Abstract
Amino acid substitutions in Escherichia coli ς70 were generated and characterized in an analysis of the role of region 1.1 in transcription initiation. Several acidic and conserved residues are tolerant of substitution. However, replacement of aspartic acid 61 with alanine results in inactivity caused by structural and functional thermolability.
Core RNA polymerase (α2ββ′) requires the variable specificity subunit, sigma (ς), to direct promoter-dependent transcription (1, 3, 4, 12, 18, 22, 23, 26). Following promoter binding, holoenzyme (α2ββ′ς) progresses through several intermediate complexes, en route to a stable initiated open complex (2, 14). ς factor has been implicated in stages of initiation beyond promoter recognition (8, 9, 13, 15, 17). Recently, we showed that the conserved amino terminal domain (region 1) of Escherichia coli ς70 is important for the process of strand melting and initiated complex formation at the λ pR promoter (24).
Region 1 is unique to the primary ς factors, yet little is known of its function. Deletion of region 1.1 (amino acids 1 to 100) from ς70 has two major consequences for holoenzyme. The first is inefficient progression from the closed to the strand-separated open complex. This can be overcome by increasing the time allowed for formation of holoenzyme-promoter complexes and is lessened by addition of region 1.1 in trans. The second and more deleterious effect is impaired transition from the strand-separated open complex to a stable initiated complex (RPinit). According to this analysis, amino acids between positions 50 and 75 of ς70 are critical for proper initiation in vitro (24).
A comparison of region 1.1 among several primary ς factors revealed conserved residues at positions 52 (glycine [G]), 53 (isoleucine [I]), 55 (valine [V]), and 61 (aspartic acid [D]), as well as a high degree of acidity (40%) within the segment from amino acids 50 to 75 (24). Here, we test whether alterations at these conserved positions or in the overall acidity of the region influence initiation by holoenzyme (Eς).
Site-directed mutagenesis (10) and the Expand high fidelity PCR system (Boehringer Mannheim) were used to create substitutions at positions 52, 53, 55, 61, 57, 58, 63, 64, and 69 (Table 1). rpoD was mutagenized in M13 phage (10) and amplified with oligonucleotides that incorporated restriction sites at the 5′ and 3′ ends of the fragment. The restricted fragments were ligated into pQE30-T (24). PCR mutagenesis was used to amplify a fragment corresponding to the 3′ end of the rpoD gene with a 5′ mutagenic oligonucleotide and a 3′ oligonucleotide that incorporated a restriction site. A concurrent round of amplification included a 5′ oligonucleotide complementary to the 5′ end of rpoD and a 3′ oligonucleotide with complementarity to an internal segment of rpoD, downstream from the genetic alteration(s). The 5′ and 3′ PCR fragments were mixed, and the full-length mutagenized rpoD gene was amplified, gel isolated, digested, ligated into pQE30-T, transformed into E. coli XL1 Blue (Stratagene), and sequenced to confirm the changes. The plasmids were transformed into E. coli 19284 (rpoD800, W3110 srl::Tn10 recA lacIq) to test for function in vivo (24). Transformation mixtures were split and spread onto Luria-Bertani plates containing ampicillin (100 mg/ml), kanamycin (30 mg/ml), and 2% glucose and then incubated at 32 and 44°C, to evaluate complementation of the rpoD800 temperature-sensitive growth defect at 44°C. Plasmids were likewise transformed into strain CAG20176 to test growth in the absence of chromosomal rpoD expression at 32°C (11, 24).
TABLE 1.
Region 1.1 amino acid substitution mutantsa
| Mutation | Complementation |
|---|---|
| Conserved | |
| G52A | + |
| V55A | + |
| V55I | + |
| Acidic | |
| D61Ab | − |
| D61E | + |
| D61S | + |
| E57,58A | + |
| D63,64A | + |
| E57,58A, D61,63–64A | − |
Results of in vivo analysis indicate complementation (+) and lack of complementation (−). The same results were obtained for two different strain backgrounds, 19284 and CAG20176.
Position 61 is both highly conserved and acidic.
Mutants were generated that replaced either acidic, conserved, or, at position 61, both conserved and acidic amino acids with alanine (A). Replacement of glutamic acid (E) at position 69 (E69) with A had no effect on ς70 function in vivo (Table 1). Double mutations replacing E at positions 57 and 58 or D at positions 63 and 64 reduced the overall acidity of region 1.1, but neither affected ς70 function in vivo (Table 1). Replacement of conserved D61 with A, however, rendered ς70 nonfunctional.
