Abstract
Inherited retinal diseases (IRD) are genotypically and phenotypically varied disorders that lead to progressive degeneration of the outer retina and the retinal pigment epithelium (RPE) eventually resulting in severe vision loss. Recent research and developments in gene therapy and cell therapy have shown therapeutic promise in these hitherto incurable diseases. In gene therapy, copies of a healthy gene are introduced into the host cells via a viral vector. Clinical trials for several genes are underway while treatment for RPE65 called voretigene neparvovec, is already approved and commercially available. Cell therapy involves the introduction of stem cells that can replace degenerated cells. These therapies are delivered to the target tissues, namely the photoreceptors (PR) and RPE via subretinal, intravitreal, or suprachoroidal delivery systems. Although there are several limitations to these therapies, they are expected to slow the disease progression and restore some visual functions. Further advances such as gene editing technologies are likely to result in more precise and personalized treatments. Currently, several IRDs such as retinitis pigmentosa, Stargardt disease, Leber congenital amaurosis, choroideremia, achromatopsia, and Usher syndrome are being evaluated for possible gene therapy or cell therapy. It is important to encourage patients to undergo gene testing and maintain a nationwide registry of IRDs. This article provides an overview of the basics of these therapies and their current status.
Keywords: Cell therapy, gene therapy, inherited retinal diseases, vectors, voretigene neparvovec
Retinal dystrophies or inherited retinal diseases (IRD) are a group of heterogeneous diseases that are characterized by progressive photoreceptor (PR) and/retinal pigment epithelium (RPE) cell loss and eventual atrophy of retinal tissue. They are a significant cause of blindness worldwide. The common IRDs include retinitis pigmentosa (RP), Stargardt disease (STGD), choroideremia, and Leber congenital amaurosis (LCA).
The management of IRDs has conventionally been limited to genetic counseling and low-vision rehabilitation. However, with the recent surge in research into gene therapy, stem cell therapy, and retinal prosthesis, several treatment options are on the horizon. The management of IRDs with gene therapy has been of interest since the discovery that biological information is passed on from one generation to the other by discrete biological material called “genes” encoded in the DNA of the cell and that mutations in various genes are responsible for the occurrence of IRDs. Cell replacement therapy is another strategy to replace dead or damaged retinal cells with cells derived from different sources including mesenchymal stem cells, peripheral or fetal RPE cells, human embryonic stem cells (hESCs), or human-induced pluripotent stem cells (hiPSCs).
This article discusses the basics of gene therapy and cell therapy and their current status in the management of retinal dystrophies.
Gene Therapy
Basics
The majority of genes mutated in RP encode proteins that are expressed either in PR cells or in the RPE.[1] Gene therapy involves gene supplementation/replacement or gene silencing. Classic gene therapy consists of gene supplementation, which enables the restoration of defective gene function or the supply of a missing gene by introducing a functional copy of the gene to target cells. This is employed for disorders due to loss-of-function mutations and is based on the delivery of a correct copy of the defective gene without removal of the endogenous mutant one. Gene silencing inhibits the expression of the mutated gene via the modification of messenger RNA (mRNA) and is applied to disorders caused by gain-of-function mutations.[1] Irrespective of mutation-dependent and -independent strategies, vectors are the key for successful gene therapy.[2] Gene therapy, therefore, uses either a viral or non-viral vector that replaces or silences the causative gene.
Vectors
Viruses are obligate parasites of cells with specific tropism for the cell type and have an efficient delivery system for the transfer of genetic material. Decades of research have helped in the development of safe and efficient viral vectors suitable for the delivery of the genes in vivo. Retroviruses, adenoviruses, adeno-associated viruses, herpes simplex viruses, and more recently with the advent of nanotechnology, nonviral gene delivery systems are being considered for gene therapy.[1,2]
Adeno-associated viruses (AAV) have become a very popular choice for the gene therapy delivery system.[3] AAV vector presents specific characteristics such as low immunogenicity and toxicity, lack of pathogenicity, long-term transgene expression, and relative ease in manipulating genetic elements, making them the safest and most effective viral vector platform for gene delivery into the retina to date. They are replication-deficient in their wild-type condition. They need infection of another virus, for example, adenovirus (and hence the name AAV) or DNA damage insult to undergo a lytic cycle in the host cell. A variety of clinical trials have been initiated worldwide using different serotypes of AAV for various IRDs. Depending on the capsid protein expressed, several serotypes of the AAV virus have been identified, among which AAV2 is the most studied. These capsid proteins allow the AAV to infect a specific cell type, thus making the in vivo cell-type specificity possible.
Two major limitations of AAVs in the retina are their slow onset of transgene expression and the vector’s limited cargo capacity of 4.7 kb.[4] Certain forms of IRDs such as Stargardt disease, Usher syndrome Type 1B and LCA10 are caused by mutations in ABCA4, MYO7A, and CEP290, respectively that have cDNA that exceeds 5 kb, and these are difficult to treat with AAV.[4]
Lentiviruses (LVs) have a large capacity of transgenes, and sequences up to 8 kb can be integrated into chromosomes of target cells, supporting long-term expression. Drawbacks of LVs are potential insertional mutagenesis, the complexity of production, and the large diameter.[5]
Nonviral vectors using a nanomaterial-based delivery system, lipid-based delivery, and more recently magnetic nanoparticles are being tried. Challenges for these systems include difficulty in delivery, instability, and short-term transient expression.[2]
To overcome some of the limitations of gene therapy, genome editing technologies have gained considerable interest. Genome editing using clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR-associated protein (Cas) have provided an accurate and efficient way to edit the human genome. The system utilizes guide RNA to direct Cas9 endonuclease to specific gene loci proximal to a protospacer adjacent motif, inducing a double-strand break. Cell machinery then repairs the CRISPR-targeted site by performing non-homologous end-joining or homology-directed repair.[6]
There are two different approaches to genome editing: an in vivo approach where the disease-causing mutations are corrected directly in the retina and an ex vivo approach in which the mutation is corrected in the patient’s cells and the autologous cells then used for cell transplantation.[7]
Treatment of autosomal dominant disorders is challenging because specific inactivation of the mutant allele is required to restore the phenotype, and gene augmentation approaches do not directly target the pathogenic gene. One promising therapeutic approach using CRISPR technology is to decrease gene transcription through a strategy known as CRISPR interference (CRISPRi).[8] Ablation of the mutant allele using the CRISPR/Cas9 technology is another strategy that has been used in dominant forms for RP due to mutations in the gene encoding rhodopsin.[9,10] These studies demonstrate the use of CRISPR/Cas systems to inactivate autosomal dominant pathogenic alleles.[7]
Diseases Treated
Gene therapy for LCA
Leber congenital amaurosis (LCA) is an infantile, autosomal recessive variant of RP that was first described by Leber in 1869. To date, mutations in 25 genes currently associated with LCA have been identified. The most common mutations include CEP290 (15%), GUCY2D (12%), CRB1 (10%), and RPE65 (6%).[11,12]
RPE65 is a gene-encoding retinoid isomerohydrolase expressed in the RPE that plays a key role in the visual cycle. Defective RPE65 gene leads to an early onset of degeneration of rods, followed by cones, causing significant visual loss. This leads to RP type 20 and LCA type 2, both of which have been extensively evaluated.[13,14] Following success in preclinical models, Russell et al.[15] (Spark Therapeutics) conducted a phase III trial enrolling 31 patients, with confirmed genetic diagnosis of biallelic RPE65 mutations, sufficient viable retina, and the ability to perform standardized multi-luminance mobility testing (MLMT). The intervention was bilateral, subretinal injection of 1·5 x 1011 vector genomes of voretigene neparvovec in 0.3 mL of total volume. The primary efficacy endpoint was a 1-year change in the MLMT performance, measuring functional vision at specified light levels. At 1 year, the mean bilateral MLMT change score was significantly higher in the intervention group than in the control group. No product-related serious adverse events or deleterious immune responses were noted. In addition, a subset of participants in this study was enrolled in a separate functional magnetic resonance imaging study that showed increased activation of the visual cortex and evidence of improved function and structure of visual pathways after the intervention.[15]
Voretigene neparvovec-rzyl is composed of human RPE65c DNA along with a cytomegalovirus enhancer and a hybrid chicken b-actin promoter incorporated into a recombinant AAV2. Following injection into the subretinal space, AAV2 enters RPE cells. While the viral vector remains in the episomal form in the nucleus, without integrating into the host DNA, the enhancer and promoter facilitate the expression of RPE65.
