Abstract
Purpose:
To compare the clinical and biometric characteristics of children presenting with nanophthalmos (NO group) with that of age-matched controls (CO group).
Methods:
Electronic medical records of 40 children (<18 years of age) with diagnosis of nanophthalmos (NO), presented to a tertiary center in Tamil Nadu between January 2010 and December 2019, were reviewed and compared with 30 age-matched controls (CO) presenting for routine eye examination between October 2019 and December 2019. Clinical parameters compared were best-corrected visual acuity (BCVA), axial length (AxL), keratometry (K), anterior chamber depth (ACD), lens thickness (LT), retinochoroidal scleral thickness (RCS), corneal diameter, central corneal thickness (CCT), intraocular pressure (IOP), lens axial length factor (LAF), and lens thickness/anterior chamber depth ratio (LT/ACD).
Results:
Mean age of the NO group was 8.95 ± 4.0 years. Mean spherical equivalent (SE) in NO group was 10.87 ± 3.1 D and was inversely correlated to AxL (r = −0.46, P value = 0.003). All biometric parameters (AxL, ACD, LT, RCS, LAF, and LT/ACD), except CCT were significantly different between NO and CO groups. NO group children had 52.5% visual impairment with BCVA ≤ 6/24 and 17.5% had esotropia. Common ocular associations in NO group were amblyopia (64.3%), primary angle-closure glaucoma (PACG) (17.8%), pigmentary retinopathy (14.3%), and retinal detachment (3.6%). Angle-closure disease was seen in 50% of NO group and 30% underwent laser peripheral iridotomy (LPI). There was a significant difference in SE, ACD, and LAF among NO children with AxL <17 mm or >17 mm. Multivariable regression analysis revealed a significant correlation of SE and ACD with AxL.
Conclusion:
Nanophthalmos in children often present as amblyopia with visual impairment and strabismus. NO group with AxL <17 mm, had angle-closure disease as a common association with significantly lower ACD, higher SE, and LAF. All morphometric characteristics, except CCT, were significantly different between NO and CO groups. Close monitoring with serial biometry in NO group is needed for the timely diagnosis and prompt intervention to avoid visual impairment, due to glaucoma.
Keywords: Amblyopia, angle-closure disease, pediatric nanophthalmos, strabismus
Nanophthalmos (NO) is a rare developmental condition resulting from the arrest of globe in all dimensions without other systemic anomalies or ocular malformations with a prevalence of <1% in most populations.[1,2,3] It typically presents as a small and highly hyperopic eye, deeply set into orbit. Hyperopia may range from +8.00 D to more than +25.00 D sphere in this disease entity.[4] Limited epidemiological data have explored the adult NO, with the few published studies describing the birth prevalence of microphthalmos varying from 0.002% to 0.017% in a British cohort to 0.0009% in a Chinese population.[5,6] However, the prevalence and clinical spectrum of NO in children have been sparsely reported.[1,2,3,4]
NO was diagnosed based on axial length <20.5 mm, retinochoroidal scleral thickness (RCS) >1.7 mm, crowded anterior chamber structures, and high hyperopia.[7] The biometric characteristics of NO in children are not well understood as there often is an overlap of diagnosis with relative anterior microphthalmos (RAM), high hyperopia, and posterior microphthalmos.[4] The morphometric analysis in such eyes reveals distinct biometric features like increase in RCS thickness, which may help in understanding the pathogenesis in addition to predicting the surgical outcomes.[8]
Owing to the small eye phenotype, they are prone to several complications like amblyopia, angle-closure glaucoma, retinal or choroidal detachments, and uveal effusions.[9] Timely diagnosis is pivotal to effectively treat or prevent these complications, for which a better understanding of ocular biometry and morphology is instrumental. The aim of our study was to characterize and differentiate the clinical and morphometric parameters in NO eyes from that of age-matched controls (CO) and to report the influence of such biometric parameters on clinical and visual outcomes in NO children.
Methods
Our study was a retrospective observational study conducted in a tertiary care eye center after approval by the institutional review board (RET201000365). Electronic medical records of children (<18 years of age), who had visited pediatric ophthalmology department at a tertiary eye care center in south Tamil Nadu, India between January 2010 and December 2019 and diagnosed to have NO, were reviewed. The diagnosis of NO was based on a shorter than average axial length (less than 20.5 mm), high hyperopia, and retinochoroidal scleral thickening greater than 1.7 mm determined by B-scan ultrasonogram.
