Abstract
A number of cyanobacteria from different taxonomic groups exhibited very low levels of uptake of 2-[U-14C]oxoglutarate. Synechococcus sp. strain PCC 7942 was transformed with DNA constructs carrying the Escherichia coli kgtP gene encoding a 2-oxoglutarate permease and a kanamycin resistance gene cassette. The Synechococcus sp. strains bearing the kgtP gene incorporated 2-oxoglutarate into the cells through an active transport process. About 75% of the radioactivity from the 2-[U-14C]oxoglutarate taken up that was recovered in soluble metabolites was found as glutamate and glutamine. 2-Oxoglutarate was, however, detrimental to the growth of a Synechococcus sp. strain bearing the kgtP gene.
The dominant mode of growth of cyanobacteria is photoautotrophy. These organisms show very simple nutritional requirements and are able to grow in water supplemented with a few mineral salts. An analysis of the putative transport proteins encoded in the chromosome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803 (11) has shown a relatively high proportion of transport systems whose predicted substrates would be ions (metals plus anions), i.e., 47% of all putative transporters, compared to 16 and 21% in Escherichia coli and Bacillus subtilis, respectively (22). On the other hand, only 6% of the putative transporters of strain PCC 6803 are predicted to have carbon compounds (carboxylates plus carbohydrates) as substrates, while carbon compound transporters represent 28 and 20% in E. coli and B. subtilis, respectively (22). One of the few carbon compound transporters experimentally identified to date in Synechocystis sp. strain PCC 6803 is the glucose permease encoded by the glcP (or gtr) gene (27, 34). This is a monocomponent permease consisting of a polypeptide of 468 amino acids with 12 putative membrane-spanning segments which is homologous to sugar transporters from several biological sources.
In cyanobacteria, the carbon compound that provides the skeleton for the assimilation of inorganic nitrogen, via the glutamine synthetase-glutamate synthase pathway, is 2-oxoglutarate (5, 18). In the unicellular cyanobacterium Synechococcus sp. strain PCC 7942, 2-oxoglutarate also appears to have a key regulatory role in the integration of carbon and nitrogen metabolisms, e.g., it determines the phosphorylation level of the PII protein (glnB gene product) (6, 10), which is required for the inhibition by ammonium of nitrate uptake to take place (12). We were interested in studying the fate and roles of 2-oxoglutarate in cyanobacteria, but found that these organisms take up 2-oxoglutarate very poorly when it is supplied to the cells at a low concentration (see below). We therefore sought the construction of a strain of Synechococcus sp. able to transport 2-oxoglutarate.
The kgtP gene of E. coli encodes a 432-amino-acid polypeptide which bears 12 putative membrane-spanning regions and mediates 2-oxoglutarate transport (28). Because the KgtP protein is similar (28% overall amino acid sequence identity) to Synechocystis sp. strain PCC 6803 GlcP, we wondered whether it could be functional in a cyanobacterium. In this note, we report the construction of some Synechococcus sp. strain PCC 7942 derivatives transformed with kgtP and show that they can efficiently transport 2-oxoglutarate.
Methods.
Cyanobacteria were grown in BG11 medium, which contains nitrate as the nitrogen source (25), at 30°C in the light, with shaking (80 to 90 rpm) for liquid cultures. When ammonium replaced nitrate as the nitrogen source, it was added as 2.5 mM NH4Cl, and the medium was buffered with 5 mM TES [N-tris(hydroxymethyl)methyl-2-aminoethanesulfonic acid]–NaOH (pH 7.5). For plates, the medium was solidified with 1% separately autoclaved agar (Difco). Synechococcus sp. strains carrying gene cassette C.K1 or C.K2 (4), both of which determine resistance to kanamycin (Kmr), were grown in medium supplemented with 10 μg of kanamycin · ml−1. Cyanobacterial cell mass was estimated by measuring the concentration of chlorophyll a (Chl), determined in methanolic extracts of the cells (15), or the concentration of protein, determined by a modified Lowry procedure (16) with bovine serum albumin as a standard. The growth rate constant, μ (μ = ln 2/td [where td represents the doubling time]) was calculated from the increase of protein concentration in the cultures. Genomic DNA from cyanobacteria was isolated as previously described (2).