In December 2017, the USFDA approved the gene therapy “Luxturna” (voretigene neparvovec-rzyl), developed by Spark Therapeutics, to treat children and adults with biallelic REP65 mutation-associated retinal dystrophy in the United States.[16] Subsequently, several countries in Europe have approved the drug for use.[17,18]
In addition to RPE65, multiple other gene therapy trials for LCA are underway for the following mutations: GUCY2D (encodes retinal guanylylcyclase-1, a protein expressed in the outer PR and leading to LCA type 1),[19] and CEP290 (encodes a centrosomal protein involved in trafficking through the connecting cilia of PR cells).[20]
Gene therapy for choroideremia
Choroideremia is an X-linked disorder that leads to progressive degeneration of the PR, RPE, and choriocapillaris, mainly affecting males. Symptoms include night blindness and progressive constriction of visual fields; most affected males are legally blind by their midlife. The causative gene has been mapped at Xq21.1-q21.3; mutations in this gene lead to the loss of function of a protein necessary for retinal cell health, Rab Escort Protein 1 (REP1).[21]
A phase I/II clinical trial was conducted by MacLaren et al.,[22] in which six patients were administered AAV2.REP1 genome particles in the subretinal space. At 6 months of follow-up, all patients showed an improvement in the visual acuity on the ETDRS chart and an increase in the retinal sensitivity on the microperimetry. Spark Therapeutics has sponsored an open-label, dose-escalating Phase 1/2 trial designed to assess the safety and preliminary efficacy of subretinal administration of investigational SPK-7001 in choroideremia.[23]
Gene therapy for RP
The prevalence of RP in India has been much higher than that noted in the Western population. Its prevalence ranges from 1 in 3,500 in the US to 1 in 1,000 in China and India.[24]
MERTK is one of the genes encoding for a tyrosine kinase, required for the phagocytosis of photoreceptor outer segments by the RPE and is associated with a rare form of autosomal recessive RP.[25] A phase-1 clinical trial utilizing an AAV2 vector with an RPE-specific promoter (VMD2) driving MERTK in six patients was published in 2016. However, the results were not sustained and further trials are awaited.[25]
The RPGR gene is involved in 70% of X-linked RP and up to 20% of all RP. RPGR encodes for a transporter protein in the cilium, connecting the inner and outer segments of photoreceptors and suspected to play a role in transporting phototransduction components across the connecting cilium. Currently, there are multiple phase 1/2 clinical trials that are going on for gene therapy for X-linked RP with RPGR mutations.[26] MeiraGTx has just concluded a phase 1/2 clinical trial using a subretinal injection of AAV2/5 vector for patients with XLRP caused by mutations in the RPGR gene (NCT03252847), the results of which are awaited. They have also started a phase 3 trial involving the AAV5 vector for patients with XLRP (NCT04671433). Applied Genetics Technologies Corp. (AGTC) is planning to conduct a phase 2/3 study involving subretinal injection of recombinant adeno-associated virus vector (rAAV2tYF-GRK1-hRPGRco- AGTC-501) in patients with XLRP caused by RPGR mutations (NCT04850118). NightstaRx Ltd is conducting a phase 1/2 clinical trial using AAV8 (BIIB112) for patients with XLRP and RPGR mutations (NCT03116113). Intravitreal injection of a biological, 4D-125 is being conducted by 4D Molecular Therapeutics in a phase 1/2 clinical trial in patients with XLRP and RPGR mutations (NCT04517149).
Gene therapy for Stargardt (STGD) disease
Pathogenesis of STGD is linked to an abnormal accumulation of lipofuscin in the RPE in the early stages, followed by slowing of the rod and cone retinoid cycles later in the disease. The commonest gene identified for STGD is ABCA4 (chromosome 1p13-21) for the ABCR retinal ATP transporter protein.[27]
The main obstacle in the development of gene therapy for STGD is the larger size of the ABCA4 gene, whose coding sequence exceeds the cargo capacity of AAV.[28] In 2019, Applied Genetic Technologies Corporation announced the development of a hybrid AAV dual vector and published preclinical data supporting the potential of this technology.[29] Promising results have also been reported in animal models with a non-viral technique relying on subretinal delivery of self-assembled nanoparticles.[30]
Gene therapy for Achromatopsia (ACHM)
Achromatopsia, or rod monochromatism, is an autosomal recessively inherited disorder that affects the cones. Nearly 90% of patients with ACHM carry mutations in CNGA3 or CNGB3, which are the genes encoding the alpha and beta subunits of the cone cyclic nucleotide-gated (CNG) channel, respectively.[31]
Reichel et al.[32] have recently reported long-term safety and efficacy outcomes of subretinal gene therapy for CNGA3-associated ACHM. Nine patients were treated in three escalating dose groups with subretinal AAV8.[33] They did not note any adverse events related to the treatment. Established endpoints for cone vision, best-corrected visual acuity, and contrast sensitivity showed consistent improvement over the 3 years; however, the fellow eye also showed a mild improvement, and no statistical significance was noted between the treated and untreated eye.[32] There are multiple clinical trials in phase1/2 testing AAV vectors in ACHM [See Table 1].