A total of 82 medical records of patients diagnosed as NO was retrieved from the database. Among them, 42 patients were excluded from this study due to a diagnosis of relative anterior microphthalmos, colobomas, microcorneas, and posterior microphthalmos. Subjects for whom both gonioscopy and biometry data were unavailable were also excluded from the analysis, as were those with other anterior segment anomalies. After excluding the above, a total of 40 children were included in NO group and were compared with 30 age-matched controls (CO group). CO group children were those who had visited the pediatric outpatient services for routine evaluation from October 2019 to December 2019. After obtaining consent from the parents, we performed the same series of tests in the CO group as in the NO group for comparison.
Electronic case records were reviewed for age at presentation, gender, family history, history of consanguinity, presenting complaints, type of strabismus, and treatment given. Unaided (UCVA) and best-corrected visual acuity (BCVA) was done for all patients using Snellen’s chart. Refraction was performed for all children and spherical equivalent (SE) was used for analysis. Slit-lamp biomicroscopic findings particularly the corneal diameter, anterior chamber depth (ACD), pupil, and lens status were retrieved. Intraocular pressure (IOP measurement recorded using Perkins or Goldmann applanation tonometer were noted and gonioscopy findings were retrieved. Angles were graded using Shaffer’s classification as open or closed with or without peripheral anterior synechiae (PAS).[10] Fundus examination and the details of the disc, background retina, and macula status were documented.
Axial length (AxL), keratometry (K), anterior chamber depth (ACD), and lens thickness (LT) were derived from the A-scan using IOL Master 500 (Carl Zeiss Meditec AG, Jena, Germany), whereas the retinochoroidal scleral (RCS) complex thickness was derived from B-scan ultrasonogram (OTI-Scan 1000, Ophthalmic Technologies Inc. [OTI], Toronto, Ontario, Canada). Corneal diameter measurement (by ruler) and central corneal thickness (CCT) measurement using a pachymeter (Pacscan 300 AP, digital biometric ruler, A-scan, Sonomed, New York, USA) were also retrieved. Optical coherence tomography (Spectralis, Heidelberg Engineering Inc., Hiedelberg, Germany) was done in children with clinical suspicion of foveal hypoplasia, pigmentary retinopathy, or macular pathology and the reports were retrieved.
Angle-closure disease was defined according to the American Academy of Ophthalmology preferred practice pattern classification as primary angle-closure suspect (PACS), primary angle closure (PAC), and primary angle-closure glaucoma (PACG).[11] A diagnosis of PACG was confirmed if the IOP was 21mmHg, with optic nerve damage in the form of rim thinning, notching, nerve fiber layer defect, or asymmetric disc cupping and gonioscopically closed angles with or without PAS. All eyes with angle-closure underwent laser peripheral iridotomy (LPI) (Visulas YAG II plus; Carl Zeiss, Oberkochen, Germany). Details of intervention like LPI and antiglaucoma medication (defined as a class of drugs used to lower the elevated IOP and reduce the risk of glaucoma) and any history of intraocular surgery were also noted. Data from the right eye was used for analysis.
Statistical methodology
Data were handled and analyzed statistically using STATA 14.0 (StataCorp, TEXAS, USA). All continuous values are presented as mean (standard deviation) or median (interquartile range) as appropriate. Categorical variables were assessed using Chi-square test or Fisher’s exact test. Before analysis, the distribution of the data was examined using Shapiro–Wilk test and Box-Whisker plot graphically for normality. For comparison between the two groups, an independent sample t-test was used to evaluate the average differences. Nonparametric, Mann–Whitney U test was used for the comparison of best-corrected visual acuity and reported in median logMAR units. Backward stepwise multivariable regression model was fitted with the variables like age, intraocular pressure, spherical equivalent, anterior chamber depth, lens thickness, and retinochoroidal scleral thickness by considering the probability value below 0.2 from the univariate model. For all statistical tests, the level of significance was considered as P < 0.05.
