Abstract
The porin composition of the Escherichia coli cell envelope was analyzed during growth at different external pHs (pHo) as a function of the acetyl phosphate (AcP) level (ΔackA pta or ackA mutant, pyruvate or glucose as the carbon source) in the presence or absence of EnvZ. Our results indicate that the AcP level is influenced by the pHo, leading to modulation of the amount of OmpR-P and subsequent pHo-dependent expression of ompF and ompC. We also propose the existence of a specific signal, independent of EnvZ and AcP, leading to OmpR phosphorylation in response to pyruvate.
The outer membrane of Escherichia coli contains two porin proteins, OmpF and OmpC, that control the permeability of small hydrophilic molecules across the outer membrane. The total amount of OmpF and OmpC proteins is fairly constant, but their relative level varies with environmental factors, including osmolarity and external pH (pHo) (14, 15). Medium with high osmolarity or low pH favors the synthesis of OmpC, and medium with low osmolarity (LO) or high pH increases the OmpF level and reduces the OmpC level (6, 7, 19, 20). Osmoregulation of the ompF and ompC genes is mediated at the transcriptional level by the EnvZ-OmpR two-component regulatory system (2). EnvZ is the osmosensor able to sense changes in external osmolarity. It undergoes autophosphorylation at His 274 and transfers the phosphate group to Asp55 of OmpR. EnvZ also acts as an OmpR phosphate (OmpR-P) phosphatase (8). OmpR-P is a transcriptional effector of both porin genes (14). The level of OmpR-P relies on the ratio of kinase to phosphatase EnvZ activity. In vivo, the level of OmpR-P increases as osmolarity is raised (3, 16). The current genetic model for porin regulation predicts that a low level of OmpR-P stimulates the transcription of the ompF gene through binding to a high-affinity site and a high level of OmpR-P represses ompF through binding to a low-affinity site (5). ompC transcription is stimulated by OmpR-P through the binding to a low-affinity site (14). The high degree of homology between sensors and regulator proteins of two-component regulatory systems favors the possibility of phosphorylation of a regulator protein by a noncognate histidine kinase but also by low-molecular-weight phosphodonor molecules (21). Such cross-regulation was demonstrated to occur in vivo between CreC and PhoB (in the absence of PhoR), EnvZ and PhoB (in the absence of OmpR), and PhoB and acetyl phosphate (AcP) (in the absence of PhoR and CreC) (10, 23). EnvZ-independent mechanisms can also lead to OmpR phosphorylation and to osmolarity-dependent ompF expression in an envZ null mutant (3). In vitro, OmpR was demonstrated to be phosphorylated by the noncognate histidine kinase CheA (9) and by low-molecular-weight phosphate donors such as AcP (5). In vivo experiments show that ompF transcription is dependent upon AcP synthesis only when EnvZ is absent (8, 11; S.-K. Kim and B. Wanner, personal communication). However, in the presence of EnvZ, the OmpC level has been demonstrated to increase as AcP accumulates (12, 18). All of these results suggest that cross-regulation would occur in vivo between OmpR and other kinase-independent, AcP-dependent mechanisms.
The role of EnvZ and OmpR-P in pHo regulation of porin expression has not yet been elucidated. Preliminary results suggest a role for EnvZ in ompF and ompC pHo regulation (19). However, in the absence of EnvZ, ompF expression is still pHo dependent, being higher during growth at low pHo than during growth at high pHo (6).
In this study, ompF and ompC transcription was analyzed under slightly acidic (pHo 6) or alkaline (pHo 7.8) growth conditions with glucose or pyruvate as the carbon source and in the presence of a pta or ackA mutation.
Bacterial strains, media, and growth conditions.
All of the strains used are derivatives of E. coli K-12 and are listed in Table 1. P1 vir lysates and transduction experiments were performed as previously described by Miller (13). Selection and identification are indicated in Table 1.
TABLE 1.
