Abstract
Background
Breast cancer-related lymphedema (BCRL) is a frequent issue that arises after mastectomy surgery in women and compromises physical and mental function. Previously published studies have shown positive effects with the use of Low-level laser therapy in another term Photo-biomodulation therapy (PBM). This research investigated the efficacy of clinical use of LLLT (PBM) in the treatment of metastatic breast cancer-related lymphedema.
Methods
PubMed, PEDro, Medline, and the Cochrane Library were searched for LLLT clinical trials published before October 2021. The methodological quality of randomized trials and the effectiveness of Laser Therapy for BCRL were evaluated. The primary objectives were arm circumference or arm volume, whereas the secondary goals were to assess shoulder mobility and pain severity.
Results
Eight clinical trials were analyzed in total. Typically, the included RCTs had good research quality. At four weeks, there was a considerable reduction in arm circumference/volume, and this continued with long-term follow-up. However, no statistically significant change in shoulder mobility or pain severity was seen between the laser and placebo groups at 0-, 1-, 2-, and 3-month short-term follow-up.
Conclusions
The findings of this comprehensive study demonstrated that LLLT (PBM) was successful in diminishing arm circumference and volume than improving shoulder mobility and pain. Data indicates that laser therapy (PBM) may be a beneficial treatment option for females with PML. Because of the scarcity of evidence, there is a strong need for well-conducted and longer-duration trials in this field.
Trial registration
Details of the protocol for this systematic review were registered on PROSPERO and can be accessed at www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42022315076.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-022-10021-8.
Keywords: Low-level laser therapy, Photo-biomodulation therapy, Laser therapy, Breast cancer, Lymphedema, Postmastectomy lymphedema, Systematic review
Background
Breast cancer is still frequent cancer in women all over the world. An aberrant expansion of cells in the breast tissue is what it is called. Milk-producing glands called lobules and ducts that link the lobules to the nipple make up the tissues. Lymphatic, connective, and fatty tissues make up the remainder of the breast. The carcinoma spreads to the lining cells (epithelial tissue) of the glandular tissue's ducts or lobules. In industrialized nations (e.g., Europe, the United States, and Japan), about 82 percent of women survive ten years after being diagnosed with breast cancer [1]. Although Asian countries have a lower incidence of breast cancer, cause-specific mortality is substantially greater in most Asian countries than in Western ones [2].
Lumpectomy (removal of the tumor) or Mastectomy (surgical removal of the entire breast) are well-known treatment options for breast cancer. These are continuing operations for woman’s survival from breast cancer, depending on the spread stage. In general, women are under-informed about the condition and its potential repercussions. Lymphedema has always been the most prevalent problem following therapy. Lymphedema is a chronic disorder in which protein-rich edema accumulates in the tissue spaces [3]. Dysfunction in the axillary drainage system induced by surgeries or laser therapy causes it to worsen. All lymph fluid drains to the axillary lymph nodes from one side of the upper body (chest, ribcage, arm, and hand). This flow is more prone to be affected when more lymph nodules and veins are removed, and could result in lymphoedema.
Women experience a variety of issues related to breast cancer therapy, aside from edema buildup in the affected arm. Patients experiences some medical conditions as a result of side effects after therapy. Cancer treatments are effective in killing cancer cells, but most of them also harm healthy cells and can alter a woman's physical or emotional state. During heavy doses of chemotherapy, radiation therapy, and hormone therapy, a woman may undergo symptoms such as poor appetite, nausea, vomiting, weakness, and hair loss. Physical appearance and psychological thoughts are both impacted by external body changes, which can cause depression, sadness, and a sense of loneliness. Exercise is one of the best strategies for managing these conditions. When used for longer than three months, a rehabilitation programme that incorporates yoga and various forms of exercise has shown to control mood swings in women [4].
As a result of long-term side effect of treatment, lymphedema causes swelling in the limbs, persistent inflammation, tissue tearing, infection, and limited motion. In addition, swelling, heaviness, hardness, tenderness, soreness, numbness, itching, and stiffness are among the signs of lymphedema in breast cancer survivors [5, 6]. Although there are many different methods for measuring arm volumes, including traditional volumetry with overflow, volumetry without overflow, and inverse volumetry, but volume based on arm circumference is still the most common one used [7]. Even though standard approach is still the best option for measuring arm fluid, a new portable three-dimension laser system (called 3DLS) for measuring upper limb volume has also produced promising findings for the diagnosis of lymphedema [8]. To be more specific, the 3DLS technology uses a triangulation process that involves projecting a laser dot onto an object (in this case, the upper limb) to represent the 3D model, and then a sensor calculates the distance to the item's surface. The 3DLS method for rapid volume measurement is a new standardized augmented reality-based technique [9].
There is no definitive medical or surgical cure for lymphedema, the fluid return is thought to be managed by standard therapy and physical activity. Surprisingly, a complete decongestive therapy (CDT) treatment can be utilized to reduce lymphedema rates. Multilayer bandaging (MLB), compression therapy, bilateral lymphatic drainage, and a healthy exercise regimen are all part of the treatment. Furthermore, secondary lymphedema can be treated conservatively without harm from the given interventions [10]. Nowadays, laser therapy has been utilized to treat (PML) postmastectomy lymphedema. Although it has been in use since past 2 decade,but due to its high level of demand it is being clinically being applied for various medical conditions. Moreover, laser treatment, also referred to as Low-level Laser Therapy (LLLT), has been demonstrated to help slow the progression of recurrent lymphedema caused by breast cancer.
Low-level laser therapy is a nonionizing light-based conservative therapy that has been utilized to treat lymphedema in women with breast cancer [11]. Photons of a specified wavelength (650 nm and 1000 nm) penetrate skin tissue to give low rays and doses to the targeted area in laser treatment or photo-biomodulation therapy (PBM). It has been implemented to help with lymphatic fluidity, redness, lymph vessel restoration, and tissue stiffness prevention [12–15]. Biochemical changes at the cellular level, on the other hand, are the critical mechanism for employing LLLT (PBM).
