Abstract
Background
In people living with HIV, much is known about chronic kidney disease, defined as a glomerular filtration rate under 60 mL/min. However, there is scarce data about prevalence and risk factors for milder impairment (60–89 mL/min).
Objective
The present study aims to assess the influence of sex, antiretroviral therapy, and classical risk factors on the occurrence of mild decreased renal function in a large Spanish cohort of HIV-infected patients.
Methods
Cross-sectional, single center study, including all adult HIV-1-infected patients under antiretroviral treatment with at least two serum creatinine measures during 2014, describing the occurrence of and the risk factors for mildly decreased renal function (eGFR by CKD-EPI creatinine equation of 60–89 mL/min).
Results
Among the 4337 patients included, the prevalence rate of mildly reduced renal function was 25%. Independent risk factors for this outcome were age older than 50 years (OR 3.03, 95% CI 2.58–3.55), female sex (OR 1.23, 95% CI 1.02–1.48), baseline hypertension (OR 1.57, 95% CI 1.25–1.97) or dyslipidemia (OR 1.48, 95% CI 1.17–1.87), virologic suppression (OR 1.88, 95% CI 1.39–2.53), and exposure to tenofovir disoproxil-fumarate (OR 1.67, 95% CI 1.33–2.08) or ritonavir-boosted protease-inhibitors (OR 1.19, 95% CI 1.03–1.39).
Conclusions
Females and patients over 50 seem to be more vulnerable to renal impairment. Potentially modifiable risk factors and exposure to tenofovir disoproxil-fumarate or ritonavir-boosted protease-inhibitors are present even in earlier stages of chronic kidney dysfunction. It remains to be determined whether early interventions including antiretroviral therapy changes (tenofovir alafenamide, cobicistat) or improving comorbidities management will improve the course of chronic kidney disease.
Keywords: HIV-infection, Antiretroviral drugs, Chronic kidney disease, Glomerular filtration rate estimates
Introduction
In most countries with broad access to antiretroviral therapy, chronic kidney disease (CKD) in people living with HIV infection is now more likely to be the result of non-HIV associated conditions,1, 2 and it might have a higher prevalence and earlier onset than in age-matched uninfected individuals.3, 4, 5 Although there is a low overall risk of developing end-stage renal disease,6, 7 decreasing GFR is related to a significantly increased risk of cardiovascular events and mortality.8, 9
Even patients with milder grades of renal dysfunction already have sizable medical costs that can be attributed to renal function impairment.10 Moreover, a considerable number of the antiretroviral drugs and antibiotics undergo renal elimination and demand dose-adjustments according to kidney function.11 Lastly, the increasing exposure to some antiretroviral drugs can lead to progression to kidney disease, even in individuals with initially normal renal function.12 Therefore, in clinical practice, it is important for the attending physician to identify the presence of incremental baseline risk factors and intervene where they are potentially modifiable and as early as possible.
Several studies have already been published evaluating the presence of risk factors for CKD stage ≥3.7, 13, 14, 15, 16, 17, 18 However, there is a lack of information regarding the factors associated with earlier stages of renal dysfunction, also associated with increased risk of complications,9, 19 but in which preventive actions would be more feasible.
The aim of the present study is therefore to assess the influence of sex, type of antiretroviral therapy (ART), and the classical risk factors on mildly decreased renal function (CKD EPI eGFR 60–89 mL/min/1.73 m2) among an urban population of stable patients with HIV-infection in a large Spanish cohort.
Methods
Study design
This was an observational, cross-sectional, single center study. The study project was reviewed and approved by the Institutional Review Board (CEIC Hospital Clinic i Provincial, Barcelona, Spain, IRB# 2014/1080). Eligible patients were all adult HIV-1-infected patients (>18 years old) with at least two serum creatinine measures during the calendar year of 2014. A description of the prevalence of the various stages of CKD of the entire cohort was published elsewhere.20 For the current analysis, patients were excluded if they presented an estimated GFR above 181 mL/min/1.73 m2, a diagnosis of chronic kidney disease (eGFR < 60 mL/min per 1.73 m2), dialysis and/or kidney transplantation, or if they were recipients of a hepatic allograft.
The main objectives of our study were to describe the occurrence of mildly decreased renal function, defined as two consecutive measures of eGFR between 60 and 89 mL/min/1.73 m2 over at least three months, and to determine the variables associated with a higher risk of this event. eGFR was obtained using the CKD-EPI creatinine equation.21 As in other publications, we considered the African-American coefficient factor as not applicable to black patients from Africa, Europe and Antilles.22, 23
The following demographic, clinical and laboratory parameters were abstracted from the HIV clinical database: age, sex, race, body mass index, hypertension (use of anti-hypertensive medication at CKD diagnosis), diabetes mellitus (glucose intolerance requiring pharmacological intervention), hyperlipidemia (use of hypolipidemic medication at CKD diagnosis), prior cardiovascular event, viruses, time of HIV-infection diagnosis, mode of transmission, AIDS stage, CD4 and viral load (current and nadir), current and previous antiretroviral treatment, hepatitis B coinfection (positive serology) and hepatitis C coinfection (positive serology + detectable HCV-RNA). Urinalysis for proteinuria was not available for the present study.
Data analysis
Demographic, clinical and laboratory parameters were described for patients with and without mildly decreased renal function. Quantitative variables were expressed as median and interquartile range. Analysis of normality of quantitative variables was performed using the Kolmogorov–Smirnov test, and because none of them displayed a normal distribution, nonparametric tests were used to compare these variables. Categorical variables were expressed as number, percentage, and 95% CI; the Chi-square test was used for comparisons. For all tests, statistical significance is considered if the p-value < 0.05. To identify risk factors associated with mildly decreased renal function, we performed a stepwise binary logistic regression analysis. Variables included in the model were those with a p-value < 0.05 in univariate analysis, or those considered relevant by other published studies. Statistical analyses were performed using the Predictive Analytics Software Statistics for Windows, v21.0 (SPSS Inc, Chicago, IL).
