Abstract
Transfusion of HLA-specific antibodies may play a role in induction of TRALI, the transfusion complication responsible for most transfusion-related deaths. In Oslo, we screen our apheresis donors and defer HLA-immunized donors from donation of plasma-rich blood components. During the second year of the Covid-19 pandemic and following the first months of SARS-CoV-2 vaccination, both the virus itself and the vaccines were suspected of inducing de novo production of antibodies to HLA class I in patients. For the blood center, the possibility of finding HLA-antibodies in an increased number of blood donors has serious implications. We therefore conducted a study to map the extent of de novo HLA-specific antibodies in representative donor groups. 106 apheresis donors were screened for antibodies to HLA class I/II following Covid-19 or vaccination with either mRNA or adenovirus-vector vaccines, and the findings were compared to pre-Covid blood samples from the same donors. In addition, we analyzed pre-Covid samples from 11 HLA-antibody-positive donors of Covid convalescence plasma. Only three established thrombapheresis donors were deferred due to vaccine-induced HLA-antibodies. In short, our findings did not support the hypothesis that SARS-CoV-2 virus or vaccination cause de novo HLA immunization in healthy blood donors. However, some donors with pre-existing antibodies showed increased antibody expression, confirming a general boost of the immune response following infection or vaccination.
1. Background
Transfusion-related acute lung injury (TRALI) is a rare but serious transfusion complication, characterized by acute lung edema and hypoxia developing within 6 h following transfusion [1]. With its high mortality, this condition is a leading cause of transfusion-related deaths, and most likely underdiagnosed. In most cases, the pathophysiology includes an immunological component, with transduction of antibodies to human leucocyte antigens (HLA) as a precipitating factor in a predisposed patient [2]. The risk of TRALI is higher after transfusion of blood products containing significant amounts of plasma from single donors, e.g., thrombocytes from apheresis or fresh frozen plasma (FFP).
Following a fatal TRALI induced by a thrombocyte concentrate collected by apheresis in the Oslo Blood Center, we implemented screening for HLA antibodies in all thrombapheresis donors from 2015. The screening is routinely performed when whole-blood donors are recruited to thrombapheresis, and only repeated after pregnancy or transfusion. Identification of donors with antibodies to HLA class I/II leads to deferral from donation of products rich in plasma. However, we allow these donors to donate whole blood for production of RBC and plasma for fractionation.
Following the SARS-CoV-2 pandemic there were contradictory reports about an increased occurrence of HLA-specific antibodies in male donors of Covid-19 convalescent plasma (CCP) [3], [4]. Further, in patients with renal transplants, Covid-19 infection induced a broad activation of B-cell subgroups [5]. In a preliminary study of seven patients with renal transplants no HLA specific antibodies were seen following Covid-19 [6]. Vaccination may induce a strong immune response and lead to production of HLA-antibodies in patients with renal transplants [7], [8].
In Norway, the proportion of blood donors with antibodies to Covid-19 increased rapidly during the spring in 2021, partly due to vaccination [9]. It was of interest to investigate whether these donors produced HLA-specific antibodies that could influence the safety of blood product recipients by increasing their risk of TRALI. This could affect our donor population and necessitate increased priority and resource allocation for the recruitment of apheresis donors. We therefore conducted the present study, aiming to explore the frequency of HLA-specific antibodies in blood donors after SARS-CoV-2 vaccine or Covid-19 infection, as compared to the frequency of positive screening samples before Covid-19.
2. Materials and methods
2.1. We defined the criteria for inclusion of donors as follows
-
1.
Thrombocyte donors with previously negative screening results for HLA-specific antibodies, who returned to donate blood or thrombocytes following Covid-19 infection or SARS-CoV-2-vaccination with any of the three vaccines in use at that time.
-
2.
Established blood donors recruited to thrombapheresis in whom HLA-specific antibodies were discovered as part of ordinary screening, who had recently suffered from Covid-19 or been vaccinated against SARS-CoV-2. For these donors, we analyzed pre-pandemic samples to check whether the HLA-specific antibodies were present at their previous blood donation (“lookback” samples are frozen for 2 years in case of later need for repeated infection testing).
-
3.
Established or inactive blood donors who were potential Covid convalescent plasma (CCP) donors, were tested as described for group 2. We analyzed pre-pandemic blood samples if they had donated blood within the previous two-year period.
The study was approved by the Data Protection Officer in Oslo University Hospital. Donors were informed and signed a consent form to participate. Samples were collected together with routine samples for blood donation.