Fibroblasts, osteoblasts, lymphocytes, and smooth cells are all altered during the therapy. These effects result from instantaneous reactions involving the absorption of specific light wavelengths. The cytochromes, cytochrome oxidase, and flavin dehydrogenases in the mitochondrial respiratory chain absorb the rays, causing changes in the reduction–oxidation reaction (REDOX) state of the cytoplasm and mitochondria, which leads to increasing the levels of adenosine triphosphate (ATP) [16]. After (ATP) synthesis, an increase in metabolic energy triggers a subsequent critical process for cellular repair. Furthermore, intracellular signalling and cytokine activation allow for various responses, including the development of new lymphatic vessels, the release of growth factors, and metabolic upregulation [17–19]. As a result, LLLT (PBM) helps enhance the immune system by facilitating the drainage of excess protein-rich fluid and increasing macrophage formation.
The usefulness of laser for the therapy of breast cancer-related lymphedema has been the subject of little published research during the last twenty years (BCRL). The most current Systematic review, published in 2017, included Randomized Control Trials (RCTs) and observational studies with a follow-up of fewer than six months conducted between 1998 and 2013, revealing the RCTs' short-term follow-up [20]. Efficacy in treating women with BCRL has been studied in several RCTs. Five short studies of appropriate methodological quality were used by Omar et al. to provide moderate to strong evidence for the benefit of laser therapy in the treatment of BCRL [21]. Another clinical investigation, done by Carati CJ et al., had two experimental groups and reported that after two cycles of LLLT, the mean impacted limb volume tended to decrease over time [22].
As a result, the goal of this study was to gather all updated clinical studies published between 2010 and 2022. In addition, this study looked at clinical trials that focused on the efficacy of LLLT (PBM) for mature females with postmastectomy lymphedema with a follow-up of 6 months or more, intending to do research on the long-term effects of laser treatment based on the literature available. Furthermore, this study analysed the findings of recently published RCTs after 2017 for the use of laser treatment for BCRL. Finally, we undertook an updated assessment of all current LLLT (PBM) evidence for BCRL to address these difficulties.
Methods
This study was performed under the guidelines by Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) [23, 24] statements.
Literature search
The research review was restricted to studies published in English between 2010 and 2022. The relevant studies were found using four databases, including PubMed, Physiotherapy Evidence Database PEDro, Medline, and Cochrane Library. The keywords (photo-biomodulation) AND (lymphedema OR lymphoedema OR edema OR edoema OR swelling) AND (low-level laser therapy OR cold laser OR cold therapy OR low energy laser OR laser therapy) AND (breast cancer) were used to search the databases, with minor modifications for specific database queries. PICO criteria was used to answer appropriate clinical questions. Further research was discovered by looking through the reference sections of all pertinent articles. If necessary, experts were called in to identify things.
Eligibility criteria
PICO criteria was used to illustrate the following criteria [25].
Inclusion criteria
If an article met the following requirements, it was considered eligible.
Types of study designs: The researchers aimed for randomized controlled trials. (Single or double-blinded)
Types of participants: Adult women older than 18 years old having unilateral lymphedema secondary to Mastectomy or radiotherapy. The circumferential limb difference was defined as more than 2 cm up to 8 cm compared with contralateral (unaffected limb) to be classified as lymphedema.
Type of Intervention applied: Low-level laser therapy/ Photo-biomodulation treatment as a single therapy or combined therapy included. The control subjects had no restrictions, including no treatment, placebo laser, or combination therapy as an active treatment other than LLLT (PBM).
Comparison: There was no comparison to low-level laser therapy.
Type of outcomes measure: Arm circumference/volume, pain intensity, shoulder mobility, and subjective symptoms are clinically linked outcome factors.
Exclusion criteria
The research excluded subjects with primary lymphedema or lymphedema caused by any disease other than breast cancer. Patients with bilateral lymphedema, active malignancy, pregnancy, or any cardiovascular disease or persistent inflammation were also excluded from the trial. Observational evaluations, suggestions, polls, remarks, columns, and emails were also excluded.
Study selection
Two reviewers first gathered the published paper by screening the titles and abstracts for their qualifying criteria. After then, the entire text was screened for final inclusion. Authors, institutions, publication journals, and study outcomes were not hidden from reviewers. The two reviewers thoroughly reviewed and graded each paper, and any disagreements between them were resolved through conversation.
Data extraction
Authors, publication year, study population and patients, treatment, co-intervention, outcomes, measurement period, findings, and inclusion criteria, and degree of evidence (if applicable) were all recorded by the reviewers using a standardized spreadsheet using Microsoft Excel for RCTs. The agreement was achieved through discussion. For further information (if needed), their respected authors were contacted.
Methodological quality appraisal
Each included trial's methodological quality was assessed using Physiotherapy Evidence Database (PEDro) scale, based on the Delphi list [26]. The PEDro scale comprises 11 components which include (eligibility criteria, random allocation, concealed allocation, baseline group comparison, blinding of the subject, blinding of the therapist, blinding of assessors, measuring time protocol, intention to treat analysis, comparison among groups and point estimate and variability). The Pedro points go from 1 to 10; the greater the PEDro score, the higher the study's quality. Because there was no established cut-off number for identifying a high-quality study, the following technique was used: a PEDro score of less than five indicated low-quality evidence, while a score of more than five indicated high-quality evidence [27, 28]. However, the scale's dependability was previously assessed and found to be acceptable (ICC00.68) by evaluators [29]. Additionally, two additional reviewers independently utilized the Cochrane Risk of Bias (2.0) tool [30] to evaluate the risk of bias for the included studies. Each domain's bias risk was categorized as high, low, or uncertain. Any dispute among two reviewers on overall rating of quality was settled with the assistance of third reviewer.
Outcomes assessments
The primary outcomes were based on the difference in the subjects' arm circumference or volume relative to the unaffected arm. Pain relief and an increase in range of motion were marked as secondary outcomes resulting from the use of LLLT (PBM). The results of the included RCTs were used in the primary analysis. The following criteria were used to separate the RCT data into control groups, outcome measures, and follow-up:
Comparison: Compression garment, manual lymphatic drainage (MLD), total decongestive therapy, and arm exercises were included in the conventional therapy group.
Outcome Measure: The primary objectives were arm volume and arm circumference, with the secondary endpoints being pain severity and shoulder mobility in the affected arm.
Measure time point: According to the accessibility of research, more than six months of long-term follow-up were included immediately after the conclusion of treatment sessions and 1- to 6 months of short-term follow-up were also considered.
