficits involving forepaw and orofacial tasks.
Fig. 1.
ak mice show both gross and fine motor functional deficits. Four- to six-month-old male rd1 and ak mice were subjected to a battery of motor function tests: (A) challenging beam test; (B) cylinder test; (C) rotarod test, (D) adhesive removal test; (E) grip strength test; and (F) nest building test. *p < 0.05; **p < 0.01; ***p < 0.001 Mean ± S.E.M, n = 7–10 for each group.
Neuroinflammation plays a critical role in neurodegenerative diseases. Inflammation itself could be a confounding factor in determining the cause of deficits related to DA cell loss. While the ak mouse motor function deficits are related to developmental failure of substantia nigra dopaminergic neurons, it remains unknown if neuroinflammation is involved in this process. To address this, we stained both the midbrain and the striatum of wild type C57BL/6 (wt), rd1 and ak mice for the astrocyte marker, GFAP, and a microglial marker, Iba1. We found no significant differences in GFAP or Iba1 staining pattern in the midbrain (Fig. S1A, C and D) or in the striatum (Fig. S1B, C and D) between adult wt, rd1 and ak mice, although ak mice had the expected lower expression of dopaminergic neurons (TH+) in the SNpc. This lack of neuroinflammatory marker activation in the midbrain or striatum associated with the SNpc DA cell deficiency of ak mice points to the lack of SNpc DA neurons itself, rather than reactive inflammation, as the cause of the motor function deficits of adult ak mice.
2.2. ak mice show deficits of olfactory function
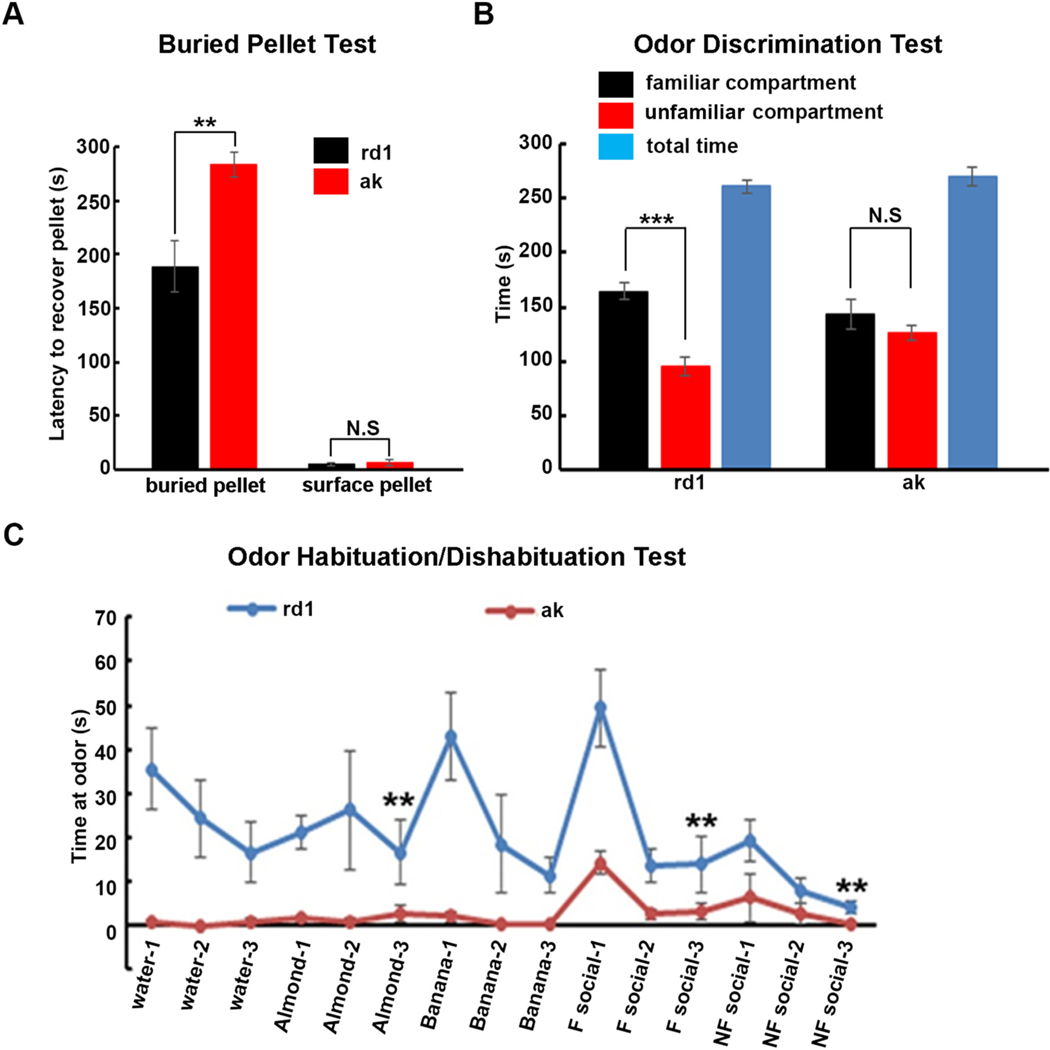
Since loss of olfactory function is one of the frequent prodromes of PD (Doty, 2017), we investigated whether the ak mouse exhibits any olfactory dysfunction. First, general olfactory function was assessed using the buried pellet test. A treat pellet was buried approximately 0.5 cm below the bedding. After treat placement, each mouse was placed in the center of the test cage and given 5 min to find and recover the treat, while the latency to sniff, dig up, and begin eating food was recorded. The tests were performed for five consecutive days. On the sixth day, the pellet was put on the surface (“surface pellet test”). Latencies were similar between ak and rd1 mice in this control surface pellet situation, thereby excluding lack of motivation as an explanation for the greater latency of ak mice with buried pellets (Lehmkuhl et al., 2014). Our data showed that latencies to recover a buried pellet by ak mice were substantially longer than by rd1 mice (283.5 vs 188.6 s, p < 0.01, Fig. 2A) while as noted above, ak mice and rd1 mice experienced similar latencies to discover a surface pellet (6.3 vs 4.8 s, p > 0.05, Fig.2A), suggesting that ak mice suffer from general olfactory dysfunction. To further assess olfactory acuity and discrimination in rd1 and ak mice, their odor discrimination ability was tested. This test relies on the fact that rodents usually prefer to stay at places impregnated with their own odor (familiar odor), versus places with unfamiliar odors. Each mouse was placed in a cage divided in two equal areas separated by an open door and allowed to choose between one compartment with fresh bedding (unfamiliar compartment) and another with unchanged bedding (familiar compartment) that the same mouse had occupied for 3 days before the test. Our data showed that rd1 mice spent more time and traveled greater distances in the familiar compartment compared to the unfamiliar compartment, while ak mice spent almost equal time and ravel distance in both familiar and unfamiliar compartments (p < 0.001, Fig. 2B). Since baseline spontaneous locomotor activity is not reduced in ak mice (Hwang et al., 2005), these deficits on the buried pellet and odor discrimination tests are best explained by olfactory perception deficit, a further indication of olfactory dysfunction in ak mice. To examine more subtle olfactory deficits, odor habituation/dishabituation paradigms were tested in rd1 and ak mice. Mice were sequentially presented with several different odors. The sniffing time directed towards each odor was recorded as a measurement of olfactory responsiveness. A typical mouse shows a decrease in response to a particular odor over repeated presentations (habituation). A novel odor presentation will elicit increased sniffing towards the new odor (dishabituation). Upon repeated presentation of the novel odor the animal again shows habituation. Here we chose water, two non-social odors (almond and banana extracts) and two social odors (familiar odor using bedding from the home cage; unfamiliar odor using fresh bedding). Each odor was presented three times for two minutes each. As shown in Fig. 2C, rd1 mice displayed odor habituation, with the sniffing time for the third presentation being shorter than the second presentation for each non-social or social odor. Sniffing time increased when a new odor was presented. However, the sniffing time curve for ak mice was almost flat, suggesting that ak mice have poor olfactory habituation/dishabituation ability. Together, our data demonstrated that the ak mouse has marked olfactory dysfunction.
