Abstract
Purpose:
To investigate the efficacy and safety of dexamethasone intravitreal implant in the treatment of relapsing posterior uveitis in patients with chronic recurrent Vogt–Koyanagi–Harada (VKH) disease.
Methods:
This was a prospective study of 29 eyes of 16 patients with posterior uveitis in chronic recurrent VKH disease. All patients received previous systemic steroid and immunosuppressive regimens. All patients underwent a comprehensive ophthalmic examination, including best-corrected visual acuity (BCVA), Indocyanine green angiography (ICGA), fundus fluorescein angiography (FFA), and spectral-domain optical coherence tomography (SD-OCT). All patients underwent intravitreal injection with sustained-release dexamethasone 0.7 mg implant (Ozurdex®). Primary outcome measures included mean change in BCVA and central foveal thickness (CFT) at 24 months of follow-up compared to the baseline.
Results:
At 24 month of follow-up, the mean BCVA improved from 0.82 ± 0.13 to 0.38 ± 0.06 logMAR (P < 0.0001). The mean CFT reduced from 505 ± 29 to 244 ± 23 um (P < 0.0001). The mean intraocular pressure (IOP) changed from 15.1 ± 2.2 to 16.9 ± 3.1 mmHg with no significant value (P-value = 0.0955). Twenty-one eyes (72.4%) received one injection, whereas eight eyes (27.6%) required two injections. The mean number of injections was 1.2 ± 0.60. The mean follow-up time was 24.75 ± 0.9 months. No serious ocular or systemic adverse events were noted during the follow-up period. Ocular hypertension was recorded in three (10.3%) eyes and controlled by IOP lowering medications. Cataract progression occurred in 11 (37.9%) eyes.
Conclusion:
Our cohort highlights the beneficial effects of the dexamethasone implant of 0.7 mg in the treatment of VKH disease relapsing posterior uveitis improving visual acuity, reducing macular edema, and minimizing the burden of systemic steroids in this sample study.
Keywords: Dexamethasone, intravitreal, macular edema, posterior uveitis, VKH disease
Vogt–Koyanagi–Harada (VKH) disease is an autoimmune disease against the melanocytes that affects the eyes, skin, and central nervous system. It is a systemic chronic inflammatory disorder characterized by a bilateral progressive asymmetrical granulomatous panuveitis often associated with exudative retinal detachment, with or without extraocular manifestations such as poliosis, vitiligo, alopecia, and hearing loss that mainly affects adults. The clinical stages of VKH disease include prodromal, uveitic, chronic, and chronic recurrent stages. Exudative retinal detachment is the hallmark of acute disease, whereas the sunset glow fundus is a unique feature of chronic disease.[1,2,3,4]
The treatment of choice for VKH disease is combined systemic high-dose corticosteroids and immunosuppressive agents followed by a slow tapering of the drugs over at least 3–6 months to suppress intraocular inflammation and prevent further recurrence or chronicity. Although appropriate corticosteroid therapy may adequately control the condition in some cases, recurrent and chronic disease is common. Long-term, high-dose systemic corticosteroid and immune-modulating therapy might result in systemic side effects such as hypertension, diabetes mellitus, osteoporosis, and long-term risks for cancer and mortality.[5,6,7,8,9]
Chronic recurrent VKH disease and the relapse of the ocular inflammation may reflect improper, insufficient, delayed initiation, rapid tapering of immune-modulatory therapy, or subclinical choroiditis. Recurrences become increasingly steroid-resistant and usually take the feature of chronic anterior uveitis and/or posterior segment involvement, which is mainly predominant as exudative retinal detachment. Relapses occur in 60% of cases in the first 4 years. The chronic recurrent form of VKH disease may last for months to years and is associated with an unfavorable long-term prognosis and vision-threatening complications including cataracts, glaucoma, optic atrophy, subretinal fibrosis, and choroidal atrophy.[10,11,12,13]
Dexamethasone 0.7 mg intravitreal implant (Ozurdex; Allergan Inc., CA, USA) is a biodegradable implant and has been approved by the Food and Drug Administration (FDA) for the treatment of noninfectious uveitis improving intraocular inflammation and visual acuity. Intravitreal dexamethasone implant provides high drug concentration in the retina, which is slowly released over an interval of 6 months with its peak levels at 2 months.[14,13,14,15,16,17]
The aim of this cohort is to investigate the efficacy and safety of dexamethasone 0.7 mg intravitreal implant in the treatment of relapsing posterior uveitis VKH disease in patients with systemic steroid and/or immune-suppressive agents’ comorbidity, dependence, or non-compliance.