For uptake assays, the cells were harvested by centrifugation at room temperature, washed with 25 mM Tricine–NaOH buffer (pH 8.1), and resuspended in the same buffer supplemented with 2.5 mM NH4Cl and 5 mM TES-NaOH buffer (pH 7.5) to give a cell density corresponding to 10 to 15 μg of Chl · ml−1. After a preincubation at 30°C in the light (100 W · m−2 [white light]) for 5 to 30 min, the assays were started by the addition of 2-[U-14C]oxoglutarate (3.84 to 280 μCi · μmol−1 [NEN]) at the concentration indicated in each experiment. After incubation for different time periods up to 30 min, 0.1- to 1-ml samples were withdrawn, filtered (0.45-μm-pore-size Millipore HA filters), and washed with 2 to 5 ml of 5 mM Tricine–NaOH buffer (pH 8.1). The filters carrying the cells were then immersed in scintillation cocktail, and their radioactivity was measured. Retention of radioactivity by boiled cells was used as a blank. In some experiments, as indicated, ammonium and 0.1 mM l-methionine-d,l-sulfoximine (MSX) were either absent or added to the cell suspension 30 min before the assay was started. To identify intracellular radioactively labelled metabolites, filters containing cells that had been used in 30-min uptake assays were immersed in 2 ml of water and incubated at 100°C for 5 min. The filters were removed, and the resulting suspensions were centrifuged. Samples from the supernatants were lyophilized and dissolved in a small volume of water. Metabolites present in these samples were resolved by thin-layer chromatography (TLC), with 0.1-mm-thick cellulose plates (20 by 20 cm; Merck). Two-dimensional separation of metabolites was effected by using the following solvents: first dimension, n-butanol–acetone–ammonium hydroxide–water (20:20:10:4 [vol/vol]); second dimension, isopropanol-formic acid-water (20:1:5 [vol/vol]). The resulting radioactive areas were quantified in an InstantImager scanner for β particles (Packard) whose efficiency in detection of 14C β particles was 1.12%. To calculate intracellular metabolite concentrations, an intracellular volume of 125 μl · mg of Chl−1 (5 μl · mg of protein−1) was assumed (9, 24, 30).
E. coli DH5α, used for all plasmid constructions, was grown in Luria-Bertani medium with, when necessary, 25 μg of kanamycin · ml−1, 50 μg of ampicillin · ml−1, 25 μg of streptomycin · ml−1, and 100 μg of spectinomycin · ml−1. Molecular biology manipulations were performed by standard procedures (1). The 2.8-kb AflII-SalI fragment from plasmid pCW28 (isolated by using polylinker restriction endonuclease sites as a BamHI-HindIII fragment), which carries the E. coli kgtP gene and its putative promoter (28), was cloned (after filling in with Klenow enzyme) together with the C.K2 or C.K1 gene cassette (4) into an EcoRV site present in the Synechococcus sp. strain PCC 7942 chromosomal DNA fragment of plasmid pCSI49b (see below) (13), rendering the constructs depicted in Fig. 1. Transformation of Synechococcus sp. strain PCC 7942 (8) and Southern analysis under high-stringency conditions with Hybond-N+ membranes (Amersham) were performed essentially as described previously (7).
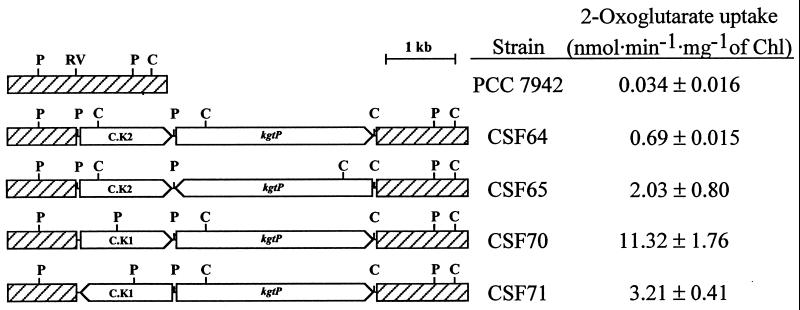
FIG. 1.
