Abstract
The Region of D eletion 2 (RD2) of Mycobacterium tuberculosis encodes reserved antigens that contribute to bacterial virulence. Among these antigens, Rv1983, Rv1986, Rv1987, and Rv1989c have been shown to be immunodominant in infected cattle; however, their diagnostic utility has not been evaluated in humans.
In this study, we screened 87 overlapping synthetic peptides encoded by five RD2 proteins for diagnosing tuberculosis epitopes in 50 active tuberculosis (TB) cases, 31 non-tuberculosis patients and 36 healthy individuals. A pool of promising epitopes was then assessed for their diagnostic value in 233 suspected TB patients using a whole blood IFN-γ release assay.
Only 10 peptides were recognized by more than 10% of active tuberculosis patients. The IFN-γ release responses to Rv1986-P9, P15, P16, Rv1988-P4, P11, and Rv1987-P11 were significantly higher in the active TB group than in the control groups (p < 0.05). The whole blood IFN-γ release assay based on these epitopes yielded a sensitivity of 51% and a specificity of 85% in diagnosing active tuberculosis, and the corresponding results using the T-SPOT.TB assay were 76% and 75%, respectively.
In conclusion, these results suggest that the six epitopes from the RD2 of M. tuberculosis have potential diagnostic value in TB.
Keywords: Mycobacterium tuberculosis, T-cell epitope, Diagnostic antigen
Introduction
Tuberculosis (TB) is the leading infectious disease, ranking above HIV/AIDS. In 2016, there were an estimated 1.7 million TB deaths and 6.3 million new cases of TB were reported, equivalent to 61% of the estimated incidence of 10.4 million.1 Worldwide, China is the country with the second largest number of tuberculosis new cases (after India) and carries an estimated 8.6% of the global burden of new cases and an estimated 12.1% of multidrug-resistant TB cases.1
After infection with the tubercle bacillus, activated CD4+ T-cells induce Th1 cytokines by the stimulation of macrophages and change innate immune responses to adaptive responses.2 Therefore, the immunological diagnostic tool, tuberculin skin test (TST), can detect Mycobacterium tuberculosis infection based on the immune response. As the antigens contained in the TST is crude and poorly refined supernatant mixture of mycobacteria, the widely or sometimes mandatorily used BCG vaccination and the exposure to environmental mycobacteria weaken the specificity of the TST.3
Interferon gamma (IFN-γ) release assays (IGRAs), including the commercially available kits QuantiFERON-TB Gold In-Tube (QFT-GIT; Qiagen, Germany) and T-SPOT.TB® (Oxford Immunotech, Abingdon, UK) have been recommended for the immunological diagnosis of M. tuberculosis infection. A meta-analysis comprising 27 studies showed that the pooled sensitivity for the diagnosis of active TB was 80% (95% CI 75-84%) for QFT-GIT and 81% (95% CI 78-84%) for T-SPOT.TB® in blood fluids. The pooled specificity was 79% (95% CI 75-82%) and 59% (95% CI 56-62%), respectively.4 Another meta-analysis focusing on mid- and low-income countries reported similar results: pooled sensitivity in HIV-uninfected persons of 88% (95% CI, 81–95%) for T-SPOT.TB and 84% (95% CI, 78–91%) for QFT-GIT. The pooled specificity was low for both assays, namely, 52% (95% CI 41–62%) and 61% (95% CI, 40–79%), respectively.5 Although both IGRAs have higher diagnostic sensitivities than TST, they were still not high enough to justify using IGRA as a test to rule out TB. The specificity limitation in diagnosing active TB could not be easily solved, which was commonly due to inability to discriminate between latent and active tuberculosis infection, in addition to cross-activation with other environmental mycobacteria. However, the sensitivity could be improved by adding more diagnostic antigens into the assays, mainly antigens encoded by the region of deletion 2 (RD2) of MTB.6, 7, 8, 9, 10, 11, 12 Peptide 4 from TB7.7 (Rv2654, encoded by RD13) was incorporated into the QFT-GIT to improve sensitivity.13, 14, 15, 16
The RD2 of MTB encodes 11 ORF (from Rv1978-Rv1989c) which contribute to bacterial virulence.17 Previously, we demonstrated that Rv1978, Rv1981c, and Rv1985c from RD2 were T-cell antigens and could be used to discriminate TB-infected individuals from healthy BCG-vaccinated controls.12, 18, 19 T-cell responses against peptide pools from other proteins in RD2, including Rv1983, Rv1986, Rv1987, and Rv1989c have been tested in M. bovis-infected and BCG-vaccinated cattle.8, 20 The percentages of positive IFN-γ releasing responders ranged from 41% to 62% in M. bovis-infected cattle, which was higher than the percentages in BCG-vaccinated cattle, which ranged from 0% to 50%. Diagnostic performance and T-cell epitopes may be different in cattle compared to humans; it is therefore necessary to identify and verify these promising T-cell epitopes in individuals. Peptides of Rv1989c have been verified, in 49 TB patients and 38 healthy individuals, but with unsatisfying results. Only 16–23% TB patients had a positive response.7 Cellular epitopes from other proteins remained unknown except for the epitopes of IL-2 responses against Rv1986.21
In this study, we initially screened overlapping synthetic peptides encoded by Rv1983p257-558, Rv1986, Rv1987, Rv1988, and Rv1989c for TB diagnostic purposes, and subsequently pooled six candidate epitopes and evaluated their joint diagnostic performance for active TB.