Fig. 2.
ak mice show deficits of olfactory function. Four- to six-month-old male rd1 and ak mice performed (A) buried pellet test; (B) odor discrimination test; and (C) odor habituation/dishabituation tests. F social = familiar social, bedding from home cage; NF social = non-familiar social, bedding from a new cage. RD1 mouse showed significant habituation (less time sniffing successive same smells), and dishabituation (more time sniffing a new smell) while ak mouse did not. **p < 0.01; ***p < 0.001. Mean ± S.E.M., n = 7–10 for each group. N.S. = no significant difference.
2.3. Potential mechanisms underlying olfactory function deficit in ak mice
There exist multiple hypotheses about the mechanisms underlying olfactory functional deficits in PD patients, such as altered numbers of dopaminergic neurons in the olfactory bulb, activation of neuroinflammation, cholinergic neuron degeneration, α-synuclein accumulation, and others (Doty, 2012a; Doty, 2012b). To explore potential mechanisms, we tested several of these possibilities. First, we performed immunohistochemistry using anti-TH antibody to determine the dopaminergic neuron numbers in the olfactory bulb of rd1 and ak mice. TH positive cells localized in the glomerular layer showed no obvious differences in numbers between rd1 and ak mice by stereological counting (Fig. S2). This suggests that ak mice do not have altered numbers of dopaminergic neurons in the olfactory bulb although they exhibit robust SNpc deficiency. Second, we tested neuroinflammatory activation by staining with antibodies against GFAP and Iba1. GFAP and Iba1 staining density in the olfactory bulb were similar between wt, rd1 and ak mice (Fig. 3), consistent with absence of neuroinflammatory markers in the midbrain and the striatum. Taken together, our data suggest that neither neuroinflammatory activation nor degeneration of dopaminergic neurons in the olfactory bulb underlie the olfactory functional deficits of ak mice. The specific mechanism(s) underlying olfactory dysfunction in ak mice therefore remain unclear and may involve downstream sensory processing related to A9 DA neurons, meriting further investigation.
Fig. 3.
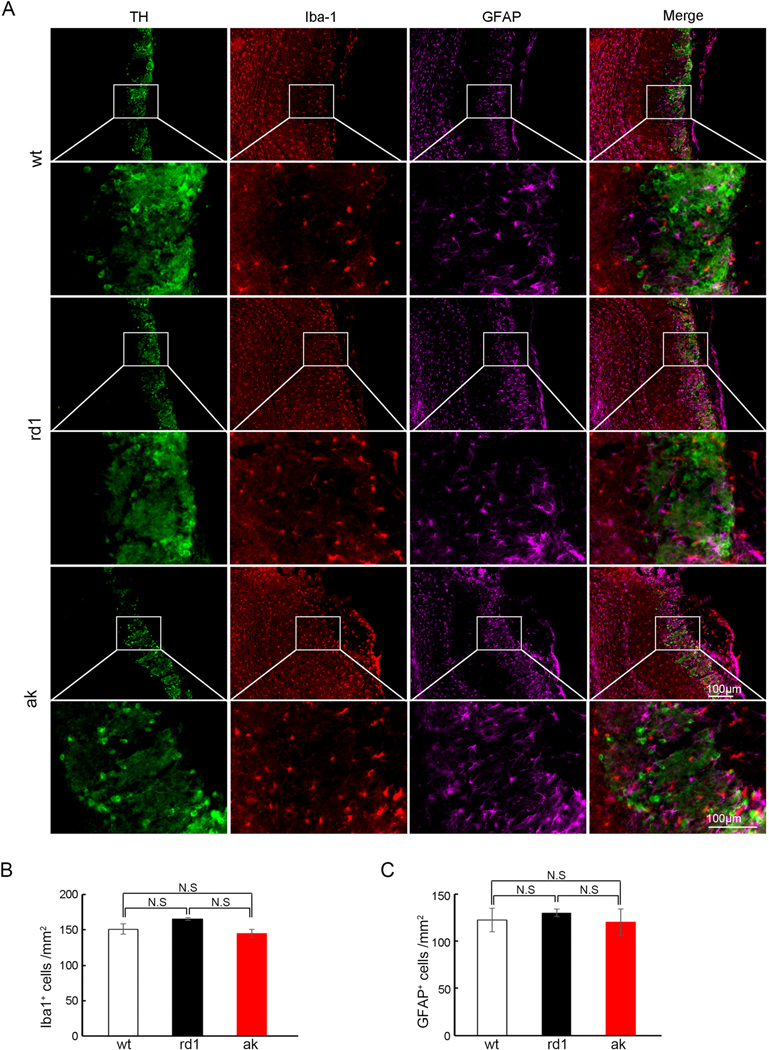
No difference in inflammatory activation in olfactory bulb between wt, rd1 and ak mice. (A) Immunofluorescence staining showed no difference of activated microglia (Iba1) or reactive astrocytes (GFAP) in the olfactory bulb of ak mice compared with wt and rd1 mice. The representative 10× and 40× images were shown. (B) Quantification of Iba1 positive cells; (C) Quantification of GFAP positive cells. The scale bar indicates 100 μm. N.S. = no significant difference. n = 4 for each group.