Additional substitutions at position 61 addressed the contribution of the amino acid side chain. Both E and serine (S) could functionally substitute for D, indicating that polarity rather than side chain charge at this position is more important for function (Table 1). D61 is found in a cluster of acidic residues; however, simultaneous substitution of D63 and D64 did not impair function in vivo, indicating that D61 does not require the flanking acidic residues. A quintuple mutation combining E57,58A with D61A and D63,64A was inactive in vivo. Since both of the double mutants were functional, the lethal phenotype is probably caused predominantly by D61A. Substitutions G52A, V55A, or V55I functioned at least as well as the wild type (Table 1). Because ς70V55A and ς70V55I function in vivo, the size and shape of the hydrophobic side chain at this position are not critical. ς70I53A was inactive in vivo and seriously defective in vitro, and its characterization has been reported elsewhere (25).
These results argue that the overall acidity of region 1.1 is not a major factor in its participation in transcription initiation. Conserved D61, however, appears to be important for ς70 structure and/or function in vivo. To address the basis for the inactivity of ς70D61A, the protein was overexpressed and purified (24) for characterization in vitro.
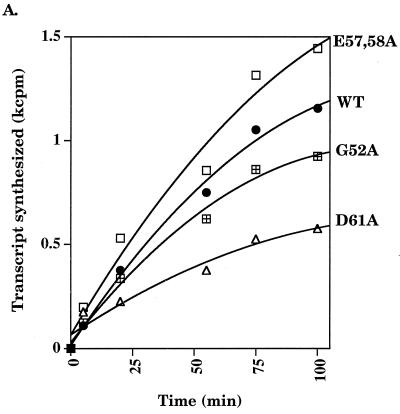
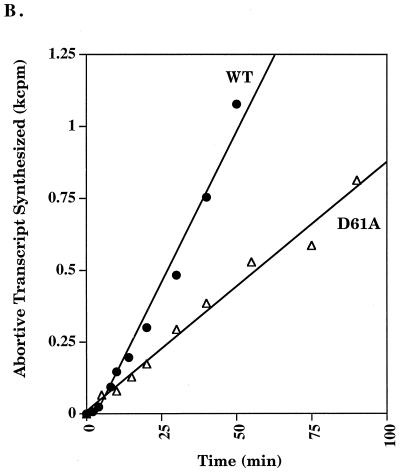
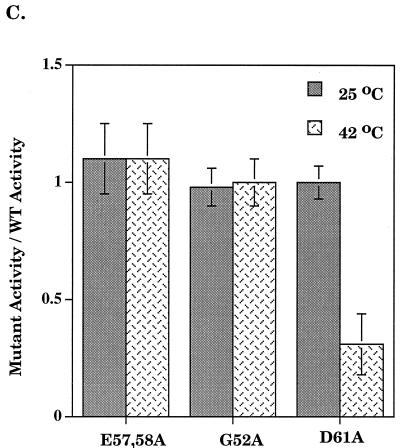
Run-off transcription analysis was performed to assess the overall effect of the D61A substitution on RNA synthesis (24). A time course at 37°C indicated that Eς70D61A was impaired in transcription, while Eς70G52A and Eς70E57,58A exhibited transcription rates similar to Eς70 (Fig. 1A). Eς70D61A was also defective for abortive transcription (24) at 37°C (Fig. 1B). One explanation for the inactivity of ς70D61A is thermolability of the protein. Thus, we examined the effect of temperature on run-off transcription. At 25°C, Eς70D61A activity was indistinguishable from Eς70. At higher temperatures, a transcriptional defect became apparent (Fig. 1C), with loss of activity as the temperature increased. The functional mutants Eς70G52A and Eς70E57,58A were not affected by increasing temperature, as compared to Eς70.
FIG. 1.
Run-off transcription analysis. (A) A representative run-off transcription time course experiment is shown for Eς70 and ς70 mutants at 37°C. Each experiment was repeated at least five times, and the error was less than 15%. (B) Abortive transcription by Eς70D61A at 37°C. Synthesis of the three nucleotide abortive RNA transcripts (ApUpG) from λ pR is shown relative to the wild type. The holoenzyme concentration was 0.008 μM and the template concentration was 1.5 nM. (C) Run-off transcription as a function of temperature. A comparison of run-off transcription activities at 60 min at 25 and 42°C is shown. Activities are normalized to the wild type, and the error indicated was less than 15%. WT, wild type.