Results
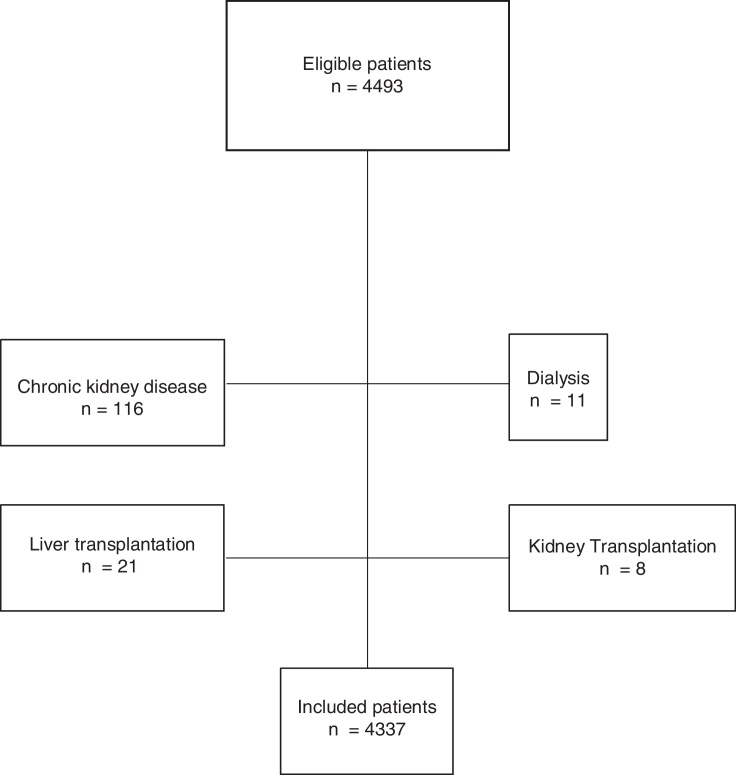
The eligible population consisted of 4493 patients. After excluding 116 subjects with chronic kidney disease, 11 patients in dialysis, eight recipients of kidney transplantation, and 21 recipients of hepatic transplantation, 4337 individuals were included for analysis (Fig. 1).
Fig. 1.
Flow diagram of the studied population.
The overall median age was 44 years-old (range 18–85), 47 (18–85) for females and 44 (18–83) for males.
The overall prevalence rate of mildly reduced renal function (eGFR 60–89 mL/min per 1.73 m2) was 25.0% (1083 patients). The median eGFR among patients with normal renal function was 103.5 (IQR 97.3–110.3) mL/min/1.73 m2; the median eGFR among patients with mildly reduced renal function was 79.7 (IQR 73.0–85.1) mL/min/1.73 m2.
Univariate analysis
Compared with patients with normal renal function, patients with mildly reduced eGFR were older, had a higher percentage of females, higher body mass index, higher proportion of heterosexuality, more prior AIDS events, and more comorbidities like hypertension, dyslipidemia, diabetes, and prior cardiovascular disease. They were also prone to longer durations of HIV-infection, higher initial and peak viral loads, lower nadir of CD4+ cell count, higher frequency of prior AIDS events, but a higher proportion of patients presented viral suppression on treatment (Table 1). The overall length of exposure to any regimen, as well as the total exposure to protease inhibitors (boosted or not), tenofovir disoproxil-fumarate, and PI plus tenofovir disoproxil-fumarate were higher among individuals with mildly reduced eGFR (Table 2).
Table 1.
Demographic and HIV-related characteristics of the global cohort and comparative analysis regarding the presence of mildly-reduced eGFR (glomerular filtration rate, estimated by CKD-EPI creatinine equation, between 60 and 89 mL/min/1.73 m2).
| Variable | Global cohort | Normal eGFR | Mildly-reduced eGFR | p-value |
|---|---|---|---|---|
| Number of patients | 4337 | 3254 | 1083 | – |
| Age, yearsa | 44 (14) | 43 (14) | 50 (13) | <0.001 |
| Female gender, n (%) | 819 (18.9) | 580 (17.8) | 239 (22.1) | 0.002 |
| Black race, n (%) (n = 2729) | 55 (2.0) | 40 (1.8) | 15 (2.5) | 0.281 |
| Body mass index, kg/m2a(n = 1373) | 22.9 (3.5) | 22.8 (3.4) | 23.2 (3.3) | 0.040 |
| Hypertension, n (%) | 530 (12.2) | 296 (9.1) | 234 (21.6) | <0.001 |
| Diabetes mellitus, n (%) | 199 (4.6) | 120 (3.7) | 79 (7.3) | <0.001 |
| Dyslipidemia, n (%) | 528 (12.2) | 302 (9.3) | 226 (20.9) | <0.001 |
| Cardiovascular events, n (%) | 168 (3.9) | 97 (3.0) | 71 (6.6) | <0.001 |
| HBV coinfection, n (%) | 145 (3.3) | 100 (3.1) | 45 (4.2) | 0.325 |
| HCV coinfection, n (%) (n = 4329) | 951 (22.0) | 708 (21.8) | 243 (22.5) | 0.639 |
| Duration of HIV infection, yearsa(n = 4313) | 10.7 (13.9) | 9.6 (13.7) | 14.1 (12.6) | <0.001 |
| Route of HIV acquisition, n (%) | ||||
| Heterosexual | 1116 (25.7) | 788 (24.2) | 328 (30.3) | <0.001 |
| Homosexual | 2590 (59.7) | 1997 (61.4) | 593 (54.8) | <0.001 |
| Injecting-drug abuse | 602 (13.9) | 445 (13.7) | 157 (14.5) | 0.681 |
| Another route | 48 (1.1) | 37 (1.1) | 11 (1.0) | 0.778 |
| Unknown | 228 (5.3) | 175 (5.4) | 53 (4.9) | 0.536 |
| Prior AIDS events, n (%) | 837 (19.3) | 578 (17.8) | 259 (23.9) | <0.001 |
| HIV suppression, n (%) | ||||
| On treatment | 3840 (88.5) | 2825 (86.8) | 1015 (93.7) | |
| Without treatment | 29 (0.7) | 23 (0.7) | 6 (0.6) | <0.001 |
| No suppression | 468 (10.8) | 406 (12.5) | 62 (5.7) | |
| Viral load, copies/mLa | ||||
| First | 23,622 (116,567) | 22,300 (105,867) | 28,670 (160,438) | 0.018 |
| Higher | 67,951 (233,350) | 62,934 (209,910) | 88,730 (286,825) | <0.001 |
| CD4 cell count, cells/mm3 | ||||
| Lower (nadir) | 252 (237) | 264 (237) | 219 (240) | <0.001 |
| Current | 633 (375) | 634 (372) | 626 (394) | 0.991 |
Results expressed as median and interquartile range (IQR).
HCV, hepatitis C virus; HBV, hepatitis B virus. Significant p < 0.05.
Table 2.
