Abstract
Osteoarthritis (OA) is an age-related chronic degenerative joint disease where the main characteristics include progressive degeneration of cartilage, varying degrees of synovitis, and periarticular osteogenesis. However, the underlying factors involved in OA pathogenesis remain elusive which has resulted in poor clinical treatment effect. Recently, glucose metabolism changes provide a new perspective on the pathogenesis of OA. Under the stimulation of external environment, the metabolic pathway of chondrocytes tends to change from oxidative phosphorylation (OXPHOS) to aerobic glycolysis. Previous studies have demonstrated that glycolysis of synovial tissue is increased in OA. The hexokinase (HK) is the first rate limiting enzyme in aerobic glycolysis, participating and catalyzing the main pathway of glucose utilization. An isoform of HKs, HK2 is considered to be a key regulator of glucose metabolism, promotes the transformation of glycolysis from OXPHOS to aerobic glycolysis. Moreover, the expression level of HK2 in OA synovial tissue (FLS) was higher than that in control group, which indicated the potential therapeutic effect of HK2 in OA. However, there is no summary to help us understand the potential therapeutic role of glucose metabolism in OA. Therefore, this review focuses on the properties of HK2 and existing research concerning HK2 and OA. We also highlight the potential role and mechanism of HK2 in OA.
Video abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-022-00943-y.
Keywords: Osteoarthritis, Glycolysis, Hexokinase 2, Chondrocyte, Metabolism
Background
As an important part of skeletal muscle diseases, osteoarthritis (OA) has been a great concern. It is a chronic degenerative bone and joint disease, which can involve in multiple joints (e.g., shoulder joint, hip joint, knee joint) [1, 2]. OA is characterized by progressive degeneration of cartilage, varying degrees of synovitis, and periarticular osteogenesis, including osteophyte formation and subchondral osteosclerosis [3], and the mainly clinical manifestations are pain and joint dysfunction [4, 5].There are many risk factors that affect the occurrence and development of OA, the main risk factors were aging [6], obesity [7], genetic susceptibility, metabolic, traumatic, inflammatory. In addition, biomechanical and epigenetic factors were also included [8]. Currently, the global epidemiological survey showed that as an important part of musculoskeletal disorders, the incidence rate of OA has been increasing gradually since 1990 [9, 10], which seriously affected the quality of life for elderly patients. However, the research on OA mainly is focused on mitochondrial dysfunction, oxidative stress and so on. At present, there are few studies on the pathogenesis of OA from the perspective of glucose metabolism.
Metabolic flexibility is significantly impaired in the OA process, including glucose metabolism [11, 12]. Glucose is metabolized to produce ATP, which is the main energy source for many cellular processes [13]. Glucose metabolism includes glycolysis, pentose phosphate pathway (PPP) and tricarboxylic acid (TCA) cycle [13]. Glycolysis is a strictly regulated process, in which a variety of enzymes (e.g., hexokinase, pyruvate kinase) play an important role in this process [14]. Manoj et al. [15] treated primary chondrocytes with IL-1β, and found that the activity and expression of lactate dehydrogenase (LDH) increased significantly. Results from mRNA sequencing showed that IL-1β induction caused a significant increase in genes expression involved in glycolysis such as pyruvate kinase (PKM), lactate dehydrogenase (LDHA) and hexokinase-2 (HK2) [15]. To determine the function of PKM2 on human OA chondrocyte glycolysis, a study reported that PKM2 was increased in OA chondrocytes compared with healthy control [16]. Furthermore, the active LDHA and lactate (LA) level in synovial fluid of TMJOA patients, were significantly higher compared to healthy control [17], suggesting that glycolysis plays an important role in the progression of OA.
The hexokinase (HK) is the first rate limiting enzyme in aerobic glycolysis, which can catalyze the conversion of glucose to glucose-6-phosphate (G-6-P). Subsequently, G-6-P initiated the main pathway of glucose utilization, including glycolysis, pentose phosphate pathway and oxidative phosphorylation pathway (OXPHOS). Therefore, HK is considered to be a key regulator of glucose metabolism [18, 19]. Hexokinase 2 (HK2) is the isoform of HKs, and more effective than others in promoting aerobic glycolysis [20]. Previous studies provided that overexpression of HK2 in various tumor cells can promote the transformation of glycolysis from OXPHOS to aerobic glycolysis or Warburg effect, which leads to increased glucose uptake and production of LA [21–24]. On the contrary, HK2 silencing could inhibit glycolysis and induce oxidative phosphorylation in hepatocellular carcinoma, and synergistically inhibit the growth of mouse tumor cells with sorafenib [25]. Glucose metabolism seems to be significantly increased in patients with arthritis. An important research question that needs to be addressed is how does glucose metabolism occur during OA initiation and progression? Overexpression of HK2 induced an increase of RNA expression levels of the pro-inflammatory cytokine such as IL-6, IL-8 and metalloproteinases (MMP) in OA FLS [26], which indicated the potential therapeutic effect of HK2 in OA. However, there is no summary to help us understand the potential therapeutic role of glucose metabolism in OA, as well as the relationship between HK2 and pathophysiology of OA remains unclear. Therefore, this review focuses on the properties of HK2 and existing research concerning HK2 and OA. We also highlight the potential role and mechanism of HK2 in OA.