2.4. ak mice show gastrointestinal function deficits
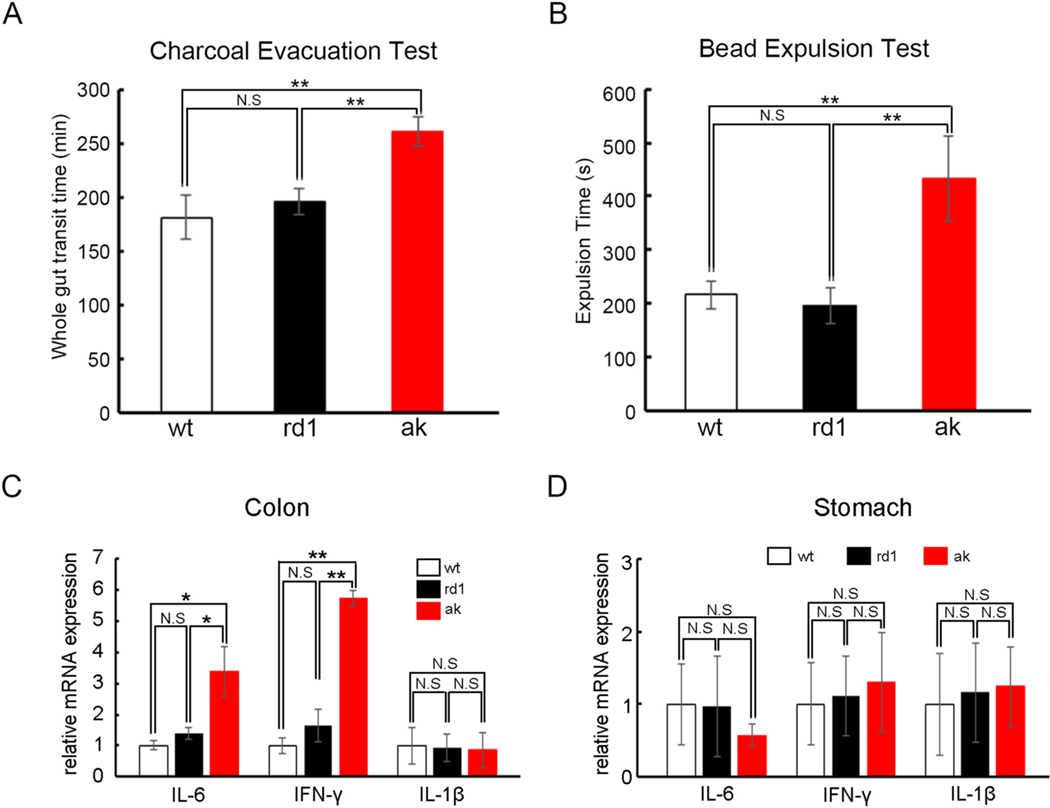
In PD patients, symptoms of gastrointestinal dysmotility include early satiety and nausea due to delayed gastric emptying, bloating related to poor coordination of small bowel motility, and constipation and defecatory dysfunction from impaired colonic transit (Fasano et al., 2015; Heetun and Quigley, 2012). To test if the ak mouse has gastrointestinal dysmotility, we first measured whole gut transit time using a charcoal meal. Interestingly, ak mice exhibited a slower whole gut transit time than wt and rd1 mice (261.8 vs 181.4 min, 261.8 vs 196.2 min, p < 0.01, Fig. 4A). To determine if the prolonged whole gut transit resulted from colonic dysmotility, we performed a bead expulsion test under isoflurane anesthesia. A 2-mm diameter glass bead was inserted into the distal colon 2 cm from the anus and the time for the bead to be expelled was recorded as a measure of colonic transit. Notably, ak mice displayed longer bead expulsion times than wt and rd1 mice (433 vs 216.7 s, 433 vs 196 s, p < 0.01, Fig. 4B), demonstrating that ak mice suffer from colonic dysmotility.
Fig. 4.
ak mice show deficits of gastrointestinal function. Four- to six-month-old male rd1 and ak mice underwent (A) charcoal evacuation tests, showing that ak mice have longer whole gut transit time than wt and rd1 mice; (B) bead expulsion tests showing that ak mice have colon motility deficits compared with wt and rd1 mice. Quantitative real time PCR showed that expression levels of certain inflammatory markers, IL-6 and IFN-γ, but not of IL-1β, are higher in ak mouse colon (C) than in wt and rd1 mice. In the stomach, none of the three markers showed significant differences between ak and wt and rd1 mice (D). *p < 0.05; **p < 0.01; Mean ± S.E.M., n = 6–10 for each group. N.S. = no significant difference.
To explore the mechanisms underlying ak mice’s colonic dysmotility, the mRNA expression levels of inflammatory markers IL-6, IFN-γ and IL-1β were tested in both colon and stomach tissues from male mice. As shown in Fig. 4C, the colonic expression levels of IL-6 and IFN-γ mRNAs were significantly higher in ak mice than in wt and rd1 mice, while IL-1β remained unchanged in all three mouse strains. In contrast, expression levels of all three inflammatory markers were similar in the stomach of wt, rd1 and ak mice (Fig.4D), suggesting that the inflammatory activation was restricted to the colon, but not the stomach, of ak mice.
2.5. Sex differences in motor and non-motor function
Our previous work has used male rd1 and ak mice for behavior experiments. To check for possible sex-related differences in function, we further compared the behavior patterns of female versus male animals.
For motor function, female rd1 and ak mice were studied using the challenging beam, cylinder, rotarod, adhesive removal, grip strength and nest building tests. Overall, female ak mice showed patterns of gross motor deficits on the challenging beam test (Fig. S3A), and fine motor deficits on adhesive removal test, grip strength test, and nest building test (Fig. S3D-S3F) similar to those of male ak mice. However, for certain tests, female mice showed larger individual variation than males, such as for cylinder and rotarod tests (Fig. S3B and C). The reason for the poor performance of the female rd1 animals in rotarod tests is unclear.
Impaired cognitive function of male ak mice has previously been demonstrated using T-maze and passive avoidance system testing (Ardayfio et al., 2008). In this study, we used the Y-maze test, which is accepted as a behavioral paradigm for evaluating spatial working memory, to compare both female and male rd1 and ak mice. We found that spontaneous alternations in both male and female ak mice were fewer than in rd1 mice (male, 51.3% vs 62.7%; female, 49.6% vs 64.6%, p < 0.01; Fig. S4A and S4B), suggesting that ak mice of both sexes exhibit significant spatial working memory deficits.
To compare olfactory function in male and female ak mice, we performed a subset of the olfactory function tests. We observed no significant differences between male and female mice. The buried pellet test, odor discrimination and odor habituation/dishabituation tests all showed that female ak mice had the same olfactory dysfunction compared with female rd1 mice as did males of the two strains (Fig. S5A-S5C). Finally, we also tested gastrointestinal function of female rd1 and ak mice. As shown in Fig. S5D and S5E, female ak mice, like male mice, presented longer whole gut transit times than female rd1 mice while there was no significant difference in bead expulsion time between female ak and rd1 mice.
Taken together, the overall performances of female and male ak mice in both motor and non-motor functions is similar, but female ak mouse behavior is more variable than that of males on certain tests.