E. coli strains used in this study
| Strain | Genotypea | Source or referenceb |
|---|---|---|
| BW13711 | ΔlacX74 | B. Wanner, Purdue University |
| BW16463 | ΔlacX74 Δ(ackA-pta-hisQ-hisP) zej-223::Tn10 | B. Wanner, Purdue University |
| BW16545 | ΔlacX74 ackA200 zej-223::Tn10 | B. Wanner, Purdue University |
| Gal5 | HfrP4X thi metB lacI spoT1 relA1 | This laboratory |
| GPH8252 | BW13711 Φ(ompF′-lacZ+)7.14 | P1/MA2946 × BW13711 (ColAr Lac+) |
| GPH8255 | GPH8252 Δ(ackA-pta-hisQ-hisP) zej-223::Tn10 | P1/BW16463 × GPH8252 (Tetr Ac+/−) |
| GPH8257 | GPH8252 ackA200 zej-223::Tn10 | P1/BW16545 × GPH8252 (Tetr Ac+/−) |
| GPH8259 | BW13711 Φ(ompC′-lacZ+)10.21 | P1/MA2948 × BW13711 (Lac+) |
| GPH8262 | SG477 malT54::Tn10 | P/TST3 × SG477 (Tetr MeIr) |
| GPH8264 | GPH8252 malT54::Tn10 envZ22(Am) | P1/GPH8262 × GPH8252 (Tetr MeIr) |
| GPH8268 | GPH8252 envZ22(Am) | P1/Gal5 × GPH8264 (Mal+ MeIr) |
| GPH8273 | GPH8268 Δ(ackA-pta-hisQ-hisP) zej-223::Tn10 | P1/BW16463 × GPH8268 (Tetr Ac+/−) |
| GPH8274 | GPH8268 ackA200 zej-223::Tn10 | P1/BW16545 × GPH8268 (Tetr Ac+/−) |
| GPH8277 | GPH8259 envZ22(Am) malT54::Tn10 | P1/GPH8262 × GPH8259 (Tetr Lac−) |
| GPH8280 | GPH8259 ackA200 zej-223::Tn10 | P1/BW16545 × GPH8259 (Tetr Ac−) |
| GPH8282 | GPH8259 Δ(ackA-pta-hisQ-hisP) zej-223::Tn10 | P1/BW16463 × GPH8259 (Tetr Ac−) |
| GPH8285 | GPH8259 envZ22(Am) | P1/Gal5 × GPH8277 (Mal+ Lac−) |
| GPH8291 | GPH8285 ackA200 zej-223::Tn10 | P1/BW16545 × GPH8285 (Tetr Ac−) |
| GPH8293 | GPH8285 Δ(ackA-pta-hisQ-hisP) zej-223::Tn10 | P1/BW16463 × GPH8285 (Tetr Ac−) |
| JC2296 | Hfr P4X thi metB1 relA1 spoT1 Δ(lac)U169 | This laboratory |
| MA2946 | JC2296 Φ(ompF′-lacZ+)7.14 | 6 |
| MA2948 | JC2296 Φ(ompC′-lacZ+)10.21 | 6 |
| MC4100 | F− Δ(lac)U169 araD139 rpsL relA thiA flbB | M. Casadaban, University of Chicago |
| SG477 | MC4100 envZ22(Am) | 4 |
| TST3 | F−araD139Δ(argF-lac)205 flbB5301 pstF25 relA1 rpsL150 malT54::Tn10 deoC1 | T. J. Silhavy, Princeton University |
Genetic nomenclature is from Berlyn (1).
P1/A × B indicates that a P1 lysate propagated on strain A was used to transduce strain B. Transductants were selected for the first phenotype in parentheses and analyzed for the second one. Tetr, ColAr, and MeIr indicate resistance to tetracycline at 15 μg/ml−1, colicin A, and bacteriophage MeI, respectively. Lac+ or Lac−, Mal+, and Ac+/−, or Ac− indicate growth or no growth with 0.2% lactose as the carbon source, growth with 0.2% maltose, and slow or no growth with 0.4% potassium acetate, respectively.
