Abstract
Microorganisms react upon hyperosmotic stress by accumulating compatible solutes. Here we report that Lactococcus lactis uses a transport system for glycine betaine that, contrary to earlier observations (D. Molenaar et al., J. Bacteriol. 175:5438–5444, 1993), is osmotically regulated at the levels of both expression and transport activity.
In their natural habitats, microorganisms are often exposed to changes in the concentrations of the solutes in their environment whereas the internal concentrations of nutrients need to be relatively constant (2, 8, 13). A sudden increase in the osmolarity of the environment results in the movement of water from the cell to the outside medium, which causes turgor pressure loss, intracellular solute concentration changes, and cell volume changes. Such hyperosmotic conditions are detrimental to any living cell. Bacteria counteract hyperosmotic stress by accumulating compatible solutes by uptake and/or synthesis. These solutes can be accumulated to high intracellular concentrations without affecting vital cellular processes, and they restore the osmotic balance of the cell. Upon hypo-osmotic stress, these compatible solutes are released from the cell, which prevents too high a turgor pressure that may ultimately lead to bursting of the cell.
Lactic acid bacteria have a limited capacity to synthesize compatible solutes, and a range of studies indicate that glycine betaine, carnitine, and proline are the most important compatible solutes in this group of organisms (3, 4, 11, 12, 19). The role of these compounds in osmoregulation in other (micro)-organisms is also well established (2, 5, 9, 13, 15, 16), but in those cases, additional molecules play a major role as well. There is a considerable amount of data that indicate that in Lactobacillus plantarum and Listeria monocytogenes, glycine betaine, carnitine, and proline are taken up via semiconstitutive transport systems that are activated upon hyperosmotic stress, whereas these compounds are rapidly released by channel-like activities upon osmotic downshock (3, 19). These studies prompted us to reinvestigate the regulation of glycine betaine transport in Lactococcus lactis, which is thought not to respond to any form of osmotic stress whereas the pool sizes during growth in different media do (11). Osmotic regulation of transport through alterations in activity has not only been shown in lactic acid bacteria but is also well documented for other bacteria (1, 10, 13, 15). In this study, we examined the regulation of glycine betaine uptake in two well-defined L. lactis strains that in many respects are paradigmatic of our knowledge of the physiology, energetics, and genetics of lactic acid bacteria. L. lactis MG1363 is a plasmid-free derivative of ML3 that was studied previously (11), whereas IL1403 is a plasmid-free strain for which the genome sequence will soon become available.
Regulation of glycine betaine uptake by osmotic upshock.
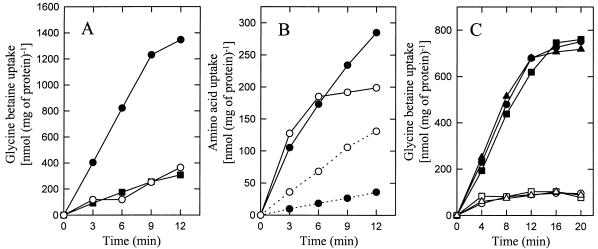
Cells were grown in CDM (14) (with or without proline) plus 25 mM glucose and 500 mM KCl, harvested by centrifugation, and washed and resuspended in 50 mM potassium phosphate, pH 6.5, plus chloramphenicol at 50 μg/ml as previously described (3, 4); details are given in the figure legends. The osmotic downshock imposed by the washing step released (most of) the organic compatible solutes from the cell. To initiate the transport reaction, the cells were incubated at 30°C in 50 mM potassium phosphate, pH 6.5, plus 10 mM glucose, and after 5 min of pre-energization, [14C]glycine betaine was added to a final concentration of 1.25 mM. To impose hyperosmotic conditions, KCl was added to the assay medium at a final concentration of 500 mM just prior to the addition of [14C]glycine betaine. Here, it is important to note that the reactions were stopped by dilution with a 20-fold excess of LiCl solutions that were iso-osmolar with the assay buffer. Figure 1A shows that the uptake of glycine betaine by L. lactis IL1403 was stimulated approximately fivefold when the cells were exposed to hyperosmotic conditions during the assay; similar results were obtained with strain MG1363. The importance of using iso-osmolar solutions to stop the reaction is evident from the observation that the “activated” uptake of glycine betaine uptake was no longer observed when the cells were exposed to a hypo-osmotic LiCl (100 instead of 500 mM) solution during the washing step. The fact that washing with a hypo-osmotic solution can obscure osmoregulatory solute transport was also established in other studies (1, 10). This is also the reason why in the earlier study by Molenaar et al. (11) no osmotic activation of glycine betaine transport was observed.