Materials and methods
Participants and study design
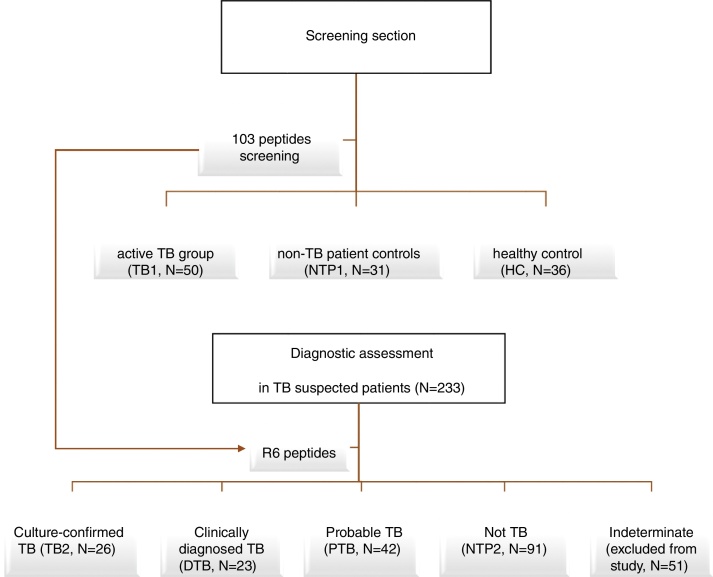
This study included a retrospective diagnostic section that screened potential diagnostic T-cell peptides from RD2 proteins and a diagnostic section that assessed the prioritized peptides for the diagnosis of active TB in patients with symptoms of tuberculosis (Figure 1).
Figure 1.
Study design. The study contains a screening section to screen potential diagnostic T-cell peptides from RD2 proteins and a diagnostic section to assess the prioritized peptides for the diagnosis of active TB in patients with symptoms of tuberculosis.
In the screening section, three groups of individuals were recruited, including 50 cases of active TB (TB1 group), 31 non-TB control patients (NTP1 group), and 36 healthy individuals (HC group). The diagnosis of TB was based on the following criteria: 1) suggestive clinical signs and symptoms including fever, cough and productive sputum; and 2) positive acid-fast bacilli (AFB) smear and/or a positive culture for M. tuberculosis. To minimize the impact of anti-TB treatment on T-cell responses, only those patients with less than three weeks of anti-TB chemotherapy were included. Non-TB controls (NTP1 group) were recruited from the inpatients at Huashan Hospital, Shanghai. These patients fulfilled the following criteria: a) fever; b) negative acid-fast bacilli (AFB) smear or culture; or c) alternative diagnosis established and clinical resolution without anti-tuberculosis treatment. Healthy adults (HC group) were recruited from among students and teachers in Fudan University, China. A questionnaire was used to collect information about exposure to mycobacteria and BCG vaccination status. Individuals with a history of contact with a TB patient or with evidence of TB infection were excluded from the study. As there is a considerable degree of latent TB infection in China, we excluded individuals with possible latent TB infection. IGRAs were performed and those with positive results were excluded from the HC group. The NTP1 and HC groups were used as the reference standard for the specificity assessment, and the TB1 group was used for the sensitivity assessment.
The diagnostic assessment was conducted at two hospitals in China. A total of 233 adult patients with persistent fever and other signs or symptoms that suggested pulmonary or extrapulmonary tuberculosis and for whom IGRAs were administered for TB diagnosis, were enrolled in this section of the study. Patients who received anti-tuberculosis therapy in the previous 12 months and who were HIV-infected were excluded.
All participants gave informed consent and the study was approved by the Ethics Committee from Huashan Hospital, Fudan University. The demographic characteristics of the study participants are described in Table 1, Table 2.
Table 1.
Demographic characteristics of the screening study population.
| Group | TB1 | HC | NTP1 |
|---|---|---|---|
| No. of individuals | n = 50 | n = 36 | n = 31 |
| Sex (male/female) | 32/18 | 14/22 | 19/12 |
| Age (median) | 16-86 (51) | 20-58 (22) | 16-86 (45) |
| BCG vaccination scars | 34 (68%) | 34 (94%) | 24 (77%) |
| Positive IGRA results | 44 (88%) | 0 (0%) | 4 (13%) |
| Geography Shanghai/Zhejiang | 18/32 | 36/0 | 31/0 |
Table 2.
Demographic characteristics and diagnostic results of the assessment study population.
| All patients (n = 233) | Culture-confirmed tuberculosis (TB2) (n = 26) | Clinically diagnosed tuberculosis (DTB) (n = 23) | Probable tuberculosis (PTB) (n = 42) | Not tuberculosis (NTP2) (n = 91) | Indeterminate (n = 51) | |
|---|---|---|---|---|---|---|
| Age (median) | 18-87 (51) | 24-80 (38) | 18-69 (41) | 18-87 (42) | 27-86 (59) | 18-87 (57) |
| Male/Female | 149/84 | 18/8 | 17/6 | 23/19 | 57/34 | 34/17 |
| Positive for R6 peptide pool by WBIGRA (Percentage) | 79 (33.9%) | 14 (53.8%) | 11 (47.8%) | 24 (57.1%) | 14(15.4%) | 14 (27.5%) |
| Positive for ESAT-6/CFP-10 peptides by WBIGRA (Percentage) | 109 (46.8%) | 22 (84.6%) | 16 (69.6%) | 31 (73.8%) | 19 (20.9%) | 16 (31.4%) |
| Positive T-SPOT.TB assay (Percentage) | 108 (46.4%) | 23 (88.5%) | 14 (60.9%) | 31 (73.8%) | 23 (25.3%) | 17 (33.3%) |
| Indeterminate T-SPOT.TB assay | 5 | 0 | 2 | 1 | 1 | 1 |
Classification standard
Classification was based on the results of the clinical and microbiological assessment after the diagnostic assays were performed. Patients were divided into a culture-confirmed TB group (TB2 group: positive AFB smear or culture for M. tuberculosis complex, confirmed by strain identification), clinically diagnosed TB group (DTB group: chest radiograph consistent with active TB, histology typical for TB), probable TB group (PTB: clinically suspected TB without confirmatory clues, as above), non-TB group (NTB2: alternative diagnosis established and clinical resolution without anti-tuberculosis treatment), or an indeterminate group (ID: any other situation). Those patients who were smear- or culture-positive but were infected with a strain identified as non-tuberculosis Mycobacterium (NTM) were included in the NTP2 group. In the TB2 group, most patients (25/26) had pulmonary TB and one had tuberculous pleurisy. In the probable TB group there were two probable lumbar vertebral TB cases and the remaining were probable pulmonary TB.