LO minimal medium was used because, as previously shown by Thomas and Booth (19), we observed that ompF transcription depends on pHo only during growth in LO minimal medium. The LO minimal medium used in this study is a derivative of both the S medium described by Thomas and Booth (19) and the A medium described by Miller (13). It contained 49.2 mM KH2PO4-K2HPO4, 1 mM trisodium citrate, 0.4 mM MgSO4, 7.6 mM (NH4)2SO4, and 3 μM thiamine hydrochloride. It was adjusted to pH 6 or 7.8 by mixing appropriate volumes of phosphate solutions (43 mM KH2PO4 and 6.2 mM K2HPO4 for pH 6 and 4.2 mM KH2PO4 and 45 mM K2HPO4 for pH 7.8). At pH 6, 40 mM NaCl was added to compensate for the change in medium osmolarity caused by the different balance of potassium and phosphate ions. Identical results were obtained whether 40 mM NaCl was added or not in the medium. Minimal media were supplemented with 0.04% glucose or 0.1% pyruvate, which was the carbon source. These sugar concentrations allow identical growth conditions with a doubling time of about 2 h and a concentration of about 5 × 108 cells ml−1 in stationary growth phase. Overnight subcultures, grown in LB broth (13) at 30°C, were used to inoculate LO medium adjusted to different pH values. These cultures were incubated at 30°C until stationary phase was reached. At this point, the different buffered cultures were diluted with the same fresh medium to an optical density at 600 nm of 0.02. These new cultures were aerated and incubated at 30°C until they reached an optical density at 600 nm of 0.2 to 0.3. Until this cell density was reached, the pH of the growth medium remained constant.
Effect of carbon source on ompF and ompC pHo regulation.
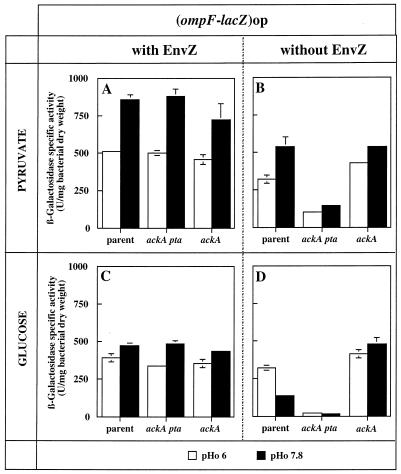
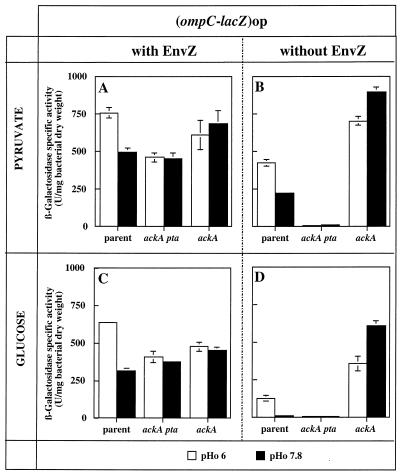
Strains GPH8252 and GPH8259, carrying the ompF-lacZ or the ompC-lacZ operon fusion, respectively, were grown in LO minimal medium adjusted to pH 6 or 7.8 with pyruvate or glucose as the carbon source. β-Galactosidase expression in toluene-treated cells was assayed (Fig. 1A and C and 2A and C; parent strains) as described by Miller (13). One unit of enzyme activity was defined as the amount of enzyme which hydrolyzed 1 nmol of substrate per min with 3 mM o-nitrophenyl-β-d-galactopyranoside as the substrate. In the parent EnvZ+ strain, with pyruvate as the carbon source, ompF transcription was induced 1.7-fold during growth at pHo 7.8 compared with growth at pHo 6 (Fig. 1A, parent strain) but this induction was not apparent with glucose (Fig. 1C, parent strain). Figure 2A and C show that in the parent EnvZ+ strain with pyruvate or glucose, ompC was induced 1.5- and 2-fold, respectively, during growth at pHo 6 compared with growth at pHo 7.8. ompC induction during growth at pHo 6 with glucose or pyruvate indicates that the OmpR-P level would be higher at pHo 6 than at pHo 7.8. Identical levels of ompF expression at both pHos in glucose are not inconsistent with different OmpR-P levels. We assume that at pHo 6, the OmpR-P level would lead to ompF repression and at pHo 7.8, a lower OmpR-P level would lead to ompF activation, resulting in the same ompF expression at both pHos.
FIG. 1.