Due to the small number of studies and clinical heterogeneity, meta-analysis was not achievable. However, to obtain the final conclusions, a high degree of evidence was considered, including methodological quality, original article results, and RCTs that revealed consistent findings.
Strong
Multiple high-quality RCTs have produced consistent results.
Moderate
Several low-quality and/or one high-quality RCT findings that are consistent.
Limited
A single poor-quality RCT.
Conflicting
Multiple RCTs produced conflicting results.
There is no proof from trials—no RCTs. [31].
Review criteria
The articles were arranged as stated by Sackett’s evidence rule [32].
Levels of evidence from Sackett’s are as follows:
Level 1, large RCTs with precise cut results.
Level 2, small RCTs with unclear results.
Level 3, non-randomized cohort, and case–control studies.
Level 4, non-randomized historical cohort, or case–control studies.
Level 5, case series without control groups.
Sackett’s level of evidence was used to rate the level of evidence to make the results more authentic.
Assessment of laser therapy treatment
Laser therapy parameters (wavelength, laser model, energy density, output power and application zone) of included RCTs were used to analyse the treatment adequacy. These parameters were acknowledged as given by World Association for Laser Therapy (WALT) guidelines [33]. Reviewers who had vast knowledge of laser therapy assessed the PBM adequacy of the treatment protocol, and any disagreements were resolved by discussion.
This study was registered with PROSPERO (number CRD42022315076).
Results
Study selection
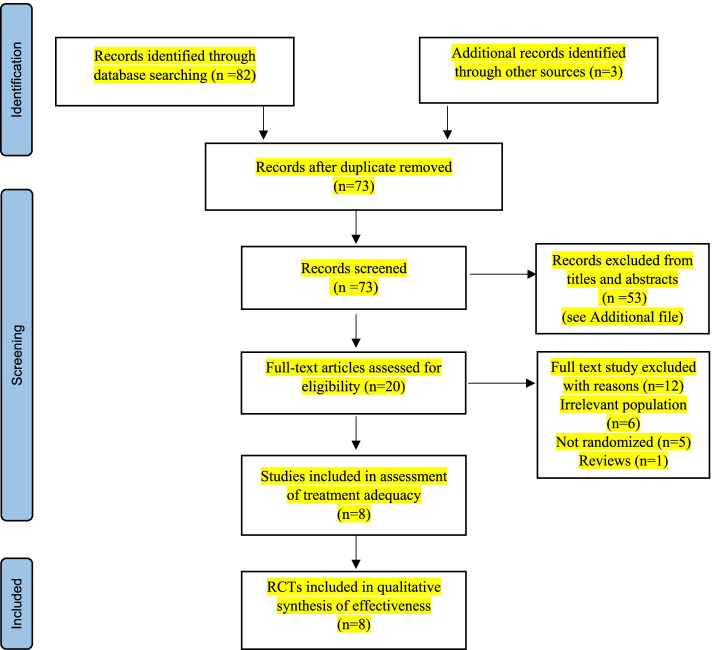
Figure 1 depicts the review procedure. Through computerized and manual searches, the initial search yielded a total of 85 studies. After screening for duplicates, 73 records were considered valid, and after screening for titles and abstracts, 53 studies were eliminated. After considering the complete content of the remaining reports, we decided to eliminate further six of them from our final analysis. As a result, eight trials [21, 34–40] were chosen to be included in this review to see how effective Laser therapy (PBM) is for postmastectomy patients with unilateral lymphedema.
Fig. 1.
Flowchart of the selected clinical trials
Participants baseline characteristics
The baseline variables for the clinical studies that are included are shown in Table 1 Data of participants baseline characteristics of both Intervention group and Control group. Some studies do not indicate any baseline characteristics; hence they have been labelled as not reported. Out of many variables, the most prevalent ones have been reported.
Table 1.
Data of participants baseline characteristics of both intervention group and control group
| Authors | Mogahed (2020) [39] | Kilmartin (2019) [38] | Baxter (2018) [37] | Storz (2017) [36] | Bramlett (2014) [35] | Ridner (2013) [40] | Lau and Cheing (2010) [34] | Omar (2011) [21] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristics | I | C | I | C | I | C | I | C | I | C | I | C | I | C | I | C |
| Age (y) | 48.4 | 48.33 | 63.64 | 59.6 | 57.9 | 64.3 | 56.12 ± 8.85 | 53.17 ± 11.42 | 54.3 | 66.1 | 66.4 | 67.5 | 50.9 ± 8.6 | 51.3 ± 8.9 | 54.76 ± 3.33 | 53.36 ± 3.56 |
| BMI (kg/cm2), Mean | NR | NR | 27.56 | 26.55 | 27.9 | 30.5 | NR | NR | NR | NR | NR | NR | NR | NR | 29.1 ± 6.6 | 25.6 ± 3.3 |
| Dominant arm | NR | NR | NR | NR | 5 | 3 | NR | NR | NR | NR | 5 | 10 | NR | NR | 20 | 19 |
| Non-dominant arm | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | 10 | 5 | NR | NR | 5 | 6 |
| Received Radiotherapy | NR | NR | 100% (11/11) | 90% (9/10) | 9 | 7 | 14 (82.4%) | 16 (84.2%) | NR | NR | 2 (14.3%) | 2(13.3%) | 90.90% | 100% | 10 | 9 |
| Received Chemotherapy | NR | NR | 90.9% (10/11) | 90% (9/10) | 8 | 6 | 13 (76.5%) | 14 (73.7%) | NR | NR | 1(7.1%) | 1(6.7%) | 54.60% | 60% | 10 | 9 |
I intervention Group
C control group
NR Not reported
Characteristics of clinical trials
The essential features of the studies considered in this review are shown in Table 2. All publications were written in English and described how LLLT (PBM) helped people with breast cancer-related lymphedema. RCTs were published between 2010 and 2022 [21, 34–40] and the sample size for the selected eight research ranged from 14 to 50 female participants [21, 34–40]. All clinical studies looked at women diagnosed with post-mastectomy lymphedema between the age of 40 to 77. All the studies examined arm circumference/volume, with two of them also measuring pain severity [37, 39] and shoulder mobility [21, 36]. Traditional treatments such as manual lymphatic drainage [39, 40] and compression garments were compared to laser therapy [21, 37, 40]. In addition, Sackett's level of evidence for each clinical trial was examined for RCTs, and eight of the RCTs were rated as level 2, as shown in Table 2. [21, 34–40]. The RCTs had different follow-up periods. The participants were monitored for three months in three trials [36, 37, 39]. One research looked at the patients for a month [34], while another trial looked at them for four months [21]. Three trials concluded the therapy with a six-month follow-up period [35, 38, 40].