Biological characteristics of HK2
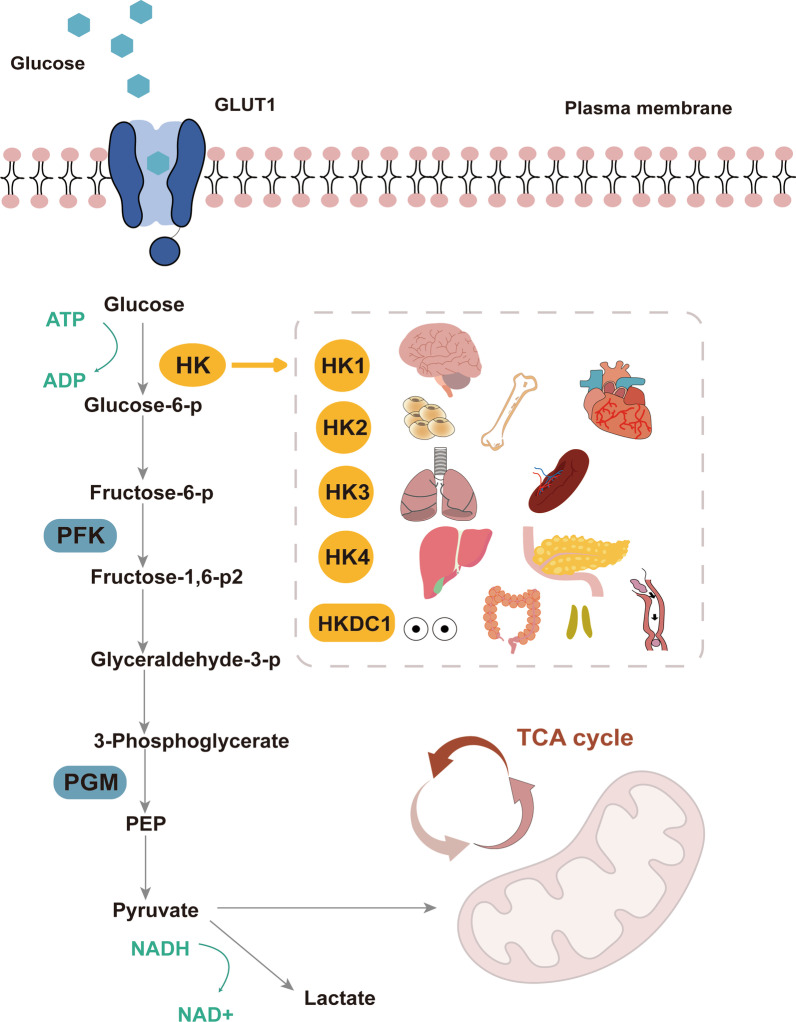
Hexokinase (HK) is a tissue-specific isoenzyme, catalyzes the first step of glucose metabolism, which is considered to be a key regulator of glucose metabolism [27, 28]. It's worth noting that the results of whole cancer analysis in multiple databases based on the Cancer Genome Atlas (TCGA) showed that abnormal expression of HK family genes was closely related to anomalous amplification, promoter hypermethylation and transcriptional activation [29]. There are five isotypes of HK family are founded in mammals: HK1, HK2, HK3, GCK (glucokinase, HK4) and HKDC1 (hexokinase domain contains 1), the expression levels in various tissues and cells are different [30, 31]. HK1 is ubiquitous in mammalian tissues and has a high content in the brain, which is called "brain hexokinase". HK1 is composed of N-terminal regulatory and C-terminal catalytic domains [32, 33]. A recent study demonstrated that HK1 is closely related to the activation of inflammation in nervous system diseases such as Alzheimer's disease (AD) [33]. HK2 is a major regulated isoform in various types of tissues cell lines, and mainly found to be expressed in musculoskeletal system and heart cells [34, 35] (Fig. 1). HK3 was mainly distributed in bone marrow, lung and spleen [35]. HK4 regulates insulin secretion, glucose uptake, glycogen synthesis and decomposition in liver, called “glucokinase” [27]. Interestingly, a novel HK-like gene called hexokinase domain containing protein-1 (HKDC1) was recently uncovered, it is the same as the other four HKs, the only difference is the last eight amino acids of C-terminal [29]. HK4 has only one kinase structural active site domain, differently, there are two kinase structural active site domains in HK1 and HK3. HK1 and HK3 have two kinase structural active site domain (N-terminal structural site domain without enzymatic activity and a C-terminal active site domain with enzymatic activity), but unlike HK1,HK3 and HK4, HK2 has high affinity for glucose, both N-terminal and C-terminal of which have catalytic activity [27, 36]. The molecular weight of HK2 protein is about 100 kDa, which is produced by replication and tandem ligation of precursor genes similar to GCK. In addition, the N-terminal and C-terminal of HK2 are sensitive to the inhibition of G-6-P, but the C-terminal of HK2 is significantly inhibited when the concentration of G-6-P is much higher than that of N-terminal [36]. These special kinetic characteristics may make HK2 play a unique role in glucose metabolism under various physiological conditions.
Fig. 1.
The pivotal role of HK2 in the glycolytic pathway. HK2 can catalyze the conversion of glucose to glucose-6-phosphate (G-6-P). Subsequently, G-6-P initiated the main pathway of glucose utilization, including glycolysis. There are five isotypes of HK family are founded in mammals: HK1, HK2, HK3, HK4 and HKDC1, the expression levels in various tissues and cells are different. HK1 is ubiquitous in mammalian tissues and has a high content in the brain. HK2 is a major regulated isoform in various types of tissues cell lines, and mainly found to be expressed in musculoskeletal system and heart cells. HK3 was mainly distributed in bone marrow, lung and spleen. HK4 regulates insulin secretion, glucose uptake, glycogen synthesis and decomposition in liver. HKDC1 is widely expressed in the pharynx, thymus, colon, and eyes
Distinctive glycolytic function of HK2
Cells under dynamic conditions (e.g., hypoxia, oxidative stress, pathological changes) need to undergo metabolic changes in order to maintain their growth, such as glucose metabolism [37, 38]. Previous studies have confirmed that HK2 plays the pivotal role in metabolic recombination [39], which is induced by carcinogens or hypoxia [39–41]. HK2 has an N-terminal active site domain (A conservative short hydrophobicity α Helical domain), which could bind to mitochondrial outer membrane voltage-dependent anion channel 1 (VDAC1) protein [42]. The binding of HK2 and VDAC1 provides a variety of "kinetic advantages" to promote hexokinase reaction, reducing the sensitivity of HK2 to the product of G-6-P, and thus increasing its affinity for ATP [43–45].