Table 1.
Current clinical trials in gene therapy for IRDs
| Target gene | Drug/Vector | Phase | Route | Sponsor | Trial ID |
|---|---|---|---|---|---|
| LCA | |||||
| RPE65 | tgAAG76 (rAAV2/2.hRPE65p. hRPE65) | I/II | SR | UCL | NCT00643747 |
| AAV OPTIRPE65 | I/II | SR | MeiraGTx UK II Ltd | NCT02946879 | |
| AAV2-hRPE65v2 (voretigene neparvovec-rzyl) | III | SR | Spark Therapeutics | NCT00999609 | |
| rAAV2-CBSB-hRPE65 | I | SR | Uni of Penn | NCT00481546 | |
| rAAV2-hRPE65 | I | SR | Hadassah Medical Organization | NCT00821340 | |
| voretigene neparvovec-rzyl | III | SR | Novartis Pharmaceuticals (Japan) | NCT04516369 | |
| AAV2-hRPE65v2 (voretigene neparvovec-rzyl | Long term (15 years) | SR | Spark Therapeutics | NCT03602820 | |
| CEP290 | Sepofarsen | II/III | IVT | ProQR Therapeutics | NCT04855045 |
| QR-110 | I/II | IVT | ProQR Therapeutics | NCT03913130 | |
| EDIT-101 | I/II | SR | Editas Medicine | NCT03872479 | |
| GUCY2D | SAR439483 | I/II | SR | Atsena Therapeutics | NCT03920007 |
| Choroideremia | |||||
| REP-1 | AAV2-REP1BIIB111 (GEMINI) | II | SR | NightstaRx Ltd | NCT03507686 |
| rAAV2.REP1 vector | I/II | SR | Ian M. MacDonald | NCT02077361 | |
| AAV2-REP1 | II | SR | Byron Lam | NCT02553135 | |
| rAAV2.REP1 | I/II | SR | University of Oxford | NCT01461213 | |
| AAV2.REP1 | II | SR | University of Oxford | NCT02407678 | |
| rAAV2.REP1 | II | SR | STZ eyetrial | NCT02671539 | |
| AAV2-hCHM | I/II | SR | Spark Therapeutics | NCT02341807 | |
| RP | |||||
| vMCO-010 Optogenetic Therapy | II | IVT | Nanoscope Therapeutics | NCT04945772 | |
| MERTK-associated RP | |||||
| MERTK | rAAV2-VMD2-hMERTK | I | SR | King Khaled Eye Specialist Hospital | NCT01482195 |
| Advanced RP | |||||
| AAV2-virally-carried Multi-Characteristic Opsin I (vMCO-I) | I/II | IVT | Nanoscope Therapeutics |
NCT04919473 | |
| RST-001 | I/II | IVT | Allergan | NCT02556736 | |
| XLRS | |||||
| RS1 | AAV-RS1 vector AAV8-scRS/IRBPhRS) | I/II | IVT | NEI | NCT02317887 |
| rAAV2tYF-CB-hRS1 | I/II | IVT | AGTC | NCT02416622 | |
| Nonsyndromic RP | |||||
| Gene therapy: GS030-DP & Medical device: GS030-MD | I/II | IVT | GenSight Biologics | NCT03326336 | |
| PDE6A-associated RP | |||||
| rAAV.hPDE6A | I/II | SR | STZ eye trial | NCT04611503 | |
| PDE6B-associated RP | |||||
| AAV2/5-hPDE6B | I/II | SR | Horama S.A. | NCT03328130 | |
| XLRP | |||||
| RPGR | AAV8-RPGR Biological BIIB112 (XIRIUS) |
I/II | SR | NightstaRx Ltd | NCT03116113 |
| AAV2/5-RPGR | I/II | SR | MeiraGTx UK II Ltd | NCT03252847 | |
| AAV5-RPGR | III | SR | MeiraGTx UK II Ltd | NCT04671433 | |
| 4D-125 | I/II | IVT | 4D Molecular Therapeutics | NCT04517149 | |
| rAAV2tYF-GRK1-hRPGRco | II/III | SR | AGTC | NCT04850118 | |
| RLBP1-associated RP | |||||
| RLBP1 | CPK850 | I/II | SR | Novartis Pharmaceuticals | NCT03374657 |
| Bietti’s crystalline dystrophy | |||||
| CYP4V2 | rAAV2/8-hCYP4V2 | Early Phase 1 | Beijing Tongren Hospital | NCT04722107 | |
| Usher syndrome | |||||
| USH1B | SAR421869 | I/II | SR | Sanofi | NCT01505062 |
| USH2A (Exon13) | QR-421a RNA antisense oligonucleotide | III | IVT | ProQR Therapeutics | NCT05158296 |
| Autosomal dominant RP | |||||
| RHO (P23mutation) | QR-1123 | I/II | IVT | ProQR Therapeutics | NCT04123626 |
| Achromatopsia | |||||
| CNGA3 | AAV- CNGA3 | I/II | SR | MeiraGTx UK II Ltd | NCT03758404 |
| CNGB3 | AAV - CNGB3 | I/II | SR | MeiraGTx UK II Ltd | NCT03001310 |
| CNGA3 | AGTC-402 | I/II | SR | AGTC | NCT02935517 |
| CNGB3 | AGTC-401 | I/II | SR | AGTC | NCT02599922 |
| CNGA3 | rAAV.hCNGA3 | I/II | SR | STZ eyetrial | NCT02610582 |
Source: www.clinicaltrials.org AGTC-Applied Genetic Technologies Corp., NEI-National Eye Institute, UCL-University College London, SR-subretinal, IVT-intravitreal, LCA-Leber Congenital Amaurosis, RP-Retinitis Pigmentosa, XLRS-X-linked Retinischisis
Gene therapy for Usher syndrome (USH)
Usher syndrome is a triad of RP, sensorineural hearing loss, and possible vestibular dysfunction. It is divided into three clinically distinct types based on the time course and degree of multi-sensory loss-USH1, USH2, and USH3. USH1 is the most severe phenotype, with patients presenting at birth with severe hearing loss, vestibular dysfunction, and early-onset RP; the commonest mutation identified is in the MYO7A gene. MYO7A is a large gene, beyond the cargo capacity of AAV vectors, necessitating the need for lenti and dual vectors.[34] Currently, Sanofi is running two trials to investigate the tolerability of subretinal administration of an EIAV-based lentiviral vector carrying MYO7A (SAR421869) for patients affected with USH1B (NCT02065011and NCT01505062), and ProQR Therapeutics is evaluating an RNA antisense oligonucleotide (QR-421a) as an intravitreal injection (NCT05158296).
Table 1 lists the current clinical trials in IRDs.