Antiretroviral treatment used by patients with normal eGFR and with mildly-reduced eGFR.
| Variable | Global cohort | Normal eGFR | Mildly-reduced eGFR | p-value |
|---|---|---|---|---|
| Number of patients | 4337 | 3254 | 1083 | |
| Currently on ART, n (%) | 4199 (96.8) | 3132 (96.3) | 1067 (98.5) | <0.001 |
| Time under current ART regimen, yearsa | 1.3 (3.6) | 1.2 (3.5) | 1.5 (3.9) | <0.001 |
| Current ART regimen, n (%) | ||||
| No treatment | 138 (3.2) | 112 (3.7) | 16 (1.5) | |
| NNRTI-based | 2336 (53.9) | 1759 (54.1) | 577 (53.3) | 0.005 |
| PI-based | 136 (3.1) | 102 (3.1) | 34 (3.1) | |
| Boosted PI-based | 1257 (29.0) | 930 (28.6) | 327 (30.2) | |
| Integrase Inh-based | 439 (10.1) | 316 (9.7) | 123 (11.4) | |
| Other | 31 (0.7) | 25 (0.8) | 6 (0.6) | |
| Patients currently using, n (%) | ||||
| Tenofovir | 2825 (65.1) | 2169 (66.7) | 656 (60.6) | <0.001 |
| Indinavir | 0 | 0 | 0 | – |
| Atazanavir | 308 (7.1) | 237 (7.3) | 71 (6.6) | 0.419 |
| Darunavir | 808 (18.6) | 586 (18.0) | 222 (20.5) | 0.068 |
| Boosted PI | 1024 (23.6) | 755 (23.2) | 269 (24.8) | 0.272 |
| Patients having used, n (%) | ||||
| Lopinavir/ritonavir | 2010 (23.5) | 711 (21.9) | 309 (28.5) | <0.001 |
| Amprenavir | 44 (1.0) | 27 (0.8) | 17 (1.6) | 0.035 |
| Atazanavir | 923 (21.3) | 632 (19.4) | 291 (26.9) | <0.001 |
| Darunavir | 1069 (24.6) | 771 (23.7) | 298 (27.5) | 0.011 |
| Fosamprenavir | 182 (4.2) | 126 (3.9) | 56 (5.2) | 0.065 |
| Indinavir | 692 (16.0) | 429 (13.2) | 263 (24.3) | <0.001 |
| Nelfinavir | 462 (10.7) | 296 (9.1) | 166 (15.3) | <0.001 |
| Ritonavir | 1788 (41.2) | 1285 (39.5) | 503 (46.4) | <0.001 |
| Saquinavir | 431 (9.9) | 313 (9.6) | 118 (10.9) | 0.224 |
| Tenofovir | 3670 (84.6) | 2711 (83.3) | 959 (88.6) | <0.001 |
| Tipranavir | 58 (1.3) | 39 (1.2) | 19 (1.8) | 0.168 |
| Any PI | 2496 (57.6) | 1769 (54.4) | 727 (67.1) | <0.001 |
| Boosted PI | 2156 (49.7) | 1554 (47.8) | 602 (55.6) | <0.001 |
| Previous/current treatment with TEN, n (%) | 3670 (84.6) | 2711 (83.3) | 959 (88.6) | <0.001 |
| Previous/current treatment with boosted PI, n (%) | 2156 (49.7) | 1554 (47.8) | 602 (55.6) | <0.001 |
| Previous/current PI or boosted PI plus TEN, n (%) | 1865 (43.0) | 1313 (40.4) | 552 (51.0) | <0.001 |
| Previous/current PI or boosted PI without TEN, n (%) | 291 (6.7) | 241 (7.4) | 50 (4.6) | 0.001 |
| Previous/current NNRTI plus TEN, n (%) | 2144 (49.4) | 1615 (49.6) | 529 (48.8) | 0.654 |
| Previous/current NNRTI without TEN, n (%) | 192 (4.4) | 144 (4.4) | 48 (4.4) | 0.992 |
| Previous/current Integrase Inh plus TEN, n (%) | 381 (8.8) | 269 (8.3) | 112 (10.3) | 0.037 |
| Previous/current Integrase Inh without TEN, n (%) | 58 (1.3) | 47 (1.4) | 11 (1.0) | 0.287 |
| Duration of the treatment, yearsa | ||||
| Lopinavir/ritonavir | 2.40 (3.8) | 2.450 (3.8) | 2.30 (3.8) | 0.534 |
| Amprenavir | 1.50 (2.1) | 1.20 (2.4) | 1.75 (2.0) | 0.642 |
| Atazanavir | 2.90 (4.1) | 2.90 (4.1) | 3.00 (3.9) | 0.555 |
| Darunavir | 2.0 (2.3) | 1.90 (2.3) | 2.20 (2.6) | 0.242 |
| Fosamprenavir | 1.90 (3.8) | 1.80 (3.5) | 2.30 (4.7) | 0.358 |
| Indinavir | 1.70 (2.4) | 1.60 (1.9) | 1.90 (2.9) | 0.015 |
| Nelfinavir | 1.80 (2.5) | 2.00 (2.7) | 1.70 (2.1) | 0.114 |
| Ritonavir | 2.60 (4.2) | 2.40 (4.0) | 3.00 (4.7) | 0.002 |
| Saquinavir | 1.80 (2.8) | 2.00 (2.9) | 1.70 (2.3) | 0.264 |
| Tenofovir | 3.20 (4.4) | 3.00 (4.1) | 3.90 (4.9) | <0.001 |
| Tipranavir | 1.80 (2.9) | 2.00 (2.8) | 1.20 (2.9) | 0.716 |
| Any PI | 4.00 (5.8) | 3.90 (5.6) | 4.30 (6.2) | 0.008 |
| Boosted PI | 3.30 (5.0) | 3.10 (4.7) | 3.75 (5.2) | 0.004 |
Results expressed as median and interquartile range (IQR). Significant p < 0.05.
ART, antiretroviral therapy; NNRTI, non-nucleoside reverse transcriptase inhibitors; PI, protease inhibitors; Inh, inhibitors; TEN, tenofovir disoproxil fumarate. All PIs were boosted with ritonavir.
Sex disparities
The median eGFR was 94.9 (IQR 81.3–105) mL/min/1.73 m2 for females and 96.6 (IQR 84.2–106.3) mL/min/1.73 m2 for males (p = 0.005). Females had a significantly higher proportion of mildly impaired renal function compared to males (29.2% vs. 23.9%, p = 0.002, Table 3).
Table 3.