Moreover, the HK2 combined with mitochondrial VDAC can take advantage of ATP produced by mitochondria to promote glucose into cells and promote glycolysis [46]. HK1 and HK2 have two equivalent domains of glucokinase, but distinct from HK1, HK2 has enzyme catalytic activity [47], potentially a promising target to positively regulate metabolism in cells, especially musculoskeletal cells under metabolic changes due to physiological or pathological conditions (Fig. 1). Furthermore, the analysis of HK2 binding to mitochondria demonstrated that HK2 located at mitochondria-endoplasmic reticulum (ER) contact sites, which called MAMs (mitochondria-associated membranes), was found to be involved in several metabolic pathway in different types of cells [37, 48].
Non glycolytic function of HK2
HK2 not only has an enzymatic function that phosphorylating of glucose to G-6-P, but also has non-glycolytic function. Studies have shown that one of the non-enzymatic functions of HK2 is directly regulate cell apoptosis, and it is considered as a potential target to prevent organelle damage and maintain cell viability after apoptosis. Specifically, HK2 seems to interfere with BCL2 family members that mediate mitochondrial membrane damage [49, 50]. The research showed that recombinant Akt phosphorylated HK2 and inhibited the release of cytochrome C in mitochondria of adult mouse heart induced by Ca2+. Akt can increase the activity of mitochondrial HK2 and cause changes in the expression of apoptotic proteins, such as Bax and Bak [51]. However, the molecular mechanism of HK2 against apoptosis is still unclear. Besides, HK2 plays an important role in transcriptional regulation by binding to transcriptional regulated metabolic enzymes [52]. Therefore, HK2 may be directly or indirectly involved in the transcriptional activation, signal transduction or phosphorylation of some signaling molecules. The specific mechanism needs to be further explored, especially in musculoskeletal tissues.
Existing evidence concerning HK2 and OA
Aerobic glycolysis refers to certain cells, including many rapidly proliferating cells, which show high fermentation rate even in the case of sufficient oxygen. This is a metabolic phenotype, which is a hot spot in the field of cancer research, but this phenotype is not unique to cancer [53, 54]. HK2 was confirmed to be expressed in RA and OA-FLS (fibroblast synovial -like cell lines), TNF and hypoxia could increase the HK2 protein level in OA FLS, meanwhile, overexpression of HK2 increases the RNA levels of pro-inflammatory cytokines such as IL-6, IL-8 and MMP in OA FLS cell lines [26]. In addition, TGF-β1, an important regulator of cartilage homeostasis, induces the increase of aerobic glycolysis flux and the decrease of oxidative phosphorylation in human articular chondrocytes (HACs) in OA. After 48–72 h treatment with TGF-β1, the expression of GLUT1 and HK2 two times higher than that in control. Interestingly, another hexokinase isoform HKI was also increased in the treated cells [55]. A present study with a focus on HK2 validated that the expression of HK2 was increased in peripheral blood mononuclear cells (PBMCs) compared with that in HCs examined by real-time PCR in an RA and OA cohort [56]. These findings highlight that HK2 may be involved in the pathogenesis of OA. Further exploration on the exact role and mechanism of HK2 in the pathogenesis of OA is of great significance to find metabolic targets in treatment development for OA.
Potential role and mechanism of HK2 in OA
HK2 is an important kinase in glycolysis, that prime glucose, whose functions in autophagy regulation, cell death and other physiological and pathological state. However, the function and mechanism of HK2 in OA development are still unclear. Chondrocytes have unique characteristics of metabolism and synthesis especially under hypoxia condition. Therefore, researches on the role of HK2 in glycolysis and the potential underlying mechanisms would help provide helpful insights into the understanding of the potential link between HK2 and OA metabolism.
Glycolysis function in OA
In the glycolysis reaction, there are several important enzymes and processes that affect the glycolytic flux, including HKs, PFKs and LA output. These key enzymes and processes play an important role in the development of chronic degenerative diseases such as OA [16, 57]. The metabolic shift in chondrocytes is critical in the development of OA, in which changes from a state of regulatory rest to an active state of high metabolism [58].
The expression of glycogen protein 1-(GYG1)-asparagine, which is an enzyme involved in the biosynthesis of glycogen, was significantly down-regulated in the metabolomics transcriptome integration analysis of RA and OA, indicating that glucose homeostasis might be implicated in the pathogenesis of OA [59]. Meanwhile, it was also found in several vivo studies that the contents of metabolites related to glycolysis (e.g., alanine, serine and LA) increased significantly in OA [60, 61]. PKM2 is a pivotal regulator in the pathogenesis of OA. A recent study showed that the expression of PKM2 was up-regulated in OA chondrocytes compared with healthy control chondrocytes, PKM2 knockdown can inhibit the proliferation and promote apoptosis of OA chondrocytes, and down regulate the expression levels of COL2A1 and SOX-9 [57]. LDHA has the function of promoting ROS formation in chondrocytes during the inflammatory state, while inhibiting the activity of LDHA was an effective therapeutic target for OA. Manoj et.al found a significant increase in expression of genes involved in glycolysis and fermentation such as HK2 and LDHA, and the level of LA in cell culture supernatant was also increased [15].