Cell Therapy
Basics
Visual impairment caused by degenerating retinal cells is irreversible because retinal cells lack the ability for self-repair. Cell replacement therapy aims at replacing damaged host cells with transplanted donor cells. This is an attractive therapeutic approach because it overcomes the limitations of gene therapy and pharmaceutical therapies. Cell therapy is mutation-independent and can work even when the cells are completely lost. The potential for RPE transplantation as a viable therapy came from studies noting improvement in visual acuity following the transplantation of autologous RPE from the peripheral retina into the submacular space.[35]
Stem cells are undifferentiated cells present at varying stages of cell formation and give rise to differentiated cells in various organs. The main characteristics of stem cells are self-renewal (the ability to proliferate extensively), clonality (usually arising from a single cell), and potency (the ability to differentiate into different cell types).[36]
Stem cells can be classified as totipotent (zygote), pluripotent (embryonic stem cells and induced pluripotent stem cells), multipotent (mesenchymal stem cells), and oligopotent. Totipotent cells form embryonic and extra-embryonic tissues. Pluripotent cells form all three germ layers, whereas multipotent cells generate cells limited to one germ layer.[37]
Embryonic stem cells (ESCs) are derived from cells from the inner cell mass of the blastocyst before implantation. They are pluripotent with an unlimited capacity for self-renewal and the ability to give rise to cells of all three embryonic germ layers, namely ectoderm, endoderm, and mesoderm, ultimately generating all tissues in the body. However, there are ethical concerns regarding the use of ESCs. They may multidifferentiate into various cell types, making it difficult to obtain cells of a specific type. They have a risk for tumor formation and require immunosuppression.[38]
Induced pluripotent stem cells (iPSCs) are pluripotent stem cells generated from somatic cells by cellular genetic reprogramming using defined transcription factors.[36] First described in 2007 by Takahashi et al.,[39] iPSCs can be derived from skin fibroblasts or peripheral blood. Because they are obtained from terminally differentiated tissues, there are no ethical concerns with using iPSCs. However, similar to ESCs, immune reactions and tumorigenicity are issues of concern. The former can be alleviated using autologous cells. However, autologous cells carry the same genetic risk factors that the patient suffers from, and therefore the cells need further genetic manipulation. A study combining gene and cell therapy using the CRISPR/Cas9 system applied to the production of iPSCs with selective HLA gene disruption has been tried.[40] Patient-derived human iPSC (hiPSCs) and their tissue-specific derivatives may be used to individually identify and test drugs for their effectiveness in a complex genetic environment. These opportunities open the scope for personalized medicines tailored to individual patients. iPSCs have also been critical in advancing our knowledge of the underlying pathogenesis because it is possible to create cell lines from patients harboring known mutations that can be then tested with small molecules and drugs.[41]
Transplantation of hESCs or allogenic hiPSCs requires systemic immunosuppression for at least 3 months.[42,43] Autologous transplantation of iPSC-derived cells has better histocompatibility than allogeneic transplantation of cells.
RPE transplantation
The RPE is a monolayer of highly specialized cuboidal cells that lie between Bruch’s membrane and the outer neurosensory retina. The RPE forms the outer blood–retinal barrier, and its polarity is important for ion transport.[44] Several factors make RPE transplantation an attractive target for cell transplantation. The cell structure and function of RPE are fairly well understood, the cells grow readily in laboratory cultures and, unlike other cell types within the retina, RPE cells do not require synaptic connections for their function. Compared to other organs in the body, the number of cells required for transplantation is relatively small. In addition, the outer retina is easily amenable to imaging via optical coherence tomography (OCT) and adaptive optics.
In in vitro cultures of differentiation of ESCs and iPSCs, the cells mirror the in vivo developmental path of RPE via the optic neuroectoderm, the eye field stage, committed RPE progenitors, immature RPE, and then to mature RPE.[45]
Although most work with RPE transplants has been done for dry AMD, early experiments in the RCS rats with an inherited RPE dystrophy (MERTK mutation) have demonstrated that subretinal injection of healthy RPE cells allowed the preservation of the outer nuclear, outer plexiform, and the photoreceptor layer and helped maintain visual function.[46]
The rationale for using cell replacement therapy in retinal dystrophies is twofold—one, that the cells integrate with the host retinal tissue and show functional benefits and second is the benefit of neurotrophic factors associated with the injection of these cells.[47,48] Unlike other cell types in the retina, the RPE cell layer does not require synaptic connections.[49]
The two major strategies for replacing atrophic RPE cells include an RPE cell suspension and an RPE cell sheet.
Injection of a suspension: RPE cells can be injected as a suspension of cells into the subretinal space. The access to the subretinal space is achieved in two ways—via a pars plana vitrectomy, detaching the posterior vitreous, and injection of the cells through a small gauge needle, or via the suprachoroidal approach and access to the subretinal space through a partial thickness sclerotomy. Although the ability to perform its essential functions depends on the RPE being a confluent monolayer with tight junctions and maintaining polarity for ion transport with a healthy Bruch’s membrane, a subretinal injection of healthy RPE cells has been shown to maintain or improve the health of the outer nuclear, outer plexiform, and photoreceptor inner/out segment layers.[46,49,50] Although the surgical implantation of these cells into the subretinal space via a parsplana vitrectomy is relatively easy, cellular efflux into the vitreous cavity through the retinotomy is a major problem. The cells then act as a scaffold for the formation of epiretinal membranes. An adequate number of cells may not remain in the subretinal space and the cells may clump and not integrate.
Transplantation of an RPE sheet: A patch of RPE cells is considered an ideal therapeutic substrate. The monolayer helps perform their physiological functions more efficiently. The formation of tight junctions between RPE cells helps optimal polarization of cells, allowing its interaction with the photoreceptor outer segments and supporting its varied functions. RPE cells are grown as a monolayer directly or on a scaffold/substrate. Mandai et al.[48] demonstrated 1-year survival of an autologous iPSC-derived RPE cell sheet injected subretinally in two patients with neovascular AMD. Multiple scaffolds such as polyethylene terephthalate, autologous sheets of cells, biomaterial-based patches, or three-dimensional structures have been tried.[51] Da Cruz et al.[52] have shown that a 6 x 3 mm patch is large enough to cover almost the entire area of a human macula. Delivery of such a large patch to the subretinal area requires a rather complex surgery, with a large retinotomy and sclerotomy. However, this surgery reduces the risk of efflux of cells into the vitreous and formation of epiretinal membranes.[53]
Another interesting development has been retinal organoids. These are three-dimensional structures developed from pluripotent stem cells that help in understanding the development of the human retina and its diseases.[54,55]
Most clinical trial work using human embryonic stem cells (hESC) or iPSC-derived RPE has been done in age-related macular degeneration and Stargardt disease. Although AMD is not an inherited retinal disease, we have included the current status of cell therapy in AMD to acknowledge the progress in this field.
