Abstract
Synthetic cathinones, such as 3,4-methylenedioxypyrovalerone (MDPV), are recreational drugs of abuse often identified in “bath salts” preparations. Humans report compulsive patterns of bath salts use, and previous work suggests that a subset of rats develop unusually high levels of MDPV self-administration. This study aims to test the hypothesis that high levels of impulsivity (e.g., inability to withhold responding for a sucrose reward) will predispose rats to high levels of MDPV self-administration relative to rats with lower levels of impulsivity. The 1-choice serial reaction time task (1-CSRTT) was used to assess impulsivity (i.e., premature responding) in 10 female and 10 male Sprague Dawley rats. Rats were then allowed to self-administer 0.032 mg/kg/inf MDPV or 0.32 mg/kg/inf cocaine, after which full dose-response curves for MDPV (0.001-0.1 mg/kg/inf) or cocaine (0.01-1 mg/kg/inf) were generated under a FR5 schedule of reinforcement. After a history of self-administering MDPV or cocaine, impulsivity was reassessed under the 1-CSRTT, prior to evaluating the acute effects of MDPV (0.032-0.32 mg/kg) or cocaine (0.1-1 mg/kg) on impulsivity. Level of impulsivity was not correlated with subsequent levels of either MDPV or cocaine self-administration, and level of drug self-administration was also not correlated with subsequent levels of impulsivity, although acute administration of MDPV and cocaine did increase premature responding. In failing to find direct relationships between either impulsivity and subsequent drug-taking behavior, or drug-taking behavior and subsequent assessments of impulsivity, these findings highlight the complexity inherent in the associations between impulsive behavior and drug-taking behavior in both animal models and humans.
Keywords: impulsivity, cocaine, MDPV, synthetic cathinones, self-administration, rats
Introduction
Impulsivity can be described as quickly responding before proper evaluation of a stimulus and/or a failure to withhold or inhibit an inappropriate response (i.e., impulsive action). 1-3 Impulsivity is thought to play a role in the development of substance use disorders, and substance use is thought to impair impulse control, but the nature of these interactions remains unclear. Studies in humans suggest that impulsive behaviors often pre-date the onset of drug use 4-7 and also can be exacerbated by drug use. 8-9 Although these findings suggest a connection between impulsive behavior and drug use, it is often difficult to establish a causal relationship in studies involving human participants. The complexity inherent in this area of research therefore necessitates the use of animal models to further clarify the possible cause-and-effect association. Interactions between impulsivity and the use of substances such as nicotine, alcohol, and cocaine have been heavily explored in animals, particularly rats. 10-12 Most of these studies examined the effects of self-administered drugs on impulsive behavior; fewer have investigated the association between basal levels of impulsivity and subsequent levels of drug taking.
The interplay between impulsivity and cocaine self-administration has been examined with the 5-choice serial reaction time test (5-CSRTT; a measure of inhibitory failure) or delay discounting procedures (a measure of impulsive choice). For instance, male rats classified as high-impulsive by the level of premature responding in the 5-CSRTT went on to self-administer higher levels of cocaine, particularly when provided extended access to cocaine. 11 Consistent with these findings, high-impulsive male and female rats, as determined by a delay discounting procedure, acquired cocaine self-administration more quickly and stabilized at higher levels of cocaine taking than their low-impulsive counterparts. 13-14 Higher levels of impulsivity, as determined by a delay-discounting task, have also been associated with less “elasticity of demand” for cocaine infusions, indicating that cocaine functions as a more effective reinforcer in high-impulsive rats relative to low-impulsive rats. 15 Impulsivity has also been linked with increased risk of relapse; high-impulsive rats, as determined by the 5-CSRTT, showed a marked reinstatement of responding for cocaine after two abstinence periods. 16 Thus, existing literature suggests that there is a positive relationship between impulsive behavior and subsequent levels of cocaine self-administration in rats; however, differences in the level of drug intake are often modest.
Conversely, others have used similar procedures to characterize the association between differences in the level of cocaine self-administration and subsequent measures of impulsivity in rats. In some studies, rats that self-administered cocaine exhibited greater levels of impulsivity on a delay discounting test than did controls. 17-18 Others have reported that high-impulsive rats, as determined by the 5-CSRTT, made fewer premature responses after cocaine self-administration, suggesting that a history of cocaine self-administration actually lowered measures of impulsivity. 19 Cocaine self- administration was also found to not impact measures of impulsivity as assessed by delay discounting. 20 It seems there is less consensus regarding how cocaine intake affects impulsive action in rats, but because there is often little inter-subject variability in cocaine self-administration in rats, it is difficult to draw strong conclusions about how impulsivity affects the levels of drug intake, and vice versa.
Recently, studies have suggested that a subset of Sprague Dawley rats develop unusually high levels of drug-taking when they are allowed to self-administer 3,4-methylenedioxypyrovalerone (MDPV) or related synthetic cathinones. 21-23 MDPV and related synthetic cathinones function as cocaine-like inhibitors of monoamine transporters 24-26 and are the primary psychoactive constituents of illicit “bath salts” preparations. 27-28 Importantly, not only is this “high-responder” phenotype persistent across time, but it also endures even when other drugs, such as cocaine, are substituted for MDPV. 21 The present study sought to exploit this natural variation in MDPV self-administration to investigate if impulsivity was positively correlated with the level of drug-taking (i.e., responder phenotype) in male and female rats allowed to self-administer either MDPV or cocaine. Serial reaction time tests have been shown to be useful in examining connections between drug effects and impulsive action in rodents 11,29-30; therefore, this study used the 1-choice serial reaction time task (1-CSRTT) to determine whether individual differences in impulsivity predicted subsequent levels of MDPV or cocaine self-administration, as well as whether individual differences in the level of drug-taking differentially impacted measures of impulsivity when reassessed after a prolonged period of MDPV or cocaine self-administration.
Materials and Methods
10 male and 10 female Sprague Dawley rats (males 295-320 g; females 200-225 g upon arrival) were purchased from Envigo (Indianapolis, IN, USA) and housed individually in a temperature- and humidity-controlled room on a 14:10-h light/dark cycle. During the 1-choice serial reaction time task (1-CSRTT), rats were lightly food restricted to facilitate responding for sucrose. Rats were provided with ad libitum access to food during the self-administration portion of the study; rats had ad libitum access to water throughout the study. All experiments were conducted in accordance with the Institutional Animal Care and Use Committees of the University of Texas Health San Antonio and the Guide for Care and Use of Laboratory Animals, eighth edition. 31
1-Choice Serial Reaction Time Task (1-CSRTT):
All experimental sessions were conducted in operant-conditioning chambers (model number: MED-NP5L-B1) equipped with five nose-poke holes and a house light on one wall. The opposite wall featured a food dispenser and a pellet trough. The nose-poke holes and pellet tray could be illuminated individually and were equipped with infrared beams to detect head entries.
The 1-CSRTT protocol has been previously documented. 32-33 Briefly, rats were habituated to the test chamber and trained to make a nose-poke response in the centermost hole when that hole was illuminated. The time between detection of a head entry in the pellet tray and the illumination of the nose-poke hole, or the time between the end of a time-out period and the illumination of the nose-poke hole was termed the inter-trial interval (ITI), marked by the illumination of the chamber house light. The amount of time rats were permitted to respond to the illuminated nose-poke hole was termed the limited hold (LH). The centermost hole was illuminated throughout the length of the LH.
Responding when the nose-poke hole was illuminated (i.e., a correct response) resulted in the delivery of a 45-mg sucrose pellet (Dustless Precision Pellets® Rodent; Bio-Serv, Flemington, NJ, USA) into the pellet trough, which was illuminated until the infrared beam detected a head entry. A response in any other hole (i.e., an incorrect response), a nose-poke in any of the 5 holes before illumination (i.e., a premature response), or no action at all (i.e., an omission) resulted in a timeout (TO), during which all lights in the operant chamber were extinguished. The number of premature responses, omissions, correct responses (reinforcers earned), and time to complete the session were recorded. Premature responses served as the measure of impulsive action. Both the number of omissions and the number of correct responses served as measures of task accuracy.
Sessions were conducted at approximately the same time of day, 7 days per week. During the initial training period, sessions were capped at 50 trials, with the LH set at 30 seconds, and the ITI and TO each set at 5 seconds. As training progressed, the LH was gradually decreased until rats were earning ≥25 reinforcers with ≤10 omissions over a period of 5 consecutive days at an LH of 5 seconds. 32 After the training period, rats completed the 1-CSRTT at ITI 5, LH 5, and TO 5 for a period of at least 5 days to assess for stability in responding, defined as premature responses differing by no more than 5 from the mean of the previous 4 sessions.