The TB1 and DTB groups were used as the TB positive population for sensitivity assessment, and the NTP2 group was used as the TB negative population for specificity assessment.
Peptides and Antigens
One hundred and three overlapping peptides spanning the complete amino acid sequence of CFP-10, ESAT-6, Rv1983p257-558, Rv1986, Rv1987, Rv1988 and Rv1989c, were designed and purchased (GL Biochem Ltd., Shanghai) (Table S1). The identity of each peptide was confirmed by mass spectrometry and the purity (>90%) was checked by high-pressure liquid chromatography. The stock concentration (20 mg/mL) of the peptides was prepared in DMSO (Sigma), and the peptides were further diluted to a working concentration in tissue culture medium RPIM-1640 (Life Technology). An ESAT-6 peptide pool containing ESAT-6 P1-P8 (Table S1) was used as the control in the epitope screening experiment.
Parallel IGRA tests: QFT-GIT and T-SPOT.TB
The QFT-GIT assay or T-SPOT.TB assays were performed in all the participants of the retrospective screening study. The T-SPOT.TB assay was performed in parallel in all the participants of the study. The assays were performed as previously described and following the manufacturer's instructions.22
T-cell diagnostic peptide screening: diluted whole blood IFN-γ release assay
All 87 peptides were initially screened in 27 TB1 group patients using a diluted whole blood IFN-γ release assay. Only candidate peptides with T-cell recognition higher than 8% in the TB1 group were further evaluated in the remaining individuals in the TB1, NTP1 and HC groups.
To reduce the quantity of patients’ whole blood that was required for screening the multiple peptides, a diluted whole blood method to measure T-cell response was performed in the study as described by Weir et al.23 Briefly, whole blood was collected via venipuncture and drawn into heparinized tubes. Within 8 h, the whole blood was diluted 1:10 with RPIM1640 tissue culture medium, and supplemented with 40 μg/mL streptomycin and 40YU/mL penicillin (Life Technologies, Paisley, UK). It was then cultured in 96-well tissue culture plates in a total volume of 200 μL with 2.5 μg/mL of PHA, RPIM1640 (negative control) or 10 μg/mL of each peptide or the peptide pool of ESAT-6. After incubation at 37 °C for 5 days, supernatants of cultured blood were collected and stored at −20 °C before testing.
IFN-γ levels in the supernatants were determined by ELISA using commercially available agents (Mabtech, Sweden), and performed according to the manufacturer's instructions. The detection range of the assay was 4-400Ypg/mL. For concentrations higher than 400Ypg/mL, the supernatants were diluted 10-fold or 100-fold and re-measured. Antigen- or peptide-specific responses are shown as Δ values (antigen-stimulated IFN-γ release minus that in the unstimulated well). Positive recognition was recoded using 17.5Ypg/mL (approximately mean concentration value of unstimulated well plus three times standard error of all the participants) as the cutoff for the peptide-specific IFN-γ levels.
Assessment of the six peptides from RD2 for TB diagnosis by a whole blood IFN-γ release assay (WBIGRA)
Six promising peptides (R6), namely, Rv1986-P9, Rv1986-P15, Rv1986-P16, Rv1988-P4, Rv1988-P11, and Rv1983-P4 (all identified by epitopes screening) were pooled together to assess the TB diagnosis potential in patients with symptoms of tuberculosis. Half-milliliter aliquots of the heparinized blood from 233 patients were stimulated in three separate 1.7 mL tubes, each with 10 μg/mL (final concentration for each peptide) of either the R6 peptide pool, the peptide pool of ESAT-6 and CFP-10 (EC), or RPIM1640 medium with DMSO (negative control). The DMSO that was added to the negative control was equal to the volume in the peptide tubes to minimize its impact on IFN-γ release assay. Stimulated blood was incubated at 37 °C for 16-24 h and the IFN-γ level was measured in the plasma by the ELISA kit mentioned above. A positive IFN-γ response was defined as (Antigen minus Negative) ≥34Ypg/mL and ≥1/2 Negative control. The cutoff value was set to optimize specificity by a receiver operator characteristic curve (ROC), which TB2 and DTB groups were set as the positive group, and NTP2 group was set as the negative group.
Statistical method
The intensity of IFN-γ released among the different groups was analyzed by the Kruskal-Wallis one-way ANOVA test and Dunn's post-test. The frequency of recognition for single peptide between TB1 group and the control group (NTP + HC) was analyzed by Chi-square or Fisher's Exact test. For the diagnostic candidate peptides, the cut-off value to optimize specificity was determined by ROC curve, in which TB1 group were set as the positive group, and NTP1 and HC groups were set as the negative group. SPSS v14 and Prism 5 (Graphpad Software 5.0, San Diego, CA, USA) were used for the analysis.
Results
Screening diagnostic T-cell epitopes against Rv1986, Rv1987, Rv1988, Rv1989c, and Rv1983 in the TB1, NTP1 and HC groups
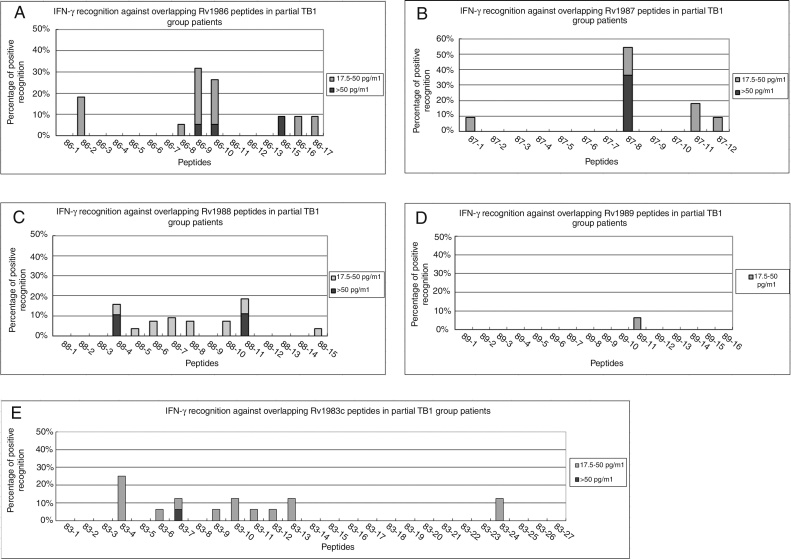
All the peptides were first screened in vitro by the diluted whole blood IFN-γ release assay in 11-27 individuals of the TB1 group. In total, 9/16 (56%), 8/11 (73%), 6/11 (55%), 14/27 (52%), and 1/16 (6%) of TB1 patients had positive responses against at least one peptide among Rv1983, Rv1986, Rv1987, Rv1988, and Rv1989c, respectively. Twenty-two peptides that were recognized by at least 8% of TB1 patients were further studied in more patients and healthy individuals for evaluating both diagnostic sensitivity and specificity (Figure 2). For the control peptides, 67% (18/27) of TB patients showed positive IFN-γ responses against the peptide pool of ESAT-6.