Impaired glucose uptake would compromise cell function and potentially result in osteoarthritis [62]. Racid et al. confirmed that both OA FLS and RA FLS showed lower respiratory rate and increased glycolysis activity [63]. On the contrary, using a glycolysis inhibitor 2-deoxy-d-glucose (2-DG), the levels of inflammatory markers were significantly decreased, indicating that glucose metabolism plays an important role in FLS metabolism and is crucial to the pathogenic function of FLS under pro-inflammatory stimulation [63]. Meanwhile, the GLUT1 expression and the level of LA in FLS of OA patients were increased, and glycolysis block inhibited the migration ability of these cells [63].
These evidences above indicate that glucose metabolism plays an important role in the development of OA. HK2 may be a potential modulator of OA metabolism, which still needs to be further clarified.
Potential protein kinase targets of HK2 in OA
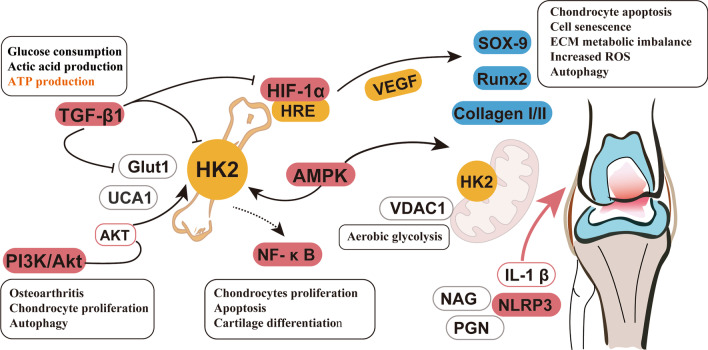
It has been clarified that the HK2 could interact with many protein molecules, but HK2 as a protein kinase in OA progress is barely elaborated. Therefore, we summarize several potential targets of HK2 in OA (Fig. 2).
Fig. 2.
The potential role and mechanism of HK2 in OA. HK2 is an important kinase in glycolysis, where it functions in autophagy regulation, cell death and other physiological and pathological state. The exact role of HK2 and aerobic glycolysis in the pathogenesis of OA also related to the certain pathway, such as HIF-1α, AMPK, TGF-β1, PI3K/Akt, NF-κB, NLRP3 inflammasome
Hypoxia inducible factor-1 (HIF-1)
Hypoxia is the allosteric regulation of glycolysis, and under conditions of hypoxia, most eukaryotic cells can change their main metabolic strategies from mitochondrial respiration towards increasing glycolysis to maintain ATP level [64]. Hypoxia inducible factor-1 (HIF-1) is an important transcription factor and a major regulator of glycolysis, which can up-regulate the transcription and expression of glucose transporters and glycolytic enzymes [64–67]. Furthermore, Steve et al. [68] clarified that HIF-1α signaling in chondrocytes regulates the synthesis and modification of collagen in chondrocytes by inducing metabolic changes. Similarly, increased expression of HIF-1α was detected in human and mouse OA chondrocytes, accompanied by chondrocyte apoptosis, cell senescence, extracellular matrix (ECM) metabolic imbalance, and increased ROS and autophagy [69]. Subsequent experiments showed that knockdown of HIF-1α partially eliminated hypoxia-induced cell damage and played an important role in ROS production [69]. The expression of HIF-1α and VEGF is also elevated in OA cartilage and IL-1β-induced chondrocytes. Conversely, the suppression of HIF-1α could reduce VEGF expression levels, which contributes to the production of collagen II, aggrecan and SOX9, further inhibit the expression of collagen I and RUNX2 [70]. One study reported that the HIF-1α and Runx2 were increased in the chondrocytes from both OA and IL-1β conditions. When HIF-1α was silenced, the glycolytic metabolism of chondrocytes was also suppressed, suggesting that HIF-1α plays a role in the self-repair of the glycolytic metabolism of OA [71]. Therefore, from the evidence above, it is worth noting that the HIF-1α may become a potential target of HK2 in chondrocyte metabolism, and further studies on that should be conducted to confirm the critical role.
AMPK
Cells need to constantly adjust their metabolic pathways to meet their energy requirements and respond to nutrient utilization. AMP-activated protein kinase (AMPK) has attracted widespread attention as a potential target for the treatment of diseases related to metabolic disorders [72, 73]. The decrease in AMPK activity was observed in human and mouse OA cartilage tissues, which was achieved by catalyzing the phosphorylation of specific threonine in AMPK (AMPKα1), suggesting that AMPK activity in chondrocytes is the key to maintaining stable state of joint function and development of OA [74, 75]. Besides, attempts to reverse AMPK dysfunction can reduce inflammation and prevent the progression of OA. Yun et al. found that AMPK activator (A-769662) significantly promoted the expression of PGC1 α in OA chondrocytes through SIRT1 signaling and rescued mitochondrial defect [76]. Another study also showed that the AMPK can limit oxidative stress and improve the mtDNA integrity and function of OA chondrocytes via activating SIRT3 [77]. The activation of AMPK is achieved in part by maintaining glycolysis. A recent study reported that blocking the level of IL1β-enhanced MMP13 by using galactose-replacement in osteoarthritic chondrocyte inversely increased bio-markers associated with p-AMPK, further confirmed AMPK is a downstream regulatory molecule in the process of glycolysis in OA [78]. The above studies provide clues to the important role of the correlation between AMPK and aerobic glycolysis in the pathogenesis of OA.