FIG. 1.
Activation of glycine betaine transport by hyperosmotic conditions. L. lactis IL1403 or MG1363 cells were grown semianaerobically at 29°C in CDM (without proline), pH 6.5, plus 25 mM glucose and 500 mM KCl, after which they were washed and resuspended in 50 mM KPi, pH 6.5. (A) Prior to the initiation of transport, IL1403 cells were pre-energized for 5 min with 10 mM glucose. Uptake of [14C]glycine betaine (1.25 mM, final concentration) was assayed in 50 mM KPi, pH 6.5, with (●, ■) or without (○) 500 mM KCl. The reaction was stopped with LiCl at 500 (●) or 100 (■, ○) mM. (B) Effect of hyperosmotic conditions on the uptake of alanine and glutamate. Experimental conditions and symbols are the same as for panel A, except that the uptake of [14C]alanine (solid lines) and [14C]glutamate (dotted lines) was assayed at 625 μM. (C) Effect of intracellular proline on the uptake of glycine betaine. Prior to the initiation of transport, MG1363 cells were pre-energized for 45 min with 10 mM glucose with (▴, ▵) or without (●, ○) 1.25 mM proline. In a parallel experiment, the proline was added at time zero, that is, simultaneously with [14C]glycine betaine (■, □). Uptake of [14C]glycine betaine was assayed in 50 mM KPi, pH 6.5, containing chloramphenicol at 50 μg/ml with (closed symbols) or without (open symbols) 500 mM KCl. The reactions were stopped with 500 (closed symbols) or 100 (open symbols) mM LiCl; this was followed by rapid filtration and washing of the filters.
Alanine and leucine uptake has previously been shown to be driven by proton motive force, whereas ATP effects that of glutamate (6). Neither of these transport systems is thought to play a role in osmoregulation. Consistent with this notion, and in contrast to the observations made for glycine betaine, the rate of uptake of alanine, glutamate, and leucine was not increased by hyperosmotic conditions (Fig. 1B). In fact, the osmotic upshift even inhibited the uptake of glutamate (Fig. 1B) and leucine (data not shown).
In L. plantarum and L. monocytogenes, it was observed that glycine betaine (and carnitine) and proline inhibited the uptake of glycine betaine in trans; that is, internal glycine betaine or proline decreased the net rate of uptake (4, 19). Glycine betaine and carnitine are taken up in L. monocytogenes by separate systems with high specificity, but both transporters are trans inhibited by glycine betaine as well as carnitine (19). In L. plantarum, glycine betaine, carnitine, and proline are taken up by one and the same system with an affinity constant for proline uptake that is almost 2 orders of magnitude higher than those for glycine betaine and carnitine (4). The trans inhibition of these systems is largely relieved when the cells are exposed to hyperosmotic stress, thereby permitting the cells to adjust their osmotic imbalance. Figure 1C shows that preloading of L. lactis MG1363 with proline under hyperosmotic conditions, which results in proline pools of at least 200 mM (11), did not affect the uptake of glycine betaine; similar results were obtained with strain IL1403. This suggests that, in contrast to that in L. plantarum, the uptake of glycine betaine is not trans inhibited by proline within this range. The control experiments show that under the experimental conditions used, the uptake of [14C]glycine betaine in L. lactis was not affected by an equal concentration of proline (compare squares and circles), which is consistent with the observation that the system has a much higher affinity for glycine betaine than for proline (11).