Age-related Macular Degeneration (AMD)
AMD is a leading cause of severe visual impairment in the elderly. Although neovascular AMD is treated by anti-VEGF injections, there is currently no cure for dry AMD. Considering the progress in RPE transplantation, cell replacement therapy for dry AMD has gained considerable attention in the last decade.
Currently, there are several clinical trials in phase 1/2 for the transplantation of hESC/iPSC-derived RPE either as suspensions or sheets in patients with dry AMD.[43,47,50] Lineage cell therapeutics are using a unique method to deliver their cells subretinally via the suprachoroidal approach through microinjection using the Orbit Subretinal Delivery System developed by Gyroscope Therapeutics (formerly Orbit Biomedical, Ltd.), which avoids the need for retinotomy and associated complications. Regenerative Patch Technologies has developed a composite subretinal implant, named the California Project to Cure Blindness-Retinal Pigment Epithelium1 (CPCB-RPE1), consisting of a polarized monolayer of human embryonic stem cell-derived RPE (hESC-RPE) on an ultrathin, synthetic parylene substrate designed to mimic Bruch’s membrane. The discussion of these clinical trials and results is outside the scope of this paper.
Stargardt disease (STGD)
Schwartz et al.[42] conducted two prospective Phase 1/2 clinical trial (NCT01469832) of hESC-derived RPE cells in patients with STGD.[43] Three doses of 50000, 100000, and 150000 cells were injected subretinally. They demonstrated medium-term to long-term safety, graft survival, and possible biological activity of pluripotent stem cells.[43] Findings from the UK site of this trial identified subretinal hyperpigmentation consistent with the survival of viable transplanted hESC-derived RPE cells. Borderline improvements in visual acuity were noted in 4 of 12 patients; however, microperimetry did not demonstrate evidence of functional benefit at 12 months.[56] Further trials are anticipated, including evaluation of combined RPE, and photoreceptor transplants.
Sung et al.[57] reported their 3-year results in three patients with STGD, who received hESC-RPE cells via a parsplana vitrectomy and subretinal injection. All patients had vision less than 20/400. Although no patient showed adverse events related to the procedure, one patient was noted to have a rhegmatogenous retinal detachment at 19 weeks of follow-up. One patient showed best-corrected visual acuity improvement, whereas the other patient had stable best-corrected visual acuity during the 3-year follow-up period. All patients received systemic immunosuppression.[57]
Currently, there are several clinical trials listed on www.clinicaltrials.gov that are using hESCs for the treatment of STGD. STGD is primarily due to mutations in the outer segment of photoreceptors. Therefore, unlike AMD, replacing RPE cells alone may not lead to sustained visual benefits.
Photoreceptor transplantation
PR precursor transplantation is a potential way to treat retinal degenerative diseases caused by PR loss. For most patients with RP, rod PR death is the primary cause of night blindness in the early disease stage. In addition, rods account for more than 90% of PR; thus, rod PR transplantation is a suitable treatment for RP.[58]
MacLaren et al.[59] in 2006 reported that the transplantation of PR precursors taken from immature mouse retinal cells into wild-type retina was able to integrate into the host PR layer with a complete mature morphology. Other groups reported successful transplantation of purified PR using mouse and human ESC/iPSC as an effective method of restoring vision in retinal degeneration models.[60,61] Others have proposed that these improvements were not necessarily from PR integration; “material exchange,” whereby biomaterials, such as proteins and/or mRNAs, are transferred from the donor to host PR, and restore some visual function by rescuing remaining PR cells.[62] JCyte, a California-based company, is testing the intravitreal delivery of retinal progenitor cells (NCT03073733). The goal for this intervention is a non-specific neurotrophic effect with emphasis on reviving cone function. Lingam et al.[63] transplanted PR precursors into the subretinal space of non-human primates and demonstrated survival and maturation into cone PR at 3 months post-transplant. Transplantation of PR and its precursors continues to be an exciting area of research.
Table 2 lists the current clinical trials using stem cells.
Table 2.
Current stem cell therapies for retinal degenerations
| Clinical disease | Intervention | Phase | Route | Sponsor | Trial ID |
|---|---|---|---|---|---|
| Macular degenerative disease | hESC-RPE cells | I/II | SR suspension | AIRM | NCT03167203 |
| AMD | Autologous iPSC-derived RPE on PGLA | I/II | SR-sheet | NEI | NCT04339764 |
| RP | UMSC | II | Sub Tenon’s space | JBRCS | NCT04763369 |
| AMD/Stargardt disease | injection of hESC-RPE in suspension/substrate | I/II | SR suspension vs. sheet | Federal University of São Paulo | NCT02903576 |
| RP | hESC-RPE cells | I | SR suspension | Qi Zhou | NCT03944239 |
| Stargardt | hESC-RPE | I/II | SR suspension | AIRM | NCT02941991 |
| RP | Human retinal progenitor cells | II | IVT suspension | jCyte, Inc | NCT04604899 |
| Dry AMD | hESC-RPE on CPCB-RPE1 | I/II | SR sheet | RPT | NCT02590692 |
| Stargardt | MA09-hRPE hESC-RPE cells | I/II | SR suspension | AIRM | NCT01469832 |
| RP | Wharton’s jelly derived MSC | III | Deep sub Tenon’s | Ankara Universitesi Teknokent | NCT04224207 |
| AMD, RP | CD34+bone marrow stem cells | I | IVT | UCD | NCT01736059 |
| Stargardt | MA09-hRPE | I/II | SR | AIRM | NCT01345006 |
| Dry AMD | MA09-hRPE | I/II | SR | AIRM | NCT01344993 |
| RP | Human Retinal progenitor cells | I/II | SR | ReNeuron Limited | NCT02464436 |
| Dry AMD | Opregen hESC-RPE cells | I/II | SR suspension | Lineage Cell Therapeutics, Inc. | NCT02286089 |
| RP | human neural progenitor cells (CNS10-NPC) | I | SR | Cedars-Sinai Medical Center | NCT04284293 |
Source: www.clinicaltrials.gov AMD- Age-Related Macular Degeneration, RP- Retinitis Pigmentosa, hESC-RPE- Human Embryonic Stem Cells- Retinal Pigment Epithelium, iPSC- Induced Pluripotent Stem Cells, PGLA- poly lactic-co-glycolic acid, UMSC-Umbilical Cord Derived Mesenchymal Stem Cells, CPCB-RPE1- California Project to Cure Blindness-Retinal Pigment Epithelium1, SR- subretinal, IVT- Intravitreal, AIRM-Astellas Institute for Regenerative Medicine, JBRCS- Jinnah Burn and Reconstructive Surgery Centre, Lahore, NEI- National Eye Institute, RPT-Regenerative Patch Technologies, SR- Subretinal, UCD- University of California, Davis
Advantages of using the eye as an end-organ for gene/cell therapy
Relative ocular immune privilege limits an immune response to the genetic/cellular material injected.