Transforming growth factor (TGF-β1)
Transforming growth factor beta1 (TGF-β1) is a multifunctional cytokine, which can regulate cell cycle, growth, development, proliferation and differentiation, ECM synthesis and immune response [79, 80]. Cell metabolism is affected by the micro-environment around, and studies have demonstrated that TGF-β1 inhibits the glycolysis process by inducing natural regulatory T cells, and also significantly down-regulates the expression of key enzymes in the glycolysis pathway (e.g., Glut1, Glucose transporter, HK2 and HIF-1α) [81]. In addition, TGF-β1 stimulation can up-regulate glycolysis in dermal fibroblasts, while inhibition of glycolysis can reduce the pro-fibrosis induced by TGF-β1 [82]. Therefore, TGF-β1 plays a dominant role in metabolic reprogramming (inducing the conversion of oxidative phosphorylation to aerobic glycolysis). It is known from the cellular and molecular level that OA is characterized by a transition from a healthy steady state to a catabolic state, and TGF- β1 signaling plays an important regulative effect in maintaining the morphology of articular cartilage and the internal environment of cartilage cells [55, 58, 83]. Wang et al. showed that in human articular chondrocytes (hAC) with OA, TGF-β1 stimulation increased glucose consumption and LA production, meanwhile reduced ATP production [55]. Under TGF-β1 treatment, Glut1 and HKII significantly increased by over than two times. Interestingly, the expression of another hexokinase isoform, HKI, also increased in cells treated with TGF-β1 [55]. Ni et al. found that the expression of HK1 and HK2 was detected in c-Kit lineage cells, and TGF-β1 significantly increased the expression of HK1 and HK2 in a time-dependent manner, and the activity of HK was also up-regulated, showing that TGF -β1 and HK may have a direct regulatory effect [84]. Findings above provide very important clues to the interaction between TGF-β1 and HK2 in the process of metabolic reprogramming, especially how to promote the pathogenesis of OA.
PI3K/Akt pathway
The phosphoinositide 3-kinase (PI3K) signaling pathway plays a key role in cell growth control and glucose metabolism. Akt (also known as protein kinase B) is a serine/threonine protein kinase directly activated by PI3K [85]. The PI3K/Akt pathway can be activated in response to insulin, growth factors and cytokines, thereby regulating a wide range of processes such as glucose metabolism, biosynthesis, and redox balance [86, 87]. Multiple control points (HK2, PFK1 and PFK2) in the glycolysis process are regulated by the PI3K/Akt pathway. Furthermore, AKT also controls the key steps of glycolysis by phosphorylation of specific glycolytic enzymes [85]. AKT activation has been found to promote HK2 activity by increasing the association with the VDAC of the outer mitochondrial membrane [36]. The intracellular localization and kinetic characteristics of HK2 is conducive to the transport and utilization of glucose during glycolysis [88]. In addition, blocking PI3K/Akt pathway inhibits the activity of key enzyme HK2 in aerobic glycolysis and cell proliferation [89]. Similarly, accumulating data have proved that PI3K/Akt can directly regulate the activity of HK2 in cells [90, 91]. The previous analysis showed that compared with healthy cartilage, the expression of phosphorylated PI3K/Akt increased in OA cartilage [92], while the inhibition of PI3K/Akt/mTOR signaling alleviated OA induced joint injury by restoring cartilage homeostasis, enhancing autophagy and inhibiting inflammation [93]. The association of PI3K/Akt and HK2 is potentially critical in the pathogenesis of OA.
NF-κB
Nuclear factor kappa B (NF-κB) is a transcription factor, which is ubiquitous in various types of cell lines, and plays a central role in the development of osteoarthritis [94]. One study found that the transient activation of NF-κB in ATDC5 chondrocytes was involved in the regulation of chondrogenic differentiation [95]. As an upstream regulator of the classic NF-κB pathway activation mechanism, IKKs are closely related to the catabolism of chondrocytes. The intra-articular administration of (BMS-345541) IKKs inhibitors significantly suppressed the expression of MMP13 and ADAMTS5, as well as prevented cartilage damage at 8 weeks [96]. Furthermore, another study also suggested that NF-κB signal transduction plays a critical role in the regulation of glycolytic activity, and activates Ca2+/NF-κB axis to promote glycolysis and microenvironment remodeling by increasing the production of downstream cytokines [97]. Wang et al. used glycolysis inhibitor 2-deoxyglucose (2-DG) to treat adjuvant arthritis (AA) rats, and found that HK2 expression was positively correlated with synovial hyperplasia, inflammatory cell infiltration and cartilage destruction, which further confirmed that the effect of HK2 glycolysis inhibitor is closely related to the activation and inhibition of NF-κB signaling [98]. The above research indicated that the role of links between HK2 and NF-κB signal transduction pathway in the process of cell metabolism for OA development although critically associated but still remains implicit, further research are required to reveal the accurate association between HK2 and NF-κB signal in OA.
NLRP3 inflammasome
Inflammation has long been identified as the driving factor of many chronic diseases and autoimmune disease, including Alzheimer's disease [99], atherosclerosis [100] and OA [101]. The NLRP3 (NLR family, containing three pyridine domains) inflammasome is a multi-component assembly of adaptor and effector proteins highly expressed in myeloid cells, consisting of (NLRP3) NOD-like receptor protein 3, adaptor protein, apoptosis associated speck- like protein (ASC) and caspase-16 [102]. Studies have demonstrated that the N-acetylglucosamine (NAG) is an activator of NLRP3 inflammasome [103]. PGN and NAG can inhibit hexokinase and induce its dissociation from mitochondria outer membranes, thus affecting the metabolism of hexokinase activity conditions to trigger the activation of inflammasomes, which indicates that the breakdown of the specific metabolism of hexokinase function also induce the activation of inflammasomes [102, 104]. There is an important connection between glucose metabolism and the activation of NLRP3 inflammasomes [103]. Ahmad et al. found that ATRA can enhance the expression of HK2 and shift the metabolism from LPS activated by macrophages to glycolysis, leading to the activation of NLRP3 inflammasome [105]. Cartilage destruction and subchondral osteosclerosis were found in OA patients and OA model rats, and the expression level of NLRP3 was significantly increased in synovial tissue [106], which indicates that NLRP3 inflammasome was involved in the pathogenesis of OA [107]. Future studies will further determine the interaction between HK2 and NLRP3 and reveal whether it plays an important role in OA.