Methods
Patient selection: This was a prospective study of 29 eyes of 16 patients with relapsing posterior uveitis in recurrent chronic VKH disease, which included patients at least 18 years of age. All patients had been previously treated with a systemic steroid regimen (at 1 mg/kg/day) and azathioprine (at 2 mg/kg/day) for at least 6 months.[7,8] All patients had systemic steroid and/or immunosuppressive comorbidity, dependency, and non-compliance. The diagnosis and classification of VKH disease were established according to the criteria of the International Workshop on VKH disease. Relapsing posterior uveitis is clinically characterized by the presence of subretinal fluid (SRF) and/or serous retinal detachment (SRD).[10,18]
Recurrent chronic VKH was defined as a relapse of intraocular inflammation after inactivity for 3 months or more without systemic steroid therapy.[19,20,21]
Exclusion criteria included a history of previous intravitreal injections (IVI) (anti-VEGF, triamcinolone acetonide), history of other causes of posterior uveitis, and chronic or recurrent anterior uveitis cases of VKH disease without posterior manifestations and patients receiving other drugs that affect their vision and/or retina.
The patient evaluation was done at baseline, 1 week, 1 month, and then every 3 months for 2 years after receiving an IVI Ozurdex implant. All patients underwent a comprehensive ophthalmic examination including best-corrected visual acuity (BCVA) measured using a logarithm of the minimum angle of resolution (logMAR) Landolt C chart at 4 m, slit-lamp biomicroscopy, intraocular pressure (IOP) measurement, and funduscopy, All patient underwent spectral-domain optical coherence tomography (SD-OCT) (3DOCT-2000, Topcon, Japan) to determine central foveal thickness (CFT) and evaluate the presence of subretinal fluid or SRD. The resolution was defined as a decrease of CFT to <260 mm and the disappearance of fluid.[22] FFA and ICGA were done for all patients.
Treatment: All patients received intravitreal injection with dexamethasone 0.7 mg implant (Ozurdex; Allergan, Inc, CA, USA) with an interval of 1 week for the bilateral patient.[9,23] Retreatment was indicated in the case of the presence of SRF with CFT ≥260 mm. Anterior uveitis was treated with topical prednisolone acetate 1% and topical cyclopentolate 1% on a withdrawal tapering pattern.
Ocular hypertension was defined as IOP measurement over 24 mmHg during the follow-up period after receiving an IVI implant.[24]
Statistical Analysis: Variables were represented as mean ± standard deviation or percentage. For statistical analysis, BCVA was expressed in logMAR. Windows SPSS software version 16.0 (SPSS, Chicago, USA) was used for statistical evaluation. Student’s paired t-test was used to analyze changes in variables and evaluate the level of significance. A P value <0.05 was considered statistically significant. The statistical significance was defined at 95% confidence intervals.
Primary Outcome Measures included mean change in BCVA and CFT at 24 months follow-up compared to baseline.
Results
Study population
This was a prospective study that included 29 eyes of 16 patients with relapsing posterior uveitis in recurrent chronic VKH disease from March 2017 to March 2021. The mean follow-up was 24.75 ± 0.9 months (range: 24–26; median: 24.5). The mean age of patients was 41.5 ± 5.1 years (range: 35–49; median: 40.5) with 11 males (68.75%) and 5 females (31.25%). The mean duration of the systemic steroid regimen and azathioprine was 9.8 ± 0.8 months (range: 9–11; median: 10). The mean duration between discontinuation of systemic regimen and IVI Ozurdex was 6.1 ± 3.8 months (range: 3.5–12; median: 4.5). The pattern of FFA includes RPE window defects, hyperfluorescent spots, late pooling of dye in SRF, and late disc staining in 24, 22, 18, and 20 eyes, respectively. The pattern of ICGA includes intermediate phase hyperfluorescence fuzzy vessels, late diffuse hyperfluorescence, and intermediate phase hypofluorescent dark in 26, 24, and 6 eyes, respectively. Bilateral IVI of dexamethasone implant was recorded in 13 patients (81.25%). At the baseline examination; anterior uveitis was recorded in 24 (28.8%) eyes. At the baseline examination, neurological, and auditory findings such as headache and tinnitus were recorded in six patients (33.3%), whereas integumentary findings such as alopecia and vitiligo were reported in five patients (31.2%).