Figure 2.
Positive T-cell responses against RD2 peptides in TB1 groups. Diluted whole blood from the active tuberculosis group patients (TB1) were stimulated with peptides from the RD2 protein of M. tuberculosis and IFN-γ responses were measured. The percentages of positive responses against Rv1986 (A), Rv1987 (B), Rv1988 (C), Rv1989c (D), and Rv1983p257-558 (E) in partial TB1 group patients are shown.
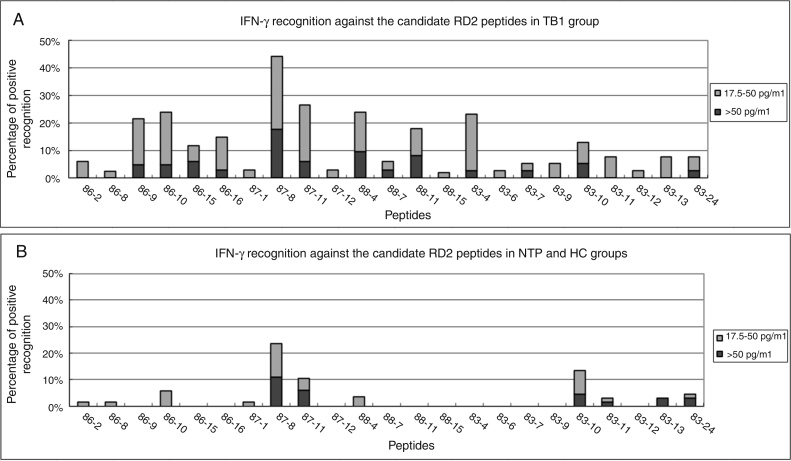
After screening all individuals of TB1 group, only 10 of 21 peptides (Rv1986-P9, P10, P15, P16, Rv1987-P8, P11, Rv1988-P4, P11 and Rv1983-P4, P10) were recognized by more than 10% of active TB patients, ranging from 12% (4/34) to 44% (15/34) of the TB1 patients (Figure 3A). In the NTP1 and HC groups, 11 peptides were not recognized by any individual (Figure 3B).
Figure 3.
Positive T-cell responses against candidate epitope peptides in TB1 and control groups. Diluted whole blood from tuberculosis group patients (TB1) and the control groups were stimulated with 22 candidate epitopes and IFN-γ responses were measured. The percentages of positive responses against the peptides in TB1 (A) and the control (HC + NTP1) groups (B) are shown.
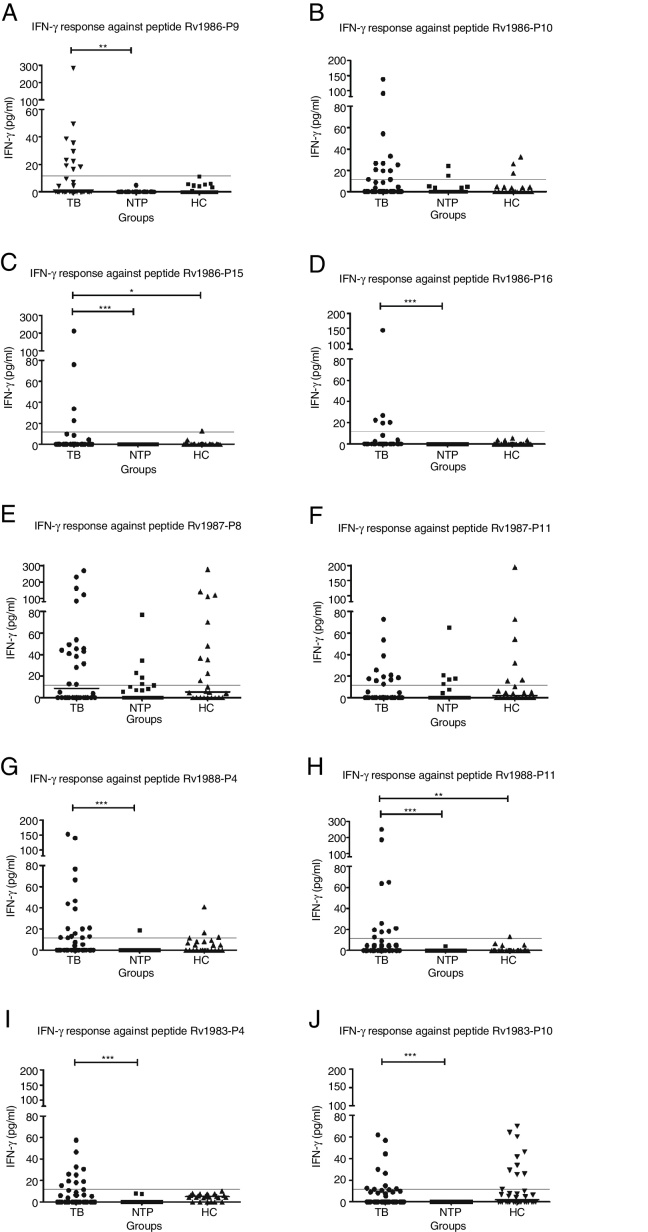
The frequency of responses to six peptides, Rv1986-P9, P15, P16, Rv1988-P4, P11, and Rv1983-P4 in the TB1 group were significantly greater than in the control groups (NTP + HC) (p < 0.05), suggesting that the peptides had the potential for diagnosing tuberculosis infection (Figure 4). Although Rv1987-P8, Rv1987-P11, and Rv1983-P10 were frequently recognized by TB1 patients, the cross-reactive response among the control groups made them unsuitable for diagnostic purposes.