Table 2.
Characteristics for eight included clinical trials
| Authors [Years] | Subjects (n) | Level of Evidence | Study Design and Blinding type | Inclusion Criteria | Interventions | Follow-up sessions | Evaluation Outcomes | Final Findings |
|---|---|---|---|---|---|---|---|---|
| Mogahed et al. [2020] [39] | 30 women, unilateral PML | Level 2 | Single blinded, controlled trial | Stage 2 or 3 BCRL |
1: Active laser (n = 15) 2: Placebo laser (n = 15) 3: MLD 4: Shoulder ROM 5: Pneumatic compression |
Pre-Tx-3 mn |
1: Arm volume 2: Shoulder pain |
Laser group was found beneficial in reducing upper arm volume and decreasing shoulder pain as a post-mastectomy complication |
| Kilmartin et al. [2019] [38] | 22 women, unilateral PML | Level 2 | Double-blinded Randomized, placebo-controlled trial |
Age ≥ 21 yr, Stage 2 or 3 unilateral lymphedema |
1: Active laser + CDT (n = 11) 2: Placebo laser + CDT (n = 11) |
Pre-Tx, 3,6,12 mn |
1: Arm volume 2: Shoulder mobility |
Laser group remarably decreases post-mastectomy arm volume and improve symptoms compared to control placebo group |
| Baxter et al. [2018] [37] | 16 women, unilateral PML | Level 2 | Double- blinded Randomized controlled trial | Age ≥ 18 yr, Diagnosis of BCRL |
1: Active laser (n = 8) 2: Placebo laser (n = 8) 3: Pressure garment 4: Massage therapy & Exe |
Pre-Tx, 6,12 wk | 1: Arm circumference | Laser group seemed to have improve arm circumference, in general LLLT was considered to treat BCRL |
| Storz et al. [2017] [36] | 40 women, unilateral PML | Level 2 | Double- blinded Randomized, Placebo-controlled trial | ≥ 3 months hx of BCRL |
1: Active laser (n = 20) 2: Placebo laser (n = 20) 3: Daily limb exe 4: Skin care |
Pre-Tx, 4,8,12 wk |
1: Arm volume 2: Shoulder pain |
Laser therapy may be effective in reduction of arm volume, but no solid results were seen |
| Bramlett et al. [2014] [35] | 14 women, unilateral PML | Level 2 | Double-blinded, Randomized placebo-controlled trial | Age ≥ 21 yr, unilateral BCRL |
1: Active laser (n = 7) 2: Placebo laser (n = 7) CDT |
Pre-Tx 3,6,12,18 mn | 1: Arm circumference and volume | 18-months of period suggested a decreasing trend in active laser group; Long-term follow-up appears to show that LLLT is useful |
| Ridner et al. [2013] [40] | 46 women, unilateral PML | Level 2 | Single blinded, Randomized controlled trial |
Age ≥ 21 yr, Stage 1 or 2 lymphedema |
1: Active laser (n = 15) 2: MLD group (n = 16) 3: Laser + MLD (n = 15) 4: Compression Bandage |
Pre-Tx, Daily and weekly, post Tx | 1: Arm circumference and volume | Active laser with bandaging was found to be effective, similarly laser with MLD also helped managed PML |
| Lau and cheing [2010] [34] | 21 women, unilateral PML | Level 2 | Single-blinded, Randomized controlled trial | Age ≥ 18 yr, undergone radical mastectomy |
1: Active laser (n = 11) 2: Placebo laser (n = 10) 3:Lymphedema education |
Pre-Tx,4wk post-Tx | 1: Arm volume | Active laser group showed notable decrease in arm volume at the 4wk follow-up |
| Omar et al. [2011] [21] | 50 women, unilateral PML | Level 2 | Double-blinded, Randomized, Placebo-controlled trial | Stage 2 or 3 breast cancer |
1: Active laser (n = 25) 2: Placebo laser (n = 25) 3: Limb exe 4: Skin protection 5: Pressure garment |
Pre-Tx 4, 8, 12, 16 wk |
1: Arm circumference 2: Shoulder mobility 3: Hand grip strength |
Laser was found to be effective to manage arm volume, shoulder ROM and hand grip strength in women with PML |
Representation in Table 2.
Hx history, MLD manual lymphatic drainage, CDT Complete decongestive therapy, Pre-Tx Pre Treatment, Post-Tx Post Treatment, Wk week, Mn month
Methodological quality
Table 3 shows the quality evaluation scores of the eight studies considered. The RCTs' quality was judged using the PEDro criteria. Three of the eight RCT publications were already evaluated for methodological quality using the PEDro scale [21, 34, 40] So, two reviewers [35–39] independently analysed the other five papers using the PEDro scale. All the publications had a score greater than the cut-off of 5, suggesting that the research was of excellent quality. As a result, research scoring 5 to 9 on the Sacketts level of evidence was designated as level 2.
Table 3.
The PEDro scale was used to assess the quality of the included RCTs
| Authors | Eligibility criteria | Random allocation | Concealed allocation | Baseline comparability | Subject were Blinded | Therapist were Blinded | Assessor were Blinded | Less than 15% dropout | Intention-to -treat analysis | Statistical comparisons between groups | Data on point measures and variability | Final points (criteria one not summed) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mogahed et al. [2020] [39] | Yes | Yes | No | Yes | Yes | No | No | Yes | No | Yes | Yes | 6 |
| Kilmartin et al. [2019] [38] | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes | 7 |
| Baxter et al. [2018] [37] | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | 9 |
| Storz et al. [2017] [36] | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes | 7 |
| Bramlett et al. [2014] [35] | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes | 7 |
| Ridner et al. [2013] [40] | Yes | Yes | No | Yes | No | No | No | Yes | Yes | Yes | Yes | 6 |
| Lau and cheing [2010] [34] | Yes | Yes | No | Yes | Yes | No | No | Yes | No | Yes | Yes | 6 |
| Omar et al. [2011] [21] | Yes | Yes | No | Yes | Yes | No | Yes | Yes | No | Yes | Yes | 7 |
| Sub-Item score | 8 | 8 | 1 | 8 | 7 | 0 | 5 | 8 | 2 | 8 | 8 |
Assessment of risk of bias for clinical trials
To improve quality of our systematic review, Cochrane risk of bias version 2 [30] tool was used. The results for Rob 2.0 figure are presented in supplementary file. We deemed eight trials [21, 34–40], out of two studies [21, 39] to be at low risk bias, two studies [34, 38] at high risk and four trials [35–37, 40] followed by unclear risk of bias. We came to conclusion that domain 1 (randomization process) for revised version of Rob was apparently fulfilled by every trials. Three studies [35, 38, 40] did not clearly mentioned details for domain 3 (Missing outcome data) therefore judged as having high-risk of bias. All studies [21, 34–40] represented with unclear risk of bias regarding domain 5 ( Selection of reported results).