Results
A total of 40 NO children (18 males and 22 females) were compared with 30 age-matched controls (CO). Mean age in NO group was 8.95 ± 4.0 years and that of the control group was 10.47 ± 3.0 years. Median log MAR uncorrected visual acuity was 1.18 (4/60) and BCVA was 0.60 (6/24). All baseline clinical and biometric parameters were significantly different between the NO and CO groups except the CCT [Table 1]. Mean spherical equivalent in the NO group was 10.87 ± 3.1D (range: 3.5D to 18.0D) compared with 0.19 ± 0.7D in the control group and was found to be statistically significant (P < 0.001). The mean keratometry in NO group was 48.56 ± 2.4 D in flat meridian (Kf) and 49.51 ± 2.2D in steep meridian (Ks), which was steeper than the control group (Kf 43.52 ± 1.3D and Ks 44.58 ± 1.7D). The ACD in NO group ranged from 2.0 to 3.2 mm (mean: 2.50 ± 0.3 mm) in contrast to the controls whose ACD ranged from 2.8 to 4.1 mm (mean: 3.47 ± 0.3 mm). The mean lens thickness (LT) in NO group was significantly higher (3.64 ± 0.5 mm) than the CO group (3.36 ± 0.2 mm). The mean RCS complex measured 2.01 ± 0.2 mm (range: 1.7 to 2.9 mm) in NO group, whereas the controls had a mean RCS thickness of 1.52 ± 0.1 mm (range: 1.3 to 1.7 mm). Lens axial length factor (LAF) and LT/ACD ratio were also significantly different between the NO and control groups.
Table 1.
Comparison of clinical and ocular biometric characteristics between nanophthalmos (NO) group and control (CO) group
| NO group | CO group | P a | |
|---|---|---|---|
| Number of subjects | 40 | 30 | - |
| Age, years | 8.95 (4.0) | 10.47 (3.0) | 0.089 |
| Female gender, n (%) | 22 (55.0) | 14 (46.7) | 0.490b |
| UCVA, logMAR | |||
| Median (Snellen’s equivalent) | 1.18 (4/60) | 0 (6/6) | <0.001c |
| IQR | 1.08 to 1.48 | 0 to 0 | |
| BCVA, logMAR | |||
| Median (Snellen’s equivalent) | 0.60 (6/24) | 0 (6/6) | <0.001c |
| IQR | 0.30 to 0.78 | 0 to 0 | |
| Spherical equivalent, D | 10.87 (3.1) | 0.19 (0.7) | <0.001 |
| Axial length, mm | 16.67 (1.3) | 22.91 (0.9) | <0.001 |
| ACD, mm | 2.50 (0.3) | 3.47 (0.3) | <0.001 |
| Lens thickness, mm | 3.64 (0.5) | 3.36 (0.2) | 0.005 |
| CCT, mm | 537.90 (43.1) | 542.57 (31.4) | 0.713 |
| RCS thickness, mm | 2.01 (0.2) | 1.52 (0.1) | <0.001 |
| Keratometry, D | |||
| Kf | 48.56 (2.4) | 43.52 (1.3) | <0.001 |
| Ks | 49.51 (2.2) | 44.58 (1.7) | <0.001 |
| Astigmatism | 1.03 (0.5) | 1.09 (0.7) | 0.785 |
| Lens axial length factor | 2.19 (0.3) | 1.47 (0.1) | <0.001 |
| LT/ACD | 1.47 (0.2) | 0.97 (0.1) | <0.001 |
UCVA, uncorrected visual acuity; BCVA, best-corrected visual acuity; Kf, keratometry in flat meridian; Ks, keratometry in steep meridian; LAF, lens axial length factor=LT/AXL ×10; ACD, anterior chamber depth; RCS, retinochoroidal scleral thickness; CCT, central corneal thickness; IQR, interquartile range; logMAR, logarithm of minimal angle of resolution. aIndependent t-test; bChi-square test/Fisher’s exact test; cMann-Whitney U test
On analyzing the NO group for presence of strabismus, lens status, angle-closure glaucoma, and retinal evaluation, it was found that most children were orthotropic (75%), with 17.5% having esotropia, 5% with esophoria, and 2.5% with exotropia on orthoptic examination. All nanophthalmic children were phakic, except one child who was aphakic as she was operated on for congenital cataract. The mean IOP in the NO group was 15.56 ± 4.3 (range: 11 to 28) mmHg. Gonioscopic evaluation revealed closed angles in 50%, open angles in 32.5,% and in the remaining 17.5%, gonio findings were not available possibly due to younger age and poor cooperation for gonioscopy. Laser peripheral iridotomy (LPI) was done in 30% of NO eyes, based on clinician discretion considering the age, IOP, occludable angles, disc status, and cooperation of the child for LPI. Fundus examination revealed 87.5% with hyperopic discs, 7.5% with pigmentary retinopathy, and 2.5% each with retinoschisis and retinal detachment. Furthermore, optical coherence tomography (OCT) was done in 10 patients who had clinical suspicion of macular pathology, which revealed presence of foveal hypoplasia in five children, retinoschisis in three children, and cystoid macular edema and drusenoid deposits in one patient each [Table 2].