2.6. L-DOPA rescues motor deficits but not non-motor deficits in ak mice
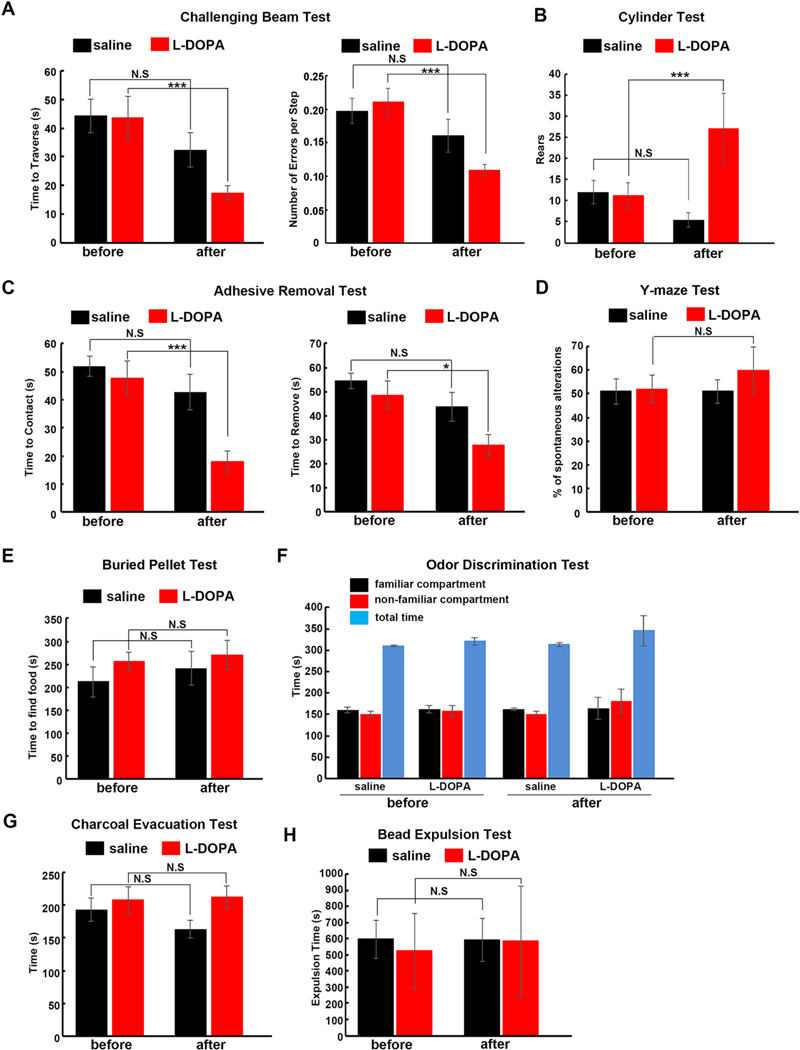
Finally, we tested how L-DOPA treatment influences the above motor and non-motor behavioral deficits observed in ak mice. Single intraperitoneal injection of L-DOPA in ak mice decreased time to traverse and number of errors per step in the challenging beam test, compared with saline injection (Fig. 5A). We also found that L-DOPA rescued gross motor deficits in the cylinder test (Fig. 5B). We further investigated whether L-DOPA could rescue fine motor deficits in ak mice. As shown in Fig. 5C, L-DOPA treatment sped up touching and removal of the adhesive patch in ak mice during the adhesive removal test (Fig. 5C), demonstrating that L-DOPA can rescue both gross and fine motor dysfunctions in ak mouse. Finally, the effects of L-DOPA on non-motor deficits were examined. ak mice received the same L-DOPA intraperitoneal injections as for the non-motor function tests. In the Y-maze test, there was no significant difference between L-DOPA and saline treatment in spontaneous alterations (Fig.5D), indicating L-DOPA did not rescue spatial working memory deficit. On the buried pellet (Fig. 5E) and odor discrimination tests (Fig. 5F), L-DOPA affected neither the time to find the pellets nor the preference for familiar versus non-familiar compartments, suggesting that L-DOPA did not rescue olfactory dysfunction. Similarly, in the charcoal evacuation test (Fig. 5G) and bead expulsion test (Fig. 5H), L-DOPA did not change the whole gut transit time or gut motility of ak mouse. There was no L-DOPA-induced dyskinesia observed after the single L-DOPA injection. In this model, we did not observe dyskinetic movements in any of the test animals in response to the L-DOPA dosage administered.
Fig. 5.
L-DOPA rescues motor but neither olfactory nor gastrointestinal function in ak mice. Four- to six-month-old ak mice were injected with either the dopamine precursor levodopa or with saline control intraperitoneally, then subjected to tests of motor, olfactory and gastrointestinal function. Challenging beam test (A), cylinder test (B) and adhesive removal tests (C) showed that ak mouse motor deficits were efficiently rescued by L-DOPA. In contrast, (D) Y-maze test showed that cognitive function deficits were not corrected by L-DOPA; (E) buried pellet test and (F) odor discrimination tests showed that olfactory functional deficits were not corrected by L-DOPA; and (G-H) charcoal evacuation and bead expulsion tests showed that gastrointestinal functional deficits were also not rescued by L-DOPA. *p < 0.05; ***p < 0.001; Mean ± S.E.M., n = 8–9 for each group.
3. Discussion
While motor symptoms are the defining diagnostic criteria and most obvious expressions of the disease, non-motor symptoms are increasingly recognized as being part of PD core dysfunctions, and indeed are frequently observed prior to the onset of overt motor impairments (Bloem et al., 2021; Poewe et al., 2017). Symptoms such as sleep disturbance, constipation, digestive dysfunction, and hyposmia may severely impact quality of life and in some patients may be the most troublesome aspects of the illness. As the disease progresses, cognitive issues may come to the fore in many patients. Dementia is not only itself a problem, but it may exacerbate efforts to control and manage motor symptoms. Cognitive symptoms of PD may include executive dysfunction as well as learning and memory deficits.
Unfortunately, most of these non-motor symptoms do not respond to standard DA replacement therapies, making them among the most difficult aspects of the disease to manage medically. Moreover, these symptoms typically also fail to improve with available surgical options such as deep brain stimulation. It is likely that some of these symptoms are non-dopaminergic in origin, and it is possible that PD, or at least some forms of it, involves degeneration or dysfunction of non-dopaminergic systems. However, models to distinguish this from chronic secondary effects of SNpc DA loss have been difficult to develop. A model showing a similar pattern of deficits or responsiveness to L-DOPA would be useful to further our understanding of the pathophysiology underlying these symptoms and to develop novel neuroprotective therapeutics.
The present study shows that the ak mouse represents a useful genetic model which permits the study of both motor and non-motor deficits. Genetic models do not require creation of pharmacological lesions as is required in the MPTP or 6-OHDA models, greatly facilitating high-throughput screening during therapeutic development. Furthermore, unlike many genetic models, ak mice show selective absence of the A9 DA neurons in the SNpc, while the A10 DA neurons in the ventral tegmental area and those in the olfactory bulb remain intact, thus closely mimicking PD’s pathological features. Finally, ak mice also show a variety of non-motor deficits such as olfactory, spatial working memory, and gastrointestinal dysfunction, analogous to symptoms seen in PD patients. The pattern of symptom responsiveness to L-DOPA also faithfully resembles that observed in human patients. Importantly, however, ak mouse is a developmental model, not an inflammatory or degenerative model. This contrast and difference may make the ak mouse model especially useful in analyzing the role of inflammation, including alterations in astrocytes and microglia, in clinical PD and in distinguishing changes arising from inflammation-related loss of DA neurons to those arising from the absence of dopaminergic function, by comparison with this non-inflammatory model. Differences in cellular and molecular pathophysiology related to such a distinction promise to be a fruitful area for future investigation.
Olfactory dysfunction is common in PD and can be considered a supportive diagnostic criterion, but the mechanism of olfactory deficit has not been well revealed. Previous studies suggested that it is related to α-synucleinopathy, activation of inflammation, or altered levels of neurotransmitters such as acetylcholine, dopamine, noradrenaline and serotonin (Doty, 2012b). A recent report demonstrated that olfactory deficits caused by depletion of the SN dopaminergic population are likely due to abnormal hyperactivity of the mitral cells in the OB (Zhang et al., 2019). A most salient finding of this study is that the ak mouse exhibits prominent olfactory dysfunction, as examined by several tests such as the buried pellet test, the odor discrimination test, and odor habituation tests, which were not rescued by L-DOPA treatment. The fact that multiple diverse olfaction-dependent behaviors were impaired suggests that the deficit was in the common sensory input underlying these responses. However, the dopaminergic neuron numbers in OB are similar in wt and rd1 mice. Behavioral tests of olfactory function require both an intact afferent sensory input mechanism and a functional network behavioral response (Breton-Provencher et al., 2009; Leinwand and Chalasani, 2011). As Pitx3 is only expressed in the midbrain dopaminergic system and is not expressed in the olfactory bulb, this suggests that olfactory dysfunction is not likely a simple consequence of dopaminergic neuronal depletion in the OB, but rather relates to loss of midbrain dopaminergic innervation of higher processing centers such as entorhinal cortex or hippocampus that may in turn disrupt coupling of oscillations between these structures required for normal function, analogous to how loss of dopamine affects oscillation coupling in the motor circuit, and that both availability and activation pattern of this dopaminergic innervation may be critical to restoring function of these circuits (Zemel et al., 2022).