Efficacy of low-level laser therapy
As there was little published research in this field, just a few RCTs were included in this systematic review. Furthermore, due to a scarcity of data, only post-treatment and three long-term follow-up studies and other short-term follow-up studies were included in this systematic review. Table 4 summarizes the findings of the various studies.
Table 4.
Results of RCTs included in subgroup analysis summarized
| Authors | Arm circumference/volume | Pain severity | Shoulder mobility | |||
|---|---|---|---|---|---|---|
| At the end of entire treatment | Follow-up (< 6 months) | At the end of entire treatment | Follow-up (< 6 months) | At the end of entire treatment | Follow-up (< 6 months) | |
| Mogahed et al. [2020] [39] | Strong | NR | Strong | NR | NR | NR |
| Kilmartin et al. [2019] [38] | Strong | Strong | Strong | NR | Strong | NR |
| Baxter et al. [2018] [37] | Strong | Weak | Strong | Strong | NR | NR |
| Storz et al. [2017] [36] | Weak | Weak | Strong | NR | NR | NR |
| Bramlett et al. [2014] [35] | Strong | Weak | NR | NR | NR | NR |
| Ridner et al. [2013] [40] | Weak | NR | NR | NR | NR | NR |
| Lau and Cheing [2010] [34] | Strong | Weak | NR | NR | NR | NR |
| Omar et al. [2011] [21] | Strong | Strong | NR | NR | Strong | Weak |
Laser therapy for arm circumference
Four trials have demonstrated the importance of low-level laser treatment in reducing the disparity in arm circumference between affected and unaffected limbs [21, 35, 37, 40]. The most popular method for measuring arm circumference was to wrap a non-stretch tape around the extremity, leaving no slack or indentation in the tissue. Measuring points were made from the ulnar styloid process to the axilla [21, 37, 40]. According to a trial, long-term investigations revealed a statistically significant reduction in arm circumference [35]. Two RCTs found that using LLLT versus placebo lasers offered moderate evidence [21, 38]. At 6- and 12-weeks after randomization, one high-quality research found that patients were satisfied with the use of LLLT for BCRL [37].
Laser therapy for arm volume
The reduction of indifference in arm volume between the affected and sound limb was determined in four high-quality clinical trials [35, 36, 38, 39] that evaluated outcomes of LLLT in patients with post-mastectomy lymphedema. Although the standardized method varied among the RCTs, one study [34] used a tank volumeter to assess the arm volume, which showed a massive reduction in volume by around 28% in the laser group, whereas in contrast; the control showed 6% increase in arm volume by the end of 4-week follow-up. Two trials [36, 40] with moderate evidence found a statistically significant effect of laser therapy regarding decreasing arm volume. However, no difference was found in such comparisons between the two groups at the end of treatment. Finally, one high-quality study [39] provided strong evidence of the comparison between affected and sound limbs when measured at the start and end of treatment.
Laser therapy for shoulder mobility
Two clinical studies [21, 38] found that using LLLT for breast cancer-related lymphedema improved patients' ranges of motion at the shoulder joint. A plastic goniometer was utilized in one high-quality study [21] to evaluate active ROM for (shoulder flexion, shoulder abduction, and external rotation), resulting in a substantial increase in ROM for two movements (shoulder flexion and abduction) at the affected arm after 8–12 weeks. However, these studies reported that there was no increase in external rotation. One low-quality RCT [38] found no convincing evidence that the use of low-level laser treatment for BCRL improved shoulder mobility.
Laser therapy for pain severity
The change in the difference in pain severity was demonstrated in two RCTs [36, 39]. One trial measured pain severity on a 10-point scale [36], and the other trial used a 0–100 mm visual analogue scale [39], which was converted into a 10-point scale. One high-quality study [36] provided strong evidence that LLLT was influential in reducing pain by 50% at the end of treatment compared to the beginning of the session. Similarly, another trial [39] provided moderate evidence supporting laser therapy to reduce pain for patients with BCRL.
Application of laser therapy for BCRL
Data extracted regarding the parameters of laser therapy from eight studies are displayed in Table 5. Major studies did not reasonably follow WALT guidelines [33] for the use of laser therapy. The wavelength commonly used was 904-nm, given in 4/8 studies [21, 35, 38, 40], whereas one trial used a combination of two wavelengths [34], and two studies used 980-nm for the treatment [36, 37]. The standard application zone was the chest wall and axillary area, or over the affected arm. Each experiment included an estimated 1 min of laser energy per location. The treatment session regimen was generally three times each week, with length ranging from four to twelve weeks.
Table 5.