Once stability criteria were met, rats completed a series of 3 test sessions over a period of 3 days in which the ITI was set to 5, 7.5, or 10 seconds, in random order (i.e., ITI Triad Set #1). The ITI was the same for the full duration of each test session and was changed with each test session. The LH and TO were both kept at 5 seconds for all 3 test sessions. An increased amount of premature responses during these sessions reflected greater response disinhibition and thus greater impulsivity. 33 After completion of ITI Triad Set #1, stability was assessed again for a 2-day period, at minimum, after which another ITI test triad was conducted (i.e., ITI Triad Set #2). A subject’s baseline was considered to be the average of premature responses over a 5-day period after reaching stability criteria and immediately before starting ITI Triad Set #1.
The area under the curve (AUC) was determined for each animal’s ITI response function (i.e., the graph consisting of the means of an animal’s premature responses at ITI 5, 7.5, and 10, respectively, during that animal’s ITI Triads #1 and #2); thus, an animal making more premature responses would have a larger AUC. The animals were then rank-ordered by AUC, irrespective of sex. High-impulsive (HI) rats were defined as the upper quartile of the ordered group; similarly, low-impulsive (LI) rats were defined as the lower quartile of the ordered group. All other rats were classified as mid-impulsive (MI).
Surgical Procedures:
Rats were implanted with indwelling catheters in the left femoral vein and with vascular access ports under anesthesia with 2% isoflurane. 21 Immediately after surgery, catheters were flushed with 0.5 ml heparinized saline (100 U/ml), and penicillin G (60,000 U/rat) was administered subcutaneously to prevent infection. Rats were given a 5-day recovery period, during which ad libitum food and water access were provided. Catheters were flushed daily with 0.5 ml heparinized (100 U/ml) saline.
Drug Self-Administration:
Using the AUC values determined for the ITI response function, rats were alternately assigned to self-administer either MDPV (n=10) or cocaine (n=10) in order to have a roughly even distribution of HI, MI, and LI rats in each drug cohort.
Self-administration sessions were conducted at approximately the same time of day, 7 days per week in standard two-level operant conditioning chambers in sound- attenuating cubicles. LED lights (red, green, and yellow) were located above each lever, with a house light located on the opposite chamber wall. Drug infusions were delivered via variable speed syringe pumps attached to a stainless steel fluid swivel and a spring tether that were held in place by a counterbalance arm.
Thirty seconds before each session’s start, rats received a catheter-loading infusion. Illumination of the yellow LED above the active lever (counterbalanced left and right) indicated drug availability and the start of the session. If the fixed ratio (FR) was completed on the active lever while the yellow LED was lit, a drug infusion occurred (0.1 ml/kg over ~1 second). Drug infusions were coincident with the start of a 5-second TO, during which all three LEDs above the active lever and the house light were illuminated. Responses made during the TO or on the inactive lever had no consequence, though they were recorded.
Initially, rats were allowed to respond under a FR1 schedule for MDPV (0.032 mg/kg/inf) or cocaine (0.32 mg/kg/inf) during daily 90-minute sessions. This schedule was in place for at least 10 sessions and until rats met acquisition criteria (>20 infusions and >80% responses on the active lever, for 2 consecutive days). Subsequently, rats responded under a FR5 schedule for at least 10 sessions and until rats met stability criteria (±20% of the mean number of infusions for 3 consecutive days, with no increasing or decreasing trends). Once responding stabilized under the FR5:TO 5-second schedule of reinforcement, full dose-response curves were generated for their respective drugs: MDPV (0.001-0.1 mg/kg/inf) or cocaine (0.01-1 mg/kg/inf). The first dose tested was always 0.032 mg/kg/inf (MDPV) or 0.32 mg/kg/inf (cocaine), with the remaining doses tested in random order. All doses were evaluated until stability criteria were met. During these sessions, drug was available for a total of 90 minutes, which was subdivided into three 30-minute components. Each daily session began and ended with a 5-minute inter-component interval during which drug was not available but lever presses were recorded. Drug-available periods were also separated by 5-minute inter-component intervals.
Catheters were flushed before (0.2 ml normal saline) and after (0.5 ml heparinized saline) each session to verify and maintain patency. If pressure was encountered during flushing, an infusion of methohexital (3 mg/kg) was used to further assess the catheter’s status. If, after the infusion, the rat did not display a rapid loss of the righting reflex, a second surgery was performed in which a catheter was inserted in the right femoral vein.
Re-Evaluation of Impulsivity (1-CSRTT):
Once all doses of an assigned drug had been evaluated under self-administration procedures, rats were once again lightly food restricted to facilitate performance in the 1-CSRTT. Impulsivity was re-evaluated to determine whether differences in drug-taking history influenced individual sensitivities to ITI manipulations (i.e., impulsive action).
Sessions were conducted at approximately the same time of day, 7 days per week. Sessions were once again capped at 50 trials, with the LH set at 5 seconds, and the ITI and TO each set at 5 seconds. If a rat failed to meet training criteria (≥25 reinforcers earned with ≤10 omissions) over a period of 3 consecutive days, the LH was increased by 5 seconds. If, after the LH adjustment, the rat still failed to meet training criteria over the next 3 days, its food ration was reduced by 10 percent.
If rats met training criteria at the parameters of ITI 5, LH 5, and TO 5, they were assessed for a period of at least 5 days to evaluate their stability in responding. Once stability criteria were met, rats underwent another ITI test triad (i.e., ITI Triad Set #3), with the ITI set to 5, 7.5, or 10 seconds, in random order. The LH and TO remained at 5 seconds. Stability was assessed again for a 2-day period, at minimum, after which another ITI triad was conducted (i.e., ITI Triad Set #4). A subject’s new baseline was considered to be the average of premature responses over a 5-day period after reaching stability criteria and immediately before starting ITI Triad Set #3.
Drug Pretreatments and the 1-CSRTT:
After rats completed ITI Triad Set #4, they were pretreated with MDPV (0.032-0.32 mg/kg; IV) or cocaine (0.1-1 mg/kg; IV), respective to their assigned drug, and 0.5 ml heparinized saline. The goal of this stage of experimentation was to evaluate the direct and acute effects of various doses of drug on impulsive action. After treatment, rats remained in their home cages for 15 minutes in order to allow for drug effects to become apparent. 35 The effects of MDPV or cocaine were evaluated using the same ITI test triad that was used to assess impulsivity (3 sessions in which the ITI was set to 5, 7.5, or 10 seconds, in random order). The LH and TO remained at 5 seconds for all sessions. The first dose tested was always 0.032 mg/kg/inf for MDPV or 0.32 mg/kg/inf for cocaine, with the remaining doses tested in ascending order; ITI test triads were separated by at least 2 days, and until the stability criteria were met.
Drugs:
MDPV HCl was synthesized by Dr. Kenner Rice in the Drug Design and Synthesis Section of the Molecular Targets and Medications Discovery branch at the National Institute on Drug Abuse Intramural Research Program (NIDA-IRP; Bethesda, MD, USA). Cocaine HCl was provided by the NIDA Drug Supply Program. All drugs were dissolved in sterile saline and administered intravenously in a volume of 0.1 ml/kg for self-administration and pre-treatment tests.
Data Analysis:
Graphical presentations of 1-CSRTT data depict the mean±SEM premature responses, pellets earned, and omissions obtained over 2 sessions each at ITI 5, 7.5, and 10 seconds. The AUC was determined for the ITI premature response function, and the animals were rank-ordered accordingly. High-impulsive (HI; n=5) rats were defined as the upper quartile of the ordered group; similarly, low-impulsive (LI; n=5) rats were defined as the lower quartile of the ordered group. All other rats were classified as mid-impulsive (MI; n=10). Repeated-measures two-way analysis of variance (ANOVA) tests were used to determine if male and female rats, and HI, MI, and LI rats made statistically different amounts of premature responses at all ITI values.
For the self-administration portion of the study, dose-response curves show the mean±SEM number of infusions of MDPV (0.001-0.1 mg/kg/inf), cocaine (0.01-1 mg/kg/inf), or saline. Repeated-measures ANOVAs were used to determine whether female and male rats differed in level of drug-taking, and also if trait impulsivity was associated with level of drug-taking. One male rat assigned to cocaine (and previously determined as LI) was excluded from these data and henceforth due to highly variable levels of self-administration observed during acquisition and maintenance of cocaine self-administration.
AUCs were calculated for each rat’s self-administration dose-response curve, and subjects were then rank-ordered within their drug group. High-responder (HR) rats (MDPV [HR-MDPV]: n=3; cocaine [HR-COC]: n=3) were defined as the upper quartile of the groups; similarly, low-responder (LR) rats (MDPV [LR-MDPV]: n=3; cocaine [LR-COC]: n=3) were defined as the lower quartile of the groups. All other rats were classified as mid-responders (MR; MDPV [MR-MDPV]: n=3; cocaine [MR-COC]: n=3). One female rat that self-administered MDPV and was classified as HI died before being able to conduct the second set of ITI triads, and thus was excluded from this and later analyses. Repeated-measures two-way ANOVAs were used to determine if HR, MR, and LR rats made statistically different amounts of premature responses at all ITI values. Data from ITI Triads Sets #3 and #4 were normalized for each animal: the mean of premature responses was calculated from an animal’s baseline at ITI 5 (i.e., the 5 days immediately prior to the start of ITI Triad Set #1; baseline mean #1). Similarly, the mean of premature responses was calculated from an animal’s new baseline at ITI 5 after drug self-administration (i.e., the 5 days immediately prior to the start of ITI Triad Set #3; baseline mean #2). Baseline mean #2 was normalized to baseline mean #1, along with the means of ITI Triad Sets #3 and #4.