Tight blood–ocular barrier, which limits the systemic spread of the injected material.
Ease of accessibility directly to the cells of interest.
Ability to monitor response to therapy noninvasively via advanced imaging systems.
Ability to use the contralateral eye as in vivo control.
A relatively small number of cells is required for cell therapy.
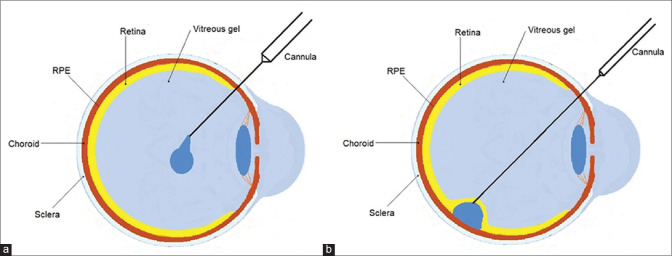
Methods of delivery [Fig. 1]
Figure 1.
Schematic diagram identifying ocular structures and location of (a) intravitreal injection and (b) subretinal injection
Two common methods are being used to introduce the gene therapy product into the eye:
Subretinal injection- This involves a parsplana vitrectomy, followed by raising a subretinal bleb close to the macula with the genetic material/cells being injected directly into the subretinal space. Although this creates a temporary retinal detachment, this has the advantage of direct delivery to the cells of interest. Procedural complications include potential retinal detachment, epiretinal membrane formation, and other complications associated with vitrectomy.
Intravitreal injection: This involves a direct injection into the vitreous cavity, without the need for a vitrectomy. Although the procedure is simpler and associated with lesser complications, the availability of genetic material/cells to the subretinal space is difficult. In addition, an induced humoral response to the intravitreally delivered material may occur, which has not been noted in subretinal injections. Intravitreal injection allows a more widespread distribution of the therapeutic agent over the retina than subretinal delivery, using a less challenging and less-invasive procedure. However, several physical barriers, such as the vitreous, the internal limiting membrane, and the inner retina, limit the diffusion of the therapeutic agent to the PR and RPE after intravitreal delivery.
Suprachoroidal approach: This involves approaching the subretinal space through a specialized suprachoroidal delivery system (Eg: Orbit delivery system, Gyroscope therapeutics). The advantages include precise delivery and lack of reflux of therapeutic material into the vitreous cavity. Disadvantages include the need for a specialized delivery system and potential suprachoroidal or subretinal hemorrhage.
Challenges
Gene therapy requires viable cells and hence will not be effective in advanced stages of IRDs with severe PR degeneration. Cell therapy is more viable in the advanced stages of the disease.
It is not definite that gene/cell therapy can prevent the progression of retinal degeneration.
Gene therapy is gene-specific, and treatment for each gene has to go through all the steps of drug development including animal studies, clinical trials, and regulatory processes.
Gene therapy is very expensive. Luxturna, the RPE-65 gene therapy product currently costs about $450,000 per injection in the US.[16]
The ability to deliver genes to PR is limited by the cargo capacity of viral vectors.
Gene and cell therapy are associated with the complications inherent to the method of delivery of the product, be it subretinal/intravitreal/suprachoroidal delivery.
Potential presence/development of anti-AAV antibodies in the eye that may reduce the efficacy of gene therapy.
Mutations in genes such as rhodopsin that cause autosomal dominant RP are associated with dominant-negative effects. Treatment of these conditions necessitates the disruption of the mutated allele along with the insertion of a functional copy of the gene.
Cell therapy requires systemic immunosuppression for prolonged periods of time with associated side effects.
Future Prospects
Science is on the cusp of being able to treat hitherto untreatable diseases. This is a major breakthrough for patients with IRDs. Future gene replacement therapy in IRDs will target patients at an early disease stage when the retinal architecture and function are still intact. Progress in retinal imaging including adaptive optics, microperimetry, and OCT-angiography should be able to identify clinical markers that identify diseases and their progression fairly early so that maximum functional vision is preserved.
Stem cell science is advancing, and although early, offers unprecedented opportunities for cell replacement strategies. Obtained from peripheral blood or a skin biopsy, iPSC offers an easy approach compared to ESC. Derivation and differentiation of human iPSCs into RPEs, PR, and retinal organoids in vitro provide exciting opportunities for cell-replacement therapy and screening small molecules for therapeutic potential. Gene therapy platforms may allow the development of multiple therapies, reducing costs considerably. Gene-editing technologies such as CRISPR/Cas9, and their combination with iPSCs to repair patient-specific mutations heralds a new era of precise and personalized medicine for our patients.
This has several implications for the clinician. Current counseling of patients with IRDs should include information on current results of gene replacement/stem cell trials. Furthermore, genetic testing and identification of the underlying mutation should be used to support the clinical diagnosis. In addition, it is important to establish a database/registry of patients with confirmed genetic mutations to inform them of forthcoming gene/cell replacement therapies and offer them the possibility of participating in future trials.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Sundaram V, Moore AT, Ali RR, Bainbridge JW. Retinal dystrophies and gene therapy. Eur J Pediatr. 2012;171:757–65. doi: 10.1007/s00431-011-1615-2. [DOI] [PubMed] [Google Scholar]
- 2.Ziccardi L, Cordeddu V, Gaddini L, Matteucci A, Parravano M, Malchiodi-Albedi F, et al. Gene therapy in retinal dystrophies. Int J Mol Sci. 2019;20:5722. doi: 10.3390/ijms20225722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buch PK, Bainbridge JW, Ali RR. AAV-mediated gene therapy for retinal disorders:From mouse to man. Gene Ther. 2008;15:849–57. doi: 10.1038/gt.2008.66. [DOI] [PubMed] [Google Scholar]
- 4.Buck TM, Wijnholds J. Recombinant Adeno-Associated Viral Vectors (rAAV)-vector elements in ocular gene therapy clinical trials and transgene expression and bioactivity assays. Int J Mol Sci. 2020;21:4197. doi: 10.3390/ijms21124197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavalieri VE, Baiamonte E, Lo Iacono M. Non-primate lentiviral vectors and their applications in gene therapy for ocular disorders. Viruses. 2018;10:316. doi: 10.3390/v10060316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doudna JA, Charpentier E. Genome. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. doi:10.1126/science. 1258096. [DOI] [PubMed] [Google Scholar]
- 7.Sanjurjo-Soriano C, Kalatzis V. Guiding lights in genome editing for inherited retinal disorders:Implications for gene and cell therapy. Neural Plast. 2018;2018:5056279. doi: 10.1155/2018/5056279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi LS, Larson MH, Gilbert LA, Doudna JA, Weissman JS, Arkin AP, et al. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell. 2013;152:1173–83. doi: 10.1016/j.cell.2013.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakondi B, Lv W, Lu B, Jones MK, Tsai Y, Kim KJ, et al. In Vivo CRISPR/Cas9 gene editing corrects retinal dystrophy in the S334ter-3 rat model of autosomal dominant retinitis pigmentosa. Mol Ther. 2016;24:556–63. doi: 10.1038/mt.2015.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Latella MC, Di Salvo MT, Cocchiarella F, Benati D, Grisendi G, Comitato A, et al. In vivo editing of the human mutant rhodopsin gene by electroporation of plasmid-based CRISPR/Cas9 in the mouse retina. Mol Ther Nucleic Acids. 2016;5:e389. doi: 10.1038/mtna.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamel CP. Gene discovery and prevalence in inherited retinal dystrophies. C R Biol. 2014;337:160–6. doi: 10.1016/j.crvi.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Cideciyan AV, Jacobson SG. Leber Congenital Amaurosis (LCA):Potential for Improvement of Vision. Invest Ophthalmol Vis Sci. 2019;60:1680–95. doi: 10.1167/iovs.19-26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–9. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 14.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Jr, Mingozzi F, Bennicelli J, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–8. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell S, Bennett J, Wellman JA, Chung DC, Yu ZF, Tillman A, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy:A randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–60. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Available from:https://www.cnbc.com/2018/01/03/spark-therapeutics-luxturna-gene-therapy-will-cost-about-850000 .