Screening for HLA-antibodies was performed with the Luminex bead assay (LABscreen, OneLambda/Thermo Fisher). All samples were analyzed with the LSM12 screening kit lot 023. The samples found to be positive (cut-off at NBG-ratio 9.9) were further investigated with Single antigen kits; LS1A04 (lot 012 or lot 013), LS2A01 (lot 014) or both. Beads with mean fluorescence intensity (MFI) above 1000 were considered positive in the single antigen assay and donors were deferred when the MFI was above 3000.
3. Results
A total of 106 apheresis donors were tested ( Table 1) between May and August 2021. We also included 11 donors of Covid-19 convalescence plasma with a positive anti-HLA screening.
Table 1.
Summary of the study groups.
| n | Gender (male/ female) | Mean age | Positive screening confirmed w/Luminex (male/female) | Neg scr Pre-Covid (deferred) | Same antibody pattern post/pre | |
|---|---|---|---|---|---|---|
| Moderna | 32 | 15/17 | 52 | 6 (2/4) | 2 | Yes |
| Pfizer | 26 | 10/16 | 50 | 1 (female) | 0 | |
| Astra-Zeneca | 40 | 7/33 | 33 | 5 (1/4) | 1a | Yes |
| Covid + vaccine(s) | 8 | 4/4 | 41 | 0 | ||
| CCP donors with pos. anti-HLA screening | 11 | 4/7 | 51 | 9b | 0 | Yes |
one new donor without pre-Covid sample was also deferred
two donors concluded as negative following specificity assay (Luminex), returned to donation
32 donors (mean age 52, 47% male,) were investigated following vaccination with the Moderna mRNA vaccine. Eight of them (five female, three male) had a positive screen. Six were confirmed with specificity assays, of whom four were also positive in the pre-Covid samples. Thus, two donors with previously negative screenings were deferred from thrombapheresis due to vaccination-induced anti-HLA antibodies above the cut-off.
Of the 26 donors (mean age 50, 38% male) vaccinated with the mRNA vaccine from Pfizer/BioNTech only two had positive screening tests (one male, one female). Specificity assays revealed that one of them was negative both before and after vaccination whereas the other had anti-HLA antibodies present in both pre- and post-vaccination samples, with the same specificity profile. Deferral of this donor was therefore not attributable to the vaccination.
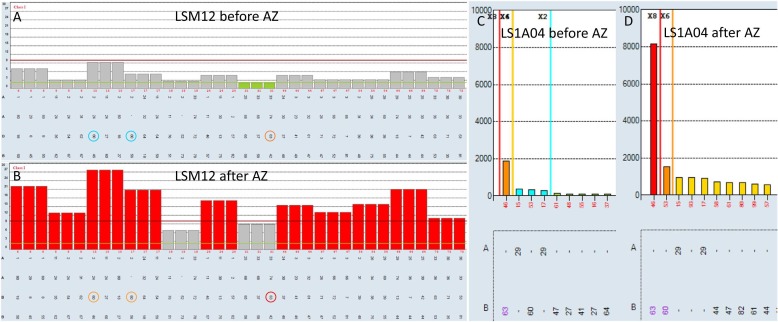
Of 40 donors (mean age 33, 18% male) vaccinated with the adenoviral vector vaccine from Astra-Zeneca, seven (six female, one male) had antibodies to HLA class I, II or both. Of these, five were confirmed in specificity assays. Three donors had positive screening also in the pre-Covid samples, one was a newly recruited donor and therefore had no pre-Covid sample available, and for the last donor the pre-Covid sample showed the same immunization pattern but weaker ( Fig. 1). The two latter donors were deferred from thrombapheresis.
Fig. 1.
Example showing increased antibody levels in samples from the same donor before and after Astra-Zeneca vaccination (AZ). A and B: Screening for anti-HLA class I. Cut-off on Y-axis set to NBG-ratio 9.9 (red line). Colored circles indicate the specificities of interest. C and D: Single antigen anti-HLA specificity analysis from same run. MFI reported on Y-axis.
We analyzed six Covid-convalescents who were vaccinated once and two donors who had received two different vaccines (mean age 41, 50% male). In this group, one donor (Covid followed by two doses of Pfizer/BioNTech vaccine) had a positive screening result indicating antibodies to HLA class II, but was negative in the specificity analysis.
In a last group of 11 donors (mean age 48, 36% male) who had positive screening following Covid-19 and an existing pre-Covid sample, the antibody patterns were similar before and after Covid. In two of them, the specificity analysis showed that the antibody levels were below the cut-off and they could return to donation of plasma-rich products.