Expression of ompF at pHos 6 and 7.8 in parental and mutant (ackA pta or ackA) strains in the presence or absence of EnvZ. Strains were grown in LO medium with pyruvate or glucose as the carbon source. Panels: A and C, GPH8252 parent strain, GPH8255 Δ(ackA pta) strain, and GPH8257 ackA200 strain; B and D, GPH8268 parent strain, GPH8273 Δ(ackA pta) strain, and GPH8274 ackA200 strain. The data are mean values ± standard deviations from three independent experiments.
FIG. 2.
Expression of ompC at pHos 6 and 7.8 in parental and mutant (ackA pta or ackA) strains in the presence or absence of EnvZ. Strains were grown in LO medium with pyruvate or glucose as the carbon source. Panels: A and C, GPH8259 parent strain, GPH8282 Δ(ackA pta) strain, and GPH8280 ackA200 strain; B and D, GPH8285 parent strain, GPH8293 Δ(ackA pta) strain, and GPH8291 ackA200 strain. The data are mean values ± standard deviations from two to four independent experiments.
In the absence of EnvZ, with pyruvate, ompF expression (strain GPH8268) was slightly decreased but still induced at pHo 7.8, as in the EnvZ+ strain (compare Fig. 1B to A, parent strains). With glucose, the absence of EnvZ led to a decrease in ompF expression mainly at pHo 7.8. Consequently, ompF expression became higher at pHo 6 than at pHo 7.8 (compare Fig. 1D to C, parent strains). These results indicate that in the absence of EnvZ, ompF expression is still influenced by the pHo and the carbon source. With pyruvate or glucose, ompC was still expressed, albeit at a lower level than in the EnvZ+ strain (compare Fig. 2B to A and D to C, parent strains). This residual expression was more important in pyruvate than in glucose and was pHo dependent. These data show that in the absence of EnvZ, ompC expression is also influenced by both the pHo and the carbon source. So, without EnvZ, OmpR would be phosphorylated by an EnvZ-independent mechanism and OmpR-P would accumulate because of the lack of EnvZ phosphatase activity. ompC expression levels indicate that the OmpR-P level would be higher at low than at high pHo and in pyruvate than in glucose. In pyruvate, OmpR phosphorylation would allow OmpR-P levels high enough to repress ompF and strongly activate ompC. In glucose, compared to pyruvate, OmpR phosphorylation would be weaker and the OmpR-P accumulation would allow ompF activation and lower ompC activation.
From our results collected with and without EnvZ, we can deduce that both EnvZ activities would not be responsible for the pHo regulation and the carbon source-dependent activation of porin genes. In addition to the kinase activity of EnvZ, OmpR phosphorylation would require pHo- and carbon source-dependent mechanisms.
Role of noncognate histidine kinase in ompF pHo regulation.
In an EnvZ-deficient strain, ompF transcription remained pHo dependent (Fig. 1B and D, parent strains). Since CheA was demonstrated to phosphorylate OmpR in vitro (9), we investigated the physiological relevance of cross-regulation between noncognate kinases (PhoR, CheA, and CreC) and OmpR when EnvZ is absent and the role that this cross-regulation may play in the pHo regulation of ompF. The β-galactosidase activities of isogenic phoR20, Δ(cheA-cheZ), and creB creC derivatives of an envZ22 strain, carrying an ompF-lacZ operon fusion, were assayed during growth in LB broth adjusted to different pHs and compared with the activities in the envZ22 mutant. ompF expression was found not to be significantly modified in the presence of these mutations (data not shown). These results indicate that in an EnvZ-deficient strain, in vivo cross-regulation between noncognate histidine kinases (PhoR, CheA, and CreC) and OmpR does not play any physiological role in ompF pHo-dependent transcription.
Role of AcP in ompF and ompC pHo regulation.