Clinical results
At 24-months follow-up, the mean BCVA improved from 0.82 ± 0.13 to 0.38 ± 0.06 log MAR (P < 0.0001). The mean CFT reduced from 505 ± 29 to 244 ± 23 mm (P < 0.0001). The mean IOP changed from 15.1 ± 2.2 to16.9 ± 3.1 mmHg without any significant value (P-value = 0.0955). Twenty-one eyes (72.4%) received one injection, whereas eight eyes (27.6%) required two injections. The relapse of posterior uveitis after Ozurdex IVI was recorded in eight eyes (27.6%). The mean number of injections was 1.2 ± 0.60 injections. The mean interval between the first and the second injection was 13.6 ± 1.70 months. The relapse of anterior uveitis after Ozurdex IVI was reported in 14 eyes (58.3%); three of these eyes (21.4%) had two attacks.
Safety
No serious ocular or systemic adverse events were recorded during the follow-up period. Ocular hypertension was recorded in three (10.3%) eyes and controlled by IOP lowering medications. Cataract progression occurred in 11 (37.9%) eyes. Intralenticular Ozurdex implant was recorded in one eye on the first follow-up visit after 1 week of receiving an IVI implant. The Ozurdex implant was removed by phacoemulsification during cataract extraction after 10 months of receiving an IVI implant. Neither capsular defect nor intra-operative complications were recorded. None of the patients received systemic steroid or immunosuppressive agents during the whole study [Table 1 and Figs. 1-4].
Table 1.
Study demography and clinical results
| Variable | Result (mean±SD), range, median or total (%) |
|---|---|
| CFT (um)* | |
| Pre IVI | 505±29 (range: 493-565; median: 507) |
| 24 months follow-up | 244±23 (range: 201-254; median: 241) |
| BCVA (log MAR)* | |
| Pre IVI | 0.82±0.13 (range: 0.8-1.3; median: 0.90) |
| 24 months follow-up | 0.38±0.06 (range: 0.2-0.4; median: 0.30) |
| IOP (mm Hg) | |
| Pre IVI | 15.1±2.2 (range: 12-19; median: 14.5) |
| 24 months follow-up | 16.9±3.1(range: 13-23; median: 16.5) |
| Number of injections (mean): | 1.2±0.6 |
| Eyes received one injection | 21 (72.4%) |
| Eyes required two injections | 8 (27.6%) |
| Follow-up time (months) | 24.75±0.9 |
| Pattern of VKH disease (patients): | |
| Complete | 3 |
| Incomplete | 5 |
| Probable | 8 |
| Pattern of Uveitis (eyes): | |
| Panuveitis | 24 (28.8%) |
| Posterior uveitis | 5 (17.2%) |
CFT (central foveal thickness), um (micrometer), BCVA (best-corrected visual acuity), IOP (intraocular pressure), IVI (intravitreal), VKH (Vogt-Koyanagi-Harada), SD (standard deviation), *A significant difference at P<0.05.
Figure 1.
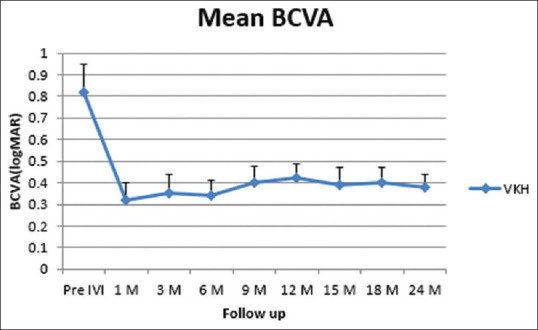
Changes in mean BCVA at follow-up. BCVA (best-corrected visual acuity), LogMAR (logarithm of the minimum angle of resolution), VKH (Vogt–Koyanagi–Harada), IVI (intravitreal), (M) Month
Figure 4.