Demographic and HIV-related characteristics according to sex.
| Female patients |
p-value | Male patients |
p-value | p-valueb | |||||
|---|---|---|---|---|---|---|---|---|---|
| Global femaleb | Normal eGFR | Mildly-reduced eGFR | Global maleb | Normal eGFR | Mildly-reduced eGFR | ||||
| Number of patients, n (%) | 819 | 580 | 239 (29.2) | – | 3518 | 2674 | 844 (23.9) | – | 0.002 |
| Age, yearsa | 47 (11) | 45 (11) | 51 (13) | <0.001 | 44 (15) | 42 (15) | 50 (13) | <0.001 | <0.001 |
| Black race, n (%) | 23 (5.3) | 18 (5.4) | 5 (4.8) | 0.802 | 32 (1.4) | 22 (1.2) | 10 (2.0) | 0.147 | <0.001 |
| Body mass index, kg/m2a | 22.4 (5.8) | 22.6 (6.5) | 22.1 (4.9) | 0.240 | 23.0 (3.3) | 22.8 (3.3) | 23.4 (3.1) | 0.004 | 0.269 |
| Hypertension, n (%) | 112 (13.7) | 58 (10.0) | 54 (22.6) | <0.001 | 418 (11.9) | 238 (8.9) | 180 (21.3) | <0.001 | 0.158 |
| Diabetes mellitus, n (%) | 39 (4.8) | 24 (4.1) | 15 (6.3) | 0.191 | 160 (4.5) | 96 (3.6) | 64 (7.6) | <0.001 | 0.792 |
| Dyslipidemia, n (%) | 89 (10.9) | 46 (7.9) | 43 (18.0) | <0.001 | 439 (12.5) | 256 (9.6) | 183 (21.7) | <0.001 | 0.204 |
| Prior cardiovascular event, n (%) | 21 (2.6) | 12 (2.2) | 8 (3.3) | 0.363 | 147 (4.2) | 84 (3.1) | 63 (7.5) | <0.001 | 0.031 |
| HBV coinfection, n (%) | 13 (1.6) | 9 (1.6) | 4 (1.7) | 0.979 | 132 (3.8) | 91 (3.4) | 41 (4.9) | 0.236 | 0.019 |
| HCV coinfection, n (%) (n = 4329) | 268 (32.8) | 176 (30.4) | 92 (38.7) | 0.022 | 683 (19.4) | 532 (19.9) | 151 (17.9) | 0.196 | <0.001 |
| Duration of HIV infection, yearsa | 17.5 (11.8) | 17.0 (12.9) | 17.7 (10.1) | 0.022 | 9.2 (13.1) | 8.4 (12.5) | 12.5 (13.0) | <0.001 | <0.001 |
| HIV acquisition, n (%) | |||||||||
| Heterosexual | 559 (68.3) | 398 (68.6) | 161 (67.4) | 0.688 | 557 (15.8) | 390 (14.6) | 167 (19.8) | 0.001 | <0.001 |
| Homosexual | 25 (3.1) | 19 (3.3) | 6 (2.5) | 0.695 | 2565 (72.9) | 1978 (74.0) | 587 (69.5) | 0.013 | <0.001 |
| Drug abuse | 175 (21.4) | 115 (19.8) | 60 (25.1) | 0.229 | 427 (12.1) | 330 (12.3) | 97 (11.5) | 0.710 | <0.001 |
| Other route | 27 (3.3) | 24 (4.1) | 3 (1.3) | 0.086 | 21 (0.6) | 13 (0.5) | 8 (0.9) | 0.289 | <0.001 |
| Unknown | 51 (6.2) | 38 (6.6) | 13 (5.4) | 0.549 | 177 (5.0) | 137 (5.1) | 40 (4.7) | 0.366 | 0.167 |
| Prior AIDS events, n (%) | 192 (23.4) | 132 (22.8) | 60 (25.1) | 0.263 | 645 (18.3) | 446 (16.7) | 199 (23.6) | <0.001 | 0.001 |
| HIV suppression, n (%) | |||||||||
| On treatment | 721 (88.0) | 504 (86.9) | 217 (90.8) | 0.199 | 3119 (88.7) | 2321 (86.8) | 798 (94.5) | <0.001 | 0.008 |
| Without treatment | 1 (1.5) | 8 (1.4) | 4 (1.7) | 17 (0.5) | 15 (0.6) | 2 (0.2) | |||
| No suppression | 86 (10.5) | 68 (11.7) | 18 (7.5) | 382 (10.9) | 338 (12.6) | 44 (5.2) | |||
| CD4 cell count, cells/mm3a | |||||||||
| Nadir | 196 (204) | 207 (208) | 178 (183) | 0.002 | 266 (245) | 277 (240) | 232 (249) | <0.001 | <0.001 |
| Current | 624 (406) | 618 (394) | 638 (434) | 0.331 | 635 (371) | 638 (369) | 625 (377) | 0.631 | 0.204 |
| Currently on ART, n (%) | 797 (97.3) | 565 (97.4) | 232 (97.1) | 0.783 | 3402 (96.7) | 2567 (96.0) | 835 (98.9) | <0.001 | 0.369 |
| Time under current ART regimen, yearsa | 1.7 (4.2) | 1.7 (4.1) | 1.7 (4.6) | 0.337 | 1.2 (3.4) | 1.2 (3.3) | 1.4 (3.7) | <0.001 | |
| Current ART regimen, n (%) | |||||||||
| No treatment | 22 (2.7) | 15 (2.6) | 7 (2.9) | 0.978 | 116 (3.3) | 107 (4.0) | 9 (1.1) | 0.001 | <0.001 |
| NNRTI-based | 356 (43.5) | 253 (43.6) | 103 (43.1) | 1980 (56.3) | 1506 (56.3) | 474 (56.2) | |||
| PI-based | 41 (5.0) | 30 (5.2) | 11 (4.6) | 95 (2.7) | 72 (2.7) | 23 (2.7) | |||
| Boosted PI-based | 290 (35.4) | 207 (35.7) | 83 (34.7) | 967 (27.5) | 723 (27.0) | 244 (28.9) | |||
| Integrase inhibitor-based | 105 (12.8) | 72 (12.4) | 33 (13.8) | 334 (9.5) | 244 (9.1) | 90 (10.7) | |||
| Other | 5 (0.6) | 3 (0.5) | 2 (0.8) | 26 (0.7) | 22 (0.8) | 4 (0.5) | |||
| Patient currently using, n (%) | |||||||||
| Tenofovir | 476 (58.1) | 355 (61.2) | 121 (50.6) | 0.005 | 2349 (66.8) | 1814 (67.8) | 535 (63.4) | 0.017 | <0.001 |
| Indinavir | 0 | 0 | 0 | – | 0 | 0 | 0 | – | – |
| Atazanavir | 85 (10.4) | 60 (10.3) | 25 (10.5) | 0.961 | 223 (6.3) | 177 (6.6) | 46 (5.5) | 0.224 | <0.001 |
| Darunavir | 161 (19.7) | 116 (20.0) | 45 (18.8) | 0.701 | 647 (18.4) | 470 (17.6) | 177 (21.0) | 0.026 | 0.402 |
| Boosted PI | 220 (26.9) | 159 (27.4) | 61 (25.5) | 0.579 | 804 (22.9) | 596 (22.3) | 208 (24.6) | 0.155 | 0.015 |
| Previous or current treatment with tenofovir, n (%) | 686 (83.8) | 487 (84.0) | 199 (83.3) | 0.804 | 2984 (84.8) | 2224 (83.2) | 760 (90.0) | <0.001 | 0.449 |
| Previous or current treatment with boosted PI, n (%) | 496 (60.6) | 347 (59.8) | 149 (62.3) | 0.503 | 1660 (47.2) | 1207 (45.1) | 453 (53.7) | <0.001 | <0.001 |
| PI or boosted PI plus TEN, n (%) | 430 (52.5) | 297 (51.2) | 133 (55.6) | 0.247 | 1435 (40.8) | 1016 (38.0) | 419 (49.6) | <0.001 | <0.001 |
| PI or boosted PI without TEN, n (%) | 66 (8.1) | 50 (8.6) | 16 (6.7) | 0.357 | 225 (6.4) | 191 (7.1) | 34 (4.0) | 0.001 | 0.087 |
| NNRTI plus TEN, n (%) | 321 (39.2) | 234 (40.3) | 87 (36.4) | 0.293 | 1823 (51.8) | 1381 (51.6) | 442 (52.4) | 0.714 | <0.001 |
| NNRTI without TEN, n (%) | 35 (4.3) | 19 (3.3) | 16 (6.7) | 0.028 | 157 (4.5) | 125 (4.7) | 32 (3.8) | 0.279 | 0.813 |
| Integrase inhibitors plus TEN, n (%) | 96 (11.7) | 64 (11.0) | 32 (13.4) | 0.341 | 285 (8.1) | 205 (7.7) | 80 (9.5) | 0.093 | 0.001 |
| Integrase inhibitors without TEN, n (%) | 9 (1.1) | 8 (1.4) | 1 (0.4) | 0.230 | 49 (1.4) | 39 (1.5) | 10 (1.2) | 0.554 | 0.510 |
Results expressed as median and interquartile range (IQR). Significant p < 0.05.