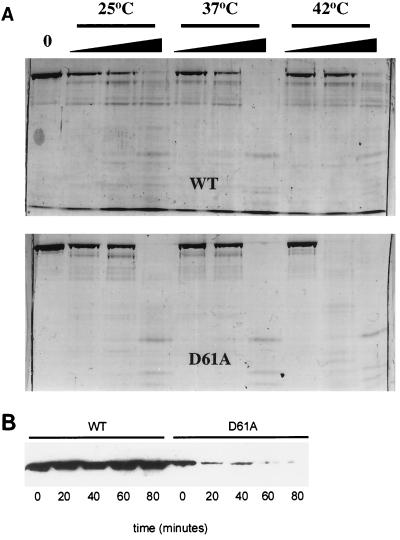
Because ς70D61A was functionally thermolabile in vitro, the possibility that it was structurally thermolabile was assessed by comparing susceptibility to trypsin digestion at 25, 37, and 42°C. Consistent with its transcriptional activity, ς70D61A exhibited increased sensitivity to trypsin digestion at 42°C (Fig. 2A). The failure of ς70D61A to complement rpoD mutant strains in vivo may therefore be caused by instability of the protein. To test this idea, we used immunoblotting to compare the levels of the wild type and ς70D61A after a shift from 37 to 44°C (Fig. 2B). At the time of the upshift, there was significantly more wild-type ς70 present, and it remained stable for longer than 80 min (Fig. 2B). Conversely, ς70D61A was much less stable, becoming nearly undetectable by 80 min. The D61A substitution appears to cause a structural disruption in ς70 that results in proteolytic instability both in vivo and in vitro and functional instability during transcription initiation. Other mutations in region 1.1 have also been reported to result in structural instability (5).
FIG. 2.
Stability of ς70D61A in vitro and in vivo. (A) Limited trypsinolysis. ς70D61A (2 μg) was subjected to trypsin digestion at three temperatures, as indicated, to assess possible structural defects. Wedges indicate increasing trypsin concentration (0.0125, 0.0625, and 0.025 μg). Digestion of wild-type (WT) ς70 is shown for reference in the top panel. Fragments were resolved on a sodium dodecyl sulfate–8% polyacrylamide gel and visualized with Coomassie brilliant blue staining. (B) Immunoblot analysis of culture lysates of strain 19284 (wild-type [WT] ς70 and ς70D61A). Exponentially growing cells at 37°C were upshifted to 44°C. Lysates were prepared from cells harvested at the indicated time points following temperature upshift, and proteins were resolved on a sodium dodecyl sulfate–8% polyacrylamide gel. Following Western transfer, histidine-tagged ς70 proteins were detected by using a six-His-tagged monoclonal antibody (Clontech).
A more thorough evaluation of the initiation properties of the mutants was conducted to determine if a particular step in the process was affected by the substitutions. The first step in initiation is promoter recognition and binding by RNA polymerase. Nitrocellulose filter retention has been used to evaluate DNA binding at λ pR, and the complexes retained are open complexes (6, 7, 16, 19, 20). Holoenzyme (1 nM) was incubated with a 32P-5′-end-labeled DNA fragment containing λ pR (0.1 nM). The binding of Eς70D61A to λ pR was indistinguishable from Eς70 as well as the functional mutants Eς70G52A and Eς70E57,58A (data not shown).
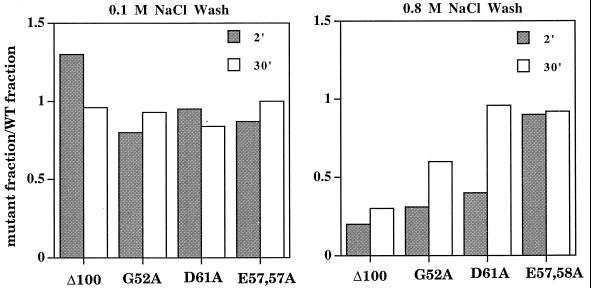
Addition of nucleoside triphosphates (NTPs) to open complexes allows progression to RPinit, which are stable to an 0.8 M NaCl wash (16, 21). The ability of NTPs to stabilize Eς70D61A-λ pR open complexes was assessed. Eς70Δ100, previously shown to be defective in RPinit formation (24), was compared for reference. Under low-stringency wash conditions (0.1 M NaCl), at 2 and 30 min after adding holoenzyme to DNA, each Eς70 derivative bound to λ pR as well as the wild type (Fig. 3). Under high-stringency wash conditions (0.8 M NaCl), the Eς70E57,58A complexes were retained as well as the wild type. Interestingly, the Eς70G52A complexes were less stable than Eς70, but this mutant was still able to complement in vivo. Eς70D61A-λ pR complexes were unstable at 2 min, but by 30 min they were indistinguishable from Eς70, indicating a slower rate of RPinit formation.