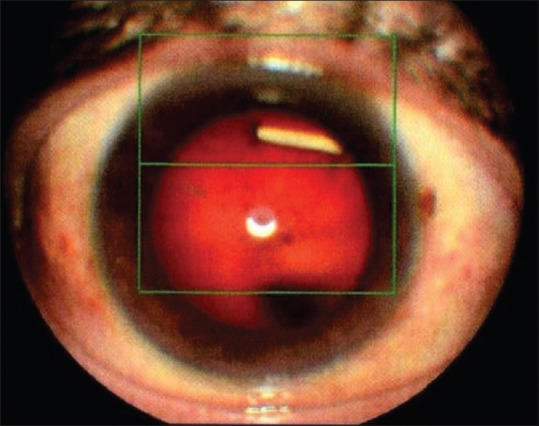
Slit-lamp photo shows intralenticular Ozurdex implant
Figure 2.
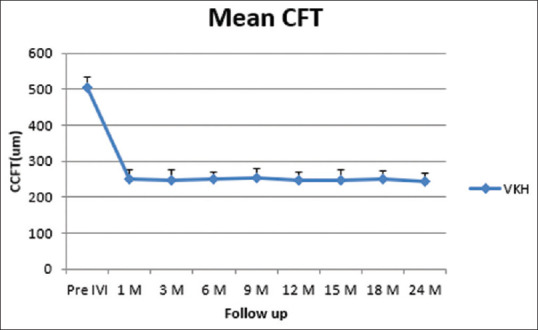
Changes in mean CFT at follow-up. CFT (Central foveal thickness), um (micrometer), VKH (Vogt–Koyanagi–Harada), SRD (serous retinal detachment), IVI (intravitreal), M (month)
Figure 3.
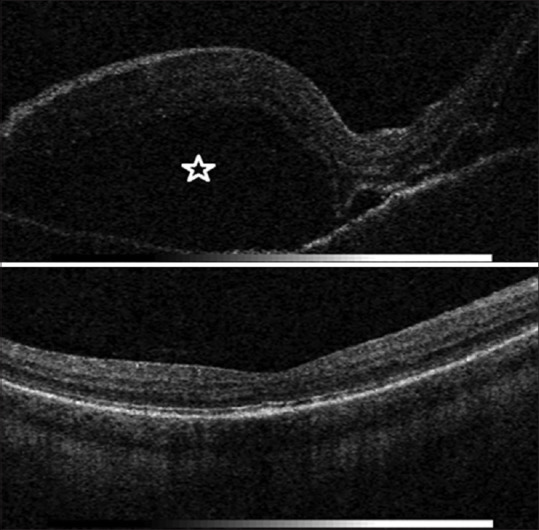
SD-OCT image of the right eye shows a serous retinal detachment (SRD) in a VKH disease patient; (left) Baseline: extensive SRD (asterisk) (right) 24 months of follow-up after intravitreal injection with dexamethasone 0.7 mg implant: none
Discussion
The treatment of chronic relapses of VKH is an enigma. Chronic recurrent VKH requires the long-term use of systemic corticosteroids and immunosuppressive drugs, which increases the risk of systemic side effects. Intravitreal steroids, anti-VEGF, and recently immunosuppressive treatment have been also reported as adjuvant options in the treatment of VKH disease. Macular edema, one of the most visions threatening complications of VKH disease, is sometimes refractory to systemic steroid therapy. Intravitreal injections of corticosteroids play an important role in the management of uveitic macular complications.[9,25,26,27,28]
Dexamethasone 0.7 mg implant has been approved to treat non-infectious posterior uveitis for controlling intraocular inflammation, especially in resistant uveitis macular edema. The dexamethasone implant provides a localized targeted therapy avoiding systemic complications; however, it is associated with local ocular side effects such as cataracts, glaucoma, risk of endophthalmitis, and retinal detachment.[29,30,31,32]
All patients in this cohort showed relapsing posterior uveitis of VKH disease after receiving a previous long-term treatment course including an oral steroid regimen (at 1.5–2 mg/kg/day) and azathioprine (at 1–2.5 mg/kg/day) for at least 9 months. Concurrent relapsing posterior uveitis with systemic steroid and/or immunosuppressive comorbidity, dependency, or non-compliance was the indication of the initiation of IVI dexamethasone 0.7 mg implant therapy. Besides, relapsing posterior uveitis was unilateral in three patients, which might give more superiority to local treatment.
The results of our cohort ensure the efficacy of dexamethasone 0.7 mg implant therapy in the treatment of relapsing posterior uveitis associated with VKH disease improving the vision with optimal control of inflammation and a remarkable systemic and immune modulator agent sparing effect.