Graphical presentations of drug pre-treatment data from the 1-CSRTT depict the mean±SEM percent change in the AUC for each ITI triad (i.e., ITIs of 5, 7.5, and 10 seconds) relative to the AUC for the ITI triads determined after self-administration. Doses tested included 0.032, 0.1, and 0.32 mg/kg MDPV; and 0.1, 0.32, and 1 mg/kg cocaine. Repeated-measures two-way ANOVAs were used to determine if AUCs differed as a function of dose or level of impulsivity, and dose or responder phenotype.
GraphPad Prism 8 (GraphPad Software Inc., La Jolla, CA, USA) was used to plot figures and perform statistical analyses.
Results
1-CSRTT:
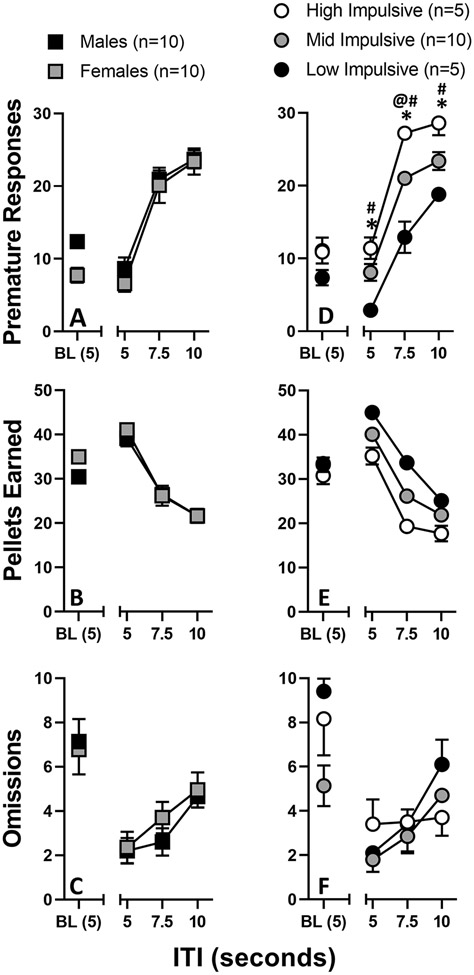
Female (n=10) rats took, on average, 15.7 ± 0.9 days and male (n=10) rats took, on average, 16.7 ± 0.6 days to satisfy stability criteria; each rat was transitioned to the next experimental stage based on individual performance, as indicated. Female and male rats did not differ significantly with regards to amount of premature responses made, pellets earned, or trials omitted at any ITI value (Figures 1A-C). Repeated measures two-way ANOVA revealed a main effect of ITI [F(2.0, 35.9) = 138.2, P < 0.0001, but no main effect of sex or ITI × sex interaction. Post-hoc analyses revealed that males, at baseline, made more premature responses than did females (P = 0.003).
Figure 1.
1-CSRTT assessed prior to drug self-administration. Premature responses (top row), pellets earned (middle row), and omissions (bottom row) were recorded twice (i.e., ITI Triad Sets #1 and #2) and averaged for each drug-naive rat at ITI values of 5, 7.5, and 10 seconds. A subject’s baseline (BL) was considered to be the average of premature responses over a 5-day period after reaching stability criteria and immediately before starting ITI Triad Set #1. Rats were subdivided by sex (left column) and impulsivity (right column). Abscissa: ITI value (seconds). Ordinate: Specific variables measured during the 1-CSRTT. &P < 0.01 between female and male points. *P < 0.05 between high impulsive and low impulsive points. #P < 0.05 between mid impulsive and low impulsive points. @P < 0.05 between high impulsive and mid impulsive points.
The AUC was determined for the ITI response function, after which the animals were rank ordered. High-impulsive (HI) rats (n=5) were defined as the upper quartile of the group; similarly, low-impulsive (LI) rats (n=5) were defined as the lower quartile of the group. All other rats were classified as mid-impulsive (MI; n=10). LI rats made significantly fewer premature responses than did HI and MI rats at all ITI levels (P < 0.008 and P < 0.0035, respectively; Figure 1D). While HI rats made more premature responses than MI rats at all ITI levels, they only differed significantly at ITI 7.5 (P < 0.0002; Figure 1D). Repeated measures two-way ANOVA revealed a main effect of impulsivity [F(2, 17) = 39.3, P < 0.0001) and ITI [F(1.9, 32.9) = 135.4, P < 0.0001), but no interaction between the two. Responder groups did not differ significantly with regards to the amount of pellets earned or trials omitted at any ITI value (Figures 1E-F).
Acquisition of Drug Self-Administration:
9/10 MDPV rats and 9/10 cocaine rats acquired responding for drug within the 10-day acquisition period. During the final 3 days of the acquisition period, rats earned a comparable number of infusions of 0.32 mg/kg/infusion cocaine (44.5 ± 3.2) and 0.032 mg/kg/infusion MDPV (54.07 ± 7.33). Infusions from female (44.5 ± 1.4) and male (38.8 ± 6.2) rats that self-administered MDPV, and infusions from female (58.5 ± 9.8) and male (51.3 ± 5.2) rats that self-administered cocaine were comparable within drug groups. The number of infusions of drug during the last 3 days of the acquisition period did not differ between impulsivity groups for either drug.
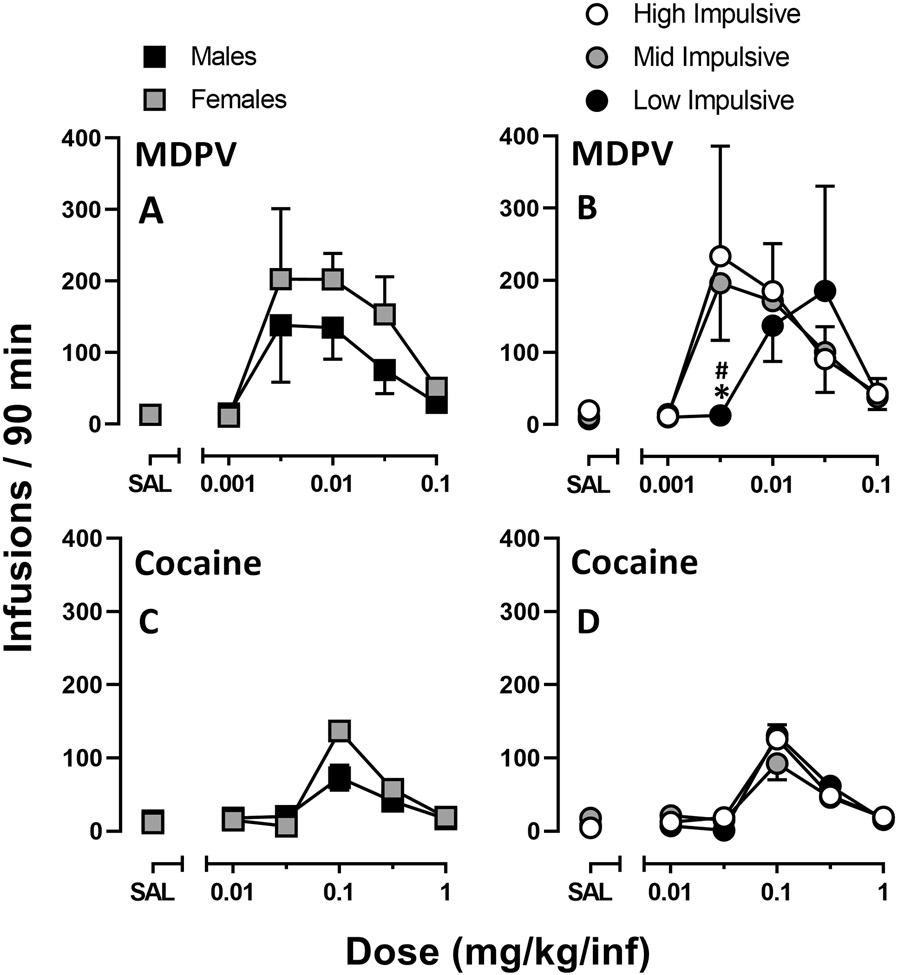
FR5 Dose-Response Curves:
Figure 2 shows the number of infusions earned per 90-minute session of each unit dose of drug under a FR5 schedule of reinforcement. Repeated measures two-way ANOVA revealed a main effect of MDPV dose [F(1.5, 11.6) = 6.3, P = 0.02], but not sex, and no dose × sex interaction (Figure 2A). Similarly, there was no main effect of impulsivity phenotype (HI-MDPV [n=3], MI-MDPV [n=5], and LI-MDPV [n=2]) on MDPV self-administration. Post-hoc analyses revealed that LI-MDPV rats self-administered fewer infusions of 0.0032 mg/kg MDPV than either MI-MDPV or HI-MDPV rats, though not at significant levels (Figure 2B). Additionally, the peak of the dose response curve for LI-MDPV rats (0.032 mg/kg MDPV) was approximately 1 log unit to the right of HI-MDPV and MI-MDPV rats (0.0032 mg/kg MDPV).