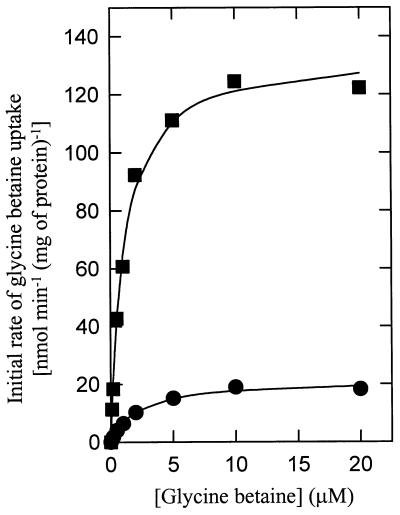
To distinguish kinetically between a single system and multiple systems and to determine the kinetic effect of osmotic activation, glycine betaine uptake was measured in the range of 0.1 μM to 1.25 mM and in the absence or presence of 500 mM KCl in L. lactis IL1403 grown under hyperosmotic conditions. Figure 2 shows that the maximal rate of uptake of glycine betaine increased from 19 to 134 nmol min−1 mg−1 of protein upon osmotic upshock, whereas the affinity constant was not significantly affected. The kinetics of glycine betaine uptake was monophasic over the entire concentration range, indicating that a single kinetically distinguishable uptake system is operative. The uptake of glycine betaine is activated by hyperosmotic stress, but the mechanism of osmotic regulation in L. lactis seems to be kinetically different from that in L. plantarum and L. monocytogenes. We conclude that the maximal rate of glycine betaine uptake in L. lactis is increased by osmotic upshock through direct activation of the system, whereas in L. plantarum and L. monocytogenes relief of trans inhibition forms a major component of the observed activation.
FIG. 2.
Kinetic parameters of glycine betaine uptake under hyperosmotic and osmostatic conditions. L. lactis IL1403 cells grown in CDM (without proline) plus 25 mM glucose and 500 mM KCl were washed and resuspended in 50 mM KPi, pH 6.5. Prior to the initiation of transport, IL1403 cells were pre-energized for 5 min with 10 mM glucose. Uptake of [14C]glycine betaine was assayed in 50 mM KPi, pH 6.5, with (■) or without (●) 500 mM KCl. The uptake rates were determined in the concentration range of 0.1 μM to 1.25 mM, but only the data up to 20 μM are shown. The transport assays were stopped after 10 s by diluting the samples 20-fold with ice-cold assay buffer of the corresponding osmolarity; this was followed by rapid filtration and washing of the filters. The data were analyzed with the Michaelis-Menten equation. The Km values were 1.7 and 1.5 μM in the presence and absence of 500 mM KCl, respectively.
Regulation of expression.
The effects of culture conditions on the regulation of expression and activity of the glycine betaine uptake system(s) were studied by cultivating strains IL1403 and MG1363 in low- and high-osmolarity media. The strains were grown in complex (GM17) or synthetic (CDM) medium with or without proline and in the presence or absence of 500 mM KCl. Although proline does stimulate the growth of L. lactis (17), the amino acid did not have significant effects on the transport activities in strain IL1403; some inhibitory effect of proline in the growth medium on the transport of glycine betaine was observed in strain MG1363 (Table 1). The data clearly indicate that in both strains glycine betaine uptake is induced by high osmolarity, provided the cells are grown in synthetic medium. Maximal transport activity was attained when the cells were grown under hyperosmotic conditions and subjected to hyperosmotic stress in the uptake assay. For reasons that are not entirely clear, the effect of hyperosmotic stress on the expression of the glycine betaine uptake system is absent in IL1403 and only moderate in MG1363 when the cells are grown in complex broth (Table 1). Opposite effects such as induction by hyperosmotic conditions and repression by high concentrations of (compatible) solutes, e.g., proline or quaternary ammonium compounds present in the complex broth, may be the cause for these observations.
TABLE 1.
Initial rates of glycine betaine uptake in L. lactis IL1403 and MG1363 grown in media of different osmotic strengths and assayed under conditions of low and high osmolarity
| Growth mediuma | Presence of 500 mM KCl in assay buffer | Avg activityb (nmol
min−1 mg−1 of protein) ± SD
|
|
|---|---|---|---|
| IL1403 | MG1363 | ||
| CDM − proline | − | <0.1 | 5 ± 1 |
| CDM − proline + 500 mM KCl | − | 3 ± 0.5 | 20 ± 2 |
| CDM | − | <0.1 | 1 ± 0.5 |
| CDM + 500 mM KCl | − | 1.5 ± 0.5 | 11 ± 2 |
| CDM − proline | + | 11 ± 2 | 24 ± 2 |
| CDM − proline + 500 mM KCl | + | 183 ± 30 | 87 ± 14 |
| CDM | + | 18 ± 3 | 5 ± 0.5 |
| CDM + 500 mM KCl | + | 131 ± 19 | 84 ± 9 |
| GM17 | − | <0.1 | 3 ± 1 |
| GM17 + 500 mM KCl | − | <0.1 | 8 ± 2 |
| GM17 | + | 10 ± 1 | 10 ± 2 |
| GM17 + 500 mM KCl | + | 9 ± 1 | 52.3 ± 11 |
CDM contains 675 mg of proline per liter. In the case of GM17, 0.5% glucose was added to the complex broth M17.