Ocular hypertension was developed in three (10.3%) eyes and was controlled by IOP-lowering medications. Episodes of ocular hypertension are recorded with dexamethasone implant therapy but are usually transient and controlled with topical treatment or no therapy.[15,24]
Cataract progression in our cohort was noted in 11 (37.9%) eyes; whereas the incidence of cataract progression was about 10% within 6 months in other studies. The study’s relatively long follow-up time might explain the higher incidence of cataract progression.[11,17,33]
Intralenticular dexamethasone 0.7 mg implant was recorded in one eye. Resolving macular edema despite intralenticular location might be explained by the partial intravitreal location of the implant. Previous studies followed the intralenticular Ozurdex implant for a duration of 7 to 12 months and they reported cataract progression and ocular hypertension.[34,35]
Our result agreed with previous studies stating that dexamethasone 0.7 mg implant is an effective therapy in patients with refractory posterior uveitis in VKH disease avoiding the side effects of systemic corticosteroids and immunosuppressive agents. Pacella et al.,[36] in a short follow-up cohort, concluded the improvement of macular edema in three eyes of three patients with VKH disease for 4–6 months with dexamethasone implant. Latronico et al.[37] reported efficacy of dexamethasone 0.7 mg implant in the treatment of refractory bilateral panuveitis in a young VKH syndrome patient.
The efficacy of other intravitreal corticosteroids, such as triamcinolone acetonide and fluocinolone 0.59 mg implant (Retisert; Bausch and Rochester, USA) in the improvement of visual acuity, macular edema, and SRDs associated with VKH disease has been proven. The advantages of dexamethasone implant over these intravitreal corticosteroids are its biodegradability, not needing to be removed, and less frequent injections. Compared to a dexamethasone implant, triamcinolone acetonide has a short duration and requires repeated injections to expose patients to a greater risk of endophthalmitis, glaucoma, and cataracts. Retisert is associated with a higher incidence rate of ocular hypertension and cataract. Besides, Retisert implant dissociation, protrusion, re-implantation in chronic uveitic patients, and explanation in case of adverse effects are other drawbacks.[38,39,40,41,42]
Fluocinolone 0.19 mg (Iluvien; Alimera Sciences, USA) intravitreal implant showed an improvement in vision, macular edema, and control of ocular inflammation secondary to noninfectious uveitis in the off-label use case report.[43] Iluvien implant reduced uveitis recurrence rate and the dosage of systemic corticosteroid and immunosuppressant requirements in patients with VKH. However, cataract and IOP elevation developed frequently.[44]
The main limitation of this study is the small cohort of patients, the absence of a control group, and non-mentioning the number of relapses before Ozurdex IVI. The long-term follow-up is one of the strong points in this cohort.
Further prospective, large comparative clinical trials are required to evaluate the efficacy and safety of different intravitreal agents such as Ozurdex implant, Iluvien implant, and intravitreal immunosuppressive agents such as sirolimus.
Conclusion
Our cohort highlights the beneficial effects of the dexamethasone implant 0.7 mg in the treatment of VKH disease relapsing posterior uveitis improving visual acuity, reducing macular edema, and providing systemic and immunosuppressive agents sparing effect, and minimizing their burden in this sample study.
Statement of ethics
The study was approved by the local ethics committee and followed the tenets of the Declaration of Helsinki. Informed consent was taken from all patients.
Financial support and sponsorship
No financial support was received for this submission. No substantial contribution was provided for this submission.
Conflicts of interest
There are no conflicts of interest.