Conclusions
Metabolism is central to maintain the function of cartilage and synovial joint. Under adverse microenvironmental conditions, energy shift to glycolysis plays pivotal roles in the progression of OA. HK2 has been proven to play multiple roles of metabolic enzymes during glycolysis in musculoskeletal tissues including bone and cartilage through the regulation of cell metabolism and many other important cellular activities, such as cell growth, proliferation, survival, autophagy, and apoptosis. Although the understanding of the role of glucose and energy metabolism in OA is incomplete, studies on HK2 have greatly expanded our understanding of the glucometabolic interaction network in the pathogenesis of OA, in which HK2 may act as regulators of transcription factors growth factors, inflammatory factors and autophagy related molecules, which potentially affect ROS level, mitochondria function, extracellular matrix remodeling, and the proliferation and differentiation of chondrocytes and synovial cells. Through investigating key factors HK2 included in aerobic glycolysis, and the regulatory pathways, such as TGF-β1, AMPK, PI3K/Akt, NF-κB, NLRP3, potential mechanisms underlying relationship between the regulation of HK2 and energy metabolism in OA will be further fueled by further investigations (Fig. 2). Potentially, new therapeutic approaches for the treatment of OA or related surrogate outcome will then be developed, especially biomarkers in energy metabolism and small molecular drugs targeting downstream protein or kinase of HK2.
Acknowledgements
Not applicable.
Abbreviations
- OA
Osteoarthritis
- OXPHOS
Oxidative phosphorylation
- HK2
Hexokinase 2
- G-6-P
Glucose-6-phosphate
- LA
Lactate
- HKDC1
Hexokinase domain contains 1
- VDAC1
Voltage-dependent anion channel 1
- HIF-1
Hypoxia inducible factor-1
- AMPK
AMP-activated protein kinase
- TGF-β1
Transforming growth factor
- PI3K
Phosphoinositide 3-kinase
- NF-κB
Nuclear factor kappa B
- NLRP3
NOD-like receptor protein 3
- RUNX2
Runt-related transcription factor 2
- ECM
Extracellular matrix
Author contributions
CQH was involved in the design of the article. CCB and SYZ drafted of the main text. KPS drafted the figures. SYZ, CCB and CQH revised the article. CCB and SYZ have contributed equally to this work. All authors read and approved the final manuscript.
Funding
This study is supported by the National Natural Science Foundation of China (81972146 to Cheng-Qi He, and 82002393 to Si-Yi Zhu), the Department of Science and Technology of Sichuan Province (2021YFS0004 to Cheng-Qi He and 2021YJ0424 to Si-Yi Zhu), China Postdoctoral Science Foundation (2020M673251).
Declarations
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Chuncha Bao and Siyi Zhu share first authorship
Contributor Information
Siyi Zhu, Email: hxkfzsy@scu.edu.cn.
Chengqi He, Email: hxkfhcq2015@126.com.
References
- 1.Hu W, Chen Y, Dou C, Dong S. Microenvironment in subchondral bone: predominant regulator for the treatment of osteoarthritis. Ann Rheum Dis. 2020;80:413–422. doi: 10.1136/annrheumdis-2020-218089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu X, Chan YT, Yung PSH, Tuan RS, Jiang Y. Subchondral bone remodeling: a therapeutic target for osteoarthritis. Front Cell Dev Biol. 2020;8:607764. doi: 10.3389/fcell.2020.607764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Volarevic V. Mesenchymal stem cell-based therapy of osteoarthritis: current knowledge and future perspectives. Biomed Pharmacother. 2019;109:2318–2326. doi: 10.1016/j.biopha.2018.11.099. [DOI] [PubMed] [Google Scholar]
- 4.Hwang HS, Kim HA. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int J Mol Sci. 2015;16:26035–26054. doi: 10.3390/ijms161125943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.An S, Hu H, Li Y, Hu Y. Pyroptosis plays a role in osteoarthritis. Aging Dis. 2020;11:1146–1157. doi: 10.14336/AD.2019.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sacitharan PK, Bou-Gharios G, Edwards JR. SIRT1 directly activates autophagy in human chondrocytes. Cell Death Discov. 2020;6:41. doi: 10.1038/s41420-020-0277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun AR, Udduttula A, Li J, Liu Y, Ren PG, Zhang P. Cartilage tissue engineering for obesity-induced osteoarthritis: physiology, challenges, and future prospects. J Orthop Translat. 2021;26:3–15. doi: 10.1016/j.jot.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grandi FC, Bhutani N. Epigenetic therapies for osteoarthritis. Trends Pharmacol Sci. 2020;41:557–569. doi: 10.1016/j.tips.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin Z, Wang D, Zhang H, Liang J, Feng X, Zhao J, Sun L. Incidence trend of five common musculoskeletal disorders from 1990 to 2017 at the global, regional and national level: results from the global burden of disease study 2017. Ann Rheum Dis. 2020;79:1014–1022. doi: 10.1136/annrheumdis-2020-217050. [DOI] [PubMed] [Google Scholar]