Table 2.
Common clinical findings in the NO group
| Frequency, n | Percentage, % | |
|---|---|---|
| Strabismus | ||
| Esophoria | 2 | 5.0 |
| Esotropia | 7 | 17.5 |
| Exotropia | 1 | 2.5 |
| Orthophoria | 30 | 75.0 |
| Lens status | ||
| Phakic | 39 | 97.5 |
| Aphakic | 1 | 2.5 |
| IOP, mmHg | ||
| Mean (SD) | 15.56 (4.3) | - |
| Min-Max | 11 to 28 | |
| BCVA | ||
| >6/24 | 19 | 47.5 |
| 6/24 to 6/60 | 16 | 40.0 |
| <6/60 | 5 | 12.5 |
| GONIO | ||
| Closed | 20 | 50.0 |
| Open | 13 | 32.5 |
| NA | 7 | 17.5 |
| Fundus | ||
| Hypermetropic | 35 | 87.5 |
| Pigmentary retinopathy | 3 | 7.5 |
| JXLR | 1 | 2.5 |
| RD | 1 | 2.5 |
| OCT findings (n=10) | ||
| Foveal hypoplasia | 5 | 50.0 |
| Retinoschisis | 3 | 30.0 |
| CME | 1 | 10.0 |
| Drusenoid | 1 | 10.0 |
IOP, intraocular pressure; BCVA, best-corrected visual acuity; NA, not available; RD, retinal detachment; JXLR, juvenile X linked retinoschisis, CME, cystoid macular edema
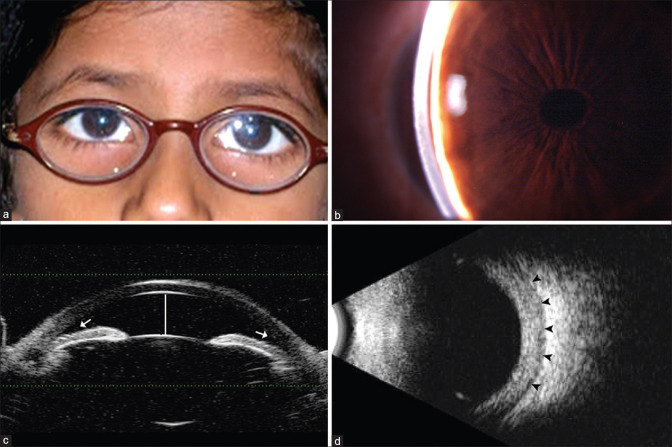
Fig. 1: Clinical photograph of nanophthalmic child showing thick hyperopic glass
Figure 1.
(a) Clinical photograph of nanophthalmic child showing thick hyperopic glass, (b) Slit-lamp photo of the same child showing shallow anterior chamber depth, (c) UBM photo showing shallow anterior chamber (white line) and crowded angle structures (white arrow), (d) B-scan image showing increased retinochoroidal scleral thickness (black arrowheads)
Fig. 2 shows the comparison of visual acuity among the various ocular associations in nanophthalmos. The common associations observed in NO group were amblyopia (64.3%, n = 18), PACG (17.8%, n = 5), pigmentary retinopathy (14.3%, n = 4), and retinal detachment (3.6%, n = 1). No significant differences were noted between the various visual acuity categories at presentation with respect to various ocular associations (P = 0.796).