Schematic representation of the Kmr-encoding gene cassette-kgtP gene constructs used to transform Synechococcus sp. strain PCC 7942 and 2-[U-14C]oxoglutarate uptake activities of the generated Synechococcus sp. strains. The orientations of the npt (in the gene cassette) and kgtP genes and the positions of some restriction endonuclease cutting sites (C, ClaI; P, PstI; RV, EcoRV) are indicated. The hatched boxes denote Synechococcus sp. strain PCC 7942 genomic DNA. Uptake of 20 μM 2-[U-14C]oxoglutarate was determined as described in the text; data are the mean and standard deviation of three to five determinations.
Uptake of 2-[U-14C]oxoglutarate.
The uptake of 20 μM 2-[U-14C]oxoglutarate was tested, in 10-min assays, in different cyanobacteria with the following results (mean of two determinations): Anabaena sp. strain PCC 7120, 0.77 nmol · mg of Chl−1; Calothrix sp. strain PCC 7601, 0.46 nmol · mg of Chl−1; Fischerella muscicola UTEX 1829, 0.65 nmol · mg of Chl−1; Pseudanabaena sp. strain PCC 6903, 0.64 nmol · mg of Chl−1; Synechocystis sp. strain PCC 6803, 0.55 nmol · mg of Chl−1; and Synechococcus sp. strain PCC 7942, 0.45 nmol · mg of Chl−1. These can be considered as relatively poor activities that are, on average, about 100 times lower than the rates of uptake of several amino acids (supplied at 10 μM) in the same cyanobacterial strains (20).
The kgtP gene from E. coli was inserted, along with a Kmr-encoding gene cassette, into a chromosomal DNA fragment from Synechococcus sp. strain PCC 7942 that has been observed to tolerate insertions of heterologous DNA (13). Four different structures were generated with two different Kmr gene cassettes, C.K1 and C.K2, positioned in different relative orientations with respect to that of the kgtP gene (Fig. 1). The four constructs were transferred to Synechococcus sp. strain PCC 7942 by transformation and selection for Kmr. The presence of the kgtP gene and the relative orientation of this gene and the C.K1 or C.K2 gene cassette were confirmed by Southern analysis for one Synechococcus sp. clone from each transformation (not shown). The names of the Synechococcus sp. strain PCC 7942 derivatives carrying the different constructs are indicated in Fig. 1.
Time course assays of 2-[U-14C]oxoglutarate uptake showed that for the four Synechococcus sp. strains carrying the kgtP gene, uptake was linear for at least 30 min. Uptake rate data are summarized in Fig. 1. Strain PCC 7942 clones transformed with similar constructs bearing only C.K1 or C.K2 showed 2-[U-14C]oxoglutarate uptake activities identical to that of the wild-type strain (not shown). The maximal level of expression attained, observed in strain CSF70, which showed an uptake activity 333-fold higher than that of strain PCC 7942, was facilitated by positioning of kgtP downstream from the C.K1 gene cassette so that the npt and kgtP genes are situated in the same orientation (Fig. 1). Because the C.K1 gene cassette does not bear any transcription terminator downstream from the npt gene (14, 17), the high uptake activity detected in strain CSF70 is likely a consequence of a high level of transcription of the kgtP gene from the npt gene promoter.