FIG. 3.
Stability of initiated complexes. Nitrocellulose filter retention under low (0.1 M NaCl)- and high (0.8 M NaCl)-stringency wash conditions is shown. The fraction of mutant complexes retained is normalized to the fraction of wild-type (WT) complexes retained after allowing formation for 2 and 30 min following mixing of RNA polymerase with DNA. Amino acid substitutions are indicated.
Impaired run-off transcription, combined with the slow rate of RPinit formation by Eς70D61A, could be caused by difficulty in open complex formation. KMnO4 footprinting analysis was performed to assess the ability of Eς70D61A to form open complexes (24). Strand melting for Eς70D61A occurred as efficiently as for Eς70 in the absence and presence of NTPs, even at the times when RPinit formation was impaired (data not shown). Open complex formation by Eς70G52A was also examined, since the RPinit were slightly less stable than they were for Eς70, but no differences relative to Eς70 were detected.
In summary, the D61A mutation renders ς70 nonfunctional in vivo and functionally and structurally thermolabile in vitro, manifested in a slow rate of RPinit formation. Alanine substitution at acidic residues 57, 58, 63, 64, and 69 has no effect on ς70 function in vivo or in vitro. Therefore, acidity of region 1.1 is not a major contributing factor to the initiation properties of ς70, but amino acids, including D61, are very important for structural stability.
Acknowledgments
This research was supported by National Institutes of Health grant GM 56453.
REFERENCES
- 1.Daniels D, Zuber R, Losick R. Two amino acids in an RNA polymerase ς factor involved in recognition of adjacent base pairs in the −10 region of a cognate promoter. Proc Natl Acad Sci USA. 1990;87:8075–8079. doi: 10.1073/pnas.87.20.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.deHaseth P L, Zupanic M L, Record M T., Jr RNA polymerase-promoter interactions: the comings and goings of RNA polymerase. J Bacteriol. 1998;180:3019–3025. doi: 10.1128/jb.180.12.3019-3025.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dombroski A J, Walter W A, Record M T, Jr, Siegele D A, Gross C A. Polypeptides containing highly conserved regions of transcription initiation factor ς70 exhibit specificity of binding to promoter DNA. Cell. 1992;70:501–512. doi: 10.1016/0092-8674(92)90174-b. [DOI] [PubMed] [Google Scholar]
- 4.Gardella T, Moyle H, Susskind M M. A mutant Escherichia coli ς70 subunit of RNA polymerase with altered promoter specificity. J Mol Biol. 1989;206:579–590. doi: 10.1016/0022-2836(89)90567-6. [DOI] [PubMed] [Google Scholar]
- 5.Gopal V, Chatterji D. Mutations in the 1.1 subdomain of Escherichia coli sigma factor ς70 and disruption of its overall structure. Eur J Biochem. 1997;244:613–618. doi: 10.1111/j.1432-1033.1997.00613.x. [DOI] [PubMed] [Google Scholar]
- 6.Hinkle D C, Chamberlin M J. Studies of the binding of Escherichia coli RNA polymerase to DNA. I. The role of the sigma subunit in site selection. J Mol Biol. 1972;70:157–185. doi: 10.1016/0022-2836(72)90531-1. [DOI] [PubMed] [Google Scholar]
- 7.Hinkle D C, Chamberlin M J. Studies of the binding of Escherichia coli RNA polymerase to DNA. II. Kinetics of the binding reaction. J Mol Biol. 1972;70:187–195. doi: 10.1016/0022-2836(72)90532-3. [DOI] [PubMed] [Google Scholar]
- 8.Huang X, Lopez de Saro F J, Helmann J D. ς factor mutations affecting the sequence-selective interaction of RNA polymerase with −10 region single-stranded DNA. Nucleic Acids Res. 1997;25:2603–2609. doi: 10.1093/nar/25.13.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juang Y, Helmann J D. A promoter melting region in the primary ς factor of Bacillus subtilis. J Mol Biol. 1994;235:1470–1488. doi: 10.1006/jmbi.1994.1102. [DOI] [PubMed] [Google Scholar]