References
- 1.He Y, Wang C, Su G, Deng B, Ye Z, Huang Y, et al. Decreased expression of A20 is associated with ocular Behcet's disease (BD) but not with Vogt-Koyanagi-Harada (VKH) disease. Br J Ophthalmol. 2018;102:1167–72. doi: 10.1136/bjophthalmol-2017-311707. [DOI] [PubMed] [Google Scholar]
- 2.Budmann GA, Franco LG, Pringe A. Long term treatment with infliximab in pediatric Vogt-Koyanagi-Harada disease. Am J Ophthalmol Case Rep. 2018;11:139–41. doi: 10.1016/j.ajoc.2018.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herbort CP, El Asrar AM, Takeuchi M, Pavésio CE, Couto C, Hedayatfar A, et al. Catching the therapeutic window of opportunity in early initial-onset Vogt–Koyanagi–Harada uveitis can cure the disease. IntOphthalmol. 2019;39:1419–25. doi: 10.1007/s10792-018-0949-4. [DOI] [PubMed] [Google Scholar]
- 4.Cuevas M, de-la-Torre A, Córdoba A. Bilateral iris depigmentation and ocular hypotony as end-stage manifestations of untreated Vogt–Koyanagi–Harada disease. OculImmunolInflamm. 2018;26:1101–6. doi: 10.1080/09273948.2017.1320411. [DOI] [PubMed] [Google Scholar]
- 5.Fabiani C, Vitale A, Emmi G, Lopalco G, Vannozzi L, Bacherini D, et al. Systemic steroid sparing effect of intravitreal dexamethasone implant in chronic noninfectious uveitic macular edema. JOcular PharmacolTher. 2017;33:549–55. doi: 10.1089/jop.2017.0034. [DOI] [PubMed] [Google Scholar]
- 6.Bouchenaki N, Herbort CP. Indocyanine green angiography guided management of Vogt-Koyanagi-Harada disease. JOphthalVis Res. 2011;6:241. [PMC free article] [PubMed] [Google Scholar]
- 7.Shen E, Rathinam SR, Babu M, Kanakath A, Thundikandy R, Lee SM, et al. Outcomes of Vogt-Koyanagi-Harada disease:A subanalysis from a randomized clinical trial of antimetabolite therapies. AmJOphthalmol. 2016;168:279–86. doi: 10.1016/j.ajo.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abu El-Asrar AM, Al Tamimi M, Hemachandran S, Al-Mezaine HS, Al-Muammar A, Kangave D. Prognostic factors for clinical outcomes in patients with Vogt-Koyanagi-Harada disease treated with high-dose corticosteroids. Acta Ophthalmol. 2013;91:e486–93. doi: 10.1111/aos.12127. [DOI] [PubMed] [Google Scholar]
- 9.Sakata VM, Da Silva FT, Hirata CE, Marin ML, Rodrigues H, Kalil J, et al. High rate of clinical recurrence in patients with Vogt–Koyanagi–Harada disease treated with early high-dose corticosteroids. Graefes ArchClin Exp Ophthalmol. 2015;253:785–90. doi: 10.1007/s00417-014-2904-z. [DOI] [PubMed] [Google Scholar]
- 10.El-Asrar AA, Dosari M, Hemachandran S, Gikandi PW, Al-Muammar A. Mycophenolate mofetil combined with systemic corticosteroids prevents progression to chronic recurrent inflammation and development of'sunset glow fundus'in initial-onset acute uveitis associated with Vogt-Koyanagi-Harada disease. Acta Ophthalmol. 2017;95:85–90. doi: 10.1111/aos.13189. [DOI] [PubMed] [Google Scholar]
- 11.Lowder C, Belfort R, Jr, Lightman S, Foster CS, Robinson MR, Schiffman RM, et al. Dexamethasone intravitreal implant for noninfectious intermediate or posterior uveitis. ArchOphthalmol. 2011;129:545–53. doi: 10.1001/archophthalmol.2010.339. [DOI] [PubMed] [Google Scholar]
- 12.O'Keefe GA, Rao NA. Vogt-Koyanagi-Harada disease. SurvOphthalmol. 2017;62:1–25. doi: 10.1016/j.survophthal.2016.05.002. doi:10.1016/j.survophthal.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 13.Lavezzo MM, Sakata VM, Morita C, Rodriguez EE, Abdallah SF, da Silva FT, et al. Vogt-Koyanagi-Harada disease:Review of a rare autoimmune disease targeting antigens of melanocytes. OrphanetJRare Dis. 2016;11:29. doi: 10.1186/s13023-016-0412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gupta A, Ram J, Gupta A, Gupta V. Intraoperative dexamethasone implant in uveitis patients with cataract undergoing phacoemulsification. OculImmunolInflamm. 2013;21:462–7. doi: 10.3109/09273948.2013.822087. [DOI] [PubMed] [Google Scholar]