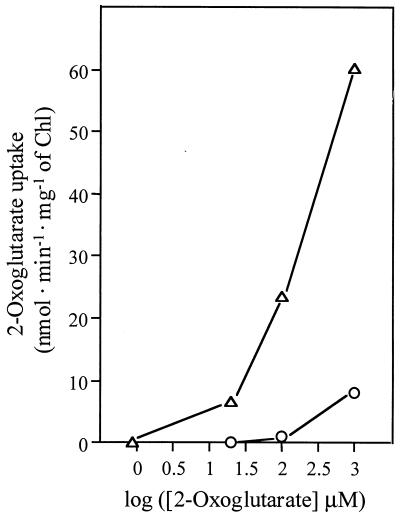
The effect of a wide range of concentrations of 2-oxoglutarate on the rate of uptake was investigated in wild-type strain PCC 7942 and in strain CSF70 (Fig. 2). Based on the data shown in Fig. 2, the following 2-oxoglutarate uptake kinetic parameters were calculated for strain CSF70: Ks, 205 μM; Vmax, 70 nmol · min−1 · mg of Chl−1 (about 2.5 nmol · min−1 · mg of protein−1). In an independent experiment in which 2-oxoglutarate was supplied at concentrations from 20 to 500 μM, the following kinetic parameters were determined: Ks, 147 μM; Vmax, 165 nmol · min−1 · mg of Chl−1 (about 6 nmol · min−1 · mg of protein−1). In E. coli MC1061 or in membrane vesicles prepared from it, the following kinetic parameters have been reported for 2-oxoglutarate uptake: Ks, 13 to 46 μM; Vmax, 8 to 11 nmol · min−1 · mg of protein−1 (29). Some retention of radioactivity by cells of Synechococcus sp. strain PCC 7942 incubated with 1 mM 2-[U-14C]oxoglutarate (see Fig. 2) was repetitively observed. Rather than taking place via a specific transport system, this low-affinity uptake might be the result of diffusion or might be mediated by another permease, e.g., the acidic amino acid permease present in Synechococcus sp. strain PCC 7942 (20).
FIG. 2.
Effect of the concentration of 2-oxoglutarate on the rate of 2-oxoglutarate uptake in Synechococcus sp. strains PCC 7942 (circles) and CSF70 (triangles). The cells were supplemented with the indicated 2-[U-14C]oxoglutarate concentrations, and uptake was determined as described in the text.
Fate of 2-[U-14C]oxoglutarate.
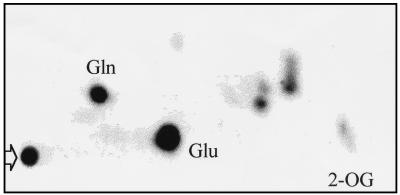
The presence of labelled metabolites was studied with cells of strain CSF70 incubated with 2-[U-14C]oxoglutarate for 30 min under the standard conditions used in this work (Fig. 3). Most label was associated with glutamate (53.9% of the radioactivity in soluble metabolites) and glutamine (18.3%), while five other metabolites together accounted for 15.0%, and a spot that could be identified as 2-oxoglutarate represented 12.8%. Although ammonium was routinely included in our standard assays to ensure metabolism of 2-oxoglutarate, this did not have a major effect on 2-oxoglutarate uptake, at least when a low concentration of 2-oxoglutarate was used (Table 1). MSX, an inhibitor of glutamine synthetase, on the other hand, blocked production of glutamine and impaired 2-oxoglutarate uptake (Table 1). This indicates that the rate of uptake of 2-oxoglutarate was dependent on its metabolism via glutamate synthase, which catalyzes the synthesis of two glutamate molecules from 2-oxoglutarate and glutamine. Synthesis of labelled glutamate from 2-[U-14C]oxoglutarate in MSX-treated cells will depend on the intracellular pool of unlabelled glutamine present in those cells before the addition of MSX.
FIG. 3.