Gastrointestinal dysfunction is another common PD symptom. Alterations in gut microbiota and enteric nervous system (ENS) inflammation are associated with PD pathogenesis (Santos et al., 2019). Gut microbial toxin-induced α-synuclein aggregation in the gut may proliferate and propagate through the vagus nerve to the brain (Dogra et al., 2022). We have shown that in ak mice, colonic motility is impaired in a fashion similar to that seen in PD patients, while some colonic inflammatory marker (IL-6 and IFN-γ) expression levels are higher than in wt and rd1 mice. This confirmed that ENS inflammation activation is associated with the gut pathophysiology underlying these symptoms. Since Pitx3 is not expressed in the gut, the mechanism responsible is also likely to be a secondary effect of the midbrain developmental defect. Importantly, as seen with PD patients, these non-motor issues did not respond to L-DOPA administration, so that for these systems, simple replacement of the absent neurotransmitter is not sufficient to restore function. This suggests that the gut-brain axis may be involved and may similarly relate to specific functional patterns of midbrain dopaminergic activity. Thus, these data suggest that the ak mouse would be a useful model for the bi-directionality of the gut-brain axis dysfunction in PD.
Finally, PD shows several significant sex-related differences. It is 1.5 times more common in males; differences also exist between sexes in the average age at onset, presenting symptoms, and rate of progression (Picillo et al., 2017). Thus, we also searched for any sex-specific effects in the parameters measured in this study. In general agreement with the human disease (Baba et al., 2005; Sauerbier et al., 2017), we found that ak mice show sex differences in some areas, including olfactory tasks and gut function, but not in others. We speculate that hormonal differences related to the estrous cycle may underlie some of these differences but need more studies to elucidate the detailed mechanisms involved.
4. Materials and methods
4.1. Animals
C57BL/6 J (strain code: 000664), rd1 mice (strain code: 004766) and Pitx3-deficient ak mice (strain code: 000942) used in this study were originally acquired from Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed in standard cages, under a 12-h light/dark cycle with ad libitum access to sterile food and water. All procedures were performed in accordance with current National Institutes of Health guidelines and McLean Hospital/Harvard University Institutional Animal Care and Use Committee protocols (2015 N000002). Males ranging in age from four- to six-month-old were used for all main experiments except those shown in the supplemental figures where female mice were also used as indicated.
4.2. Challenging beam test
The challenging beam test was performed based on previous reports from our group and others (Chung et al., 2011; Ekmark-Lewen et al., 2018; Hwang et al., 2005). Briefly, the beam (length, 1 m) starts at a width of 3.5 cm and gradually narrows to 0.5 cm in 1 cm decrements. Animals were trained for 2 days to traverse the length of the beam, starting at the widest section and ending at the narrowest section. On the third day, a mesh grid (1 cm square) of corresponding width was placed over the beam surface, leaving a ∼ 1 cm space between the grid and the beam surface. Animals were videotaped while traversing the gridsurfaced beam for a total of three trials. There was at least a 30 min gap between successive trials. Videotapes were viewed and rated in slow motion to evaluate each animal: the number of steps taken, the time to traverse, and the error numbers from each trial were recorded. An error was defined as when a forelimb or hindlimb slipped through the grid and became visible between the grid and the beam surface or on the side of the grid during a forward movement. The measurements were calculated by an investigator blinded to the mouse genotype.
4.3. Cylinder test
The cylinder test was performed as described in our previous reports (Chung et al., 2014; Chung et al., 2011). In brief, mice were put in a transparent cylinder (height, 15.5 cm: diameter, 12.7 cm) with a mirror behind it. Spontaneous activity was videotaped for 5 mins. Videotapes were viewed in slow motion to rate and count rear events. A rear was counted when an animal made a vertical movement with both forelimbs removed from the ground.
4.4. Rotarod test
The rotarod test was performed based on our previous report with slight modifications (Ardayfio et al., 2008). Mice were placed on a rotating cylinder within a rotarod apparatus at a constant rotation speed (2 rpm). Over the span of 5 mins, the rod began rotating at an increasingly faster rate (2–12 rpm on the first two days of training, 2–30 rpm on the third day of testing). The latency to fall off was recorded. The increase in latency over 3 consecutive days of testing is a measure of procedural motor learning.
4.5. Adhesive removal test
The adhesive removal test was performed as described in previous reports (Bouet et al., 2009; Fleming et al., 2004). Each mouse was brought into the testing room and allowed to acclimate to the environment for one hour. After this period, the animal was removed from its home cage and placed in the testing box, for a habituation period of 60 s. The animal was gently removed from the testing box and a small circular adhesive patch was placed on its snout, between the bridge of its nose and eyes, with forceps. For each mouse, the time between placement of the adhesive patch and when the mouse first made forepaw contact with it was recorded. Additionally, the time was recorded when the mouse succeeded in removing the adhesive patch. The adhesive was manually removed if the mouse failed to remove the adhesive within 60 s. Two trials were used for each mouse and the inter-trial interval was about ∼2 mins.
4.6. Grip strength test
The grip strength test was performed as described (Deacon, 2013) with slight modifications. Briefly, the apparatus consisted of four weighted chains attached to a fine mesh for mice to grip onto. Mice were suspended by the mid-base of their tail and slowly brought down to the bench to grasp the wire mesh and chains. Mice were required to pick up the mesh and weights and to be fully suspended off the table for 3 s to achieve a passing score for that weight level. Mice from each cage were tested one at a time on picking up the fine wire mesh (WM) without added weight, WM with one chain link, WM with two chain links, WM with three chain links, and WM with four chain links, weighing 18.8 g, 39.3 g, 59.8 g, 80.8 g and 115.8 g respectively. Once a mouse passed a weight level the other mice from that cage were tested on that weight level, and then all mice were tested on the next weight level. A mouse was allowed three trials to hold the weight successfully; failure to hold the weight successfully resulted in the previous weight level being assigned as its outcome score. Score (Max chain links X 3 s) Max seconds on failed weight level. Example: A mouse successfully holding two-weight chains but only holding the three weight chains for 2 s. Score = (2 chains × 3 s) + 2 s = 8.
4.7. Nest building test
The nest building test was performed as previously described (Deacon, 2006). Mice were individually placed in a new cage. One pressed-cotton square was placed in each cage. The next morning, a photograph was taken of each cage and nests were assessed on a rating scale 1–5 by a blinded evaluator.