Figure 2.
Dose-response curves for MDPV (top row) and cocaine (bottom row) self- administration obtained under the FR5 schedule of reinforcement. Rats were subdivided by sex (left column) and impulsivity (right column). Abscissa: Drug (MDPV or cocaine) dose (mg/kg/inf). Ordinate: Infusions earned per 90-minute session. Total MDPV rats: n=10 (females n=5, males n=5; HI-MDPV = 3, MI-MDPV = 5, LI-MDPV = 2). Total cocaine rats: n=9 (females n= 5, males n= 4; HI-COC = 2, MI-COC = 5, LI-COC = 2).
Regarding the cocaine data, one male rat assigned to cocaine (and previously determined as LI) was excluded from these data and henceforth due to highly variable levels of drug-taking during acquisition and maintenance of self-administration. Repeated measures two-way ANOVA revealed a main effect of cocaine dose [F(1.9, 13.0) = 78.5, P < 0.0001]. Although there was no main effect of sex, there was a dose × sex interaction [F(4.0, 28.0) = 11.9, P < 0.0001] (Figure 2C). Unlike their MDPV counterparts, impulsivity phenotype (HI-COC [n=2], MI-COC [n=5], and LI-COC [n=2]) did not significantly impact the number of cocaine infusions earned at any dose (Figure 2D).
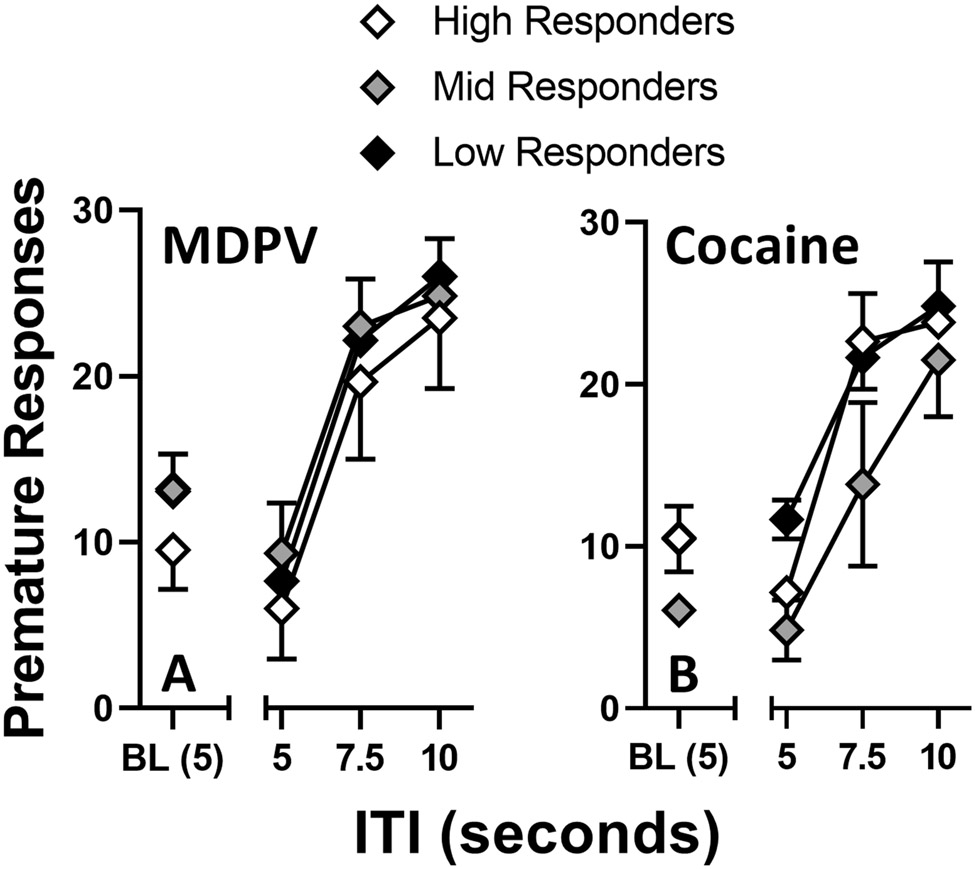
AUCs calculated for individual subject dose-response curves allowed rats to be classified as high-responders (HR; upper quartile) for MDPV (HR-MDPV; n=3) or cocaine (HR-COC; n=3), or low-responders (LR; lower quartile) for MDPV (LR-MDPV; n=3) or cocaine (LR-COC; n=3); all other rats were classified as mid-responders (MR; MDPV [MR-MDPV]: n=3; cocaine [MR-COC]: n=3). Data from the initial 1-CSRTT triads were reorganized by responder type to examine interactions between initial measures of impulsivity and the subsequent emergence of the high-responder phenotype. Post-hoc analyses revealed that LR-COC rats had higher baseline premature responses than MR-COC (P = 0.02). Repeated measures two-way ANOVA of the MDPV data revealed a main effect of ITI [F(1.9, 11.5) = 57.9, P < 0.0001] , but not responder type, and no ITI × responder type interaction. Repeated measures two-way ANOVA of the cocaine data revealed a main effect of ITI [F(2.0, 11.8) = 64.6, P < 0.0001], but not responder type, and no ITI × responder type interaction. HR-MDPV rats, on average, made fewer premature responses than did MR-MDPV and LR-MDPV rats at all ITI values (Figure 3A); however, these differences failed to reach significance. MR-COC rats, on average, made fewer premature responses than did HR-COC and LR-COC rats at all ITI values (Figure 3B); however, these differences failed to reach significance.
Figure 3.
Premature responses from the initial ITI triads arranged by drug self-administration responder group (high, middle, and low) and separated by drug (MDPV and cocaine, respectively). A subject’s baseline (BL) was considered to be the average of premature responses over a 5-day period after reaching stability criteria and immediately before starting ITI Triad Set #1. Abscissa: ITI value (seconds). Ordinate: Premature responses. #P < 0.05 between mid responder and low responder points. Total MDPV rats: n=10 (HR-MDPV = 4, MR-MDPV = 3, LR-MDPV = 3). Total cocaine rats: n=9 (HR-COC = 3, MR-COC = 3, LR-COC = 3).
Return to the 1-CSRTT:
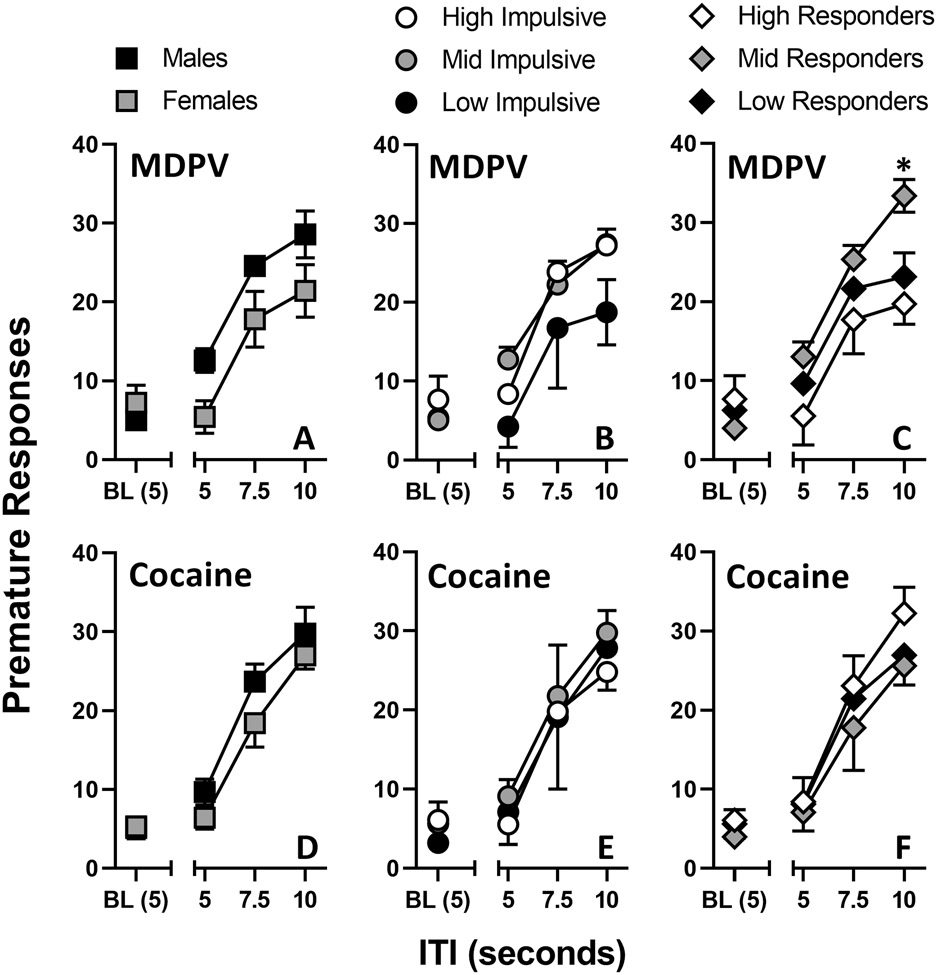
Female (n=4) rats that previously self-administered MDPV took, on average, 27.8 ± 11.6 days and male (n=5) rats that previously self-administered MDPV took, on average, 14.4 ± 7.2 days to satisfy stability criteria. Female (n=5) rats that previously self-administered cocaine took, on average, 32.8 ± 9.4 days and male (n=4) rats that previously self-administered cocaine took, on average, 15.3 ± 4.4 days to satisfy stability criteria. Each rat was moved along individually to the next experimental stage when appropriate. Female and male rats that previously self-administered MDPV, and female and male rats that previously self-administered cocaine did not differ significantly with regards to amount of premature responses made at any ITI value during the second set of 1-CSRTT triads (Figure 4A; 4D). Males, on average, made more premature responses than females for all ITI values, regardless of drug previously self-administered.