p-value for comparisons between global female and global male.
HBV, hepatitis B virus; HCV, hepatitis C virus; ART, antiretroviral; PI, protease inhibitors; NNRTI, non-nucleoside reverse transcriptase inhibitors; TEN, tenofovir disoproxil fumarate. All PIs were boosted with ritonavir.
Compared to men, women were older, had higher proportion of black race, higher prevalence of HCV-coinfection, longer duration of HIV-infection, higher proportion of heterosexual route of acquisition, of drug injecting drugs and prior AIDS events, a lower nadir of CD4+ cell count, a more frequent exposure to PI or boosted-PI with tenofovir disoproxil-fumarate, and a higher proportion of current therapy with PI, but a lower proportion of current ART with tenofovir disoproxil-fumarate.
Multivariate analysis
After multivariate adjustment for demographics, traditional risk factors for kidney disease, and HIV-related characteristics, female sex was associated with a 23% increased risk for renal dysfunction (Table 4). Individuals older than 50 years had a three-fold higher risk for mildly reduced eGFR compared to the under 50s. Hypertension and dyslipidemia were also independently associated with this outcome. Subjects under virological suppression had an almost 2.0-fold greater risk of renal impairment. Previous tenofovir disoproxil-fumarate and protease-inhibitor exposure were also significant risk factors for mild impaired renal function.
Table 4.
Multivariate analysis of risk factors independently associated with mildly decreased renal function in patients with HIV-infection.
| Risk factors | OR (95% CI) | p-value |
|---|---|---|
| Baseline age | ||
| ≤50 y | 1.00 (reference) | – |
| >50 y | 3.03 (2.58–3.55) | <0.001 |
| Gender | ||
| Male | 1.00 (reference) | – |
| Female | 1.23 (1.02–1.48) | 0.031 |
| Baseline hypertension | 1.57 (1.25–1.97) | <0.001 |
| Diabetes mellitus | 0.99 (0.71–1.37) | 0.955 |
| Dyslipidemia | 1.48 (1.17–1.87) | 0.001 |
| Previous cardiovascular events | 0.77 (0.53–1.13) | 0.186 |
| HCV coinfection | 0.84 (0.69–1.01) | 0.387 |
| Duration of HIV infection (years)a | 1.01 (0.99–1.02) | 0.283 |
| HIV suppression | ||
| No | 1.00 (reference) | – |
| Yes | 1.88 (1.39–2.53) | <0.001 |
| CD4+ cell count nadir | 1.00 (0.99–1.00) | 0.467 |
| Current and/or previous exposure to tenofovira | 1.67 (1.33–2.08) | <0.001 |
| Current and/or previous exposure to boosted PIb | 1.19 (1.03–1.39) | 0.023 |
HCV, hepatitis C virus; PI, protease inhibitors.
Tenofovir disoproxil fumarate.
All PIs were boosted with ritonavir. Significant p < 0.05.
Analyses were also stratified by sex to evaluate possible effect modification: among men, older age (OR 2.57; 95% CI 2.17–3.04), dyslipidemia (OR 1.56; 95% CI 2.17–3.04), viral suppression (OR 2.05; 95% CI 1.47–2.84), each additional year of exposure to tenofovir disoproxil-fumarate (OR 1.08; 95% CI 1.05–1.11), and coinfection with hepatitis B (OR 1.48; 95% CI 1.01–2.17) remained independently associated with increased risk for mild renal impairment, whereas coinfection with hepatitis C (OR 0.74; 95% CI 0.57–0.95) was related to a lower risk for this event. On the other hand, among women, older age (OR 2.84; 95% CI 2.07–3.89), hypertension (OR 1.62; 95% CI 1.05–2.50), viral suppression (OR 2.19; 95% CI 1.20–4.03), and each additional year of exposure to protease inhibitors (OR 1.05; 95% CI 1.01–1.08) remained as independent variables associated with renal dysfunction.
Discussion
The main findings of this study, which involved a large sample of patients with well-controlled HIV-infection, were: (a) one quarter of the patients had mildly decreased renal function; (b) women appear to be more susceptible to changes in renal function than men; and (c) traditional and non-traditional risk factors were associated with this outcome.
Renal involvement among patients with HIV-infection is highly variable, but there is a higher risk of developing end-stage renal disease (ESRD) in comparison with the non-infected population. A recent report from the North American AIDS Cohort Collaboration on Research and Design (NAACCORD) in USA and Canada, including 38,354 HIV-infected adults from 2000 to 2009, showed a three-fold higher incidence of ESRD than in the general population. Patients with HIV-infection and ESRD were more likely to be of black race, have diabetes mellitus or hypertension, inject drugs, or have a prior AIDS-defining illness.24 In the same way, in Europe, a German cohort study of 9198 patients observed that the incidence of ESRD was more than two times that of the general population. Risk factors for ESRD were black ethnicity, use of injecting drugs, and HCV-coinfection. Interestingly, the prevalence of ESRD increased over time, especially among Caucasian patients, and ESRD was associated with a high overall mortality.25 The overall prevalence of ESRD ranged from 0.3% to 0.5%.20, 25
There is significant clinical information on moderate chronic renal failure (GFR < 60 mL/min) among patients with HIV-infection.13, 14, 15, 16, 17, 18, 26 However, there is scant information on milder degrees of renal function impairment, especially with GFR 60–89 mL/min.9, 27 The reason is the relatively recent use of the estimated glomerular filtration rate (eGFR) with the MDRD-equation which is not validated to discriminate GFR over 60 mL/min. In addition, the eGFR with the new Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation allows for more detailed analysis of renal function and discriminates patients with mildly reduced GFR (60–89 mL/min/1.73 m2).20
In the present analysis, there was a significant prevalence of mildly reduced renal function (eGFR 60–89 mL/min/1.73 m2) in 25% of the patients. The overall prevalence of mild renal impairment found in this study is in accordance with the rates of 25–40% found in other urban, ambulatory and well-controlled populations in the modern era of antiviral therapy.28, 29, 30 Due to the asymptomatic character of this condition, these patients were often not recognized as having kidney disease by the caring clinicians and thus missed opportunities for early preventive measures.