Laser protocols used in clinical trials
| RCTs | Laser Unit | Wavelength (nm) | Treatment Sessions | Application Zone |
|---|---|---|---|---|
| Mogahed et al. [39] | Bravo Terza Serie, Model D | 905 | 36 sessions, 3times/week/12 weeks | Scanner 50 cm perpendicular to treatment area |
| Kilmartin et al. [38] | Rian-Corp Laser | 904 | 8–16 sessions, | 1 min/spot/10 sites in Axilla and a portion of chest wall |
| Baxter et al. [37] | Light-Force EX, LTS-1500 | 980 | 12 sessions, 2times/week/6 weeks | 10 points from axilla to wrist on affected side |
| Storz et al. [36] | TIMELAS Vital | 980 | 8 sessions, 2times/week/4 weeks | Spot covered 4.9cm2, 10 min each session |
| Bramlett et al. [35] | Rian-Corp Laser | 904 | 16 sessions, 2time/week/8 weeks | 1 min/10spots over the axillary region |
| Ridner et al. [40] | Rian-Corp Laser | 904 | 10 Sessions, 20 min each | 20-30 s/spot, over the area to be treated |
| Lau and Cheing [34] | Comby 3 Terza Serie, Model D | 808 | 12 sessions, 3times/week/4 weeks | Entire Axillary Region (144cm2) |
| 905 × 2 | ||||
| Omar et al. [21] | Rian-Corp, Ga-As Laser | 904 | 36 Sessions,3times/week/12 weeks | 2 min/spot, 20 min, over the affected arm |
Dicussion
In the last two decades, LLLT has attracted much attention. It has been used to treat lymphedema and a variety of other ailments, including musculoskeletal issues. In addition, since the LLLT has been employed in clinical settings, it has improved patient satisfaction following treatment. When compared to other procedures used to treat postmastectomy lymphedema, laser therapy has, according to the results of RCTs, not only shown to be successful but also time efficient. This review sought to determine the best outcomes for the therapeutic application of low-level laser therapy since it supported the conclusion of previously published literature [20], and demonstrated the efficiency of LLLT in the management of BCRL.
Even though, manual lymphatic drainage (MLD) [41], when compared to laser therapy, has proved a beneficial therapeutic and rehabilitative strategy. Rehabilitative exercise, complex decongestive therapy (CDT), and multi-layer bandaging for compression also helps to reduce arm volume [42]. however, when these interventions are combined with low-level laser treatment, a significant difference in arm volume is obtained.
As a result, the primary goal of this systematic review was to see how effective LLLT (PBM) is for BCRL. Once all studies were qualified to be included in the inclusion criteria, eight published RCTs were chosen based on the best evidence synthesis. This systematic review assesses recent RCTs on the efficacy of LLLT that were published after 2017 and had not previously been included in any systematic review. As a result, we tried to incorporate freshly published research and past trials in our analysis.
Secondly, we focused on conducting a systematic review on long-term follow-up [35, 38, 40], with a duration of more than six months, to survey whether that would make a difference in the results compared with the short-term follow-up to treat females with BCRL. Thirdly, this review examined the quality of RCTs by assessing them on Sackett’s level of evidence [32]. Eight of these randomized controlled trials were graded on level 2. The treatment approaches used, and their respective results in this randomized controlled trial were apparent to some extent. However, none of the studies stated well-defined results to be marked at level 1 on Sackett’s level of evidence.
With the sheer random assignment of individuals and the control of external factors, the reported trials offered the greatest evidence for treatment effectiveness. This has strengthened the experimental design and made the results less biased. (Four high quality trials) supporting LLLT (PBM) over placebo laser group showed a reduction in arm circumference at four weeks follow up. In comparison, this review provided moderate evidence in support of LLLT for the decline in arm volume seen in (four high quality trials), which eventually favoured the laser therapy group over the placebo group and (two low quality trials) supported laser therapy over the placebo group when compared for affected arm shoulder mobility.
Considering RCTs are the high benchmark in the medical field, the findings of the eight RCTs on the usefulness of Laser therapy were assessed in a critical appraisal (PBM). The methodological quality of the RCTs under investigation was graded as 'strong' on the PEDro scale, with four trials receiving a score of 7/10 and one study receiving a score of 9/10. Despite this, clinical variation in treatment techniques was evident throughout all eight studies. Unfortunately, a solid comparison to establish the treatment regime was unattainable due to the small number of published publications.
Despite WALT [33] unambiguous wordings suggesting a regulated variable for the wavelength, appropriate dose, and duration of laser therapy, the authors could not describe the treatment parameters completely in RCTs. This is not unusual, as other reviewers have also pointed out these flaws [43, 44]. Furthermore, variations in treatment processes, methodologies, application sites, and variability among the factors make it difficult to pool information on the usage of LLLT (PBM). Unless a comparable WALT suggestion is adequately followed, this will contradict outcomes.
All our reviewed RCTs, commonly wavelengths between (808 nm and 980 nm), and energy densities between 1.5–4.89 per centimetre squared (J/cm2) were reported in included studies. Similarly, depending on the location of the tendon, efficient power concentrations for tendinopathy vary between 1.8 to 19.2 J/cm2 [43]. Treatment sessions were typically four weeks long. However, trials with extended durations are also discussed in this review to assess the efficacy of Laser therapy for BCRL.
To reduce the element of bias, our systematic review closely adhered to a robust strategy. To begin, in terms of external validity, the review process followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis 2020 (PRISMA) recommendations [23]. Second, Sackett's level of evidence was utilised to describe the research based on the methodological quality and validity of their design [32]. Third, subgroup analyses were conducted to assess clinical heterogeneity for result synthesis. Finally, the review's results were derived from eight high-quality techniques research.
Limitations
This systematic review's major limitation is that it primarily comprises of free online papers, not paid or grey literature. Moreover, some of the RCTs that were examined had shorter follow-up durations due to the absence of published literature on the long-term effects of low-level laser therapy; therefore, a review with a all RCTs with longer follow-up may anticipate positive outcomes. Time constraints hindered the conduct of a meta-analysis, which would have improved understanding and helped this field form new trends. This evaluation concludes that further well-designed studies are required to fully confirm the efficacy of laser therapy in the treatment of lymphedema in breast cancer patients.
Conclusions
This systematic review revealed that LLLT (PBM) in a reduction in arm circumference and volume was more successful than improving shoulder mobility and pain. Data covey that the application of laser therapy (PBM) may be a positive approach for females with PML. Due to the scarcity of data, there is a strong need for well-conducted studies in this field.
Supplementary Information
Acknowledgements
I would like to express my heartfelt appreciation to my brother, Mr. Hamza Mahmood, a student at Simon Fraser University in British Columbia, Canada. I absolutely value his willingness to volunteer his time so generously to enhance the excellence of this review. Lastly, I want to express my endless thanks to my parents for encouraging me.