Figure 4.
1-CSRTT assessed after drug self-administration. Premature responses were recorded twice (i.e., ITI Triad Sets #3 and #4) and averaged for each rat at ITI values of 5, 7.5, and 10 seconds. Rats are subdivided into sex (left column), impulsivity (middle column), drug responder (right column), and self-administered drug (MDPV, top row; cocaine, bottom row). A subject’s baseline was considered to be the average of premature responses over a 5-day period after reaching stability criteria and immediately before starting ITI Triad Set #3. Abscissa: ITI value (seconds). Ordinate: Premature responses. *P < 0.05 between mid-responder and high-responder points. Total MDPV rats: n=9 (females n=4, males n=5; HI-MDPV = 3, MI-MDPV = 4, LI-MDPV = 2; HR-MDPV = 3, MR-MDPV = 3, LR-MDPV = 3). Total cocaine rats: n=9 (females n= 5, males n= 4; HI-COC = 2, MI-COC = 5, LI-COC = 2; HR-COC = 3, MR-COC = 3, LR-COC = 3).
For MDPV, repeated measures two-way ANOVA revealed an effect of ITI (F[1.9, 13.6] = 46.7, P < 0.0001) and an effect of sex (F[1, 7] = 5.8, P = 0.04, but no ITI × sex interaction. For cocaine, there was an effect of ITI (F[1.4, 9.8] = 73.1, P < 0.0001 but not sex, and no ITI × sex interaction. While the 1-CSRTT triads prior to drug self-administration showed a clear stratification between HI, MI, and LI rats with regards to amount of premature responses made, these differences were no longer apparent after the redetermination of the 1-CSRTT following either MDPV or cocaine self-administration (Figure 4B; 4E). For MDPV, repeated measures two-way ANOVA revealed an effect of ITI (F[1.8, 10.6] = 47.4, P < 0.0001), but not of impulsivity, and no interaction between the two. For cocaine, there was an effect of ITI (F[1.4, 8.6] = 52.5, P < 0.0001), but not of impulsivity, and no interaction between the two.
HR-MDPV, MR-MDPV, and LR-MDPV rats did not differ significantly with regards to amount of premature responses made at most ITI values during the second set of 1-CSRTT triads (Figure 4C). Repeated measures two-way ANOVA revealed an effect of ITI (F[1.9, 11.5] = 57.9, P < 0.0001), but not of impulsivity, and no interaction between the two. MR-MDPV rats did make significantly more premature responses than HR-MDPV rats at ITI 10 (P = 0.03). Like the first set of 1-CSRTT triads, HR-MDPV rats made the fewest amount of premature responses at every ITI value.
HR-COC, MR-COC, and LR-COC rats that previously self-administered cocaine also did not differ significantly with regards to amount of premature responses made at any ITI value during the second set of 1-CSRTT triads (Figure 4F). Repeated measures two-way ANOVA revealed an effect of ITI (F[1.2, 7.2] = 73.8, P < 0.0001), but not of impulsivity, and no interaction between the two. Like the first set of 1-CSRTT triads before drug self-administration, MR-COC rats made the fewest amount of premature responses at every ITI value. HR-COC rats made the most premature responses at every ITI value.
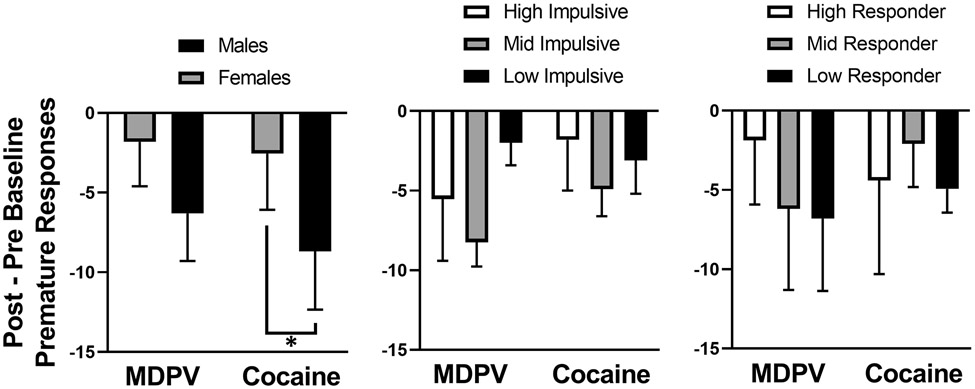
Figure 5 depicts differences in the amount of premature responding observed before and after drug self-administration. To do so, baseline measures (i.e., average premature responses) were calculated for each subject over the 5-day period prior to initiating 1-CSRTT triads, both before and after drug self-administration, with the pre-drug baseline subtracted from the post-drug baseline. Overall, rats tended to make fewer premature responses after drug self-administration. Repeated measures two-way ANOVA revealed an effect of sex (F[1.0, 8.0] = 7.0, P < 0.05), but none of drug, impulsivity type, or responder type. Post-hoc analyses revealed that male rats that self-administered cocaine had a significantly greater change in baseline premature responding than did female rats (P = 0.03).
Figure 5.
Change scores of premature responses. A subject’s baseline (i.e. average premature responses over a 5-day period before starting 1-CSRTT triads) was calculated both before drug self-administration and after drug self-administration. Then the pre-drug baseline was subtracted from the post-drug baseline. Abscissa: MDPV or cocaine grouping. Ordinate: Difference in baseline premature responses. *P < 0.05 between cocaine females and cocaine males. Total MDPV rats: n=9 (females n=4, males n=5; HI-MDPV = 3, MI-MDPV = 4, LI-MDPV = 2; HR-MDPV = 3, MR-MDPV = 3, LR-MDPV = 3). Total cocaine rats: n=9 (females n= 5, males n= 4; HI-COC = 2, MI-COC = 5, LI-COC = 2; HR-COC = 3, MR-COC = 3, LR-COC = 3).