AcP is synthesized from acetyl coenzyme A and Pi with the release of free coenzyme A by phosphotransacetylase. AcP and ADP are then converted to acetate and ATP by acetate kinase. An ackA mutant is expected to accumulate AcP because its breakdown is blocked. An ackA pta mutant is expected to display a low AcP level because its synthesis no longer occurs and the phosphotransacetylase-acetate kinase pathway cannot be inverted (10). Use of pyruvate as a carbon source was described to lead to a fourfold higher AcP level than the use of glucose during the exponential growth phase (12). To determine the role of AcP in OmpR activation, we analyzed the expression of ompF-lacZ and ompC-lacZ operon fusions with glucose or pyruvate as the carbon source and in the presence of an ackA pta or ackA mutation. β-Galactosidase activities were assayed during the growth of isogenic strains (Table 1) in LO minimal medium adjusted to pH 6 or 7.8 (Fig. 1A and C and 2A and C).
In the presence of EnvZ, with a low AcP level, and in pyruvate or glucose, ompC was not significantly induced at pHo 6, leading to pHo-independent expression (Fig. 2A and C, ackA pta strain). This result indicates that in the presence of EnvZ, AcP would contribute to OmpR phosphorylation during growth at pHo 6. Without this contribution, OmpR-P levels would be the same at both pHos. Unexpectedly, the 1.7-fold induction of ompF during growth with pyruvate at pHo 7.8 compared to pHo 6 was not significantly modified when the AcP level was decreased (Fig. 1A, ackA pta strain). When AcP was high, whatever the carbon source, ompC expression was almost the same at both pHos, meaning that OmpR-P levels were the same at both pHos (Fig. 2A and C, ackA strain). ompF expression was not modified, whatever the pHo, by a high AcP level (Fig. 1A and C, compare parent and ackA strains).
We next tested if OmpR activation requires AcP in the absence of EnvZ. We assayed β-galactosidase activities of isogenic envZ22, envZ22 ackA pta, and envZ22 ackA derivatives carrying an ompF-lacZ or ompC-lacZ operon fusion (Table 1) during growth in LO medium adjusted to pHo 6 or 7.8 and supplemented with pyruvate or glucose as the carbon source (Fig. 1B and D and 2B and D). In the absence of EnvZ and AcP, ompF was no longer expressed in glucose (Fig. 1D, ackA pta strain) but was still expressed in pyruvate although at a lower level than in the presence of AcP (Fig. 1B, compare ackA pta to parent strains). This expression was slightly pHo dependent. These results indicate that in the absence of EnvZ, ompF transcription is controlled by an AcP-dependent mechanism. Moreover, ompF residual expression during growth in pyruvate evidences apparently pyruvate-dependent activation of ompF (Fig. 1B, ackA pta strain). This mechanism, independently of EnvZ and AcP, would allow OmpR phosphorylation only during growth in pyruvate. Pyruvate-dependent activation of the phosphate regulon was previously reported by Wanner and Wilmes-Riesenberg (22). In the absence of the Pi sensor (PhoR) and its homolog CreC, pyruvate led to the activation of alkaline phosphatase synthesis. When the AcP level was high, ompF expression increased up to the parental level and became pHo independent (Fig. 1B and D, ackA strain). In this strain, lacking EnvZ phosphatase activity, the OmpR-P formed by the AcP-dependent mechanism accumulates to a level corresponding to ompF activation (8).
In the absence of EnvZ and AcP, ompC was not expressed (Fig. 2B and D, ackA pta strain). This result indicates that whatever the carbon source, AcP is responsible for ompC expression when EnvZ is absent. We assume that the pyruvate-dependent OmpR phosphorylation evidenced with ompF would yield an OmpR-P level too low to activate ompC. With an increased AcP level, ompC expression was increased, with slightly higher expression at pHo 7.8 than at pHo 6 (Fig. 2B and D, ackA strain). These results correlate well with the resistance of the envZ22 ackA pta derivative (strain GPH8273) and the sensitivity of the envZ22 ackA derivative (strain GPH8274) to bacteriophage MeI, which uses OmpC as a receptor.
Unexpectedly, growth of an EnvZ-deficient strain with pyruvate and a high level of AcP was associated with high expression of both the ompF and ompC genes (Fig. 1B and 2B, ackA strains). Such a phenotype was previously described by McCleary and Stock (12) for an envZ+ pta strain during growth with sodium acetate and by Russo et al. (17) for a mutant with an OmpR protein unable to repress ompF.