Fate of 2-[U-14C]oxoglutarate in Synechococcus sp. strain CSF70. The cells were supplemented with 30 μM 2-[U-14C]oxoglutarate and incubated for 30 min under standard conditions (i.e., in the presence of ammonium). Metabolites were then extracted and analyzed by TLC as described in the text. The amount of extract loaded onto the TLC plate corresponded to an amount of cells containing 0.52 μg of Chl. The arrow points to the origin of the chromatogram, where some radioactive material accumulated (Table 1). Glu, glutamate; Gln, glutamine; 2-OG, 2-oxoglutarate. The glutamate and glutamine spots were identified by cochromatography with unlabelled amino acids that were revealed by the standard ninhydrin reaction. The 2-oxoglutarate spot was identified by cochromatography with labelled 2-oxoglutarate. Controls run with authentic 2-[U-14C]oxoglutarate indicated that some decomposition of 2-oxoglutarate took place during the development of the chromatography, so that the radioactivity in the spot identified as 2-oxoglutarate accounted for 24.7% of the total radioactivity of the 2-[U-14C]oxoglutarate applied to the TLC.
TABLE 1.
Effect of ammonium and MSX on the uptake and fate of 2-[U-14C]oxoglutarate in Synechococcus sp. strain CSF70a
| Addition(s) | 2-Oxoglutarate uptake (nmol · min−1 · mg of Chl−1) | Radioactivity in soluble metabolites (cpm · μg of Chl−1)
|
|||
|---|---|---|---|---|---|
| 2-Oxoglutarateb | Glutamate | Glutamine | Others | ||
| None | 7.0 ± 1.0 | 33.4 ± 21.5 | 189.6 ± 18.1 | 149.5 ± 52.3 | 60.7 ± 5.6 |
| MSX | 1.4 ± 0.3 | 43.9 ± 9.3 | 100.9 ± 35.3 | 0.0 | 17.8 ± 4.9 |
| NH4+c | 8.4 ± 1.6 | 49.8 ± 18.4 | 210.6 ± 23.4 | 71.6 ± 25.3 | 58.5 ± 17.1 |
| NH4+, MSX | 0.5 ± 0.1 | 14.2 ± 4.0 | 31.8 ± 11.1 | 0.0 | 9.6 ± 1.6 |
The cells were supplemented with the indicated additions (see the text) and incubated for 30 min. 2-[U-14C]oxoglutarate was then added at 30 μM, and after a further incubation of 30 min, metabolites were extracted and analyzed by TLC as described in the text and in the legend to Fig. 3. Data are the means and standard deviations of three independent experiments. In all cases, uptake was linear for the 30-min incubation period.
Values corrected for the fraction of 2-oxoglutarate decomposed during the chromatography (see the legend to Fig. 3).
Only for the cells incubated with ammonium and no MSX, was about 25% of the radioactivity loaded into the TLC found in the origin of the chromatogram. This value was lower than 4% in the other cases.
The highest levels of 2-[U-14C]oxoglutarate accumulated (Table 1) corresponded to intracellular concentrations of about 50 to 60 μM. Considering a membrane potential of −120 mV (21, 26), the electrochemical potential of 2-oxoglutarate (intracellular concentration of labelled 2-oxoglutarate, 60 μM; extracellular concentration at the time of sampling, 27 μM) would correspond to +260 mV, implying that 2-oxoglutarate is actively transported into the cell. Moreover, this figure was derived without taking into account the intracellular pool of unlabelled 2-oxoglutarate, which can be in the order of 16 to 340 μM (calculated from data in references 3 and 19). The KgtP permease is a proton symporter (29), and photosynthetically active cyanobacterial cells maintain, under incubation conditions similar to those used in this work, an electrochemical proton potential of about −100 mV (26). The active transport of 2-oxoglutarate in Synechococcus sp. can therefore proceed, as is the case in E. coli (29), through a proton symport mechanism.
Growth rates.