Drug Pretreatments:
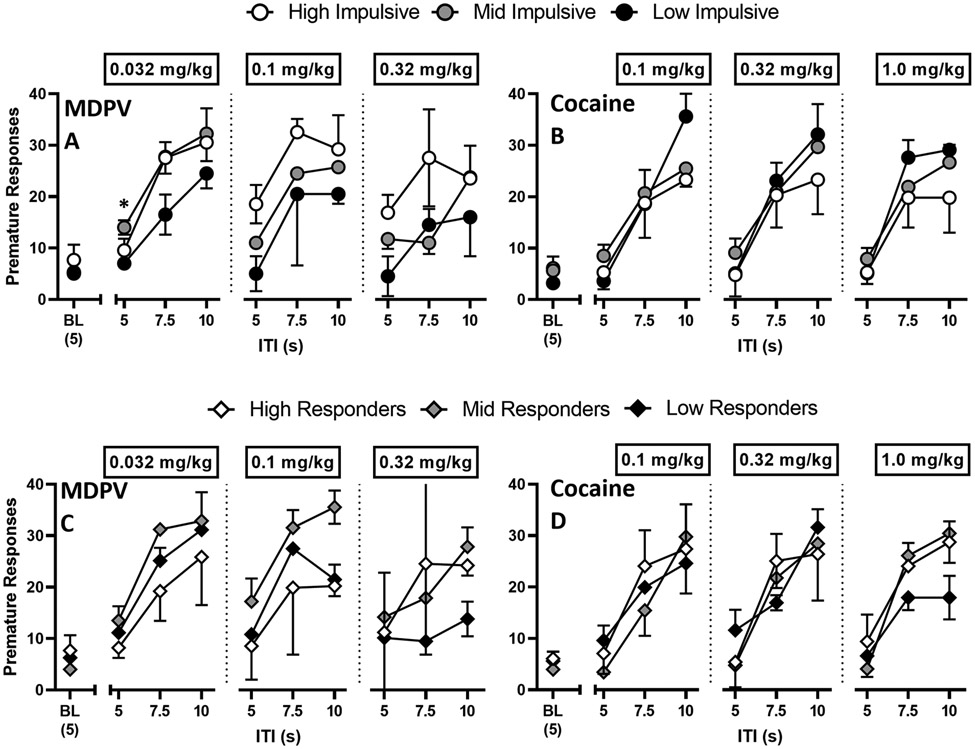
The aim of these experiments was to determine if acute non-contingent injections of MDPV or cocaine impacted performance under the 1-CSRTT. These experiments were conducted after rats (n=18: F=9, M=9) returned to the 1-CSRTT. When premature responses were analyzed by impulsivity group (Figures 6A-6B), there was an effect of ITI for all doses of MDPV: 0.032 mg/kg (F[1.4, 8.4] = 33.0, P < 0.001), 0.1 mg/kg (F[1.5, 9.0] = 19.3, P < 0.001), and 0.32 mg/kg (F[1.8, 11.0] = 5.2, P = 0.02); and cocaine: 0.1 mg/kg (F[1.7, 10.3] = 39.8, P < 0.0001), 0.32 mg/kg (F[1.5, 8.8] = 27.9, P < 0.001), and 1 mg/kg (F[1.6, 9.4] = 42.5, P < 0.0001). However, there was no effect of impulsivity or interaction between impulsivity and ITI.
Figure 6.
1-CSRTT assessed 15 minutes after pretreatments with MDPV (0.032, 0.1, and 0.32 mg/kg), or cocaine (0.1, 0.32, and 1.0 mg/kg). Premature responses were recorded twice and averaged for each rat at ITI values of 5, 7.5, and 10 seconds. Rats are subdivided into impulsivity (top row) and responder (bottom row). Abscissa: ITI value (s). Ordinate: Premature responses. *P < 0.05 between mid impulsive and low impulsive points. Total MDPV rats: n=9 (females n=4, males n=5; HI-MDPV = 3, MI-MDPV = 4, LI-MDPV = 2; HR-MDPV = 3, MR-MDPV = 3, LR-MDPV = 3). Total cocaine rats: n=9 (females n= 5, males n= 4; HI-COC = 2, MI-COC = 5, LI-COC = 2; HR-COC = 3, MR-COC = 3, LR-COC = 3).
When premature responses were analyzed by responder type (Figures 6C-6D), there was an effect of ITI for all doses of MDPV: 0.032 mg/kg (F[1.5, 9.0] = 36.7, P < 0.0001), 0.1 mg/kg (F[1.4, 8.6] = 26.0, P < 0.001), and 0.32 mg/kg (F[1.4, 8.4] = 4.9, P = 0.04); and cocaine: 0.1 mg/kg (F[1.8, 11.0] = 36.5, P < 0.0001), 0.32 mg/kg (F[1.6, 9.8] = 49.3, P < 0.0001), and 1 mg/kg (F[1.9, 11.6] = 89.3, P < 0.0001). Although there was an ITI × responder type interaction for 1 mg/kg cocaine (F[4.0, 12.0] = 4.4, P = 0.02), no other interactions were observed.
HI-MDPV tended to make more premature responses compared to their MI-MDPV and LI-MDPV counterparts for the higher 2 doses tested (0.1 and 0.32 mg/kg MDPV) and all ITI values tested, though these effects failed to reach significance (Figure 6A). LI-MDPV rats tended to make the fewest premature responses for all doses and all ITI values tested. MI-MDPV rats made significantly more premature responses than LI-MDPV rats at ITI 5 for 0.032 mg/kg MDPV (P = 0.02). There was also no significant difference in premature responses between responder groups (Figure 6C). MR-MDPV rats made the most premature responses and HR-MDPV rats made the fewest premature responses for the lower 2 doses tested (0.032 and 0.1 mg/kg MDPV).
HI-COC rats tended to make the fewest number of premature responses compared to their counterparts at all doses and ITI values tested, though these differences failed to reach significance (Figure 6B). There was also no significant difference in premature responses between HR-COC, MR-COC, and LR-COC groups (Figure 6D).
Discussion
The two primary goals of this study were to determine if individual differences in impulsive action were predictive of subsequent levels of drug self-administration, and whether individual differences in drug-taking were positively correlated with reassessments of impulsive action. There were three main findings: (1) high measures of impulsivity at baseline did not predispose female or male rats to high levels of cocaine or MDPV self-administration; (2) high levels of cocaine or MDPV self-administration did not result in rats becoming more impulsive (i.e., increases in premature responding) in the 1-CSRTT; (3) pretreatment with cocaine or MDPV tended to increase a measure of impulsivity in HR rats.
Rats underwent impulsivity phenotyping with the 1-CSRTT prior to self- administration of MDPV or cocaine to determine if high impulsivity would predispose a rat to higher levels of drug self-administration. Individual differences in impulsive action were not predictive of levels of MDPV or cocaine self-administration; thus, this study failed to identify an association between impulsivity and subsequent levels of drug self-administration, although LI rats were less sensitive to the reinforcing effects of MDPV. To our knowledge, there have not been any previous studies examining the connection between impulsivity phenotype and subsequent MDPV self-administration, but some work has been done examining interactions between impulsivity and the self- administration of cocaine. HI male Lister hooded rats, as measured by the 5-CSRTT, did indeed self-administer higher levels of cocaine. 11 However, those rats self-administered cocaine under extended-access (8-hour) sessions, whereas the current study evaluated drug-taking behavior under relatively short, 90-minute sessions. Similarly, measures of impulsivity and the escalation of cocaine self-administration have been positively correlated in female rats, given either short (2 hours) or extended access (6 hours) to cocaine. 36 Thus, it is possible that a similar interaction between impulsivity and drug-taking would have emerged if the current study had evaluated MDPV and cocaine self-administration under extended, rather than short access, procedures.
A subset of male and female rats that self-administer MDPV has been shown to demonstrate unusual drug-taking behavior, including high levels of drug intake in short periods of time and high levels of responding during periods when drug is unavailable. 21,23 The current study replicated this finding and took advantage of this natural variation in MDPV self-administration to investigate if the level of drug-taking in male and female rats allowed to self-administer either MDPV or cocaine was associated with subsequent measures of impulsivity. HR rats in either drug group did not make more premature responses in the 1-CSRTT as compared to other responder types. Omissions and pellets earned were similar between sexes and drug groups. Thus, the current study failed to identify an association between levels of drug self-administration and subsequent measures of impulsivity with the 1-CSRTT. Consensus regarding how drug self-administration, particularly cocaine, affects measures of impulsivity is mixed. Although Dalley et al. (2005) reported an interaction between impulsivity and subsequent cocaine self-administration, cocaine self-administration did not significantly impact premature responding under the 5-CSRTT. 37 Dalley et al. (2007) also found that differences in premature responding in the 5-CSRTT between HI-COC and LI-COC rats were not present after cocaine self-administration. 11 Similarly, HI rats, as determined by the 5-CSRTT, did not differ from LI rats in acquisition of cocaine self-administration. 38 These discrepancies suggest that impulsive behavior may not necessarily be a consequence of drug use, or the association observed may be affected by the type of assay chosen to assess impulsivity. The fact that HR rats failed to show increases in subsequent measures of impulsivity finding could have been influenced by prolonged periods of training on the impulsivity task. In addition, the 1-CSRTT has a low working memory load, which may have explained the lack of premature responding in the trials after drug self-administration; different results may have been achieved by using the 5-CSRTT. 37 While drug self-administration may have contributed to changes in premature responding, it is also possible that motivation also had a hand, as animals had unlimited access to food during drug self-administration. However, this potential confounder was mitigated by reinstating food restriction upon starting the second round of 1-CSRTT testing.