The risk factors associated with the presence of mildly reduced renal function in HIV-infected patients have been poorly studied. In the last several years, there has been growing attention to sex in HIV epidemiology, prevention and treatment. Although the association of female sex and chronic kidney disease was not found in the classic EuroSida study,31 several other publications,32, 33 including a modern prospective analysis of the large cohort from the D:A:D study,34 have found contrary results. Several reasons may explain why female sex was a strong independent risk factor for mildly impaired renal function in the multivariate analysis. Firstly, in the present study, women presented a higher prevalence of black race and injecting drug use. Although not sex-specific, these factors may interact with sex and create a structural barrier to prevention, testing, and treatment services, as already addressed in a recent review.35 The higher prevalence of prior AIDS events and lower nadir CD4 cell count support this affirmation, as they indicate more advanced stages of HIV-infection at diagnosis. Finally, there are sex differences in the antiretroviral pharmacokinetic parameters, and in general, women have been found to be more susceptible than men to developing ART-associated toxicities.36 In fact, the longer duration of HIV-infection in women, and the more frequent exposure to boosted-PI with or without tenofovir disoproxil-fumarate are in accordance with this hypothesis.
Consistent with other studies,16, 26, 27, 33, 34 older age was the most important risk factor for the occurrence of renal dysfunction. On the one hand, as HIV patients are living longer, they are also getting older. In 2013, individuals aged 55 and older comprised 26% of the people living with HIV in the USA.37 Moreover, although much attention has been paid to preventing HIV-infection in young people, many patients are infected later in life. For example, 12.9% of newly reported cases of HIV in Western Europe in 2007 were in people aged 50 years or older.38 On the other hand, chronic HIV-infection is associated with accelerated aging despite apparent viral control, manifested as increased genetic instability, enhanced T-cell senescence, diminished naïve T-cell regeneration, and altered intracellular communication. It is therefore, associated with early onset of diseases linked to aging, including renal impairment.39, 40
In this cohort, coinfection with HCV was not found to be an independent risk factor for renal dysfunction. Among male patients, this condition was even regarded as a protective variable. Certainly, this topic remains an unsolved issue. A pooled analysis of more than 18,000 patients with HIV-infection found a 50% increased risk of chronic kidney disease among individuals with HCV-coinfection,41 and a more recent meta-analysis of more than 13,000 subjects confirmed these findings.42 However, both authors acknowledged that all the available studies were retrospective and subject to heterogeneity in the design and in the quality of data, as many confounding variables were not reported. Although some investigators have linked HCV to atherosclerosis and atherosclerotic diseases at the extra-hepatic level including kidneys,43 and although there is an association between HCV infection and several types of glomerulonephritis,44 the often observed association between HCV infection and increased risk for kidney disease may still reflect confounding variables such as older age, injecting drug use, poor socioeconomic status, and exposure to nephrotoxic medications.
In this study, experiencing HIV-suppression was a strong independent risk factor for renal impairment. It is true that, on the one hand, an untreated HIV-infection may be related to acute and chronic kidney disease due to direct viral kidney injury (HIVAN and other manifestations), chronic inflammation, opportunistic infections, and their potentially nephrotoxic treatments. However, on the other hand, to achieve adequate control of HIV-infection, patients are exposed to prolonged periods on potentially nephrotoxic antiretrovirals and accumulate several adverse events.45
Tenofovir disoproxil-fumarate, the first-choice standard of care treatment, constitutes a risk factor even for milder grades of renal dysfunction.16, 46 The primary mechanism by which tenofovir causes renal toxicity may involve drug accumulation within proximal renal tubules, leading to mitochondrial injury and depletion. Tenofovir renal injury may present as partial or full Fanconi syndrome and acute tubular necrosis, eventually leading to tubulointerstitial scarring, which may account for the lack of reversibility of tenofovir renal toxicity in some individuals.47 Nephrotoxicity due to protease inhibitors (PIs), mainly indinavir and atazanavir, is related to the formation of urolithiasis and intratubular precipitation, obstructive nephropathy, and acute or chronic interstitial nephritis. Ritonavir toxicity is more likely the result of drug interactions than of a direct kidney effect.48 Other PIs such as nelfinavir, amprenavir, saquinavir, ritonavir, and darunavir have also been reported to cause urolithiasis.49
This finding has strong implications for clinical practice. Women and middle-aged patients are a population associated with an increased risk of alterations in renal function even in the initial stages of renal injury. This new scenario involves performing a specific clinical management in this group of patients, especially with the use of certain antiretroviral drugs with potentially nephrotoxic effects (as tenofovir disoproxil-fumarate or ritonavir-boosted protease-inhibitors), and intensifying the control of cardiovascular risk factors. Prospective studies are needed to assess whether it is possible to stabilize or reverse the mild decline of renal function in HIV-infected patients.
Our study has two limitations: first, currently most patients are being treated with tenofovir alafenamide, which can improve renal failure.50 We have not evaluated in our study the impact of occult chronic renal failure of the switching from tenofovir disoproxil-fumarate to tenofovir alafenamide. Second, in this study protease-inhibitors were boosted with ritonavir and now cobicistat is the booster agent. We do not know if cobicistat can also enhance tenofovir tubular toxicity as ritonavir does.50 Finally, due to the retrospective nature of the study, and due the fact that information was obtained from databases, ii should acknowledged that some information could have been missed (for example, minor information regarding hypertensive patients, once hypertension diagnosis was based on the use of antihypertensive medication).
Conclusion
This study found a 25% prevalence of already established renal impairment, albeit in the initial stages, among stable, ambulatory patients with well-controlled HIV-infection. Older subjects and female patients are the most susceptible population. Modifiable risk factors, such as hypertension and dyslipidemia, and exposure to potentially nephrotoxic antiretrovirals, such as tenofovir disoproxil-fumarate and ritonavir-boosted protease-inhibitors, were also associated with this outcome. It remains to be determined whether early interventions including antiretroviral therapy switch (tenofovir alafenamide, cobicistat) or improving comorbidities management will improve the course of mild chronic kidney disease.