Abbreviations
- BCRL
Breast cancer related lymphedema
- LLLT
Low-level laser therapy
- PML
Post-mastectomy lymphedema
- PBM
Photo-biomodulation
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-analysis
- RCT
Randomized Controlled Trials
- WALT
World Association for laser therapy
- ATP
Adenosine triphosphate
- REDOX
Reduction–oxidation reaction
Authors’ contributions
D.M: study idea and design, data search, data extraction, drafting and reviewing, review of RCTs, laser therapy variables, appraisals of RCTs. F.S preparing tables and figures, result interpretations and reviewing the manuscript. S.A and A.A: assessed the outcomes and concluded the final findings. D.M and F.S wrote the discussion and conclusion. All authors reviewed the final manuscript. The author(s) read and approved the final manuscript.
Funding
There is no funding for this research.
Availability of data and materials
All information gathered or analysed during this study are given in this article [and its additional files].
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
There are no potential conflicts of interest declared by the author(s).
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dania Mahmood, Email: danyamehmud@gmail.com.
Ashfaq Ahmad, Email: ashfaq.ahmad@uipt.uol.edu.pk.
Faiza Sharif, Email: faiza.sharif@uipt.uol.edu.pk.
Syed Asadullah Arslan, Email: asad.arslan@uipt.uol.edu.pk.
References
- 1.Pinto AC, de Azambuja E. Improving quality of life after breast cancer: dealing with symptoms. Maturitas. 2011;70(4):343–348. doi: 10.1016/j.maturitas.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Shibuya K, Mathers CD, Boschi-Pinto C, Lopez AD, Murray CJ. Global and regional estimates of cancer mortality and incidence by site: II. Results for the global burden of disease 2000. BMC Cancer. 2002;2:37. doi: 10.1186/1471-2407-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clark B, Sitzia J, Harlow W. Incidence and risk of arm oedema following treatment for breast cancer: a three-year follow-up study. QJM. 2005;98(5):343–348. doi: 10.1093/qjmed/hci053. [DOI] [PubMed] [Google Scholar]
- 4.Olsson Möller U, Beck I, Rydén L, Malmström M. A comprehensive approach to rehabilitation interventions following breast cancer treatment - a systematic review of systematic reviews. BMC Cancer. 2019;19(1):472. doi: 10.1186/s12885-019-5648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Armer JM, Radina ME, Porock D, Culbertson SD. Predicting breast cancer-related lymphedema using self-reported symptoms. Nurs Res. 2003;52(6):370–379. doi: 10.1097/00006199-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Fu MR, Rosedale M. Breast cancer survivors' experiences of lymphedema-related symptoms. J Pain Symptom Manage. 2009;38(6):849–859. doi: 10.1016/j.jpainsymman.2009.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Vrieze T, Gebruers N, Tjalma WA, Nevelsteen I, Thomis S, De Groef A, et al. What is the best method to determine excessive arm volume in patients with breast cancer-related lymphoedema in clinical practice? Reliability, time efficiency and clinical feasibility of five different methods. Clin Rehabil. 2019;33(7):1221–1232. doi: 10.1177/0269215519835907. [DOI] [PubMed] [Google Scholar]
- 8.de Sire A, Losco L, Cigna E, Lippi L, Gimigliano F, Gennari A, et al. Three-dimensional laser scanning as a reliable and reproducible diagnostic tool in breast cancer related lymphedema rehabilitation: a proof-of-principle study. Eur Rev Med Pharmacol Sci. 2020;24(8):4476–4485. doi: 10.26355/eurrev_202004_21030. [DOI] [PubMed] [Google Scholar]
- 9.Invernizzi M, Runza L, De Sire A, Lippi L, Blundo C, Gambini D, et al. Integrating Augmented Reality Tools in Breast Cancer Related Lymphedema Prognostication and Diagnosis. J Vis Exp. 2020;(156):e60093. [DOI] [PubMed]
- 10.Oremus M, Dayes I, Walker K, Raina P. Systematic review: conservative treatments for secondary lymphedema. BMC Cancer. 2012;12:6. doi: 10.1186/1471-2407-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moseley AL, Carati CJ, Piller NB. A systematic review of common conservative therapies for arm lymphoedema secondary to breast cancer treatment. Ann Oncol. 2007;18(4):639–646. doi: 10.1093/annonc/mdl182. [DOI] [PubMed] [Google Scholar]
- 12.Nouri K, Jimenez GP, Harrison-Balestra C, Elgart GW. 585-nm pulsed dye laser in the treatment of surgical scars starting on the suture removal day. Dermatol Surg. 2003;29(1):65–73. doi: 10.1046/j.1524-4725.2003.29014.x. [DOI] [PubMed] [Google Scholar]
- 13.Lievens PC. The effect of a combined HeNe and i.r. laser treatment on the regeneration of the lymphatic system during the process of wound healing. Lasers Med Sci. 1991;6(2):193–9. [Google Scholar]
- 14.Jang DH, Song DH, Chang EJ, Jeon JY. Anti-inflammatory and lymphangiogenetic effects of low-level laser therapy on lymphedema in an experimental mouse tail model. Lasers Med Sci. 2016;31(2):289–296. doi: 10.1007/s10103-015-1854-y. [DOI] [PubMed] [Google Scholar]
- 15.Assis L, Moretti AI, Abrahão TB, de Souza HP, Hamblin MR, Parizotto NA. Low-level laser therapy (808 nm) contributes to muscle regeneration and prevents fibrosis in rat tibialis anterior muscle after cryolesion. Lasers Med Sci. 2013;28(3):947–955. doi: 10.1007/s10103-012-1183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karu TIHL. Ten lectures on basic science of laser phototherapy. Grängesberg: Prima Books; 2007. [Google Scholar]
- 17.Hou JF, Zhang H, Yuan X, Li J, Wei YJ, Hu SS. In vitro effects of low-level laser irradiation for bone marrow mesenchymal stem cells: proliferation, growth factors secretion and myogenic differentiation. Lasers Surg Med. 2008;40(10):726–733. doi: 10.1002/lsm.20709. [DOI] [PubMed] [Google Scholar]
- 18.Saygun I, Karacay S, Serdar M, Ural AU, Sencimen M, Kurtis B. Effects of laser irradiation on the release of basic fibroblast growth factor (bFGF), insulin like growth factor-1 (IGF-1), and receptor of IGF-1 (IGFBP3) from gingival fibroblasts. Lasers Med Sci. 2008;23(2):211–215. doi: 10.1007/s10103-007-0477-3. [DOI] [PubMed] [Google Scholar]