Figure 2.
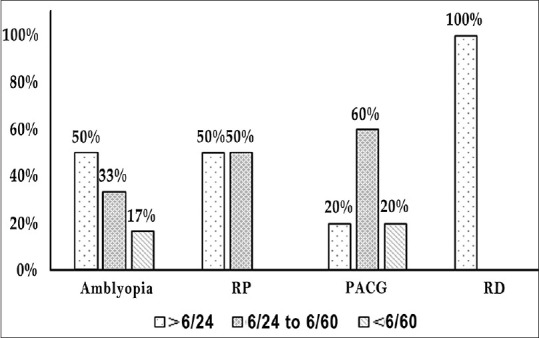
Showing various visual acuity categories with common ocular associations in NO group. PACG, primary angle-closure glaucoma; RD, retinal detachment
Table 3 compares the ocular biometry variables within the NO group children with AxL less than 17 mm and more than 17 mm. On studying the clinical spectrum of small eye phenotypes based on axial length, it was found that ACD was lower, SE was significantly higher and the LAF was also higher in the group with AxL <17 mm (P = 0.039, P = 0.005, and P = 0.031, respectively). To understand the influence of short axial length on various clinical and biometric factors a stepwise multivariable regression analysis was performed to determine the association [Table 4].
Table 3.
Comparison of ocular biometry variables in NO group with axial length (AXL)
| AXL, ≤17 mm | AXL, >17 mm | P a | |
|---|---|---|---|
| Number of subjects | 26 | 14 | - |
| Age, years | 8.61 (3.5) | 9.57 (4.9) | 0.484 |
| IOP, mmHg | 15.00 (3.8) | 16.54 (4.9) | 0.305 |
| BCVA, logMAR | |||
| Median (IQR) | 0.60 (0.48 to 0.78) | 0.39 (0.30 to 0.78) | 0.330b |
| Spherical equivalent, D | 11.87 (2.3) | 9.09 (3.6) | 0.005 |
| ACD, mm | 2.43 (0.2) | 2.61 (0.3) | 0.039 |
| Lens thickness, mm | 3.60 (0.5) | 3.71 (0.6) | 0.531 |
| CCT, mm | 517.80 (33.5) | 558.00 (45.3) | 0.149 |
| RCS thickness, mm | 2.01 (0.2) | 2.02 (0.3) | 0.861 |
| LAF | 2.26 (0.3) | 2.00 (0.3) | 0.031 |
| LT/ACD | 1.49 (0.2) | 1.43 (0.3) | 0.492 |
| Gonio, n (%) | |||
| Closed | 14 (53.8) | 6 (42.9) | 0.745c |
| Open | 8 (30.8) | 5 (35.7) | |
| NA | 4 (15.4) | 3 (21.4) | |
| YAG PI, n (%) | |||
| Done | 9 (34.6) | 3 (21.4) | 0.484c |
IOP, intraocular pressure; BCVA, best-corrected visual acuity; ACD, anterior chamber depth; CCT, central corneal thickness; RCS, retinochoroidal scleral thickness; LAF, lens axial length factor; LT, lens thickness; YAG PI, YAG peripheral iridotomy. aIndependent t-test; bMann-Whitney U test; cFisher’s exact test
Table 4.
Factors associated with axial length multivariable regression model
| Univariate analysis | Multivariable analysis | |||
|---|---|---|---|---|
|
|
|
|||
| β (95% CI) | P | β (95% CI) | P | |
| Age, years | −0.003 (−0.11 to 0.11) | 0.956 | - | - |
| IOP, mmHg | 0.02 (−0.09 to 0.13) | 0.686 | - | - |
| SE, D | −0.20 (−0.33 to−0.07) | 0.003 | −0.14 (−0.26 to−0.03) | 0.016 |
| ACD, mm | 2.94 (1.62 to 4.27) | <0.001 | 2.32 (0.94 to 3.71) | 0.002 |
| LT, mm | 0.71 (−0.11 to 1.54) | 0.086 | 0.46 (−0.24 to 1.16) | 0.193 |
| RCS, mm | −0.04 (−2.10 to 2.02) | 0.969 | - | - |
IOP, intraocular pressure; SE, spherical equivalent; ACD, anterior chamber depth; LT, lens thickness; RCS, retinochoroidoscleral thickness; β, regression coefficient; CI, confidence interval
The regression model included factors like age, IOP, SE, ACD, LT, and RCS thickness. SE (β = −0.14, 95% CI − 0.26 to − 0.03; P = 0.016) and ACD (β = 2.32, 95% CI 0.94 to 3.71, P = 0.002) were correlated most commonly with AxL.