To test any possible effects of the presence of the KgtP permease on the growth of Synechococcus sp., the growth rate of strain CSF70 was determined under the conditions summarized in Table 2. As a control, a strain, PCC 7942::C.K1, carrying the C.K1 gene cassette in the same location and orientation as in CSF70, was used. The presence of the permease itself was not detrimental to the growth of Synechococcus sp. Addition of 1 mM 2-oxoglutarate, however, slowed down the growth of the kgtP-bearing strain, but not that of the control strain. This effect was more pronounced when ammonium was used as the nitrogen source (Table 2). Nonetheless, the cells were not killed by 2-oxoglutarate and kept growing for a long period of time. Whether the observed negative effect is due to an imbalance of metabolites within the cells, to a bioenergetic shortage caused by 2-oxoglutarate transport and affecting other membrane transport functions, or to a general effect on the physiology of the cell such as a change of intracellular pH caused by the influx of a dicarboxylate, is currently unknown.
TABLE 2.
Effect of 2-oxoglutarate on the growth of Synechococcus sp.a
| N source | μb (day−1) for strain:
|
|||
|---|---|---|---|---|
| PCC 7942::C.K1
|
CSF70
|
|||
| −2-OG | +2-OG | −2-OG | +2-OG | |
| Nitrate | 0.39 ± 0.05 | 0.37 ± 0.06 | 0.37 ± 0.05 | 0.083 ± 0.07 |
| Ammonium | 0.29 ± 0.02 | 0.28 ± 0.04 | 0.40 ± 0.11 | 0.018 ± 0.01 |
The cells were grown with the indicated nitrogen source in the presence of kanamycin (10 μg · ml−1) and, when indicated, 1 mM 2-oxoglutarate (2-OG). Data are the mean and standard deviation of three independent experiments.
μ = ln 2/td, where td is doubling time.
Conclusions.
The results presented above show the feasibility of expressing a secondary active transporter from a heterologous biological source in a cyanobacterium. Expression of heterologous genes in cyanobacteria is well documented: e.g., the antibiotic resistance genes widely used in molecular genetics work (32, 33), the gene encoding ribulose bisphosphate carboxylase from Rhodospirillum rubrum that has been expressed in Synechocystis sp. strain PCC 6803 (23), or the sacB gene encoding a levansucrase that has been expressed in Anabaena sp. strain PCC 7120 (2). A derivative of Synechococcus sp. strain PCC 7942 transformed with the glcP gene from Synechocystis sp. strain PCC 6803 has been shown to take up glucose (35). We are not aware, however, of any previous expression in a cyanobacterium of a permease from a noncyanobacterial source.
Cyanobacteria lack 2-oxoglutarate dehydrogenase and bear an incomplete citric acid cycle that produces 2-oxoglutarate, which is not futher catabolized and is used for biosynthetic purposes (31). Consistently, the fate of 2-[U-14C]oxoglutarate observed in this work mainly consisted of its incorporation into glutamate through the reaction catalyzed by glutamate synthase. This limited use of 2-oxoglutarate may be a rationale to explain why the cyanobacteria, which when provided with an appropriate permease can transport 2-oxoglutarate, appear not to have acquired transporters specific for this compound. Additionally, the metabolism and/or transport capabilities of Synechococcus sp. strain PCC 7942 appear to have been tuned in a way that makes this organism not tolerate well exposure to 2-oxoglutarate when it has also been provided with a 2-oxoglutarate permease.
Acknowledgments
We thank W. Seol and A. J. Shatkin for kindly providing us with E. coli kgtP clones and M. M. Allen for useful discussion.
This work was supported by grant PB95-1267 from the Dirección General de Enseñanza Superior (Spain). M.F.V.-B. was the recipient of a fellowship from the Plan de Formación de Personal Investigador, Ministerio de Educación y Cultura (Spain).