- 15.Bahadorani S, Krambeer C, Wannamaker K, Tie W, Jansen M, Espitia J, Sohn JH, et al. The effects of repeated Ozurdex injections on ocular hypertension. ClinOphthalmol. 2018;12:639–42. doi: 10.2147/OPTH.S148990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whitcup SM, Robinson MR. Development of a dexamethasone intravitreal implant for the treatment of noninfectious posterior segment uveitis. Ann N YAcad Sci. 2015;1358:1–2. doi: 10.1111/nyas.12824. doi:10.1111/nyas. 12824. [DOI] [PubMed] [Google Scholar]
- 17.Zarranz-Ventura J, Carreño E, Johnston RL, Mohammed Q, Ross AH, Barker C, et al. Multicenter study of intravitreal dexamethasone implant in noninfectious uveitis:Indications, outcomes, and reinjection frequency. AmJOphthalmol. 2014;158:1136–45. doi: 10.1016/j.ajo.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Read RW, Holland GN, Rao NA, Tabbara KF, Ohno S, Arellanes-Garcia L, et al. Revised diagnostic criteria for Vogt-Koyanagi-Harada disease:Report of an international committee on nomenclature. AmJOphthalmol. 2001;131:647–52. doi: 10.1016/s0002-9394(01)00925-4. [DOI] [PubMed] [Google Scholar]
- 19.Lai TY, Chan RP, Chan CK, Lam DS. Effects of the duration of initial oral corticosteroid treatment on the recurrence of inflammation in Vogt-Koyanagi-Harada disease. Eye. 2009;23:543–8. doi: 10.1038/eye.2008.89. [DOI] [PubMed] [Google Scholar]
- 20.Tan JJ, Rao NA. Posterior Uveitis. Cham: Springer; 2019. Vogt-Koyanagi-Harada disease and sympathetic ophthalmia; pp. 39–56. [Google Scholar]
- 21.Du L, Kijlstra A, Yang P. Vogt-Koyanagi-Harada disease:Novel insights into pathophysiology, diagnosis, and treatment. ProgRetinEye Res. 2016;52:84–111. doi: 10.1016/j.preteyeres.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Sugar EA, Jabs DA, Altaweel MM, Lightman S, Acharya N, Vitale AT, et al. Multicenter uveitis steroid treatment (MUST) trial research group. Identifying a clinically meaningful threshold for change in uveitic macular edema evaluated by optical coherence tomography. AmJOphthalmol. 2011;152:1044–52. doi: 10.1016/j.ajo.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moisseiev E, Regenbogen M, Rabinovitch T, Barak A, Loewenstein A, Goldstein M. Evaluation of pain during intravitreal Ozurdex injections vs intravitreal bevacizumab injections. Eye. 2014;28:980–5. doi: 10.1038/eye.2014.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malclès A, Dot C, Voirin N, Vié AL, Agard É, Bellocq D, et al. Safety of intravitreal dexamethasone implant (Ozurdex):the SAFODEX study. Incidence and risk factors of ocular hypertension. Retina. 2017;37:1352–9. doi: 10.1097/IAE.0000000000001369. [DOI] [PubMed] [Google Scholar]
- 25.Dolz-Marco R, Gallego-Pinazo R, Díaz-Llopis M. Rituximab in refractory Vogt–Koyanagi–Harada disease. JOphthalmic InflammInfect. 2011;1:177–80. doi: 10.1007/s12348-011-0027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park HS, Nam KY, Kim JY. Intravitreal bevacizumab injection for persistent serous retinal detachment associated with Vogt–Koyanagi–Harada disease. Graefe's Arch ClinExp Ophthalmol. 2011;249:133–6. doi: 10.1007/s00417-010-1477-8. [DOI] [PubMed] [Google Scholar]
- 27.sakata VM, da Silva FT, Hirata CE, de Carvalho JF, Yamamoto JH. Diagnosis and classification of Vogt–Koyanagi–Harada disease. AutoimmunRev. 2014;13:550–5. doi: 10.1016/j.autrev.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Errera MH, Fardeau C, Cohen D, Navarro A, Gaudric A, Bodaghi B, et al. Effect of the duration of immunomodulatory therapy on the clinical features of recurrent episodes in Vogt–Koyanagi–Harada disease. Acta Ophthalmologica. 2011;89:e357–66. doi: 10.1111/j.1755-3768.2010.02055.x. [DOI] [PubMed] [Google Scholar]