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Szczech L.A., Gupta S.K., Habash R., et al. The clinical epidemiology and course of the spectrum of renal diseases associated with HIV infection. Kidney Int. 2004;66:1145–1152. doi: 10.1111/j.1523-1755.2004.00865.x. [DOI] [PubMed] [Google Scholar]
- 2.Mallipattu S.K., Salem F., Wyatt C.M. The changing epidemiology of HIV-related chronic kidney disease in the era of antiretroviral therapy. Kidney Int. 2014;86:259–265. doi: 10.1038/ki.2014.44. [DOI] [PubMed] [Google Scholar]
- 3.Schouten J., Wit F.W., Stolte I.G., et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV cohort study. Clin Infect Dis. 2014;59:1787–1797. doi: 10.1093/cid/ciu701. [DOI] [PubMed] [Google Scholar]
- 4.Althoff K.N., McGinnis K.A., Wyatt C.M., et al. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin Infect Dis. 2015;60:627–638. doi: 10.1093/cid/ciu869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kooij K.W., Vogt L., Wit F.W.N.M., et al. Higher prevalence and faster progression of chronic kidney disease in human immunodeficiency virus-infected middle-aged individuals compared with human immunodeficiency virus-uninfected controls. J Infect Dis. 2017;216:622–631. doi: 10.1093/infdis/jix202. [DOI] [PubMed] [Google Scholar]
- 6.Mocroft A., Kirk O., Reiss P., et al. Estimated glomerular filtration rate, chronic kidney disease and antiretroviral drug use in HIV-positive patients. AIDS. 2010;24:1667–1678. doi: 10.1097/QAD.0b013e328339fe53. [DOI] [PubMed] [Google Scholar]
- 7.Jotwani V., Li Y., Grunfeld C., Choi A.I., Shlipak M.G. Risk factors for ESRD in HIV-infected individuals: traditional and HIV-related factors. Am J Kidney Dis. 2012;59:628–635. doi: 10.1053/j.ajkd.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George E., Lucas G.M., Nadkarni G.N., Fine D.M., Moore R., Atta M.G. Kidney function and the risk of cardiovascular events in HIV-1-infected patients. AIDS. 2010;24:387–394. doi: 10.1097/QAD.0b013e3283359253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bandera A., Gori A., Sabbatini F., et al. Evaluation of the prognostic value of impaired renal function on clinical progression in a large cohort of HIV-infected people seen for care in Italy. PLOS ONE. 2015;10:e0124252. doi: 10.1371/journal.pone.0124252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Honeycutt A.A., Segel J.E., Zhuo X., Hoerger T.J., Imai K., Williams D. Medical costs of CKD in the Medicare population. J Am Soc Nephrol. 2013;24:1478–1483. doi: 10.1681/ASN.2012040392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryom L., Mocroft A., Lundgren J. HIV therapies and the kidney: some good, some not so good? Curr HIV/AIDS Rep. 2012;9:111–120. doi: 10.1007/s11904-012-0110-3. [DOI] [PubMed] [Google Scholar]
- 12.Mocroft A., Lundgren J.D., Ross M., et al. Cumulative and current exposure to potentially nephrotoxic antiretrovirals and development of chronic kidney disease in HIV-positive individuals with a normal baseline estimated glomerular filtration rate: a prospective international cohort study. Lancet HIV. 2016;3:e23–e32. doi: 10.1016/S2352-3018(15)00211-8. [DOI] [PubMed] [Google Scholar]
- 13.Ryom L., Mocroft A., Kirk O., et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis. 2013;207:1359–1369. doi: 10.1093/infdis/jit043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flandre P., Pugliese P., Cuzin L., et al. Risk factors of chronic kidney disease in HIV-infected patients. Clin J Am Soc Nephrol. 2011;6:1700–1707. doi: 10.2215/CJN.09191010. [DOI] [PubMed] [Google Scholar]
- 15.Campbell L.J., Ibrahim F., Fisher M., Holt S.G., Hendry B.M., Post F.A. Spectrum of chronic kidney disease in HIV-infected patients. HIV Med. 2009;10:329–336. doi: 10.1111/j.1468-1293.2008.00691.x. [DOI] [PubMed] [Google Scholar]
- 16.Scherzer R., Gandhi M., Estrella M.M., et al. A chronic kidney disease risk score to determine tenofovir safety in a prospective cohort of HIV-positive male veterans. AIDS. 2014;28:1289–1295. doi: 10.1097/QAD.0000000000000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menezes AM1, Torelly J., Jr., Real L., Bay M., Poeta J., Sprinz E. Prevalence and risk factors associated to chronic kidney disease in HIV-infected patients on HAART and undetectable viral load in Brazil. PLoS ONE. 2011;6:e26042. doi: 10.1371/journal.pone.0026042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Juega-Mariño J., Bonjoch A., Pérez-Alvarez N., et al. Prevalence, evolution, and related risk factors of kidney disease among Spanish HIV-infected individuals. Medicine (Baltimore) 2017;96:e7421. doi: 10.1097/MD.0000000000007421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orlando L.A., Belasco E.J., Patel U.D., Matchar D.B. The chronic kidney disease model: a general purpose model of disease progression and treatment. BMC Med Inform Decis Mak. 2011;11:41. doi: 10.1186/1472-6947-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cristelli M.P., Cofán F., Rico N., et al. Estimation of renal function by CKD-EPI versus MDRD in a cohort of HIV-infected patients: a cross-sectional analysis. BMC Nephrol. 2017;18:58. doi: 10.1186/s12882-017-0470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levey A.S., Stevens L.A., Schmid C.H., et al. CKD-EPI (chronic kidney disease epidemiology). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delanaye P., Mariat C., Maillard N., Krzesinski J.M., Cavalier E. Are the creatinine-based equations accurate to estimate glomerular filtration rate in African American populations? Clin J Am Soc Nephrol. 2011;6:906–912. doi: 10.2215/CJN.10931210. [DOI] [PubMed] [Google Scholar]
- 23.Gagneux-Brunon A., Delanaye P., Maillard N., et al. Performance of creatinine and cystatin C-based glomerular filtration rate estimating equations in a European HIV-positive cohort. AIDS. 2013;27:1573–1581. doi: 10.1097/QAD.0b013e32835fac30. [DOI] [PubMed] [Google Scholar]
- 24.Abraham A.G., Althoff K.N., Jing Y., et al. End-stage renal disease among HIV-infected adults in North America. Clin Infect Dis. 2015;60:941–949. doi: 10.1093/cid/ciu919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bickel M., Marben W., Betz C., et al. End-stage renal disease and dialysis in HIV-positive patients: observations from a long-term cohort study with a follow-up of 22 years. HIV Med. 2013;14:127–135. doi: 10.1111/j.1468-1293.2012.01045.x. [DOI] [PubMed] [Google Scholar]