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing and Wiley Interscience; 1998. [Google Scholar]
- 2.Cai Y, Wolk C P. Use of a conditionally lethal gene in Anabaena sp. strain PCC 7120 to select for double recombinants and to entrap insertion sequences. J Bacteriol. 1990;172:3138–3145. doi: 10.1128/jb.172.6.3138-3145.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronil T, Lara C, Guerrero M G. Shift in carbon flow and stimulation of amino-acid turnover induced by nitrate and ammonium assimilation in Anacystis nidulans. Planta. 1993;189:461–467. doi: 10.1007/BF00194446. [DOI] [PubMed] [Google Scholar]
- 4.Elhai J, Wolk C P. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene. 1988;68:119–138. doi: 10.1016/0378-1119(88)90605-1. [DOI] [PubMed] [Google Scholar]
- 5.Flores E, Herrero A. Assimilatory nitrogen metabolism and its regulation. In: Bryant D A, editor. The molecular biology of cyanobacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 487–517. [Google Scholar]
- 6.Forchhammer K, Tandeau de Marsac N. Phosphorylation of the PII protein (glnB gene product) in the cyanobacterium Synechococcus sp. strain PCC 7942: analysis of in vitro kinase activity. J Bacteriol. 1995;177:5812–5817. doi: 10.1128/jb.177.20.5812-5817.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frías J E, Mérida A, Herrero A, Martín-Nieto J, Flores E. General distribution of the nitrogen control gene ntcA in cyanobacteria. J Bacteriol. 1993;175:5710–5713. doi: 10.1128/jb.175.17.5710-5713.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Golden S S, Sherman L A. Optimal conditions for genetic transformation of the cyanobacterium Anacystis nidulans R2. J Bacteriol. 1984;158:36–42. doi: 10.1128/jb.158.1.36-42.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ihlenfeldt M J A, Gibson J. CO2 fixation and its regulation in Anacystis nidulans (Synechococcus) Arch Microbiol. 1975;102:13–21. doi: 10.1007/BF00428339. [DOI] [PubMed] [Google Scholar]
- 10.Irmler A, Sanner S, Dierks H, Forchhammer K. Dephosphorylation of the phosphoprotein PII in Synechococcus PCC 7942: identification of an ATP and 2-oxoglutarate-regulated phosphatase activity. Mol Microbiol. 1997;26:81–90. doi: 10.1046/j.1365-2958.1997.5521918.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assigment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 12.Lee H-M, Flores E, Herrero A, Houmard J, Tandeau de Marsac N. A role for the signal transduction protein PII in the control of nitrate/nitrite uptake in a cyanobacterium. FEBS Lett. 1998;427:291–295. doi: 10.1016/s0014-5793(98)00451-7. [DOI] [PubMed] [Google Scholar]
- 13.Luque, I. Unpublished results.
- 14.Luque I, Flores E, Herrero A. Nitrate and nitrite transport in the cyanobacterium Synechococcus sp. PCC 7942 are mediated by the same permease. Biochim Biophys Acta. 1994;1184:296–298. [Google Scholar]
- 15.Mackinney G. Absorption of light by chlorophyll solutions. J Biol Chem. 1941;140:315–322. [Google Scholar]
- 16.Markwell M A K, Haas S M, Bieber L L, Tolbert N E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 17.Mazodier P, Cossart P, Giraud E, Gasser F. Completion of the nucleotide sequence of the central region of Tn5 confirms the presence of three resistance genes. Nucleic Acids Res. 1985;13:195–205. doi: 10.1093/nar/13.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meeks J C, Wolk C P, Lockau W, Schilling N, Shaffer P W, Chien W-S. Pathways of assimilation of [13N]N2 and 13NH4+ by cyanobacteria with and without heterocysts. J Bacteriol. 1978;134:125–130. doi: 10.1128/jb.134.1.125-130.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mérida A, Candau P, Florencio F J. Regulation of glutamine synthetase activity in the unicellular cyanobacterium Synechocystis sp. strain PCC 6803 by the nitrogen source: effect of ammonium. J Bacteriol. 