Discussion
Nanophthalmic eyes are typically characterized by a short axial length, high hyperopia, and reduced ocular volume.[1,2,3,4] It is often associated with a normal-sized crystalline lens, leading to a high lens/eye volume ratio and crowded anterior segment.[12] In our study, we observed distinct biometric parameters like short AxL, narrow ACD, high RCS, increased LT and LAF, high LT/ACD ratio in the NO group. Furthermore, we also observed significant difference in SE, ACD, and LAF among NO children with AxL <17 mm or >17 mm. Ametropic amblyopia (64.3%) and primary angle-closure disease (50%) were predominant association in our study cohorts.
Visual impairment was mostly due to ametropic amblyopia (64.3%) in our cohort with a BCVA of 20/70 compared with 20/40 in a study by Agarkar et al. (35%) and Relhan et al. (7.69%).[4,13] Strabismus, high hyperopia, angle-closure glaucoma, and pigmentary retinopathy were other reasons for visual impairment in our cohort. In those with strabismus, esotropia (17.5%) was more common, and the same was reported earlier by Agarkar et al. (18.6%).[13] High hyperopia in nanophthalmic eyes occurs mainly due to the short axial length and increased lens/eye volume ratio, causing objects to be imaged behind the retina. Excess accommodation stimulated by marked hypermetropia in these eyes, precipitates convergence, that overwhelms the maximum divergence amplitude causing esotropia and may further worsen the amblyopia process.[4,14] In addition, we observed a few posterior segment anomalies like foveal hypoplasia and retinoschisis as documented by OCT, which possibly could be other reasons for poor vision in these eyes.[15,16,17]
NO group individuals had high risk for developing chronic angle closure.[18] In our study, angle closure was seen in 50% of NO children compared with 22.7% in Agarkar et al. study.[13] Also, 17.9% of our NO group had synechial angle closure and laser PI was done in 30% of our cohort compared with 18.6% in Agarkar et al. study.[13] The decision to perform LPI, was not based on gonioscopic findings alone, but rather a combination of various other factors like age of the child, family history of glaucoma, presenting IOP, and ability to cooperate for a LPI.[19] The mechanism of angle closure in these eyes was predominantly due to relative pupillary block secondary to crowded anterior segment or a posterior pushing mechanism.
Although 50% had angle closure, the incidence of angle-closure glaucoma was relatively low in our study. The low incidence of ACG in our cohort could be attributed to the younger age in our series, as normal lens might attain significance only later in life and precipitate angle-closure glaucoma. LT/AxL ratio that defines the relationship between iris lens diaphragm and cornea, is a definite indicator of angle status. These values was found to be age dependent and were greater than normal for most age groups.[20] According to George et al.,[8] LT/AXL ratio was 0.192 in normal adults and 0.199 in adults with occludable angles in south India. Moreover, Agarkar et al.[13] had suggested a high LT/AxL ratio of above 0.239 to be significantly associated with risk of developing angle closure. Likewise, the LT/AXL ratio in our study was 0.219 in the NO group, which significantly was higher, contributing to angle closure in NO children.
Our previous observation on adult clinical spectrum of nanophthalmic population found that meticulous gonioscopic evaluation is a key to detect PAS, which had 3.66 times higher odds of developing ACG.[9] Performing a gonioscopy in children though invaluable is not always possible due to younger age and poor cooperation. Therefore, it is necessary to closely monitor all NO children with serial biometry and noncontact anterior segment OCT (ASOCT) for timely diagnosis and prompt treatment to avoid visual impairment due to glaucoma.