In the absence of EnvZ, pHo-dependent expression of ompF and ompC was clearly demonstrated to depend upon the AcP amount, which would be higher at pHo 6 than at pHo 7.8. To obtain direct evidence of pHo modulation of the AcP level, we tried to directly measure AcP by enzymatic methods during growth at different pHos but the concentration of this compound in cells proved to be too low under our conditions to be quantitatively assayed.
Conclusion.
In the absence of EnvZ, our results show that ompF and ompC expression is influenced by pHo and AcP. A higher OmpR-P level at low pHo than at high pHo might be responsible for this regulation. We propose that with or without EnvZ, the AcP amount is influenced by pHo, leading to modulation of the OmpR-P level and, consequently, pHo-dependent expression of ompF and ompC. According to our results obtained with and without EnvZ, both EnvZ activities are not involved in pHo regulation of the porin regulon. We do not know whether OmpR is phosphorylated directly by AcP or indirectly through a noncognate kinase, as suggested by Kim et al. (10). AcP was also shown to be responsible for ompF and ompC osmoregulation in the absence of EnvZ (11; Kim and Wanner, personal communication). Our study also suggests that the OmpR-P level is higher during growth in pyruvate than during growth in glucose, when EnvZ is absent. Indeed, under our assay conditions, ompF and ompC expression depends on the nature of the carbon source. Similar results were described by Kim et al. (10). The link between the carbon source and the expression of ompF and ompC might simply be explained by the modulation of the AcP level as a function of the carbon source, this hypothesis cannot account for all of our results. Indeed, ompF residual expression, in the absence of AcP and EnvZ, was still higher in pyruvate than in glucose (compare Fig. 1B to D, ackA pta strain), so it may be that a specific signal leads to OmpR phosphorylation in response to pyruvate. This pyruvate-dependent signal would not depend on EnvZ and AcP. Pyruvate-dependent activation of the phosphate (22) and porin regulons should involve different mechanisms. Indeed, AcP is required for pyruvate-dependent activation of the phosphate regulon but not for pyruvate-dependent activation of the porin regulon. This regulon constitutes another two-component regulatory system affected by a control linked to the use of pyruvate as a carbon source. This control might be of more general interest.
Acknowledgments
We thank S.-K. Kim and B. Wanner for unpublished information; M. Berlyn, S. Garrett, D. E. Koshland, and B. Wanner for generous gift of strains; and B. Dequatre for excellent technical assistance.
This work was supported by grants from the Centre National de la Recherche Scientifique (UMR5577) and the University Claude Bernard Lyon I.
REFERENCES
- 1.Berlyn M K. Linkage map of Escherichia coli K-12, edition 10: the traditional map. Microbiol Mol Biol Rev. 1998;62:814–984. doi: 10.1128/mmbr.62.3.814-984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forst S, Roberts D. Signal transduction by the EnvZ-OmpR phosphotransfer system in bacteria. Res Microbiol. 1994;145:363–373. doi: 10.1016/0923-2508(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 3.Forst S, Delgado J, Rampersaud A, Inouye M. In vivo phosphorylation of OmpR, the transcription activator of the ompF and ompC genes in Escherichia coli. J Bacteriol. 1990;172:3473–3477. doi: 10.1128/jb.172.6.3473-3477.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garrett S, Taylor R K, Silhavy T J. Isolation and characterization of chain-terminating nonsense mutation in a porin regulator gene, envZ. J Bacteriol. 1983;156:62–69. doi: 10.1128/jb.156.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Head C G, Tardy A, Kenney L J. Relative binding affinities of OmpR and OmpR-phosphate at the ompF and ompC regulatory sites. J Mol Biol. 1998;281:857–870. doi: 10.1006/jmbi.1998.1985. [DOI] [PubMed] [Google Scholar]