4.8. Y-maze test
The Y-maze was purchased from Maze Engineers (Cambridge, MA, USA). The Y-maze has three arms of 35 cm length, 6 cm width and 20 cm height. The Y-maze test followed the description from our and other laboratories’ reports (Kraeuter et al., 2019; Moon et al., 2019). Spontaneous alternation was measured in two separate phases, an exposure phase, and a test phase. Each mouse was pseudo-randomly assigned two arms (the “start arm” and the “other/familiar arm”) to which they were exposed and allowed to freely explore for 5 min (“exposure” phase). The entrance to the third “novel” arm was blocked by the presence of a large opaque insert. At the end of the 5-min period, the mouse was removed from the maze and placed back in its home cage for 1 min, following which the “test” phase started. The insert was removed; mice were placed back into the start arm and allowed to explore the entire maze (i. e., all three arms) for 2 min. Videos were recorded and manually rated using Noldus Ethovision XT 7.0 tracking software. Spontaneous alternation was defined as successive entries into three different arms without repetition (e.g., ABC or BCA but not ABA). The spontaneous alternation percentage was calculated using the following equation: Spontaneous alternation (%) = (# spontaneous alternations/total number of arm entries −2) × 100.
4.9. Buried pellet test
The buried pellet test was performed as previously described (Lehmkuhl et al., 2014). Two days before testing, mice were weighed, their weight recorded and then food was restricted to 90% of body weight. Prior to testing and during food restriction, each mouse had access to 1–2 pellets of the type to be used during the test (Reese’s Puffs, General Mills, MN, USA). On testing days, mice were habituated in a clean cage for one hour prior to testing. A pellet was buried approximately 0.5 cm under the bedding in one of six different equally spaced positions in the cage. Once the mouse was placed in the test cage the latency time was measured until the mouse recovered a pellet. If a mouse failed to find a pellet within the predetermined time of 300 s the experiment was terminated and a latency of 5 min was recorded. The test was repeated for 5 days. The location of the pellet was changed for every test. On the sixth day, the test was repeated using the same scheme but now the pellet was placed on the surface (surface pellet test). Latencies for recovery of the buried pellets were averaged over the five daily trials and recorded as mean ± SEM. These means were compared to the latency for recovery of the surface pellet to ensure that olfactory sensation and not lack of motivation explained differences between ak and rd1.
4.10. Odor discrimination test
The odor discrimination test was performed as described in previous studies (Prediger et al., 2010; Zhang et al., 2015). Mice were placed in a cage divided into two equal areas separated by an open door, where it could choose between one compartment with fresh bedding (unfamiliar compartment) and another with unchanged bedding (familiar compartment) that the same mouse had occupied for 3 days before the test. Performances were recorded using Noldus Ethovision XT 7.0 tracking software and time (seconds) and distance (cm) traveled in each compartment (familiar versus non-familiar) were analyzed.
4.11. Odor habituation/dishabituation test
The odor habituation/dishabituation test was performed as previously described (Arbuckle et al., 2015; Zhang et al., 2015). In addition to water, two non-social odors (almond and banana extracts, 1:100 dilution) and two social odors (familiar odor consisting of bedding from the home cage; unfamiliar odor consisting of fresh bedding) were applied. These samples were stored at room temperature and used within 4–6 h to maintain strong and consistent odors. Mice were acclimated to the new cage for 45 min in a room other than the testing room. Both acclimation and testing room were assured to be free of other significant odors, loud noises, and bright light.
4.12. Charcoal evacuation test
Whole gut transit time was measured by charcoal evacuation test based on previous report (Marona and Lucchesi, 2004) with slight modifications. Mice were gavage fed with 0.3 ml of a 5% charcoal solution (Sigma-Aldrich, #242276). The time from the administration of charcoal until the first appearance of one black fecal pellet was recorded.
4.13. Bead expulsion test
The bead expulsion test was performed as described in previous studies (Kuo et al., 2019; Wang et al., 2008). Mice were briefly anesthetized with isoflurane to insert a glass bead (2 mm diameter) into the distal colon 2 cm from the anus. The time at which the bead was expelled was recorded as a measure of colonic transit.
4.14. L-DOPA treatment
ak mice were divided into two groups receiving either intraperitoneal injections of saline or 25 mg/kg L-DOPA and 12.5 mg/kg Benserazide (Hwang et al., 2005). Behavioral testing began 20 min after the L-DOPA/Benserazide injection. All subsequent behavioral tests were performed during the daytime. To avoid L-DOPA-induced dyskinesia, different ak mice had been used for the battery of behavioral evaluations.
4.15. Immunohistochemistry and stereological counting
Mice were euthanized by intraperitoneal injection of Ketamine/Xylazine (75 mg/kg/7.5 mg/kg), followed by intracardiac perfusion with ice-cold phosphate buffered saline (PBS; 0.01 M, pH 7.4) for 8 min, followed by perfusion with 4% formaldehyde for 20 min at 10 ml/min. The nose and skull containing the olfactory bulb were removed as a block and post-fixed overnight in the same fixative. Specimens were immersed for 1 week in a 10% EDTA solution (pH 7.4) in 0.1 M phosphate buffer for decalcification. Then, brains were cryopreserved after successive incubations in 20% and 30% sucrose, frozen in O.C.T. compound and sectioned at 30 μm intervals covering the entire olfactory bulb using a Leica CM1950 cryostat. Brain slices were first incubated with PBS containing 30% H2O2 for 30 min and were then incubated with rabbit anti-TH antibody (1:2000, Pel-Freez, #P40101–0) overnight. The next day, after washing with 0.3% Triton X-100 in PBS, samples were stained with HRP-labeled anti-rabbit antibody for 1 h. Finally, sections were visualized using the 3, 3-diaminobenzidine (DAB) peroxidase substrate kit (Vector Labs, #SK-4100) according to the manufacturer’s protocol.
To count olfactory bulb TH+ neuron numbers, coronal sections (30 μm) encompassing the entire olfactory bulb (AP + 5.5 to +3.1 mm) were serially collected. Every sixth section was used for TH staining, corresponding to approximately 12 sections from each mouse. After first making a contour tracing of the outline of the olfactory bulb on the section, the numbers of TH positive neurons were counted using a Stereo Investigator (MicroBrightField Inc.; Williston, VT) optical fractionator probe under a 63 X oil lens with counting frame 50 × 50 μm and grid size 200 × 200 μm (Fig. S5). Final counts were corrected for series number (1:6) to yield an estimate of the total number of TH+ neurons.
4.16. Immunofluorescence
Immunofluorescence of brain sections were performed as described in our previous report (Song et al., 2020). Briefly, free-floating coronal sections of olfactory bulb, striatum and substantial nigra were preincubated in blocking solution containing 5% normal donkey serum, 3% BSA and 0.3% Triton X-100 in PBS at room temperature for 1 h. For TH, Iba1 and GFAP triple-staining, the primary antibodies included mouse anti-TH (Millipore, MAB318, 1:2000), rabbit anti-Iba1 (Abcam, Ab178846, 1:1000), chicken anti-GFAP (Abcam, Ab4674,1:1000) and a M.O.M ™ staining kit (Fluorometric) (Vector Labs, FMK-2001) was applied to avoid mouse endogenous IgG background. All sections were counterstained with Hoechst 33342. Following three additional washes, a cover slip was applied over the sections with mounting medium and the sections were visualized with a fluorescence microscope (KEYENCE, Osaka, Japan).
To count the numbers of positive neurons, coronal sections (30 μm) encompassing the entire olfactory bulb (AP + 5.5 to +3.1 mm), striatum (AP +1.34 to −0.82) and substantial nigra (AP −2.7 to −3.8) were serially collected. Every sixth section was used for TH/Iba1/GFAP staining, corresponding to 6–12 sections from each mouse. The total number of Iba1 or GFAP positive cells in a region of interest were counted manually.