Figure 4.
Diluted whole blood IFN-γ responses against 10 epitopes from RD2 among TB1, NTP1 and HC groups. Diluted whole blood from the tuberculosis patient group (TB) and non-TB patient group (NTP) and BCG-vaccinated healthy control group (HC) were stimulated with 10 candidate peptides and IFN-γ responses were measured. The result of the IFN-γ responses in the three groups against peptides Rv1986-P9 (A), Rv1986-P10 (B), Rv1986-P15 (C), Rv1986-P16 (D), Rv1987-P8 (E), Rv1987-P11 (F), Rv1988-P4 (G), Rv1988-P11 (H), Rv1983-P4 (I), and Rv1983-P10 (J) are shown. Horizontal lines indicate the median of IFN-gamma in each group. For all the peptides, IFN-gamma responses above 11.6Ýpg/mL are considered to be positive, as determined by ROC analysis (indicated by the dotted line). *, ** and *** indicates statistically significant difference among groups by the Kruskal-Wallis one-way ANOVA test and followed by 2 groups nonparametric comparison. *, 0.01 ≤ p< 0.05; **, 0.001 ≤ p< 0.01; ***, p< 0.001.
All the enrolled participants agreed to a parallel IGRA test being performed. In total, 45 of 50 (90%) of TB patients and six of 31 (19%) NTP1 patients showed a positive IGRA result, which indicated uncompromised T-cell responses against specific tuberculosis antigens among the study population.
In order to better evaluate sensitivities and specificities for the TB diagnostic tests, a ROC curve was drawn combining the IFN-γ responses to the six promising peptides. The area under the curve was only 0.623 (95% CI: 0.576-0.670, p < 0.0001). Using 11.6Ypg/mL as the IFN-γ cut-off, which produces the optimal specificity of 92.7–100%, the sensitivity of the IFN-γ assay for the peptides Rv1986-P9, Rv1986-P15, Rv1986-P16, Rv1988-P4, Rv1988-P11, and Rv1983-P4 were 24% (10/42), 12% (4/34), 15% (5/34), 37% (16/43), 20% (10/51), and 26% (10/39), respectively (Figure 4).
Apparently, the sensitivity of any of the six peptides alone was not sufficient to diagnose tuberculosis. In combination or as an adjunct to RD1 epitopes these epitopes could be used for clinical diagnostic purposes. Therefore, we assessed the prioritized peptide pool in parallel to RD1 antigens for diagnosis of active TB in patients with symptoms of the disease.
Assessment of the six peptides from RD2 for TB diagnosis by a whole blood IFN-γ release assay
The diagnostic performance of the RD2 epitope pool was assessed in 233 patients with suspected TB. As shown in Table 2, 26 (11.3%) patients were classified in the TB2 group, 23 (10%) in the DTB group, 42 (18.3%) in the PTB group, and 88 (38.3%) in the NTB2 group (including four with NTM infection), and 51 (22.2%) in the ID group.
Fourteen (54%) and 22 (85%) of 26 TB2 patients had a positive WBIGRA result against R6 and EC antigens, respectively (Table 2). Twenty-three of 26 (88.5%) patients had a positive T-SPOT.TB assay result. Fourteen (61%), 11 (48%), and 16 (70%) of 23 DTB patients had a positive T-SPOT.TB, and WBIGRA results against R6 and EC antigens, respectively. Thirty-one (74%), 24 (57%), and 31 (74%) of 42 probable TB patients had positive T-SPOT.TB and WBIGRA results against R6 and EC antigens, respectively (Table 2).
Most patients in the NTB2 group were diagnosed with cryptococcosis, COPD, lung cancer, viral infections, candidiasis, bacterial infections, and NTM. Twenty-three (25%), 16 (15.4%), and 19 (20.9%) of 91 NTB2 patients had positive T-SPOT.TB and WBIGRA results against R6 and EC antigens, respectively.
Overall, the sensitivity of the R6 peptide assay was 51% (25/49) for tuberculosis, and the specificity reached 85% (77/91). For tuberculosis patients, the sensitivities of the EC peptides and T-SPOT.TB assay were 78% (38/49) and 76% (37/49), respectively, and the specificities were 79% (72/91) and 75% (68/91), respectively. The difference in sensitivity between the R6 peptide assay and T-SPOT.TB was statistically significant (p = 0.023), but the difference in the specificity was not (p = 0.093).
Discussion
In recent years, there has been great progress in developing new approaches for the immune-diagnosis of tuberculosis, which includes the measurement of alternative biomarkers secreted as part of cell-mediated immune responses,24, 25 the identification of novel antigens,12, 26, 27 monitoring of T-cell subgroup changes 28, 29 and the detection of host biomarkers of immune response.27, 30 Using a peptide-based approach to identify new antigens and epitopes from the old immune-dominant proteins can be an effective way to screen and improve the performance of the current methods.