Rats were given pretreatments of either MDPV or cocaine 15 minutes prior to a 1-CSRTT trial in order to investigate the acute effects of drug on impulsivity. Pretreatments with cocaine or MDPV tended to increase measures of impulsivity in high impulsive rats, but not consistently and not at significant levels. This finding is similar to what has been reported before in experiments with cocaine. 39-40 Hyatt et al. (2019), one of the first papers to describe the effects of MDPV on impulsivity, found that acute administration of MDPV, but not cocaine, led to increases in choice impulsivity in a delay discounting task. 41 Hyatt et al. (2020) then reported that both MDPV and cocaine consistently increased impulsive action in a differential reinforcement of low rates of responding task, similar to the effects observed in the 1-CSRTT. 42 While the current study mostly aligns with the results of these papers, the increase in impulsive action after acute administration of increasing doses of each drug was not as robust, which is different from that found in previous studies. 39, 41-42 In addition, the amount of premature responses and food reinforcers earned decreased (and thus omissions increased) at the largest doses of cocaine and MDPV tested. Similar results were reported in Hyatt et al. (2020), but operant responding suppression in the current study occurred at lower doses than what was observed in the Hyatt study. 42 This might be due to a rate-decreasing and/or behavioral disruptive effect of the drug. Additionally, differences in the route of administration could have accounted for discrepancies in responding to increasing doses of drug. Marked increases in premature responses with larger doses of cocaine, administered intraperitoneally, have been observed. 40 Hyatt et al. (2020) injected MDPV subcutaneously and cocaine intraperitoneally, 42 whereas the current study administered both drugs intravenously. Additionally, these animals were essentially withdrawing from their respective drug during the pretreatment tests; this state may have influenced their behavior in the 1-CSRTT. Winstanley et al. (2009) found that rats become more impulsive during withdrawal from cocaine and did not find any changes in the amount of omissions made; both of these findings were not found in the present study. 43 Another caveat regarding these results could be the prolonged history of MDPV or cocaine self-administration prior to assessing the acute effects of these drugs on impulsivity. Hyatt et al. (2020) tested both the acute effects of MDPV and cocaine on impulsivity with drug-naïve rats, and then again after those rats had been exposed to the highest doses of MDPV and cocaine used in that study over a period of 10 days. 42 While the number of earned reinforcers decreased as MDPV dose increased for both rat groups, the drop was less precipitous in the drug-naïve rats. It is possible that these animals, with a history of self-administration of either MDPV or cocaine, were sensitized to higher doses of that drug and went on to exhibit stereotypies for prolonged periods, which may explain why so many animals responded less in the 1-CSRTT after acute administration of higher doses of drug. 41
The current study included both male and female rats in order to investigate potential differences between sex in measures of impulsivity and drug self-administration. Post-hoc analyses revealed that males made significantly more premature responses than females at baseline, but this difference did not persist throughout the rest of the study. Furthermore, post-hoc analyses did not reveal sex differences with regards to premature responses during ITI Triad Sets, pellets earned, and omissions made—both before and after drug exposure—and levels of drug intake. There have been conflicting studies regarding the impact of sex differences on measures of impulsive behavior in preclinical models. Female rats have been reported to make more premature responses than males in the variable delay-to-signal task (an assay similar to the 5-CSRTT), 44 as well as in the 2-CSRTT at ITI 9. 45 Conversely, male rats were found to make significantly more premature responses in the 5-CSRTT at ITI 17 than female rats. 46 When taken together with the current studies, these findings suggest that sex differences are not consistently observed when impulsivity is assessed using serial reaction time tasks. However, several important differences among these studies should be noted, including the use of the 5-CSRTT and sample sizes. The 1-CSRTT differs from the 5-CSRTT in that the former assesses impulsive behavior independent of visuospatial relationships. Additionally, the sample size of the current study was smaller than those studies previously cited.
Sex differences in drug intake have also been reported, with female rats showing a correlation between higher levels of novelty-induced locomotion and subsequent higher levels of cocaine self-administration. 47 Similarly, female rats have been found to self-administer more cocaine than male rats under both progressive ratio and extended access conditions, 48 suggesting a difference in reinforcing effectiveness. However, when tested under short access conditions, sex differences in the number of cocaine infusions earned were not observed, 49-50 a finding that is consistent with the current study. Cocaine self-administration levels were compared between male and female rats during extended and short access conditions, and female rats self-administered more cocaine during 4 out of the 21 days than did male rats under long-access conditions 51; there was no difference found between sexes for short-access conditions. 51 Therefore, studies show that female rats tend to self-administer more cocaine than do male rats, but mostly under extended access conditions. This study only used short access conditions, and thus it is unclear whether the female rats would have displayed higher drug intake levels as compared to their male counterparts if longer sessions were employed.
A subset of Sprague Dawley rats develop unusually high levels of drug-taking when allowed to self-administer MDPV, and this natural variation in MDPV self- administration was exploited to investigate interactions between impulsivity and aberrant drug-taking behaviors. The results of this study indicate that impulsivity does not appear to be a predisposing factor to, or consequence of, high levels of cocaine or MDPV self-administration under short access conditions, and thus cannot explain why the MDPV high responder phenotype emerges. However, further studies utilizing extended access conditions may be useful in further investigations of the connection between impulsivity and drug-taking behavior.
Acknowledgements
Supported by NIH grants R01DA039146 (GTC), T32NS082145 (MRD), and R36DA050955 (MRD) as well as the NIH IRPs of NIDA and NIAAA.
Abbreviations:
- AUC
area under the curve
- ANOVA
analysis of variance
- FR
fixed ratio
- HI
high-impulsive
- HR
high-responder
- ITI
inter-trial interval
- LH
limited hold
- LI
low-impulsive
- LR
low-responder
- MDPV
3,4-methylenedioxypyrovalerone
- MI
mid-impulsive
- MR
mid-responder
- TO
timeout
- 1-CSRTT
1-choice serial reaction time task
- 5-CSRTT
5-choice serial reaction time task
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Chuang C-WI, Sussman S, Stone MD, et al. Impulsivity and history of behavioral addictions are associated with drug use in adolescents. Addict Behav. 2017;74:41–47. doi: 10.1016/j.addbeh.2017.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dougherty DM, Marsh-Richard DM, Hatzis ES, Nouvion SO, Mathias CW. A test of alcohol dose effects on multiple behavioral measures of impulsivity. Drug Alcohol Depend. 2008;96(1- 2):111–120. doi: 10.1016/j.drugalcdep.2008.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weafer J, Mitchell SH, de Wit H. Recent translational findings on impulsivity in relation to drug abuse. Current Addiction Reports. 2014;1(4):289–300. doi: 10.1007/s40429-014-0035-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argyriou E, Um M, Carron C, Cyders MA. Age and impulsive behavior in drug addiction: A review of past research and future directions. Pharmacol Biochem Behav. 2018;164:106–117. doi: 10.1016/j.pbb.2017.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nat Neurosci. 2005;8(11):1458–1463. doi: 10.1038/nn1584 [DOI] [PubMed] [Google Scholar]
- 6.Sher KJ, Bartholow BD, Wood MD. Personality and substance use disorders: a prospective study. J Consult Clin Psychol. 2000;68(5):818–829. [PubMed] [Google Scholar]
- 7.Tarter RE, Kirisci L, Feske U, Vanyukov M. Modeling the pathways linking childhood hyperactivity and substance use disorder in young adulthood. Psychol Addict Behav. 2007;21(2):266–271. doi: 10.1037/0893-164X.21.2.266 [DOI] [PubMed] [Google Scholar]
- 8.Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry. 2010;68(8):770–773. doi: 10.1016/j.biopsych.2010.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA Psychiatry. 2015;72(6):584. doi: 10.1001/jamapsychiatry.2015.1 [DOI] [PubMed] [Google Scholar]
- 10.Diergaarde L, Pattij T, Poortvliet I, et al. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63(3):301–308. doi: 10.1016/j.biopsych.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 11.Dalley JW, Fryer TD, Brichard L, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315(5816):1267–1270. doi: 10.1126/science.1137073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlton AJ, May C, Luikinga SJ, et al. Chronic voluntary alcohol consumption causes persistent cognitive deficits and cortical cell loss in a rodent model. Sci Rep. 2019;9(1):18651. doi: 10.1038/s41598-019-55095-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology. 2005;178(2-3):193–201. doi: 10.1007/s00213-004-1994-4 [DOI] [PubMed] [Google Scholar]
- 14.Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol. 2008;16(2):165–177. doi: 10.1037/1064-1297.16.2.165 [DOI] [PubMed] [Google Scholar]