4. Discussion
Altogether, 18 of 106 donors in this study had a positive screening for anti-HLA antibodies following vaccination or Covid-infection. Of these, 12 were confirmed by specificity analysis (Luminex Single Assay). For four (three established donors and one new) of the 12, the antibody level in the pre-Covid-sample was considered negative and they were deferred due to increased antibody levels following vaccination. The specificity analysis revealed that the same antibody profile could be identified before and after vaccine or infection in each of the 12 donors, but following immunization their antibody levels increased above the cut-off value. Thus, we could not show the occurrence of any de novo HLA-specific antibodies in this population of healthy blood donors, but a few cases in which the general immune activation had stimulated an increased production of antibody.
In a sample of 11 potential CCP donors deferred for anti-HLA antibodies, all had a positive pre-Covid screening. The hypothesis of SARS-CoV-2-infection as an inducer of anti-HLA antibodies with new specificities is therefore not supported by this study. Researchers from the Mayo clinic initially reported a high rate of HLA-antibodies among Covid19-convalescent plasma donors [3], but they were later unable to reproduce these findings in a larger population [4]. Although our study is limited by a small sample size, the access to pre-Covid plasma samples lend strength to the results.
Based on these findings, it seems unnecessary to investigate all donors following the pandemic and mass vaccination, and we can maintain our current routines for sampling of new apheresis donors only, without worrying about an increased number of antibody-induced TRALIs in patients in the near future. However, it is important to remember the difference between healthy blood donors and patients, particularly patients who already have alloantibodies and may be considered as “responders”. The current study does not exclude immunization events in these individuals.
Acknowledgements
Thanks to Karoline Furnes for valuable help with handling of samples and donors.
References
- 1.Steinsvåg T.T., Espinosa A. and Flesland Ø. Overvåking av blod i Norge 2012. Transfusjonskomplikasjoner. Rapport fra Hemovigilansgruppen ved Nasjonalt kunnskapssenter for helsetjenesten. 〈https://www.fhi.no/globalassets/dokumenterfiler/rapporter/2014/overvaking-av-blod-i-norge-2012〉 (summary in English).
- 2.Gupta A., Yan M. Transfusion-related acute lung injury (TRALI) | Professional Education (blood.ca) 〈https://professionaleducation.blood.ca/en/transfusion/publications/transfusion-related-acute-lung-injury-trali〉.
- 3.Juskewitch J.E., Stubbs J.R., Gandhi M.J. Elevated rate of HLA antibodies in male COVID-19 convalescent plasma donors: a risk factor for transfusion-related acute lung injury. Mayo Clin Proc. 2021;96(2):500–502. doi: 10.1016/j.mayocp.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juskewitch J.E., Senefeld J.W., Johnson P.W., Mills J.R., Joyner M.J., Gandhi M.J. HLA antibody rates are not increased in a regional group of male COVID-19 convalescent plasma donors. Mayo Clin Proc. 2021;96(10):2727–2728. doi: 10.1016/j.mayocp.2021.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartzell S., Bin S., Benedetti C., Haverly M., Gallon L., Zaza G., et al. Evidence of potent humoral immune activity in COVID-19-infected kidney transplant recipients. Am J Transplant. 2020;20:3149–3161. doi: 10.1111/ajt.16261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandolfini I., Zanelli P., Palmisano A., Salvetti D., Parmigiani A., Maltzman J.S., et al. Anti-HLA and anti-SARS-CoV-2 antibodies in kidney transplant recipients with COVID-19. Transpl Int. 2021;34:596–599. doi: 10.1111/tri.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katerinis I., Hadaya K., Duquesnoy R., Ferrari-Lacraz S., Meier S., van Delden C., et al. De novo anti-HLA antibody after pandemic H1N1 and seasonal influenza immunization in kidney transplant recipients. Am J Transplant. 2011;11:1727–1733. doi: 10.1111/j.1600-6143.2011.03604.x. [DOI] [PubMed] [Google Scholar]
- 8.HOOKIPA Announces Positive Phase 2 Interim Safety, Immunogenicity, and Efficacy Data for its Cytomegalovirus Vaccine Candidate HB-101. 〈https://ir.hookipapharma.com/node/7361/pdf〉.
- 9.Hvalryg M., Nissen-Meyer L.S.H. Sero-prevalence of SARS-CoV-2 antibodies in blood donors during the third wave of infection in Norway, winter/spring 2021. Transfus Apher Sci. 2021;60(5) doi: 10.1016/j.transci.2021.103256. [DOI] [PMC free article] [PubMed] [Google Scholar]