- 17.Viriato D, Bennett N, Sidhu R, Hancock E, Lomax H, Trueman D, et al. An economic evaluation of voretigene neparvovec for the treatment of biallelic RPE65-mediated inherited retinal dystrophies in the UK. Adv Ther. 2020;37:1233–47. doi: 10.1007/s12325-020-01243-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uhrmann MF, Lorenz B, Gissel C. Cost effectiveness of voretigene neparvovec for RPE65-mediated inherited retinal degeneration in Germany. Transl Vis Sci Technol. 2020;9:17. doi: 10.1167/tvst.9.9.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mihelec M, Pearson RA, Robbie SJ, Buch PK, Azam SA, Bainbridge JW, et al. Long-term preservation of cones and improvement in visual function following gene therapy in a mouse model of leber congenital amaurosis caused by guanylate cyclase-1 deficiency. Hum Gene Ther. 2011;22:1179–90. doi: 10.1089/hum.2011.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cideciyan AV, Rachel RA, Aleman TS, Swider M, Schwartz SB, Sumaroka A, et al. Cone photoreceptors are the main targets for gene therapy of NPHP5 (IQCB1) or NPHP6 (CEP290) blindness:Generation of an all-cone Nphp6 hypomorph mouse that mimics the human retinal ciliopathy. Hum Mol Genet. 2011;20:1411–23. doi: 10.1093/hmg/ddr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pennesi ME, Birch DG, Duncan JL, Bennett J, Girach A. Choroideremia:Retinal degeneration with an unmet need. Retina. 2019;39:2059–69. doi: 10.1097/IAE.0000000000002553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLaren RE, Groppe M, Barnard AR, Cottriall CL, Tolmachova T, Seymour L, et al. Retinal gene therapy in patients with choroideremia:Initial findings from a phase 1/2 clinical trial. Lancet. 2014;383:1129–37. doi: 10.1016/S0140-6736(13)62117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spark Therapeutics. SPK-7001:Choroideremia. 2017. Available from:http://sparktx.com/scientific-platform-programs/
- 24.Sen P, Bhargava A, George R, Ve Ramesh S, Hemamalini A, Prema R, et al. Prevalence of retinitis pigmentosa in South Indian population aged above 40 years. Ophthalmic Epidemiol. 2008;15:279–81. doi: 10.1080/09286580802105814. [DOI] [PubMed] [Google Scholar]
- 25.Ghazi NG, Abboud EB, Nowilaty SR, Alkuraya H, Alhommadi A, Cai H, et al. Treatment of retinitis pigmentosa due to MERTK mutations by ocular subretinal injection of adeno-associated virus gene vector:Results of a phase I trial. Hum Genet. 2016;135:327–43. doi: 10.1007/s00439-016-1637-y. [DOI] [PubMed] [Google Scholar]
- 26.Cehajic Kapetanovic J, McClements ME, Martinez-Fernandez de la Camara C, MacLaren RE. Molecular strategies for RPGR gene therapy. Genes (Basel) 2019;10:674. doi: 10.3390/genes10090674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koenekoop RK. The gene for Stargardt disease, ABCA4, is a major retinal gene:A mini-review. Ophthalmic Genet. 2003;24:75–80. doi: 10.1076/opge.24.2.75.13996. [DOI] [PubMed] [Google Scholar]
- 28.Amato A, Arrigo A, Aragona E, Manitto MP, Saladino A, Bandello F, et al. Gene therapy in inherited retinal diseases:An update on current state of the art. Front Med (Lausanne) 2021;8:750586. doi: 10.3389/fmed.2021.750586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyka FM, Molday LL, Chiodo VA, Molday RS, Hauswirth WW. Dual ABCA4-AAV vector treatment reduces pathogenic retinal A2E accumulation in a mouse model of autosomal recessive stargardt disease. Hum Gene Ther. 2019;30:1361–70. doi: 10.1089/hum.2019.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun D, Schur RM, Sears AE, Gao SQ, Vaidya A, Sun W, et al. Non-viral gene therapy for Stargardt disease with ECO/pRHO-ABCA4 self-assembled nanoparticles. Mol Ther. 2020;28:293–303. doi: 10.1016/j.ymthe.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohl S, Varsanyi B, Antunes GA, Baumann B, Hoyng CB, Jagle H, et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet. 2005;13:302–8. doi: 10.1038/sj.ejhg.5201269. [DOI] [PubMed] [Google Scholar]
- 32.Reichel FF, Michalakis S, Wilhelm B, Zobor D, Muehlfriedel R, Kohl S, et al. Three-year results of phase I retinal gene therapy trial for CNGA3-mutated achromatopsia:Results of a non randomised controlled trial. Br J Ophthalmol. 2021 doi: 10.1136/bjophthalmol-2021-319067. bjophthalmol-2021-319067. doi:10.1136/bjophthalmol-2021-319067. [DOI] [PubMed] [Google Scholar]
- 33.Fischer MD, Michalakis S, Wilhelm B, Zobor D, Muehlfriedel R, Kohl S, et al. Safety and vision outcomes of subretinal gene therapy targeting cone photoreceptors in achromatopsia:A nonrandomized controlled trial. JAMA Ophthalmol. 2020;138:643–51. doi: 10.1001/jamaophthalmol.2020.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nuzbrokh Y, Ragi SD, Tsang SH. Gene therapy for inherited retinal diseases. Ann Transl Med. 2021;9:1278. doi: 10.21037/atm-20-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Binder S, Stolba U, Krebs I, Kellner L, Jahn C, Feichtinger H, et al. Transplantation of autologous retinal pigment epithelium in eyes with foveal neovascularization resulting from age-related macular degeneration:A pilot study. Am J Ophthalmol. 2002;133:215–25. doi: 10.1016/s0002-9394(01)01373-3. [DOI] [PubMed] [Google Scholar]
- 36.Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85:3–10. doi: 10.1159/000345615. [DOI] [PubMed] [Google Scholar]