- 10.Kunkel T A, Roberts J D, Zakour R A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- 11.Lonetto M A, Rhodius V, Lamberg K, Kiley P, Busby S, Gross C. Identification of a contact site for different transcriptional activators in region 4 of the Escherichia coli RNA polymerase ς70 subunit. J Mol Biol. 1998;284:1353–1365. doi: 10.1006/jmbi.1998.2268. [DOI] [PubMed] [Google Scholar]
- 12.Losick R, Pero J. Cascades of sigma factors. Cell. 1981;25:582–584. doi: 10.1016/0092-8674(81)90164-1. [DOI] [PubMed] [Google Scholar]
- 13.Marr M T, Roberts J W. Promoter recognition as measured by binding of polymerase to nontemplate strand oligonucleotide. Science. 1997;276:1258–1260. doi: 10.1126/science.276.5316.1258. [DOI] [PubMed] [Google Scholar]
- 14.Record M T, Jr, Reznikoff W S, Craig M L, McQuade K L, Schlax P J. Escherichia coli RNA polymerase (Eς70), promoters, and the kinetics of the steps of transcription initiation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 792–820. [Google Scholar]
- 15.Roberts C W, Roberts J W. Base-specific recognition of the nontemplate strand of promoter DNA by E. coli RNA polymerase. Cell. 1996;86:495–501. doi: 10.1016/s0092-8674(00)80122-1. [DOI] [PubMed] [Google Scholar]
- 16.Roe J, Burgess R R, Record M T., Jr Kinetics and mechanism of the interaction of Escherichia coli RNA polymerase with the λPR promoter. J Mol Biol. 1984;176:495–521. doi: 10.1016/0022-2836(84)90174-8. [DOI] [PubMed] [Google Scholar]
- 17.Rong J C, Helmann J D. Genetic and physiological studies of Bacillus subtilis ςA mutants defective in promoter melting. J Bacteriol. 1994;176:5218–5224. doi: 10.1128/jb.176.17.5218-5224.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegele D A, Hu J C, Walter W A, Gross C A. Altered promoter recognition by mutant forms of the ς70 subunit of Escherichia coli RNA polymerase. J Mol Biol. 1989;206:591–603. doi: 10.1016/0022-2836(89)90568-8. [DOI] [PubMed] [Google Scholar]
- 19.Strauss H S, Burgess R R, Record M T., Jr Binding of Escherichia coli ribonucleic acid polymerase holoenzyme to a bacteriophage T7 promoter-containing fragment: evaluation of promoter binding constants as a function of solution conditions. Biochemistry. 1980;19:3504–3515. doi: 10.1021/bi00556a015. [DOI] [PubMed] [Google Scholar]
- 20.Strauss H S, Burgess R R, Record M T., Jr Binding of Escherichia coli ribonucleic acid polymerase holoenzyme to a bacteriophage T7 promoter-containing fragment: selectivity exists over a wide range of solution conditions. Biochemistry. 1980;19:3496–3504. doi: 10.1021/bi00556a014. [DOI] [PubMed] [Google Scholar]
- 21.Taylor W E, Burgess R R. Escherichia coli RNA polymerase binding and initiation of transcription on fragments of λrifd 18 DNA containing promoters for λ genes and for rrnB, tufB, rplK,A, rplJ,L, and rpoB,C genes. Gene. 1979;6:331–365. doi: 10.1016/0378-1119(79)90073-8. [DOI] [PubMed] [Google Scholar]
- 22.Travers A A, Burgess R R. Cyclic re-use of the RNA polymerase sigma factor. Nature (London) 1969;222:537–540. doi: 10.1038/222537a0. [DOI] [PubMed] [Google Scholar]
- 23.Waldburger C, Gardella T, Wong R, Susskind M M. Changes in conserved region 2 of Escherichia coli ς70 affecting promoter recognition. J Mol Biol. 1990;215:267–276. doi: 10.1016/s0022-2836(05)80345-6. [DOI] [PubMed] [Google Scholar]
- 24.Wilson C, Dombroski A J. Region 1 of ς70 is required for efficient isomerization and initiation of transcription by Escherichia coli RNA polymerase. J Mol Biol. 1997;267:60–74. doi: 10.1006/jmbi.1997.0875. [DOI] [PubMed] [Google Scholar]
- 25.Wilson Bowers C, Dombroski A J. A mutation in region 1.1 of ς70 affects promoter DNA binding by E. coli RNA polymerase holoenzyme. EMBO J. 1999;18:101–108. doi: 10.1093/emboj/18.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zuber P, Healy J, Carter III H L, Cutting S, Moran C P, Jr, Losick R. Mutation changing the specificity of an RNA polymerase sigma factor. J Mol Biol. 1989;206:605–614. doi: 10.1016/0022-2836(89)90569-x. [DOI] [PubMed] [Google Scholar]