The mean axial length in our study was 16.67 ± 1.3 mm, which was lower than Agarkar et al.[13] (16.88 ± 1.48 mm) and Relhan et al.[4] (17.20 ± 1.64 mm) studies. Likewise, our NO group children were younger, with lower mean ACD (2.50 ± 0.3 mm) and higher RCS thickness (2.01 ± 0.2 mm) compared with earlier published literature.[4,13]
Our study was unique from previously published studies, as we not only compared the NO group with age-matched controls, we also stratified the NO group based on AxL < 17 mm or >17 mm. The subgroup analysis was mainly done to understand the differences in ocular biometric parameters among the NO group children. Interestingly, we found SE, ACD, and LAF to be statistically different between the two groups. Agarkar et al.[13] in their study compared the biometric factors among the NO children with occludable and open angles. They reported a greater risk of angle-closure if ACD was <3 mm, LT >4 mm, LT/AxL ratio >0.239. Similarly, in our cohort, eyes with AxL <17 mm had significantly higher SE, lower ACD, and greater LAF contributing to angle-closure disease. NO is a complex entity and multiple biometric factors may influence the risk of developing angle closure disease. The merit of the study is that it highlights the importance of a clinically important condition that can be overlooked by pediatric ophthalmologists.
To the best of our knowledge, this is the largest study comparing the morphometric features between NO eyes and age-matched controls. In addition, this study also compares the ocular biometry between eyes with AxL <17 or >17 mm, which has not been reported earlier. The limitations of this study are inherent to retrospective nature of the study. Secondly, gonioscopy could not be done in all children due to younger age and poor cooperation. Thirdly, several pediatric ophthalmologists were involved in the care of these patients, which could have led to some bias.
Conclusion
A clinician who encounters high hyperopia needs to be vigilant about nanophthalmos and record the baseline ocular biometric factors like AxL, ACD, LT, LAF, LT/ACD ratio, keratometry, and RCS thickness. Visual impairment due to amblyopia, strabismus, and angle-closure are common associations. Early detection of angle closure is crucial to avoid needless blindness. Hence, serial biometry may be extremely useful to identify NO children at risk of developing angle-closure disease and glaucoma. Furthermore, use of noncontact imaging modalities like AS-OCT may augment the detection of angle closure in pediatric population whenever gonioscopy was not possible. Future prospective studies with a longer follow-up and serial biometry may help us unravel the complex pathophysiological mechanisms that exist in these eyes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Singh OS, Simmons RJ, Brockhurst RJ, Trempe CL. Nanophthalmos:A perspective on identification and therapy. Ophthalmology. 1982;89:1006–12. [PubMed] [Google Scholar]
- 2.Othman MI, Sullivan SA, Skuta GL, Cockrell DA, Stringham HM, Downs CA, et al. Autosomal dominant nanophthalmos (NNO1) with high hyperopia and angle-closure glaucoma maps to chromosome 11. Am J Hum Genet. 1998;63:1411–8. doi: 10.1086/302113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Altintaş AK, Acar MA, Yalvaç IS, Koçak I, Nurözler A, Duman S. Autosomal recessive nanophthalmos. Acta Ophthalmol Scand. 1997;75:325–8. doi: 10.1111/j.1600-0420.1997.tb00788.x. [DOI] [PubMed] [Google Scholar]
- 4.Relhan N, Jalali S, Pehre N, Rao HL, Manusani U, Bodduluri L. High-hyperopia database, part I:Clinical characterisation including morphometric (biometric) differentiation of posterior microphthalmos from nanophthalmos. Eye (Lond) 2016;30:120–6. doi: 10.1038/eye.2015.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah SP, Taylor AE, Sowden JC, Ragge NK, Russell-Eggitt I, Rahi JS, et al. Anophthalmos, microphthalmos, and typical coloboma in the United Kingdom:A prospective study of incidence and risk. Invest Ophthalmol Vis Sci. 2011;52:558–64. doi: 10.1167/iovs.10-5263. [DOI] [PubMed] [Google Scholar]