- 10.Peat G, Thomas MJ. Osteoarthritis year in review 2020: epidemiology and therapy. Osteoarthritis Cartilage. 2021;29:180–189. doi: 10.1016/j.joca.2020.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Smith RL, Soeters MR, Wüst RCI, Houtkooper RH. Metabolic flexibility as an adaptation to energy resources and requirements in health and disease. Endocr Rev. 2018;39:489–517. doi: 10.1210/er.2017-00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalmao-Fernández A, Lund J, Hermida-Gómez T, Vazquez-Mosquera ME, Rego-Pérez I, Blanco FJ, Fernández-Moreno M. Impaired metabolic flexibility in the osteoarthritis process: a study on transmitochondrial cybrids. Cells. 2020;9:809. doi: 10.3390/cells9040809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.June RK, Liu-Bryan R, Long F, Griffin TM. Emerging role of metabolic signaling in synovial joint remodeling and osteoarthritis. J Orthop Res. 2016;34:2048–2058. doi: 10.1002/jor.23420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenberg L, Eisenberg-Bord M, Eisenberg-Lerner A, Sagi-Eisenberg R. Metabolic alterations in the tumor microenvironment and their role in oncogenesis. Cancer Lett. 2020;484:65–71. doi: 10.1016/j.canlet.2020.04.016. [DOI] [PubMed] [Google Scholar]
- 15.Arra M, Swarnkar G, Ke K, Otero JE, Ying J, Duan X, Maruyama T, Rai MF, O'Keefe RJ, Mbalaviele G, et al. LDHA-mediated ROS generation in chondrocytes is a potential therapeutic target for osteoarthritis. Nat Commun. 2020;11:3427. doi: 10.1038/s41467-020-17242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, Chen W, Zhao X, Chen L, Li W, Ran J, Wu L. Pyruvate kinase M2 modulates the glycolysis of chondrocyte and extracellular matrix in osteoarthritis. DNA Cell Biol. 2018;37:271–277. doi: 10.1089/dna.2017.4048. [DOI] [PubMed] [Google Scholar]
- 17.Li HM, Guo HL, Xu C, Liu L, Hu SY, Hu ZH, Jiang HH, He YM, Li YJ, Ke J, Long X. Inhibition of glycolysis by targeting lactate dehydrogenase A facilitates hyaluronan synthase 2 synthesis in synovial fibroblasts of temporomandibular joint osteoarthritis. Bone. 2020;141:115584. doi: 10.1016/j.bone.2020.115584. [DOI] [PubMed] [Google Scholar]
- 18.Lee HJ, Li CF, Ruan D, He J, Montal ED, Lorenz S, Girnun GD, Chan CH. Non-proteolytic ubiquitination of Hexokinase 2 by HectH9 controls tumor metabolism and cancer stem cell expansion. Nat Commun. 2019;10:2625. doi: 10.1038/s41467-019-10374-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varghese E, Samuel SM, Líšková A, Samec M, Kubatka P, Büsselberg D. Targeting glucose metabolism to overcome resistance to anticancer chemotherapy in breast cancer. Cancers Basel. 2020;12:2252. doi: 10.3390/cancers12082252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng J, Li J, Wu L, Yu Q, Ji J, Wu J, Dai W, Guo C. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J Exp Clin Cancer Res. 2020;39:126. doi: 10.1186/s13046-020-01629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, Liu T, Sun H, Weng W, Zhang Q, Liu C, Han Y, Sheng W. Pim1 supports human colorectal cancer growth during glucose deprivation by enhancing the Warburg effect. Cancer Sci. 2018;109:1468–1479. doi: 10.1111/cas.13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shangguan C, Gan G, Zhang J, Wu J, Miao Y, Zhang M, Li B, Mi J. Cancer-associated fibroblasts enhance tumor (18)F-FDG uptake and contribute to the intratumor heterogeneity of PET-CT. Theranostics. 2018;8:1376–1388. doi: 10.7150/thno.22717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen C, Xuan B, Yan T, Ma Y, Xu P, Tian X, Zhang X, Cao Y, Ma D, Zhu X, et al. m(6)A-dependent glycolysis enhances colorectal cancer progression. Mol Cancer. 2020;19:72. doi: 10.1186/s12943-020-01190-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siu MKY, Jiang YX, Wang JJ, Leung THY, Han CY, Tsang BK, Cheung ANY, Ngan HYS, Chan KKL. Hexokinase 2 regulates ovarian cancer cell migration, invasion and stemness via FAK/ERK1/2/MMP9/NANOG/SOX9 signaling cascades. Cancers (Basel) 2019;11:813. doi: 10.3390/cancers11060813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeWaal D, Nogueira V, Terry AR, Patra KC, Jeon SM, Guzman G, Au J, Long CP, Antoniewicz MR, Hay N. Hexokinase-2 depletion inhibits glycolysis and induces oxidative phosphorylation in hepatocellular carcinoma and sensitizes to metformin. Nat Commun. 2018;9:446. doi: 10.1038/s41467-017-02733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bustamante MF, Oliveira PG, Garcia-Carbonell R, Croft AP, Smith JM, Serrano RL, Sanchez-Lopez E, Liu X, Kisseleva T, Hay N, et al. Hexokinase 2 as a novel selective metabolic target for rheumatoid arthritis. Ann Rheum Dis. 2018;77:1636–1643. doi: 10.1136/annrheumdis-2018-213103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu S, Herschman HR. A tumor agnostic therapeutic strategy for hexokinase 1-null/hexokinase 2-positive cancers. Cancer Res. 2019;79:5907–5914. doi: 10.1158/0008-5472.CAN-19-1789. [DOI] [PubMed] [Google Scholar]