In our screening study, four epitopes from Rv1986, and two epitopes each from Rv1987, Rv1988, and Rv1983 were recognized by more than 10% of TB patients (Figure 3A). Rv1986c, a probable LysE family protein involved in the transport of lysine, may contribute to bacillus persistence, as it is up-regulated at later time points (4-7 days) when mycobacteria is treated with hypoxia, developing what is known as the enduring hypoxic response (EHR).31 Rv1986c, together with other EHR-induced antigens, can stimulate an immune response that leads to the secretion of both IFN-γ and IL-2 in TB-infected cattle.32 One identified epitope region, P15 and P16 (amino acid 157-187) was identified previously,21 but the other region P9 and P10 (amino acid 91-120) was not. The most dominant epitopes were Rv1987-P8 and Rv1987-P11, with median intensity reached at 38Ypg/mL and 12Ypg/mL, respectively, in the TB1 group. Despite the high intensity, they were unsuitable for diagnostic purposes due to high cross-reactivity in the control population. Rv1987 encodes a possible chitinase family protein that is distributed widely among bacteria, fungi and even animals, and which may underlie the high cross-reactivity of Rv1987. The recognition in healthy individuals was in agreement with a previous study with M. bovis-infected cattle: 57% recognition in infected cattle and 50% in BCG-vaccinated cattle.8 Rv1983 (PE_PGRS35), which belongs to the PE/PPE protein family of Mycobacterium, is another antigen that is definitively recognized in both infected and BCG-vaccinated cattle.8 In order to avoid cross-reactivity maximally, the region of PE_PGRS35p1–p256, which had common motifs shared among the PE/PPE family members, was not chosen for epitope screening. In the screening experiments, 56% (9/16) of TB patients had a positive response to at least one peptide of Rv1983. The percentage that was recognized was equivalent to that in the cattle study (59%).8 TB patients preferentially recognized Rv1983-P4, P6-P13 (amino acid 312-408), and P24, but the percentage that was recognized was low for any single peptide. Among them, two were demonstrated to be immune-dominant epitopes and one, Rv1983-P4 (P290-309), had potential diagnostic value. Rv1988 encodes an intrinsic macrolide-resistant gene named erm,37, 33 probably through its methyltransferase function. In this study, Rv1988 was recognized by 52% of TB patients and identified as a T-cell antigen. Two dominant epitopes, Rv1988-P4 and P11, had relatively high recognition in TB patients but less or none in NTP1 or healthy controls, which makes them potentially useful for the diagnosis of TB. To our knowledge, this is the first time they have been studied. It is less common for an antibiotic-resistant gene to be identified as a diagnostic antigen with high specificity; however, it has been found that a high similarity homolog of the erm family is mainly restricted to the tuberculosis complex and limited species of Mycobacterium, including Mycobacterium abscessus and Mycobacterium bolletii.34
In this study, T-cell responses were measured by using a diluted whole blood assay and the incubation period was lengthened to 5 days instead of overnight to ensure the production of a sufficient amount of secreted IFN-γ.35 Although the antigen-specific cytokines were reported to progressively decrease when whole blood was diluted, the assay required much less blood than expected, considering that screening included 87 peptide subgroups for each patient. The data showed that the amount of cytokine secreted from 20 μL of blood was sufficient to determine the T-cell epitopes. All but three of the 117 NTP1 individuals had positive IFN-γ responses to the positive control antigen, PHA, and the results ranged from 10 to>400Ypg/mL, with the median value exceeding 400Ypg/mL. Similarly, all but one participant had a positive T-cell response to PHA in an undiluted parallel IGRA test. The patient who had negative responses to PHA had a positive response to TB antigen, suggesting that most patients were immune-competent at a cellular level. However, we noted that the 5-day incubation was not suitable for clinical diagnosis. Therefore, overnight incubation and an undiluted whole blood assay were performed in the assessment study. Prolonged incubation can stimulate more memory T-cells, but short-time incubation mainly stimulates effector T-cells. In addition, a longer stimulation duration (6 days) was shown to be necessary for the optimal induction of IFN-γ and TNF-β, and was practically convenient for the detection of IL-10, IL-1-β, TNF-α, and IL-6.36
In the assessment study, it is inspiring that a pool of six peptides from RD2 could detect more than half of all active TB patients, although this is lower than the number detected by the T-SPOT.TB assay. A total of 57% of the patients also had positive results for the peptides in the PTB group. Patients in this group all had symptoms, radiographic signs or histological evidence that suggested a high possibility of TB infection, while 60% (25/42) of them received empirical anti-tuberculosis therapy and most showed clinical improvement. Positive detection in this group is also meaningful and instructive as microbiological investigation failed to provide conclusive results. In the preliminary screen, only 10 peptides were recognized by more than 10% of TB patients. The peptides with less frequent recognition would be useful for diagnostic purposes and would have potential to increase sensitivity, if the recognition pattern to these peptides were complementary to the more frequently recognized peptides. However, considering the budget limitation and work load, the recognition value in TB1 group was set to 8%, which included 22 peptides (25%) in the latter screen. Although the sensitivity is not high enough to allow them to be used alone, the peptides have shown potential diagnostic value, and to be adjunctive antigens to the current immunological diagnostic methods.
In the NTP2 group and the ID group, in which TB was less likely, the positive results for the R6 assay were lower than in the highly likely TB groups, as expected. Interestingly, patients diagnosed with cancer, especially lung cancer, were the major reasons for false positive results in the R6 assay in the NTP2 group. In total, 10 of 16 patients with false positive results by the R6 assay had cancer. After excluding those patients whose condition was complicated by cancer, the specificity could be improved to 90.3% (56/62). Lung cancer may cause an autoimmune disorder that results in frequent positive IGRA results37; therefore, one should be very careful when interpreting IGRA positive results from lung cancer patients. For the remainder, false positive results in the NTP2 group and latent TB infection could be the most feasible explanation. Peptide Rv1988-P4, which was recognized by control individuals in screening assay (Figure 4G), could be another reason for reducing the specificity of the peptide pool. As all the patients are from China, which is a high TB epidemic country, the 10-20% positive IGRA results in the NTP group are reasonable and close to the previously reported positive rate in healthy individuals in China.38 Although we assumed that latent TB infection could be the reason for false positivity in the study population, as these antigens are not specifically expressed in latent infection, we did not design a group to test whether latent TB could be recognized by the R6 assay or any different recognition between active and latent TB individuals, which is worthy investigating in the future study. Four patients were culture-positive but confirmed to have NTM disease by selective PNB culture media. None had any positive results for the R6 assay, suggesting that the peptides may also be suitable for differentiating NTM from TB. Despite this, more cases need to be evaluated.