- 19.Rocha Júnior AM, Vieira BJ, de Andrade LC, Aarestrup FM. Low-level laser therapy increases transforming growth factor-beta2 expression and induces apoptosis of epithelial cells during the tissue repair process. Photomed Laser Surg. 2009;27(2):303–307. doi: 10.1089/pho.2008.2277. [DOI] [PubMed] [Google Scholar]
- 20.Baxter GD, Liu L, Petrich S, Gisselman AS, Chapple C, Anders JJ, et al. Low level laser therapy (Photobiomodulation therapy) for breast cancer-related lymphedema: a systematic review. BMC Cancer. 2017;17(1):833. doi: 10.1186/s12885-017-3852-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed Omar MT, Abd-El-Gayed Ebid A, El Morsy AM. Treatment of post-mastectomy lymphedema with laser therapy: double blind placebo control randomized study. J Surg Res. 2011;165(1):82–90. doi: 10.1016/j.jss.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 22.Carati CJ, Anderson SN, Gannon BJ, Piller NB. Treatment of postmastectomy lymphedema with low-level laser therapy: a double blind, placebo-controlled trial. Cancer. 2003;98(6):1114–1122. doi: 10.1002/cncr.11641. [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed) 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS ONE. 2013;8(12):e83138. doi: 10.1371/journal.pone.0083138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schardt C, Adams MB, Owens T, Keitz S, Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verhagen AP, de Vet HC, de Bie RA, Kessels AG, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol. 1998;51(12):1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- 27.Mt EL, Jg EL, de Andrade MF, Bergmann A. Low-level laser therapy in secondary lymphedema after breast cancer: systematic review. Lasers Med Sci. 2014;29(3):1289–1295. doi: 10.1007/s10103-012-1240-y. [DOI] [PubMed] [Google Scholar]
- 28.Omar MT, Shaheen AA, Zafar H. A systematic review of the effect of low-level laser therapy in the management of breast cancer-related lymphedema. Support Care Cancer. 2012;20(11):2977–2984. doi: 10.1007/s00520-012-1546-0. [DOI] [PubMed] [Google Scholar]
- 29.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–721. [PubMed] [Google Scholar]
- 30.Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 31.van Tulder M, Furlan A, Bombardier C, Bouter L. Updated method guidelines for systematic reviews in the cochrane collaboration back review group. Spine. 2003;28(12):1290–1299. doi: 10.1097/01.BRS.0000065484.95996.AF. [DOI] [PubMed] [Google Scholar]
- 32.Sackett DL. Rules of evidence and clinical recommendations on the use of antithrombotic agents. Chest. 1989;95(2 Suppl):2s–4s. [PubMed] [Google Scholar]
- 33.World Association of Laser Therapy (WALT). Consensus agreement on the design and conduct of clinical studies with low-level laser therapy and light therapy for musculoskeletal pain and disorders. Photomed Laser Surg. 2006;24(6):761–2. [DOI] [PubMed]
- 34.Lau RW, Cheing GL. Managing postmastectomy lymphedema with low-level laser therapy. Photomed Laser Surg. 2009;27(5):763–769. doi: 10.1089/pho.2008.2330. [DOI] [PubMed] [Google Scholar]
- 35.Bramlett O, Daysudov I, Odaira T, Rodriguez BP, editors. The Long-Term Effects of Low Level Laser Therapy (LLLT) Combined with Complex Decongestive Therapy (CDT) in the Treatment of Breast Cancer Lymphedema: A Double-Blind, Randomized, Placebo-Controlled Study. 2014.
- 36.Storz MA, Gronwald B, Gottschling S, Schöpe J, Mavrova R, Baum S. Photobiomodulation therapy in breast cancer-related lymphedema: a randomized placebo-controlled trial. Photodermatol Photoimmunol Photomed. 2017;33(1):32–40. doi: 10.1111/phpp.12284. [DOI] [PubMed] [Google Scholar]
- 37.Baxter GD, Liu L, Tumilty S, Petrich S, Chapple C, Anders JJ. Low level laser therapy for the management of breast cancer-related lymphedema: A randomized controlled feasibility study. Lasers Surg Med. 2018;50(9):924–932. doi: 10.1002/lsm.22947. [DOI] [PubMed] [Google Scholar]
- 38.Kilmartin L, Denham T, Fu MR, Yu G, Kuo TT, Axelrod D, et al. Complementary low-level laser therapy for breast cancer-related lymphedema: a pilot, double-blind, randomized, placebo-controlled study. Lasers Med Sci. 2020;35(1):95–105. doi: 10.1007/s10103-019-02798-1. [DOI] [PubMed] [Google Scholar]
- 39.Mogahed HGH, Badawy MM, Education NMAAJJOAP, Research. Low-Level laser Diode on post modified Radical Mastectomy Lymphedema: a randomized controlled trial. J Adv Pharm Educ Res. 2020;10(4):105-9.
- 40.Ridner SH, Poage-Hooper E, Kanar C, Doersam JK, Bond SM, Dietrich MS. A pilot randomized trial evaluating low-level laser therapy as an alternative treatment to manual lymphatic drainage for breast cancer-related lymphedema. Oncol Nurs Forum. 2013;40(4):383–393. doi: 10.1188/13.ONF.383-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martín ML, Hernández MA, Avendaño C, Rodríguez F, Martínez H. Manual lymphatic drainage therapy in patients with breast cancer related lymphoedema. BMC Cancer. 2011;11:94. doi: 10.1186/1471-2407-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Sire A, Fusco N, Sajjadi E, Lippi L, Cisari C, Invernizzi M. Lymphedema Rehabilitation Using Self-Adaptive Inelastic Compression in Breast Cancer: A Proof-of-Principle Study. Appl Sci. 2021;11(4):1901. [Google Scholar]
- 43.Tumilty S, Munn J, McDonough S, Hurley DA, Basford JR, Baxter GD. Low level laser treatment of tendinopathy: a systematic review with meta-analysis. Photomed Laser Surg. 2010;28(1):3–16. doi: 10.1089/pho.2008.2470. [DOI] [PubMed] [Google Scholar]
- 44.Law D, McDonough S, Bleakley C, Baxter GD, Tumilty S. Laser acupuncture for treating musculoskeletal pain: a systematic review with meta-analysis. J Acupunct Meridian Stud. 2015;8(1):2–16. doi: 10.1016/j.jams.2014.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All information gathered or analysed during this study are given in this article [and its additional files].