- 6.Hu Z, Yu C, Li J, Wang Y, Liu D, Xiang X, et al. A novel locus for congenital simple microphthalmia family mapping to 17p12-q12. Invest Ophthalmol Vis Sci. 2011;52:3425–9. doi: 10.1167/iovs.10-6747. [DOI] [PubMed] [Google Scholar]
- 7.Wu W, Dawson DG, Sugar A, Elner SG, Meyer KA, McKey JB, et al. Cataract surgery in patients with nanophthalmos:Results and complications. J Cataract Refract Surg. 2004;30:584–90. doi: 10.1016/j.jcrs.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 8.George R, Paul PG, Baskaran M, Ramesh SV, Raju P, Arvind H, et al. Ocular biometry in occludable angles and angle closure glaucoma:A population based survey. Br J Ophthalmol. 2003;87:399–402. doi: 10.1136/bjo.87.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajendrababu S, Shroff S, Uduman MS, Babu N. Clinical spectrum and treatment outcomes of patients with nanophthalmos. Eye (Lond) 2021;35:825–30. doi: 10.1038/s41433-020-0971-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaffer RN. Primary glaucomas. Gonioscopy, ophthalmoscopy and perimetry. Trans Am Acad Ophthalmol Otolaryngol. 1960;64:112–27. [PubMed] [Google Scholar]
- 11.American Academy of Ophthalmology Glaucoma Panel. Primary angle closure glaucoma. San Francisco, CA: American Academy of Ophthalmology; 2020. [Last accessed on 2022 May 07]. Preferred practice pattern guidelines. Available from:https://www.aao.org/preferred-practice-pattern/primary-angle-closure-disease-ppp . [Google Scholar]
- 12.Weiss AH, Kousseff BG, Ross EA, Longbottom J. Simple microphthalmos. Arch Ophthalmol. 1989;107:1625–30. doi: 10.1001/archopht.1989.01070020703032. [DOI] [PubMed] [Google Scholar]
- 13.Agarkar S, Koladiya N, Kumar M, Vijaya L, Raman R. Nanophthalmos in children:Morphometric and clinical characterization. J AAPOS. 2020;24:27.e1–5. doi: 10.1016/j.jaapos.2019.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Sener EC, Mocan MC, Saraç OI, Gedik S, Sanaç AS. Management of strabismus in nanophthalmic patients:A long-term follow-up report. Ophthalmology. 2003;110:1230–6. doi: 10.1016/S0161-6420(03)00267-7. [DOI] [PubMed] [Google Scholar]
- 15.Proença H, Castanheira-Dinis A, Monteiro-Grillo M. Bilateral nanophthalmos and pigmentary retinal dystrophy--An unusual syndrome. Graefes Arch Clin Exp Ophthalmol. 2006;244:1203–5. doi: 10.1007/s00417-005-0230-1. [DOI] [PubMed] [Google Scholar]
- 16.Ayala-Ramirez R, Graue-Wiechers F, Robredo V, Amato-Almanza M, Horta-Diez I, Zenteno JC. A new autosomal recessive syndrome consisting of posterior microphthalmos, retinitis pigmentosa, foveoschisis, and optic disc drusen is caused by a MFRP gene mutation. Mol Vis. 2006;12:1483–9. [PubMed] [Google Scholar]
- 17.Khairallah M, Messaoud R, Zaouali S, Ben Yahia S, Ladjimi A, Jenzri S. Posterior segment changes associated with posterior microphthalmos. Ophthalmology. 2002;109:569–74. doi: 10.1016/s0161-6420(01)00996-4. [DOI] [PubMed] [Google Scholar]
- 18.Yalvac IS, Satana B, Ozkan G, Eksioglu U, Duman S. Management of glaucoma in patients with nanophthalmos. Eye (Lond) 2008;22:838–43. doi: 10.1038/sj.eye.6702742. [DOI] [PubMed] [Google Scholar]
- 19.Singh OS, Belcher CD, Simmons RJ. Nanophthalmic eyes and neodymium-YAG laser iridectomies. Arch Ophthalmol. 1987;105:455–6. doi: 10.1001/archopht.1987.01060040025008. [DOI] [PubMed] [Google Scholar]
- 20.Markowitz SN, Morin JD. The ratio of lens thickness to axial length for biometric standardization in angle-closure glaucoma. Am J Ophthalmol. 1985;99:400–2. doi: 10.1016/0002-9394(85)90005-4. [DOI] [PubMed] [Google Scholar]