For the parallel T-SPOT.TB assay, the sensitivity and specificity were 76% (37/49) and 75% (68/91), respectively, for the two groups. Unlike the assessments in healthy controls or in some retrospective studies, the unsatisfactory results reflect the real-life diagnostic challenge in highly epidemic regions. The diagnosing performance in active TB was close to that summarized in meta-analysis studies,4, 5 and reinforces the conclusion that the sensitivity is not high enough to rule out TB. Like WBIGRA for the R6 peptides, more than half of the false positive results (13/23) in the NTP2 group could be attributed to the diagnosis of cancer. If patients diagnosed with cancer were excluded, the specificity improved to 83.9% (52/62).
Conclusions
In this study, the diagnostic performance of the six promising epitopes, Rv1986-P9, P15, P16, Rv1988-P4, P11, and Rv1983-P4, was assessed on a panel of 233 patients suspected of having TB for the first time. Our data suggest that these epitopes from RD2 of M. tuberculosis may have potential diagnostic value in the immunological diagnosis of TB.
Conflict of interest
The authors have applied a patent for five peptides, including Rv1986-P9, Rv1986-P15, Rv1986-P16, Rv1988-P4, Rv1988-P11, in TB diagnosis.
Acknowledgments
This work was sponsored by the Major Project of the Twelfth Five-Year Plan (2013ZX10003001-002), National Natural Science Foundation of China (81101226) and the Shanghai Science and Technology Commission (12DZ1941402).
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.bjid.2018.10.280.
Appendix A. Supplementary data
References
- 1.Organization WH. Global tuberculosis report 2017. 2017.
- 2.Kaufmann S.H. How can immunology contribute to the control of tuberculosis? Nat Rev Immunol. 2001;1:20–30. doi: 10.1038/35095558. [DOI] [PubMed] [Google Scholar]
- 3.Fine P.E., Bruce J., Ponnighaus J.M., Nkhosa P., Harawa A., Vynnycky E. Tuberculin sensitivity: conversions and reversions in a rural African population. Int J Tuberc Lung Dis. 1999;3:962–975. [PubMed] [Google Scholar]
- 4.Sester M., Sotgiu G., Lange C., Giehl C., et al. Interferon-gamma release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2011;37:100–111. doi: 10.1183/09031936.00114810. [DOI] [PubMed] [Google Scholar]
- 5.Metcalfe J.Z., Everett C.K., Steingart K.R., et al. Interferon-gamma release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: systematic review and meta-analysis. J Infect Dis. 2011;204(Suppl 4):S1120–S1129. doi: 10.1093/infdis/jir410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones G.J., Hewinson R.G., Vordermeier H.M. Screening of predicted secreted antigens from Mycobacterium bovis identifies potential novel differential diagnostic reagents. Clin Vaccine Immunol. 2010;17:1344–1348. doi: 10.1128/CVI.00261-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X.Q., Dosanjh D., Varia H., et al. Evaluation of T-cell responses to novel RD1- and RD2-encoded Mycobacterium tuberculosis gene products for specific detection of human tuberculosis infection. Infect Immun. 2004;72:2574–2581. doi: 10.1128/IAI.72.5.2574-2581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cockle P.J., Gordon S.V., Lalvani A., Buddle B.M., Hewinson R.G., Vordermeier H.M. Identification of novel Mycobacterium tuberculosis antigens with potential as diagnostic reagents or subunit vaccine candidates by comparative genomics. Infect Immun. 2002;70:6996–7003. doi: 10.1128/IAI.70.12.6996-7003.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Attiyah R., Mustafa A.S. Characterization of human cellular immune responses to novel Mycobacterium tuberculosis antigens encoded by genomic regions absent in Mycobacterium bovis BCG. Infect Immun. 2008;76:4190–4198. doi: 10.1128/IAI.00199-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Attiyah R., Mustafa A.S. Characterization of human cellular immune responses to Mycobacterium tuberculosis proteins encoded by genes predicted in RD15 genomic region that is absent in Mycobacterium bovis BCG. FEMS Immunol Med Microbiol. 2010;59:177–187. doi: 10.1111/j.1574-695X.2010.00677.x. [DOI] [PubMed] [Google Scholar]
- 11.Mustafa A.S., Al-Saidi F., El-Shamy A.S., Al-Attiyah R. Cytokines in response to proteins predicted in genomic regions of difference of Mycobacterium tuberculosis. Microbiol Immunol. 2011;55:267–278. doi: 10.1111/j.1348-0421.2011.00307.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen J., Su X., Zhang Y., et al. Novel recombinant RD2- and RD11-encoded Mycobacterium tuberculosis antigens are potential candidates for diagnosis of tuberculosis infections in BCG-vaccinated individuals. Microbes Infect. 2009;11(10–11):876–885. doi: 10.1016/j.micinf.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Lalvani A. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest. 2007;131:1898–1906. doi: 10.1378/chest.06-2471. [DOI] [PubMed] [Google Scholar]
- 14.Pai M., Zwerling A., Menzies D. Systematic review: T-cell-based assays for the diagnosis of latent tuberculosis infection: an update. Ann Intern Med. 2008;149:177–184. doi: 10.7326/0003-4819-149-3-200808050-00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aagaard C., Brock I., Olsen A., Ottenhoff T.H., Weldingh K., Andersen P. Mapping immune reactivity toward Rv2653 and Rv2654: two novel low-molecular-mass antigens found specifically in the Mycobacterium tuberculosis complex. J Infect Dis. 2004;189:812–819. doi: 10.1086/381679. [DOI] [PubMed] [Google Scholar]