- 6.Heyde M, Portalier R. Regulation of major outer membrane porin proteins of Escherichia coli K 12 by pH. Mol Gen Genet. 1987;208:511–517. doi: 10.1007/BF00328148. [DOI] [PubMed] [Google Scholar]
- 7.Heyde M, Lazzaroni J-C, Magnouloux-Blanc B, Portalier R. Regulation of porin gene expression over a wide range of extracellular pH in Escherichia coli K-12: influence of a tolA mutation. FEMS Microbiol Lett. 1988;52:59–66. [Google Scholar]
- 8.Hsing W, Silhavy T J. Function of conserved histidine-243 in phosphatase activity of EnvZ, the sensor for porin osmoregulation in Escherichia coli. J Bacteriol. 1997;179:3729–3735. doi: 10.1128/jb.179.11.3729-3735.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Igo M M, Ninfa A J, Stock J B, Silhavy T J. Phosphorylation and dephosphorylation of a bacterial transcriptional activator by a transmembrane receptor. Genes Dev. 1989;3:1725–1734. doi: 10.1101/gad.3.11.1725. [DOI] [PubMed] [Google Scholar]
- 10.Kim S-K, Wilmes-Riesenberg M R, Wanner B L. Involvement of the sensor kinase EnvZ in the in vivo activation of the response-regulator PhoB by acetyl phosphate. Mol Microbiol. 1996;22:135–147. doi: 10.1111/j.1365-2958.1996.tb02663.x. [DOI] [PubMed] [Google Scholar]
- 11.Leonardo M R, Forst S. Re-examination of the role of the periplasmic domain of EnvZ in sensing of osmolarity signals in Escherichia coli. Mol Microbiol. 1996;22:405–413. doi: 10.1046/j.1365-2958.1996.1271487.x. [DOI] [PubMed] [Google Scholar]
- 12.McCleary W R, Stock J B. Acetyl phosphate and the activation of two-component response regulators. J Biol Chem. 1994;269:31567–31572. [PubMed] [Google Scholar]
- 13.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- 14.Pratt L A, Silhavy T J. Porin regulon of Escherichia coli. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: American Society for Microbiology; 1995. pp. 105–127. [Google Scholar]
- 15.Pratt L A, Hsing W, Gibson K E, Silhavy T J. From acids to osmZ: multiple factors influence synthesis of the OmpF and OmpC porins in Escherichia coli. Mol Microbiol. 1996;20:911–917. doi: 10.1111/j.1365-2958.1996.tb02532.x. [DOI] [PubMed] [Google Scholar]
- 16.Russo F D, Silhavy T J. EnvZ controls the concentration of phosphorylated OmpR to mediate osmoregulation of the porin genes. J Mol Biol. 1991;222:567–580. doi: 10.1016/0022-2836(91)90497-t. [DOI] [PubMed] [Google Scholar]
- 17.Russo F D, Slauch J M, Silhavy T J. Mutations that affect separate functions of OmpR the phosphorylated regulator of porin transcription in Escherichia coli. J Mol Biol. 1993;231:261–273. doi: 10.1006/jmbi.1993.1281. [DOI] [PubMed] [Google Scholar]
- 18.Shin S, Park C. Modulation of flagellar expression in Escherichia coli by acetyl phosphate and the osmoregulator OmpR. J Bacteriol. 1995;177:4696–4702. doi: 10.1128/jb.177.16.4696-4702.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas A D, Booth I R. The regulation of expression of the porin gene ompC by acid pH. J Gen Microbiol. 1992;138:1829–1835. doi: 10.1099/00221287-138-9-1829. [DOI] [PubMed] [Google Scholar]
- 20.van Alphen W, Lugtenberg B. Influence of osmolarity of the growth medium on the outer membrane protein pattern of Escherichia coli. J Bacteriol. 1977;131:623–630. doi: 10.1128/jb.131.2.623-630.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wanner B L. Is cross regulation by phosphorylation of two-component response regulator proteins important in bacteria? J Bacteriol. 1992;174:2053–2058. doi: 10.1128/jb.174.7.2053-2058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wanner B L, Wilmes-Riesenberg M R. Involvement of phosphotransacetylase, acetate kinase, and acetyl phosphate synthesis in control of the phosphate regulon in Escherichia coli. J Bacteriol. 1992;174:2124–2130. doi: 10.1128/jb.174.7.2124-2130.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanner B L. Signal transduction and cross regulation in the Escherichia coli phosphate regulon by PhoR, CreC, and acetyl phosphate. In: Hoch J A, Silhavy T J, editors. Two-component signal transduction. Washington, D.C.: American Society for Microbiology; 1995. pp. 203–221. [Google Scholar]