- 29.Yap YC, Papathomas T, Kamal A. Results of intravitreal dexamethasone implant 0.7 mg (Ozurdex®) in non-infectious posterior uveitis. IntJOphthalmol. 2015;8:835. doi: 10.3980/j.issn.2222-3959.2015.04.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karim R, Sykakis E, Lightman S, Fraser-Bell S. Interventions for the treatment of uveitic macular edema:A systematic review and meta-analysis. ClinOphthalmol (Auckland, NZ) 2013;7:1109–44. doi: 10.2147/OPTH.S40268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. InvestigOphthalmolVisSci. 2011;52:80–6. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- 32.McCartney M, McCluskey P, Zagora S. Intravitreal dexamethasone implants for non-infectious uveitis. ClinExpOphthalmol. 2019;47:1156–63. doi: 10.1111/ceo.13611. [DOI] [PubMed] [Google Scholar]
- 33.Cao JH, Mulvahill M, Zhang L, Joondeph BC, Dacey MS. Dexamethasone intravitreal implant in the treatment of persistent uveitic macular edema in the absence of active inflammation. Ophthalmology. 2014;121:1871–6. doi: 10.1016/j.ophtha.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 34.Regan KA, Blake CR, Lukowski ZL, Iyer SSR. IntralenticularOzurdex®–one year later. Case RepOphthalmol. 2017;8:590–4. doi: 10.1159/000485318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chhabra R, Kopsidas K, Mahmood S. Accidental insertion of dexamethasone implant into the crystalline lens—12 months follow-up. Eye. 2014;28:624–5. doi: 10.1038/eye.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacella F, Smaldone G, Albanese G, Campagna O, Turchetti P, Pacella E. Treatment chronic macular edema in Vogt-Koyanagi Harada syndrome with dexamethasone intravitreal implant:Description of three case. Senses Sci. 2015;2:57–63. [Google Scholar]
- 37.Latronico ME, Rigante D, Caso F, Cantarini L, Costa L, Nieves-Martín L, et al. Bilateral dexamethasone intravitreal implant in a young patient with Vogt-Koyanagi-Harada disease and refractory uveitis. ClinRheumatol. 2015;34:1145–8. doi: 10.1007/s10067-014-2623-1. [DOI] [PubMed] [Google Scholar]
- 38.Byon IS, Kim JH, Lee JE, Oum BS. Intravitreal triamcinolone acetonide for rebound phenomenon after high-dose intravenous steroid treatment in Vogt-Koyanagi-Harada disease. ClinOphthalmol (Auckland, NZ) 2011;5:1589–91. doi: 10.2147/OPTH.S25477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khalifa Y, Loh AR, Acharya NR. Fluocinolone acetonide intravitreal implants in Vogt-Koyanagi-Harada disease. Ocular ImmunolInflamm. 2009;17:431–3. doi: 10.3109/09273940903267936. [DOI] [PubMed] [Google Scholar]
- 40.Myung JS, Aaker GD, Kiss S. Treatment of noninfectious posterior uveitis with dexamethasone intravitreal implant. ClinOphthalmol (Auckland, NZ) 2010;4:1423–6. doi: 10.2147/OPTH.S15696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bollinger K, Kim J, Lowder CY, Kaiser PK, Smith SD. Intraocular pressure outcome of patients with fluocinolone acetonide intravitreal implant for noninfectious uveitis. Ophthalmology. 2011;118:1927–31. doi: 10.1016/j.ophtha.2011.02.042. [DOI] [PubMed] [Google Scholar]
- 42.Nobre-Cardoso J, Champion E, Darugar A, Fel A, Lehoang P, Bodaghi B, et al. Treatment of non-infectious uveitic macular edema with the intravitreal dexamethasone implant. Ocul Immunol Inflamm. 2017;25:447–54. doi: 10.3109/09273948.2015.1132738. [DOI] [PubMed] [Google Scholar]
- 43.Reddy AK, Burkholder BM, Khan IR, Thorne JE. Iluvien implantation for uveitis and uveitic macular edema. Ocul Immunol Inflamm. 2018;26:315–6. doi: 10.1080/09273948.2016.1215472. [DOI] [PubMed] [Google Scholar]
- 44.Heo JA, Cho BJ, Goldstein DA, Sepah YJ, Do DV, Nguyen QU. Fluocinolone acetonide implant for Vogt–Koyanagi–Harada disease. Retina. 2016;36:2124–31. doi: 10.1097/IAE.0000000000001094. [DOI] [PubMed] [Google Scholar]