4.17. Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
After intracardiac perfusion of six-month-old wt, rd1 and ak mice, the colon and stomach tissues were collected in Trizol (Thermo Fisher). Total RNA was extracted, and reverse transcribed with oligo dT primer using Superscript II. For real time quantitative RT-PCR we used SsoAdvanced Universal SYBR Green Supermix and reactions were performed on a CFX Connect Real-Time System (Bio-Rad). PCR amplification was performed using gene-specific primers. Target gene expression was normalized to endogenous ACTIN expression using the comparative cycle time method. Primers used in this study included the following:
ACTIN: forward: 5′-GGCTGTATTCCCCTCCATCG-3′;
reverse: 5′-CCAGTTGGTAACAATGCCATGT-3′;
IL-6: forward: 5′-GACAAAGCCAGAGTCCTTCAGAGAG-3′;
reverse: 5′-CTAGGTTTGCCGAGTAGATCTC-3′.
IFN-γ: forward: 5′-CGGCACAGTCATTGAAAGCCTA-3′.
reverse: 5′-GTTGCTGATGGCCTGATTGTC-3′.
IL-1β: forward: 5′-GAAGTTGACGGACCCCAAAA-3′.
reverse: 5′-TGAGTGATACTGCCTGCCTGA-3′
4.18. Quantification and statistical analysis
All experiments were performed with biological triplicates unless otherwise indicated. The “n” for each experiment can be found in the figure legends and represents independently generated samples for all experiments. Prism 8 for MacOS (GraphPad Software, Inc. La Jolla, CA) was used for statistical analyses using two-way ANOVA with Tukey’s post-test. *p < 0.05; **p < 0.01; ***p < 0.001.
Supplementary Material
Acknowledgments
We would like to thank all members of the molecular neurobiology laboratory for their help and feedback. This work was supported by NIH grants (OD024622), the National Natural Science Foundation of China (32170807) and the Natural Science Foundation of Shanghai (21ZR1406300).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nbd.2022.105777
Credit authorship statement
K.-S.K. and B.S. conceived and supervised the project. B.S., J.W.F., S. C., M. F., Y. K., W.K., and A.S. designed and performed the experiments. B.S., J.W.F., and P.L. analyzed the data. B.S., J.S.S. and K.-S.K. wrote the paper with input from all co-authors.
Conflict of interest statement
The authors declare no competing financial interests.
References
- Alberquilla S, et al. , 2020. Dopamine regulates spine density in striatal projection neurons in a concentration-dependent manner. Neurobiol. Dis. 134, 104666. [DOI] [PubMed] [Google Scholar]
- Arbuckle EP, et al. , 2015. Testing for odor discrimination and habituation in mice. J. Vis. Exp. e52615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardayfio P, et al. , 2008. Impaired learning and memory in Pitx3 deficient aphakia mice: a genetic model for striatum-dependent cognitive symptoms in Parkinson’s disease. Neurobiol. Dis. 31, 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong MJ, Okun MS, 2020. Diagnosis and treatment of Parkinson disease: a review. JAMA 323, 548–560. [DOI] [PubMed] [Google Scholar]
- Baba Y, et al. , 2005. Gender and the Parkinson’s disease phenotype. J. Neurol. 252, 1201–1205. [DOI] [PubMed] [Google Scholar]
- Balestrino R, Schapira AHV, 2020. Parkinson disease. Eur. J. Neurol. 27, 27–42. [DOI] [PubMed] [Google Scholar]
- Blandini F, Armentero MT, 2012. Animal models of Parkinson’s disease. FEBS J. 279, 1156–1166. [DOI] [PubMed] [Google Scholar]
- Bloem BR, et al. , 2021. Parkinson’s disease. Lancet 397, 2284–2303. [DOI] [PubMed] [Google Scholar]
- Bouet V, et al. , 2009. The adhesive removal test: a sensitive method to assess sensorimotor deficits in mice. Nat. Protoc. 4, 1560–1564. [DOI] [PubMed] [Google Scholar]
- Brandt MD, et al. , 2017. Early postnatal but not late adult neurogenesis is impaired in the Pitx3-mutant animal model of Parkinson’s disease. Front. Neurosci. 11, 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton-Provencher V, et al. , 2009. Interneurons produced in adulthood are required for the normal functioning of the olfactory bulb network and for the execution of selected olfactory behaviors. J. Neurosci. 29, 15245–15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri KR, et al. , 2006. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 5, 235–245. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Richter F, 2011. Modelling of Parkinson’s disease in mice. Lancet Neurol. 10, 1108–1118. [DOI] [PubMed] [Google Scholar]
- Chung S, et al. , 2011. ES cell-derived renewable and functional midbrain dopaminergic progenitors. Proc. Natl. Acad. Sci. U. S. A. 108, 9703–9708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, et al. , 2014. Improvement of neurological dysfunctions in aphakia mice, a model of Parkinson’s disease, after transplantation of ES cell-derived dopaminergic neuronal precursors. Methods Mol. Biol. 1213, 285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer W, Przedborski S, 2003. Parkinson’s disease: mechanisms and models. Neuron 39, 889–909. [DOI] [PubMed] [Google Scholar]
- Dawson TM, et al. , 2010. Genetic animal models of Parkinson’s disease. Neuron 66, 646–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lau LM, Breteler MM, 2006. Epidemiology of Parkinson’s disease. Lancet Neurol. 5, 525–535. [DOI] [PubMed] [Google Scholar]
- Deacon RM, 2006. Assessing nest building in mice. Nat. Protoc. 1, 1117–1119. [DOI] [PubMed] [Google Scholar]
- Deacon RM, 2013. Measuring the strength of mice. J. Vis. Exp. 76, e2610. 10.3791/2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio-Martin A, et al. , 2019. Hypomorphic expression of Pitx3 disrupts circadian clocks and prevents metabolic entrainment of energy expenditure. Cell Rep. 29, 3678–3692 e4. [DOI] [PubMed] [Google Scholar]
- Ding Y, et al. , 2007. Chronic 3,4-dihydroxyphenylalanine treatment induces dyskinesia in aphakia mice, a novel genetic model of Parkinson’s disease. Neurobiol. Dis. 27, 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogra N, et al. , 2022. The gut-brain Axis: two ways signaling in Parkinson’s disease. Cell. Mol. Neurobiol. 42, 315–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, 2012a. Olfaction in Parkinson’s disease and related disorders. Neurobiol. Dis. 46, 527–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL, 2012b. Olfactory dysfunction in Parkinson disease. Nat. Rev. Neurol. 8, 329–339. [DOI] [PubMed] [Google Scholar]
- Doty RL, 2017. Olfactory dysfunction in neurodegenerative diseases: is there a common pathological substrate? Lancet Neurol. 16, 478–488. [DOI] [PubMed] [Google Scholar]
- Ekmark-Lewen S, et al. , 2018. Early fine motor impairment and behavioral dysfunction in (Thy-1)-h[A30P] alpha-synuclein mice. Brain Behav. 8, e00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A, et al. , 2015. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 14, 625–639. [DOI] [PubMed] [Google Scholar]
- Fernandez-Perez A, et al. , 2022. Restricting feeding to dark phase fails to entrain circadian activity and energy expenditure oscillations in Pitx3-mutant Aphakia mice. Cell Rep. 38, 110241. [DOI] [PubMed] [Google Scholar]
- Fleming SM, et al. , 2004. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J. Neurosci. 24, 9434–9440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heetun ZS, Quigley EM, 2012. Gastroparesis and Parkinson’s disease: a systematic review. Parkinsonism Relat. Disord. 18, 433–440. [DOI] [PubMed] [Google Scholar]