- 16.Brock I., Weldingh K., Leyten E.M., Arend S.M., Ravn P., Andersen P. Specific T-cell epitopes for immunoassay-based diagnosis of Mycobacterium tuberculosis infection. J Clin Microbiol. 2004;42:2379–2387. doi: 10.1128/JCM.42.6.2379-2387.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kozak R.A., Alexander D.C., Liao R., Sherman D.R., Behr M.A. Region of difference 2 contributes to virulence of Mycobacterium tuberculosis. Infect Immun. 2011;79:59–66. doi: 10.1128/IAI.00824-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J., Wang S., Zhang Y., et al. Rv1985c, a promising novel antigen for diagnosis of tuberculosis infection from BCG-vaccinated controls. BMC Infect Dis. 2010;10:273. doi: 10.1186/1471-2334-10-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S., Chen J., Zhang Y., et al. Mycobacterium tuberculosis region of difference (RD) 2 antigen Rv1985c and RD11 antigen Rv3425 have the promising potential to distinguish patients with active tuberculosis from M. bovis BCG-vaccinated individuals. Clin Vaccine Immunol. 2013;20:69–76. doi: 10.1128/CVI.00481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chuang Meng T.W., Zhengzhong Xu, Yan Liu, Fa Shan, Lin Sun, Yuelan Yin, Xiang Chen∗ X.J. Screening putative antigens as stimulators in the Mycobacterium bovis interferon-gamma release assay for cattle. Vet Immunol Immunopathol. 2015:111–117. doi: 10.1016/j.vetimm.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Gideon H.P., Wilkinson K.A., Rustad T.R., et al. Hypoxia induces an immunodominant target of tuberculosis specific T cells absent from common BCG vaccines. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001237. e1001237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ewer K., Deeks J., Alvarez L., et al. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003;361:1168–1173. doi: 10.1016/S0140-6736(03)12950-9. [DOI] [PubMed] [Google Scholar]
- 23.Weir R.E., Morgan A.R., Britton W.J., Butlin C.R., Dockrell H.M. Development of a whole blood assay to measure T cell responses to leprosy: a new tool for immuno-epidemiological field studies of leprosy immunity. J Immunol Methods. 1994;176:93–101. doi: 10.1016/0022-1759(94)90353-0. [DOI] [PubMed] [Google Scholar]
- 24.Ruhwald M., Bodmer T., Maier C., et al. Evaluating the potential of IP-10 and MCP-2 as biomarkers for the diagnosis of tuberculosis. Eur Respir J. 2008;32:1607–1615. doi: 10.1183/09031936.00055508. [DOI] [PubMed] [Google Scholar]
- 25.Wang S., Diao N., Lu C., et al. Evaluation of the diagnostic potential of IP-10 and IL-2 as biomarkers for the diagnosis of active and latent tuberculosis in a BCG-vaccinated population. PLoS One. 2012;7:e51338. doi: 10.1371/journal.pone.0051338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu L., Zhang W.J., Zheng J., et al. Exploration of novel cellular and serological antigen biomarkers in the ORFeome of Mycobacterium tuberculosis. Mol Cell Proteomics. 2014;13:897–906. doi: 10.1074/mcp.M113.032623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chegou N.N., Essone P.N., Loxton A.G., et al. Potential of host markers produced by infection phase-dependent antigen-stimulated cells for the diagnosis of tuberculosis in a highly endemic area. PLoS One. 2012;7:e38501. doi: 10.1371/journal.pone.0038501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marin N.D., Paris S.C., Rojas M., Garcia L.F. Reduced frequency of memory T cells and increased Th17 responses in patients with active tuberculosis. Clin Vaccine Immunol. 2012;19:1667–1676. doi: 10.1128/CVI.00390-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geldmacher C., Ngwenyama N., Schuetz A., et al. Preferential infection and depletion of Mycobacterium tuberculosis-specific CD4 T cells after HIV-1 infection. J Exp Med. 2010;207:2869–2881. doi: 10.1084/jem.20100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sutherland J.S., Lalor M.K., Black G.F., et al. Analysis of host responses to Mycobacterium tuberculosis antigens in a multi-site study of subjects with different TB and HIV infection states in sub-Saharan Africa. PLoS One. 2013;8:e74080. doi: 10.1371/journal.pone.0074080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rustad T.R., Harrell M.I., Liao R., Sherman D.R. The enduring hypoxic response of Mycobacterium tuberculosis. PLoS One. 2008;3:e1502. doi: 10.1371/journal.pone.0001502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones G.J., Pirson C., Gideon H.P., et al. Immune responses to the enduring hypoxic response antigen Rv0188 are preferentially detected in Mycobacterium bovis infected cattle with low pathology. PLoS One. 2011;6:e21371. doi: 10.1371/journal.pone.0021371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andini N., Nash K.A. Intrinsic macrolide resistance of the Mycobacterium tuberculosis complex is inducible. Antimicrob Agents Chemother. 2006;50:2560–2562. doi: 10.1128/AAC.00264-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nash K.A., Brown-Elliott B.A., Wallace R.J., Jr. A novel gene, erm(41), confers inducible macrolide resistance to clinical isolates of Mycobacterium abscessus but is absent from Mycobacterium chelonae. Antimicrob Agents Chemother. 2009;53:1367–1376. doi: 10.1128/AAC.01275-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Attiyah R., El-Shazly A., Mustafa A.S. Comparative analysis of spontaneous and mycobacterial antigen-induced secretion of Th1, Th2 and pro-inflammatory cytokines by peripheral blood mononuclear cells of tuberculosis patients. Scand J Immunol. 2012 doi: 10.1111/j.1365-3083.2012.02692.x. [DOI] [PubMed] [Google Scholar]
- 36.Petrovsky N., Harrison L.C. Cytokine-based human whole blood assay for the detection of antigen-reactive T cells. J Immunol Methods. 1995;186:37–46. doi: 10.1016/0022-1759(95)00127-v. [DOI] [PubMed] [Google Scholar]
- 37.Bordignon V., Bultrini S., Prignano G., et al. High prevalence of latent tuberculosis infection in autoimmune disorders such as psoriasis and in chronic respiratory diseases, including lung cancer. J Biol Regul Homeost Agents. 2011;25:213–220. [PubMed] [Google Scholar]
- 38.Zhang S., Shao L., Mo L., et al. Evaluation of gamma interferon release assays using Mycobacterium tuberculosis antigens for diagnosis of latent and active tuberculosis in Mycobacterium bovis BCG-vaccinated populations. Clin Vaccine Immunol. 2010;17:1985–1990. doi: 10.1128/CVI.00294-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.