- 26.Cheung J., Puhr R., Petoumenos K., et al. Chronic kidney disease in Australian HIV-infected patients: analysis of the Australian HIV Observational Database. Nephrology (Carlton) 2017 doi: 10.1111/nep.13100. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Limkunakul C., Srinithiwat P., Lochinda B., Sawanyawisuth K. Close monitoring of eGFR should be performed in HIV-infected patients aged over 37 years. Jpn J Infect Dis. 2017:656–659. doi: 10.7883/yoken.JJID.2016.370. [DOI] [PubMed] [Google Scholar]
- 28.Calza L., Vanino E., Magistrelli E., et al. Prevalence of renal disease within an urban HIV-infected cohort in northern Italy. Clin Exp Nephrol. 2014;18:104–112. doi: 10.1007/s10157-013-0817-5. [DOI] [PubMed] [Google Scholar]
- 29.Overton E.T., Nurutdinova D., Freeman J., Seyfried W., Mondy K.E. Factors associated with renal dysfunction within an urban HIV-infected cohort in the era of highly active antiretroviral therapy. HIV Med. 2009;10:343–350. doi: 10.1111/j.1468-1293.2009.00693.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasch M.G., Engsig F.N., Feldt-Rasmussen B., et al. Renal function and incidence of chronic kidney disease in HIV patients: a Danish cohort study. Scand J Infect Dis. 2012;44:689–696. doi: 10.3109/00365548.2012.673730. [DOI] [PubMed] [Google Scholar]
- 31.Mocroft A., Kirk O., Gatell J., et al. Chronic renal failure among HIV-1-infected patients. AIDS. 2007;21:1119–1127. doi: 10.1097/QAD.0b013e3280f774ee. [DOI] [PubMed] [Google Scholar]
- 32.Sorlí M.L., Guelar A., Montero M., González A., Rodriguez E., Knobel H. Chronic kidney disease prevalence and risk factors among HIV-infected patients. J Acquir Immune Defic Syndr. 2008;48:506–508. doi: 10.1097/QAI.0b013e31817bbecb. [DOI] [PubMed] [Google Scholar]
- 33.Lucas G.M., Lau B., Atta M.G., Fine D.M., Keruly J., Moore R.D. Chronic kidney disease incidence, and progression to end-stage renal disease, in HIV-infected individuals: a tale of two races. J Infect Dis. 2008;197:1548–1557. doi: 10.1086/587994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mocroft A., Lundgren J.D., Ross M., et al. Development and validation of a risk score for chronic kidney disease in HIV infection using prospective cohort data from the D:A:D study. PLoS Med. 2015;12:e1001809. doi: 10.1371/journal.pmed.1001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer J.P., Womack J.A., Gibson B. Beyond the Pap smear: gender-responsive HIV care for women. Yale J Biol Med. 2016;89:193–203. [PMC free article] [PubMed] [Google Scholar]
- 36.Loutfy M.R., Sherr L., Sonnenberg-Schwan U., et al. Women for positive. Caring for women living with HIV: gaps in the evidence. J Int AIDS Soc. 2013;16:18509. doi: 10.7448/IAS.16.1.18509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention . 2017. HIV among people aged 50 and over. https://www.cdc.gov/hiv/group/age/olderamericans/index.html [cited: 02.07.17] [Google Scholar]
- 38.Lazarus J.V., Nielsen K.K. HIV and people over 50 years old in Europe. HIV Med. 2010;11:479–481. doi: 10.1111/j.1468-1293.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- 39.Deeks S.G., Lewin S.R., Havlir D.V. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382:1525–1533. doi: 10.1016/S0140-6736(13)61809-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Van Epps P., Kalayjian R.C. Human immunodeficiency virus and aging in the era of effective antiretroviral therapy. Infect Dis Clin N Am. 2017;31:791–810. doi: 10.1016/j.idc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 41.Wyatt C.M., Malvestutto C., Coca S.G., Klotman P.E., Parikh C.R. The impact of hepatitis C virus coinfection on HIV-related kidney disease: a systematic review and meta-analysis. AIDS. 2008;22:1799–1807. doi: 10.1097/QAD.0b013e32830e0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fabrizi F., Dixit V., Martin P., Messa P. Hepatitis C virus increases the risk of kidney disease among HIV-positive patients: systematic review and meta-analysis. J Med Virol. 2016;88:487–497. doi: 10.1002/jmv.24353. [DOI] [PubMed] [Google Scholar]
- 43.Bedimo R., Abodunde O. Metabolic and cardiovascular complications in HIV/HCV-co-infected patients. Curr HIV/AIDS Rep. 2016;13:328–339. doi: 10.1007/s11904-016-0333-9. [DOI] [PubMed] [Google Scholar]
- 44.Kupin W.L. Viral-associated GN: hepatitis C and HIV. Clin J Am Soc Nephrol. 2017;12:1337–1342. doi: 10.2215/CJN.04320416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ryom L., Mocroft A., Lundgren J.D. Antiretroviral therapy, immune suppression and renal impairment in HIV-positive persons. Curr Opin HIV AIDS. 2014;9:41–47. doi: 10.1097/COH.0000000000000023. [DOI] [PubMed] [Google Scholar]
- 46.Scherzer R., Estrella M., Li Y., et al. Association of tenofovir exposure with kidney disease risk in HIV infection. AIDS. 2012;26:867–875. doi: 10.1097/QAD.0b013e328351f68f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jafari A., Khalili H., Dashti-Khavidaki S. Tenofovir-induced nephrotoxicity: incidence, mechanism, risk factors, prognosis and proposed agents for prevention. Eur J Clin Pharmacol. 2014;70:1029–1040. doi: 10.1007/s00228-014-1712-z. [DOI] [PubMed] [Google Scholar]
- 48.Jotwani V., Atta M.G., Estrella M.M. Kidney disease in HIV: moving beyond HIV-associated nephropathy. J Am Soc Nephrol. 2017;28:3142–3154. doi: 10.1681/ASN.2017040468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Izzedine H., Lescure F.X., Bonnet F. HIV medication-based urolithiasis. Clin Kidney J. 2014;7:121–126. doi: 10.1093/ckj/sfu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mills A., Arribas J.R., Andrade-Villanueva J., et al. Switching from tenofovir disoproxil fumarate to tenofovir alafenamide in antiretroviral regimens for virologically suppressed adults with HIV-1 infection: a randomised, active-controlled, multicentre, open-label, phase 3, non-inferiority study. Lancet Infect Dis. 2016;16:43–52. doi: 10.1016/S1473-3099(15)00348-5. [DOI] [PubMed] [Google Scholar]