- 37.Miotti G, Parodi PC, Zeppieri M. Stem cell therapy in ocular pathologies in the past 20 years. World J Stem Cells. 2021;13:366–85. doi: 10.4252/wjsc.v13.i5.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coco-Martin RM, Pastor-Idoate S, Pastor JC. Cell replacement therapy for retinal and optic nerve diseases:Cell sources, clinical trials and challenges. Pharmaceutics. 2021;13:865. doi: 10.3390/pharmaceutics13060865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 40.Xu H, Wang B, Ono M, Kagita A, Fujii K, Sasakawa N, et al. Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell. 2019;24:566–78.e7. doi: 10.1016/j.stem.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 41.Konala VBR, Nandakumar S, Battu R, Pal R. Derivation of three induced pluripotent stem cell lines under feeder-free culture conditions from peripheral blood mononuclear cells (PBMC) of Indian patients suffering from inherited retinal diseases carrying different mutations. Stem Cell Res. 2020;45:101757. doi: 10.1016/j.scr.2020.101757. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz SD, Hubschman JP, Heilwell G, Franco-Cardenas V, Pan CK, Ostrick R, et al. Embryonic stem cell trials for macular degeneration:A preliminary report. Lancet. 2012;379:713–20. doi: 10.1016/S0140-6736(12)60028-2. [DOI] [PubMed] [Google Scholar]
- 43.Schwartz SD, Regillo CD, Lam BL, Eliott D, Rosenfeld PJ, Gregori NZ, et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt's macular dystrophy:Follow-up of two open-label phase 1/2 studies. Lancet. 2015;385:509–16. doi: 10.1016/S0140-6736(14)61376-3. [DOI] [PubMed] [Google Scholar]
- 44.Caceres PS, Rodriguez-Boulan E. Retinal pigment epithelium polarity in health and blinding diseases. Curr Opin Cell Biol. 2020;62:37–45. doi: 10.1016/j.ceb.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bharti K, Miller SS, Arnheiter H. The new paradigm:Retinal pigment epithelium cells generated from embryonic or induced pluripotent stem cells. Pigment Cell Melanoma Res. 2011;24:21–34. doi: 10.1111/j.1755-148X.2010.00772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li LX, Turner JE. Inherited retinal dystrophy in the RCS rat:Prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp Eye Res. 1988;47:911–7. doi: 10.1016/0014-4835(88)90073-5. [DOI] [PubMed] [Google Scholar]
- 47.Bharti K. Patching the retina with stem cells. Nat Biotechnol. 2018;36:311–3. doi: 10.1038/nbt.4118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mandai M, Fujii M, Hashiguchi T, Sunagawa GA, Ito SI, Sun J, et al. iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice. Stem Cell Rep. 2017;8:69–83. doi: 10.1016/j.stemcr.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alexander P, Thomson HA, Luff AJ, Lotery AJ. Retinal pigment epithelium transplantation:Concepts, challenges, and future prospects. Eye (Lond) 2015;29:992–1002. doi: 10.1038/eye.2015.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Surendran H, Nandakumar S, Reddy K VB, Stoddard J, Mohan KV, Upadhyay PK, et al. Transplantation of retinal pigment epithelium and photoreceptors generated concomitantly via small molecule-mediated differentiation rescues visual function in rodent models of retinal degeneration. Stem Cell Res Ther. 2021;12:70. doi: 10.1186/s13287-021-02134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhong X, Gutierrez C, Xue T, Hampton C, Vergara MN, Cao LH, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5:4047. doi: 10.1038/ncomms5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Da Cruz L, Fynes K, Georgiadis O, Kerby J, Luo YH, Ahmado A, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36:328–37. doi: 10.1038/nbt.4114. [DOI] [PubMed] [Google Scholar]
- 53.Liu Z, Parikh BH, Tan QSW, Wong DSL, Ong KH, Yu W, et al. Surgical transplantation of human RPE stem cell-derived RPE monolayers into non-human primates with immunosuppression. Stem Cell Reports. 2021;16:237–51. doi: 10.1016/j.stemcr.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O'Hara-Wright M, Gonzalez-Cordero A. Retinal organoids:A window into human retinal development. Development. 2020;147:dev189746. doi: 10.1242/dev.189746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fligor CM, Langer KB, Sridhar A, Ren Y, Shields PK, Edler MC, et al. Three-dimensional retinal organoids facilitate the investigation of retinal ganglion cell development, organization and neurite outgrowth from human pluripotent stem cells. Sci Rep. 2018;8:14520. doi: 10.1038/s41598-018-32871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Georgiou M, Fujinami K, Michaelides M. Inherited retinal diseases:Therapeutics, clinical trials and end points-A review. Clin Exp Ophthalmol. 2021;49:270–88. doi: 10.1111/ceo.13917. [DOI] [PubMed] [Google Scholar]
- 57.Sung Y, Lee MJ, Choi J, Jung SY, Chong SY, Sung JH, et al. Long-term safety and tolerability of subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium in Asian Stargardt disease patients. Br J Ophthalmol. 2021;105:829–37. doi: 10.1136/bjophthalmol-2020-316225. [DOI] [PubMed] [Google Scholar]
- 58.Jin ZB, Gao ML, Deng WL, Wu KC, Sugita S, Mandai M, et al. Stemming retinal regeneration with pluripotent stem cells. Prog Retin Eye Res. 2019;69:38–56. doi: 10.1016/j.preteyeres.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 59.MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–7. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- 60.Lamba DA, Gust J, Reh TA. Transplantation of human embryonic stem cell-derived photoreceptors restores some visual function in Crx-deficient mice. Cell Stem Cell. 2009;4:73–9. doi: 10.1016/j.stem.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gonzalez-Cordero A, West EL, Pearson RA, Duran Y, Carvalho LS, Chu CJ, et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol. 2013;31:741–7. doi: 10.1038/nbt.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nickerson PEB, Ortin-Martinez A, Wallace VA. Material exchange in photoreceptor transplantation:Updating our understanding of donor/host communication and the future of cell engraftment science. Front Neural Circuits. 2018;12:17. doi: 10.3389/fncir.2018.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lingam S, Liu Z, Yang B, Wong W, Parikh BH, Ong JY, et al. cGMP-grade human iPSC-derived retinal photoreceptor precursor cells rescue cone photoreceptor damage in non-human primates. Stem Cell Res Ther. 2021;12:464. doi: 10.1186/s13287-021-02539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]