- Huang X, et al. , 2019. Non-motor symptoms in early Parkinson’s disease with different motor subtypes and their associations with quality of life. Eur. J. Neurol. 26, 400–406. [DOI] [PubMed] [Google Scholar]
- Hwang DY, et al. , 2003. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res. Mol. Brain Res. 114, 123–131. [DOI] [PubMed] [Google Scholar]
- Hwang DY, et al. , 2005. 3,4-dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: behavioral characterization of a novel genetic model of Parkinson’s disease. J. Neurosci. 25, 2132–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katunina E, Titova N, 2017. The epidemiology of nonmotor symptoms in Parkinson’s disease (cohort and other studies). Int. Rev. Neurobiol. 133, 91–110. [DOI] [PubMed] [Google Scholar]
- Kim KS, et al. , 2014. Pitx3 deficient mice as a genetic animal model of co-morbid depressive disorder and parkinsonism. Brain Res. 1552, 72–81. [DOI] [PubMed] [Google Scholar]
- Konno T, et al. , 2017. Biomarkers of nonmotor symptoms in Parkinson’s disease. Int. Rev. Neurobiol. 133, 259–289. [DOI] [PubMed] [Google Scholar]
- Kraeuter AK, et al. , 2019. The Y-maze for assessment of spatial working and reference memory in mice. Methods Mol. Biol. 1916, 105–111. [DOI] [PubMed] [Google Scholar]
- Kuo YM, et al. , 2019. Translational inhibition of alpha-synuclein by Posiphen normalizes distal colon motility in transgenic Parkinson mice. Am. J. Neurodegener. Dis. 8, 1–15. [PMC free article] [PubMed] [Google Scholar]
- Langley MR, et al. , 2021. Characterization of nonmotor behavioral impairments and their neurochemical mechanisms in the MitoPark mouse model of progressive neurodegeneration in Parkinson’s disease. Exp. Neurol. 341, 113716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmkuhl AM, et al. , 2014. Olfactory assays for mouse models of neurodegenerative disease. J. Vis. Exp. e51804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinwand SG, Chalasani SH, 2011. Olfactory networks: from sensation to perception. Curr. Opin. Genet. Dev. 21, 806–811. [DOI] [PubMed] [Google Scholar]
- Marona HR, Lucchesi MB, 2004. Protocol to refine intestinal motility test in mice. Lab. Anim. 38, 257–260. [DOI] [PubMed] [Google Scholar]
- McDowell K, Chesselet MF, 2012. Animal models of the non-motor features of Parkinson’s disease. Neurobiol. Dis. 46, 597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon J, et al. , 2013. Stem cell grafting improves both motor and cognitive impairments in a genetic model of Parkinson’s disease, the aphakia (ak) mouse. Cell Transplant. 22, 1263–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon M, et al. , 2019. Nurr1 (NR4A2) regulates Alzheimer’s disease-related pathogenesis and cognitive function in the 5XFAD mouse model. Aging Cell 18, e12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes I, et al. , 2003. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc. Natl. Acad. Sci. U. S. A. 100, 4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumier KL, et al. , 2013. Behavioral characterization of A53T mice reveals early and late stage deficits related to Parkinson’s disease. PLoS One 8, e70274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picillo M, et al. , 2017. The relevance of gender in Parkinson’s disease: a review. J. Neurol. 264, 1583–1607. [DOI] [PubMed] [Google Scholar]
- Poewe W, et al. , 2017. Parkinson disease. Nat. Rev. Dis. Prime. 3, 17013. [DOI] [PubMed] [Google Scholar]
- Prediger RD, et al. , 2010. Single intranasal administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in C57BL/6 mice models early preclinical phase of Parkinson’s disease. Neurotox. Res. 17, 114–129. [DOI] [PubMed] [Google Scholar]
- Santos SF, et al. , 2019. The gut and Parkinson’s disease-a bidirectional pathway. Front. Neurol. 10, 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerbier A, et al. , 2017. Nonmotor symptoms in Parkinson’s disease: gender and ethnic differences. Int. Rev. Neurobiol. 133, 417–446. [DOI] [PubMed] [Google Scholar]
- Scarpa LL, et al. , 2022. Mice hypomorphic for Pitx3 show robust entrainment of circadian behavioral and metabolic rhythms to scheduled feeding. Cell Rep. 38, 109865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer E, Berg D, 2017. Dopaminergic therapies for non-motor symptoms in Parkinson’s disease. CNS Drugs 31, 551–570. [DOI] [PubMed] [Google Scholar]
- Schapira AHV, et al. , 2017. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 18, 435–450. [DOI] [PubMed] [Google Scholar]
- Semina EV, et al. , 2000. Deletion in the promoter region and altered expression of Pitx3 homeobox gene in aphakia mice. Hum. Mol. Genet. 9, 1575–1585. [DOI] [PubMed] [Google Scholar]
- Singh B, et al. , 2007. Motor deficits and altered striatal gene expression in aphakia (ak) mice. Brain Res. 1185, 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smidt MP, et al. , 2004. Early developmental failure of substantia nigra dopamine neurons in mice lacking the homeodomain gene Pitx3. Development 131, 1145–1155. [DOI] [PubMed] [Google Scholar]
- Solis O., et al., 2015. Nitric oxide synthase inhibition decreases l-DOPA-induced dyskinesia and the expression of striatal molecular markers in Pitx3(−/−) aphakia mice. Neurobiol. Dis. 73, 49–59. [DOI] [PubMed] [Google Scholar]
- Song B, et al. , 2020. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J. Clin. Invest. 130, 904–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez LM., et al., 2018. Differential synaptic remodeling by dopamine in direct and indirect striatal projection neurons in Pitx3(−/−) mice, a genetic model of Parkinson’s disease. J. Neurosci. 38, 3619–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieu K, 2011. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb Perspect. Med. 1, a009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Munckhof P, et al. , 2003. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development 130, 2535–2542. [DOI] [PubMed] [Google Scholar]
- van den Munckhof P, et al. , 2006. Striatal neuroadaptation and rescue of locomotor deficit by L-dopa in aphakia mice, a model of Parkinson’s disease. J. Neurochem. 96, 160–170. [DOI] [PubMed] [Google Scholar]
- Vingill S, et al. , 2018. Are rodent models of Parkinson’s disease behaving as they should? Behav. Brain Res. 352, 133–141. [DOI] [PubMed] [Google Scholar]
- Wang L, et al. , 2008. Abnormal colonic motility in mice overexpressing human wild-type alpha-synuclein. Neuroreport 19, 873–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel D, et al. , 2022. Dopamine depletion selectively disrupts interactions between striatal neuron subtypes and LFP oscillations. Cell Rep. 38, 110265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenchak JR, et al. , 2020. Bioluminescence-driven optogenetic activation of transplanted neural precursor cells improves motor deficits in a Parkinson’s disease mouse model. J. Neurosci. Res. 98, 458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, et al. , 2015. Olfactory dysfunction and neurotransmitter disturbance in olfactory bulb of transgenic mice expressing human A53T mutant alpha-synuclein. PLoS One 10, e0119928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, et al. , 2019. Partial depletion of dopaminergic neurons in the substantia nigra impairs olfaction and alters neural activity in the olfactory bulb. Sci. Rep. 9, 254. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.