1991;173:4095–4100. doi: 10.1128/jb.173.13.4095-4100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montesinos M L, Herrero A, Flores E. Amino acid transport in taxonomically diverse cyanobacteria and identification of two genes encoding elements of a neutral amino acid permease putatively involved in recapture of leaked hydrophobic amino acids. J Bacteriol. 1997;179:853–862. doi: 10.1128/jb.179.3.853-862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paschinger H. DCCD induced sodium uptake by Anacystis nidulans. Arch Microbiol. 1977;113:285–291. doi: 10.1007/BF00492037. [DOI] [PubMed] [Google Scholar]
- 22.Paulsen I T, Sliwinski M K, Saier M H., Jr Microbial genome analyses: global comparisons of transport capabilities based on phylogenies, bioenergetics and substrate specificities. J Mol Biol. 1998;277:573–592. doi: 10.1006/jmbi.1998.1609. [DOI] [PubMed] [Google Scholar]
- 23.Pierce J, Carlson T J, Williams J G K. A cyanobacterial mutant requiring the expression of ribulose bisphosphate carboxylase from a photosynthetic anaerobe. Proc Natl Acad Sci USA. 1989;86:5753–5757. doi: 10.1073/pnas.86.15.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raboy B, Padan E. Active transport of glucose and α-methylglucoside in the cyanobacterium Plectonema boryanum. J Biol Chem. 1978;253:3287–3291. [PubMed] [Google Scholar]
- 25.Rippka R, Deruelles J, Waterbury J B, Herdman M, Stanier R Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J Gen Microbiol. 1979;111:1–61. [Google Scholar]
- 26.Ritchie R J. Membrane potential and pH control in the cyanobacterium Synechococcus R-2 (Anacystis nidulans) PCC 7942. J Plant Physiol. 1991;137:409–418. [Google Scholar]
- 27.Schmetterer G R. Sequence conservation among the glucose transporter from the cyanobacterium Synechocystis sp. PCC 6803 and mammalian glucose transporters. Plant Mol Biol. 1990;14:697–706. doi: 10.1007/BF00016502. [DOI] [PubMed] [Google Scholar]
- 28.Seol W, Shatkin A J. Escherichia coli kgtP encodes an α-ketoglutarate transporter. Proc Natl Acad Sci USA. 1991;88:3802–3806. doi: 10.1073/pnas.88.9.3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seol W, Shatkin A J. Escherichia coli α-ketoglutarate permease is a constitutively expressed proton symporter. J Biol Chem. 1992;267:6409–6413. [PubMed] [Google Scholar]
- 30.Shelp B J, Canvin D T. Evidence for bicarbonate accumulation by Anacystis nidulans. Can J Bot. 1984;62:1398–1403. [Google Scholar]
- 31.Smith A J, London J, Stanier R Y. Biochemical basis of obligate autotrophy in blue-green algae and thiobacilli. J Bacteriol. 1967;94:972–983. doi: 10.1128/jb.94.4.972-983.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Hondel C A M J J, Verbeek S, van der Ende A, Weisbeek P, Borrias W E, van Arkel G A. Introduction of transposon Tn901 into a plasmid of Anacystis nidulans: preparation for cloning in cyanobacteria. Proc Natl Acad Sci USA. 1980;77:1570–1574. doi: 10.1073/pnas.77.3.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wolk C P, Vonshak A, Kehoe P, Elhai J. Construction of shuttle vectors capable of conjugative transfer from Escherichia coli to nitrogen-fixing filamentous cyanobacteria. Proc Natl Acad Sci USA. 1984;81:1561–1565. doi: 10.1073/pnas.81.5.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang C-C, Durand M-C, Jeanjean R, Joset F. Molecular and genetical analysis of the fructose-glucose transport system in the cyanobacterium Synechocystis PCC6803. Mol Microbiol. 1989;3:1221–1229. doi: 10.1111/j.1365-2958.1989.tb00272.x. [DOI] [PubMed] [Google Scholar]
- 35.Zhang C-C, Jeanjean R, Joset F. Obligate phototrophy in cyanobacteria: more than a lack of sugar transport. FEMS Microbiol Lett. 1998;161:285–292. doi: 10.1111/j.1574-6968.1998.tb12959.x. [DOI] [PubMed] [Google Scholar]