Presented are initial rates of glycine betaine uptake. The data are for duplicate experiments.
In addition to the use of iso-osmolar buffers to stop the transport reaction in the filtration assay, the experimental conditions used here also differed from those of Molenaar et al. (11) in a few other respects. In the previous study, the cells were washed with iso-osmotic buffers and, as a result, the intracellular pool of proline and other compatible solutes was considerable at the start of the uptake assay. Moreover, the effect of an osmotic upshift was only studied in cells grown and washed at low osmolarity, which involved suboptimal expression levels, as shown in Table 1. The present study shows that, in addition to the appropriate assay conditions, the history of the cells in terms of the medium used for growth and preparation of the cells for the uptake experiments strongly influences observations made with regard to the osmotic regulation of transport.
Regulation of glycine betaine efflux by osmotic downshock.
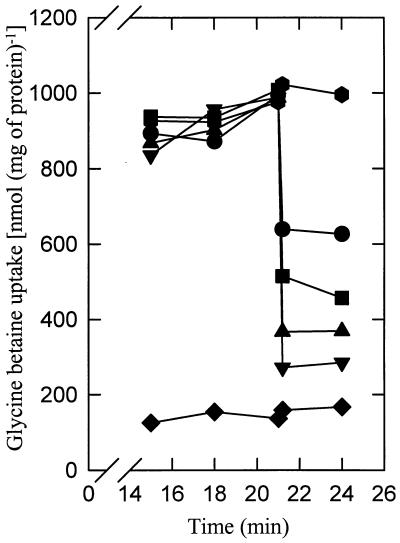
As suggested by the experiment whose results are shown in Fig. 1A, most of the accumulated glycine betaine was released when the cells were washed with hypo-osmotic “stop” solutions. To investigate this efflux in further detail, we diluted glycine betaine-accumulating cells of L. lactis to media of various osmolarities (Fig. 3). As observed in L. plantarum and L. monocytogenes (3, 4, 19), efflux is instantaneous and occurs in proportion to the osmotic downshock. The glycine betaine levels after osmotic downshock were reached within the sampling time of the experiment, that is, a few seconds. The very rapid efflux of glycine betaine is indicative of channel-like activities, as the rates are far too high for catalysis by an “ordinary” transport system (7, 13, 18).
FIG. 3.
Glycine betaine efflux is triggered by hypo-osmotic conditions. L. lactis IL1403 cells grown in CDM (without proline) plus 25 mM glucose and 500 mM KCl were washed and resuspended in 50 mM KPi, pH 6.5. Prior to the initiation of transport, IL1403 cells were pre-energized for 5 min with 10 mM glucose. Uptake of [14C]glycine betaine was assayed in 50 mM KPi, pH 6.5, with ( , ●, ■, ▴, ▾) or without (⧫) 500 mM KCl. The reaction mixtures were either not diluted ( , ⧫) or diluted 3 (●)-, 5 (■)-, 10 (▴)-, or 20 (▾)-fold with 50 mM KPi, pH 6.5, after 21 min of uptake. The reactions were stopped as described in the legend to Fig. 1, by using LiCl solutions of the appropriate osmolarity.
Concluding remarks.
Glycine betaine uptake in L. lactis is subject to osmotic regulation at two levels, that is, expression and transport activity. In L. plantarum, glycine betaine uptake is regulated mainly at the level of transport activity, which involves inhibition by accumulated solute(s) in dependence on the osmotic status of the cells (4). This phenomenon of trans inhibition is not evident from our studies on the osmotic regulation of glycine betaine uptake in L. lactis.
Acknowledgments
The research was supported by a grant from the Netherlands Foundation of Life Sciences, which is subsidized by the Netherlands Organization for Scientific Research.