- 15.Koffarnus MN, Woods JH. Individual differences in discount rate are associated with demand for self-administered cocaine, but not sucrose: Discounting and cocaine demand. Addict Biol. 2013;18(1):8–18. doi: 10.1111/j.1369-1600.2011.00361.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High Impulsivity Predicts Relapse to Cocaine-Seeking After Punishment-Induced Abstinence. Biol Psych. 2009;65(10):851–856. doi: 10.1016/j.biopsych.2008.12.008 [DOI] [PubMed] [Google Scholar]
- 17.Mendez IA, Simon NW, Hart N, et al. Self-administered cocaine causes long-lasting increases in impulsive choice in a delay discounting task. Behav Neurosci. 2010;124(4):470–477. doi: 10.1037/a0020458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell MR, Weiss VG, Ouimet DJ, Fuchs RA, Morgan D, Setlow B. Intake-dependent effects of cocaine self-administration on impulsive choice in a delay discounting task. Behav Neurosci. 2014;128(4):419–429. doi: 10.1037/a0036742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caprioli D, Hong YT, Sawiak SJ, et al. Baseline-dependent effects of cocaine pre-exposure on impulsivity and D2/3 receptor availability in the rat striatum: possible relevance to the attention-deficit hyperactivity syndrome. Neuropsychopharmacology. 2013;38(8):1460–1471. doi: 10.1038/npp.2013.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moschak TM, Carelli RM. Impulsive rats exhibit blunted dopamine release dynamics during a delay discounting task independent of cocaine history. eNeuro. 2017;4(2):ENEURO.0119-17.2017. doi: 10.1523/ENEURO.0119-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gannon BM, Galindo KI, Rice KC, Collins GT. Individual differences in the relative reinforcing effects of 3,4-methylenedioxypyrovalerone under fixed and progressive ratio schedules of reinforcement in rats. J Pharmacol Exp Ther. 2017;361(1):181–189. doi: 10.1124/jpet.116.239376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gannon BM, Baumann MH, Walther D, et al. The abuse-related effects of pyrrolidine- containing cathinones are related to their potency and selectivity to inhibit the dopamine transporter. Neuropsychopharmacology. 2018a;43(12):2399–2407. doi: 10.1038/s41386-018-0209-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doyle MR, Sulima A, Rice KC, Collins GT. MDPV self-administration in female rats: influence of reinforcement history. Psychopharmacology. 2021a;238(3):735–744. doi: 10.1007/s00213-020-05726-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumann MH, Partilla JS, Lehner KR, et al. Powerful cocaine-like actions of 3,4- methylenedioxypyrovalerone (MDPV), a principal constituent of psychoactive “bath salts” products. Neuropsychopharmacology. 2013;38(4):552–562. doi: 10.1038/npp.2012.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eshleman AJ, Wolfrum KM, Reed JF, et al. Structure-activity relationships of substituted cathinones, with transporter binding, uptake, and release. J Pharmacol Exp Ther. 2017;360(1):33–47. doi: 10.1124/jpet.116.236349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gannon BM, Galindo KI, Mesmin MP, Sulima A, Rice KC, Collins GT. Relative reinforcing effects of second-generation synthetic cathinones: acquisition of self-administration and fixed ratio dose-response curves in rats. Neuropharmacology. 2018b;134(Pt A):28–35. doi: 10.1016/j.neuropharm.2017.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seely KA, Patton AL, Moran CL, et al. Forensic investigation of K2, Spice, and “bath salt” commercial preparations: a three-year study of new designer drug products containing synthetic cannabinoid, stimulant, and hallucinogenic compounds. Forensic Sci Int. 2013;233(1-3):416–422. doi: 10.1016/j.forsciint.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 28.Spiller HA, Ryan ML, Weston RG, Jansen J. Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol. 2011;49(6):499–505. doi: 10.3109/15563650.2011.590812 [DOI] [PubMed] [Google Scholar]
- 29.Sholler DJ, Stutz SJ, Fox RG, et al. The 5-HT2A receptor (5-HT2A R) regulates impulsive action and cocaine cue reactivity in male sprague-dawley rats. J Pharmacol Exp Ther. 2019;368(1):41–49. doi: 10.1124/jpet.118.251199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Winstanley CA. The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders: Rodent models of impulsivity and drug discovery. Br J Pharmacol.2011;164(4):1301–1321. doi: 10.1111/j.1476-5381.2011.01323.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.National Research Council (U.S.), Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.), eds. Guide for the Care and Use of Laboratory Animals. 8th ed. National Academies Press; 2011 [Google Scholar]
- 32.Anastasio NC, Stoffel EC, Fox RG, et al. Serotonin (5-hydroxytryptamine) 5-HT2A receptor: association with inherent and cocaine-evoked behavioral disinhibition in rats. Behav Pharmacol. 2011;22(3):248–261. doi: 10.1097/FBP.0b013e328345f90d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunningham KA, Anastasio NC, Fox RG, et al. Synergism between a serotonin 5-HT 2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem Neurosci. 2013;4(1):110–121. doi: 10.1021/cn300072u [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amitai N, Markou A. Comparative effects of different test day challenges on performance in the 5-choice serial reaction time task. Behav Neurosci. 2011;125(5):764–774. doi: 10.1037/a0024722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Doyle MR, Sulima A, Rice KC, Collins GT. Interactions between reinforcement history and drug-primed reinstatement: studies with MDPV and mixtures of MDPV and caffeine. Addict Biol. 2021b;26(2):e12904. doi: 10.1111/adb.12904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anker JJ, Perry JL, Gliddon LA, Carroll ME. Impulsivity predicts the escalation of cocaine self-administration in rats. Pharmacol Biochem Behav. 2009;93(3):343–348. doi: 10.1016/j.pbb.2009.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dalley JW, Lääne K, Pena Y, Theobald DEH, Everitt BJ, Robbins TW. Attentional and motivational deficits in rats withdrawn from intravenous self-administration of cocaine or heroin. Psychopharmacology. 2005;182(4):579–587. doi: 10.1007/s00213-005-0107-3 [DOI] [PubMed] [Google Scholar]
- 38.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320(5881):1352–1355. doi: 10.1126/science.1158136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barlow RL, Dalley JW, Pekcec A. Differences in trait impulsivity do not bias the response to pharmacological drug challenge in the rat five-choice serial reaction time task. Psychopharmacology. 2018;235(4):1199–1209. doi: 10.1007/s00213-018-4836-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Terry AV, Callahan PM, Schade R, Kille NJ, Plagenhoef M. Alpha 2A adrenergic receptor agonist, guanfacine, attenuates cocaine-related impairments of inhibitory response control and working memory in animal models. Pharmacol Biochem and Behav. 2014;126:63–72. doi: 10.1016/j.pbb.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hyatt WS, Berquist MD, Chitre NM, et al. Repeated administration of synthetic cathinone 3,4-methylenedioxypyrovalerone persistently increases impulsive choice in rats. Behav Pharmacol. 2019;30(7):555–565. doi: 10.1097/FBP.0000000000000492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hyatt WS, Hirsh CE, Russell LN, et al. The synthetic cathinone 3,4- methylenedioxypyrovalerone increases impulsive action in rats: Behav Pharmacol. 2020;31(4):309–321. doi: 10.1097/FBP.0000000000000548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Winstanley CA, Bachtell RK, Theobald DEH, et al. Increased Impulsivity during Withdrawal from Cocaine Self-Administration: Role for FosB in the Orbitofrontal Cortex. Cereb Cortex. 2009;19(2):435–444. doi: 10.1093/cercor/bhn094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soares AR, Esteves M, Moreira PS, et al. Trait determinants of impulsive behavior: a comprehensive analysis of 188 rats. Sci Rep. 2018;8(1). doi: 10.1038/s41598-018-35537-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burton CL, Fletcher PJ. Age and sex differences in impulsive action in rats: the role of dopamine and glutamate. Behav Brain Res. 2012;230(1):21–33. doi: 10.1016/j.bbr.2012.01.046 [DOI] [PubMed] [Google Scholar]
- 46.Bayless DW, Darling JS, Stout WJ, Daniel JM. Sex differences in attentional processes in adult rats as measured by performance on the 5-choice serial reaction time task. Behav Brain Res. 2012;235(1):48–54. doi: 10.1016/j.bbr.2012.07.028 [DOI] [PubMed] [Google Scholar]
- 47.Davis BA, Clinton SM, Akil H, Becker JB. The effects of novelty-seeking phenotypes and sex differences on acquisition of cocaine self-administration in selectively bred high-responder and low-eesponder rats. Pharmacol Biochem Behav. 2008;90(3):331–338. doi: 10.1016/j.pbb.2008.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lynch WJ. Acquisition and maintenance of cocaine self-administration in adolescent rats: effects of sex and gonadal hormones. Psychopharmacology. 2008;197(2):237–246. doi: 10.1007/s00213-007-1028-0 [DOI] [PubMed] [Google Scholar]
- 49.Caine SB, Bowen CA, Yu G, Zuzga D, Negus SS, Mello NK. Effect of gonadectomy and gonadal hormone replacement on cocaine self-administration in female and male rats. Neuropsychopharmacology. 2004;29(5):929–942. doi: 10.1038/sj.npp.1300387 [DOI] [PubMed] [Google Scholar]
- 50.Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2005;179(3):662–672. doi: 10.1007/s00213-004-2080-7 [DOI] [PubMed] [Google Scholar]
- 51.Roth ME, Carroll ME. Sex differences in the escalation of intravenous cocaine intake following long- or short-access to cocaine self-administration. Pharmacol Biochem Behav. 2004;78(2):199–207. doi: 10.1016/j.pbb.2004.03.018 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.