REFERENCES
- 1.Cairney J, Booth I R, Higgins C F. Salmonella typhimurium proPencodes a transport system for the osmoprotectant betaine. J Bacteriol. 1985;164:1218–1223. doi: 10.1128/jb.164.3.1218-1223.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Csonka L N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989;53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Glaasker E, Konings W N, Poolman B. Glycine betaine fluxes in Lactococcus plantarumduring osmostasis and hyper- and hypo-osmotic shock. J Biol Chem. 1996;271:10060–10065. doi: 10.1074/jbc.271.17.10060. [DOI] [PubMed] [Google Scholar]
- 4.Glaasker E, Heuberger E H M L, Konings W N, Poolman B. Mechanism of osmotic activation of the quaternary ammonium compound transporter (QacT) of Lactococcus plantarum. J Bacteriol. 1998;180:5540–5546. doi: 10.1128/jb.180.21.5540-5546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gouesbet G, Trautweller A, Bonassie S, Wu L F, Blanco C. Characterization of the Erwinia chrysanthemi osmoprotectant transporter gene ousA. J Bacteriol. 1996;178:447–455. doi: 10.1128/jb.178.2.447-455.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konings W N, Driessen A J M, Poolman B. Bioenergetics and solute transport in lactococci. CRC Crit Rev Microbiol. 1989;16:419–476. doi: 10.3109/10408418909104474. [DOI] [PubMed] [Google Scholar]
- 7.Lambert C, Erdmann A, Eikmanns M, Krämer R. Triggering glutamate excretion in Corynebacterium glutamicumby modulating the membrane state with local anesthetics and osmotic gradients. Appl Environ Microbiol. 1995;61:4334–4342. doi: 10.1128/aem.61.12.4334-4342.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lucht J M, Bremer E. Adaptation of Escherichia colito high osmolarity environments: osmoregulation of the high-affinity glycine betaine transport system ProU. FEMS Microbiol Rev. 1994;14:3–20. doi: 10.1111/j.1574-6976.1994.tb00067.x. [DOI] [PubMed] [Google Scholar]
- 9.McLaggan D, Naprstek J, Buurman E T, Epstein W. Interdependence of K+ and glutamate accumulation during osmotic adaptation of Escherichia coli. J Biol Chem. 1994;269:1911–1917. [PubMed] [Google Scholar]
- 10.Milner J L, McClellan D J, Wood J M. Factors reducing and promoting the effectiveness of proline as an osmoprotectant in Escherichia coliK12. J Gen Microbiol. 1987;133:1851–1860. doi: 10.1099/00221287-133-7-1851. [DOI] [PubMed] [Google Scholar]
- 11.Molenaar D, Hagting A, Alkema H, Driessen A J, Konings W N. Characteristics and osmoregulatory roles of uptake systems for proline and glycine betaine in Lactococcus lactis. J Bacteriol. 1993;175:5438–5444. doi: 10.1128/jb.175.17.5438-5444.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patchett R A, Kelly A F, Kroll R G. Transport of glycine betaine by Listeria monocytogenes. Arch Microbiol. 1994;162:205–210. doi: 10.1007/BF00314476. [DOI] [PubMed] [Google Scholar]
- 13.Poolman B, Glaasker E. Regulation of compatible solute accumulation in bacteria. Mol Microbiol. 1998;29:397–407. doi: 10.1046/j.1365-2958.1998.00875.x. [DOI] [PubMed] [Google Scholar]
- 14.Poolman B, Konings W N. Relation of growth of Streptococcus lactis and Streptococcus cremoristo amino acid transport. J Bacteriol. 1988;170:700–707. doi: 10.1128/jb.170.2.700-707.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pourkomalian B, Booth I R. Glycine betaine transport by Staphylococcus aureus: evidence for feedback regulation of the activity of two transport systems. Microbiology. 1994;140:3131–3138. doi: 10.1099/13500872-140-11-3131. [DOI] [PubMed] [Google Scholar]
- 16.Record M T, Jr, Courtenay E S, Cayley D S, Guttman H J. Responses of E. colito osmotic stress: large changes in amounts of cytoplasmic solutes and water. Trends Biochem Sci. 1998;23:143–148. doi: 10.1016/s0968-0004(98)01196-7. [DOI] [PubMed] [Google Scholar]
- 17.Smid E J, Konings W N. Relationship between utilization of proline and proline-containing peptides and growth of Lactococcus lactis. J Bacteriol. 1989;172:5286–5292. doi: 10.1128/jb.172.9.5286-5292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sukharev S I, Blount P, Martinac B, Kung C. Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Annu Rev Physiol. 1997;59:633–657. doi: 10.1146/annurev.physiol.59.1.633. [DOI] [PubMed] [Google Scholar]
- 19.Verheul A, Glaasker E, Poolman B, Abee T. Betaine and l-carnitine transport by Listeria monocytogenesScott A in response to osmotic signals. J Bacteriol. 1997;179:6979–6985. doi: 10.1128/jb.179.22.6979-6985.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]