- 28.Yang T, Ren C, Qiao P, Han X, Wang L, Lv S, Sun Y, Liu Z, Du Y, Yu Z. PIM2-mediated phosphorylation of hexokinase 2 is critical for tumor growth and paclitaxel resistance in breast cancer. Oncogene. 2018;37:5997–6009. doi: 10.1038/s41388-018-0386-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Q, Feng J, Wu J, Yu Z, Zhang W, Chen Y, Yao P, Zhang H. HKDC1 C-terminal based peptides inhibit extranodal natural killer/T-cell lymphoma by modulation of mitochondrial function and EBV suppression. Leukemia. 2020;34:2736–2748. doi: 10.1038/s41375-020-0801-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi T, Ma Y, Cao L, Zhan S, Xu Y, Fu F, Liu C, Zhang G, Wang Z, Wang R, et al. B7–H3 promotes aerobic glycolysis and chemoresistance in colorectal cancer cells by regulating HK2. Cell Death Dis. 2019;10:308. doi: 10.1038/s41419-019-1549-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- 32.Okur V, Cho MT, van Wijk R, van Oirschot B, Picker J, Coury SA, Grange D, Manwaring L, Krantz I, Muraresku CC, et al. De novo variants in HK1 associated with neurodevelopmental abnormalities and visual impairment. Eur J Hum Genet. 2019;27:1081–1089. doi: 10.1038/s41431-019-0366-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Wang R, Hu D, Sun X, Fujioka H, Lundberg K, Chan ER, Wang Q, Xu R, Flanagan ME, et al. Oligodendroglial glycolytic stress triggers inflammasome activation and neuropathology in Alzheimer's disease. Sci Adv. 2020;6:eabb8680. doi: 10.1126/sciadv.abb8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuo J, Tang J, Lu M, Zhou Z, Li Y, Tian H, Liu E, Gao B, Liu T, Shao P. Glycolysis rate-limiting enzymes: novel potential regulators of rheumatoid arthritis pathogenesis. Front Immunol. 2021;12:779787. doi: 10.3389/fimmu.2021.779787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin YH, Wu Y, Wang Y, Yao ZF, Tang J, Wang R, Shen L, Ding SQ, Hu JG, Lü HZ. Spatio-temporal expression of Hexokinase-3 in the injured female rat spinal cords. Neurochem Int. 2018;113:23–33. doi: 10.1016/j.neuint.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 36.Robey RB, Hay N. Mitochondrial hexokinases, novel mediators of the antiapoptotic effects of growth factors and Akt. Oncogene. 2006;25:4683–4696. doi: 10.1038/sj.onc.1209595. [DOI] [PubMed] [Google Scholar]
- 37.Ciscato F, Filadi R, Masgras I, Pizzi M, Marin O, Damiano N, Pizzo P, Gori A, Frezzato F, Chiara F, et al. Hexokinase 2 displacement from mitochondria-associated membranes prompts Ca(2+)-dependent death of cancer cells. EMBO Rep. 2020;21:e49117. doi: 10.15252/embr.201949117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vander Heiden MG, DeBerardinis RJ. Understanding the intersections between metabolism and cancer biology. Cell. 2017;168:657–669. doi: 10.1016/j.cell.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Xiong H, Wu F, Zhang Y, Wang J, Zhao L, Guo X, Chang LJ, Zhang Y, You MJ, et al. Hexokinase 2-mediated Warburg effect is required for PTEN- and p53-deficiency-driven prostate cancer growth. Cell Rep. 2014;8:1461–1474. doi: 10.1016/j.celrep.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhalla K, Jaber S, Nahid MN, Underwood K, Beheshti A, Landon A, Bhandary B, Bastian P, Evens AM, Haley J, et al. Role of hypoxia in diffuse large B-cell Lymphoma: metabolic repression and selective translation of HK2 facilitates development of DLBCL. Sci Rep. 2018;8:744. doi: 10.1038/s41598-018-19182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikeda S, Abe F, Matsuda Y, Kitadate A, Takahashi N, Tagawa H. Hypoxia-inducible hexokinase-2 enhances anti-apoptotic function via activating autophagy in multiple myeloma. Cancer Sci. 2020;111:4088–4101. doi: 10.1111/cas.14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xie GC, Wilson JE. Rat brain hexokinase: the hydrophobic N-terminus of the mitochondrially bound enzyme is inserted in the lipid bilayer. Arch Biochem Biophys. 1988;267:803–810. doi: 10.1016/0003-9861(88)90090-2. [DOI] [PubMed] [Google Scholar]
- 43.Bustamante E, Pedersen PL. Mitochondrial hexokinase of rat hepatoma cells in culture: solubilization and kinetic properties. Biochemistry. 1980;19:4972–4977. doi: 10.1021/bi00563a006. [DOI] [PubMed] [Google Scholar]
- 44.Pedersen PL. Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers' most common phenotypes, the "Warburg Effect", i.e., elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr. 2007;39:211–222. doi: 10.1007/s10863-007-9094-x. [DOI] [PubMed] [Google Scholar]
- 45.Mazure NM. VDAC in cancer. Biochim Biophys Acta Bioenerg. 2017;1858:665–673. doi: 10.1016/j.bbabio.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Arora KK, Pedersen PL. Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J Biol Chem. 1988;263:17422–17428. doi: 10.1016/S0021-9258(19)77853-3. [DOI] [PubMed] [Google Scholar]
- 47.Wilson JE. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J Exp Biol. 2003;206:2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 48.Ciscato F, Ferrone L, Masgras I, Laquatra C, Rasola A. Hexokinase 2 in cancer: a prima donna playing multiple characters. Int J Mol Sci. 2021;22:4716. doi: 10.3390/ijms22094716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gall JM, Wong V, Pimental DR, Havasi A, Wang Z, Pastorino JG, Bonegio RG, Schwartz JH, Borkan SC. Hexokinase regulates Bax-mediated mitochondrial membrane injury following ischemic stress. Kidney Int. 2011;79:1207–1216. doi: 10.1038/ki.2010.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pastorino JG, Shulga N, Hoek JB. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J Biol Chem. 2002;277:7610–7618. doi: 10.1074/jbc.M109950200. [DOI] [PubMed] [Google Scholar]
- 51.Miyamoto S, Murphy AN, Brown JH. Akt mediates mitochondrial protection in cardiomyocytes through phosphorylation of mitochondrial hexokinase-II. Cell Death Differ. 2008;15:521–529. doi: 10.1038/sj.cdd.4402285. [DOI] [PubMed] [Google Scholar]
- 52.Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci. 2005;30:142–150. doi: 10.1016/j.tibs.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 53.Luengo A, Li Z, Gui DY, Sullivan LB, Zagorulya M, Do BT, Ferreira R, Naamati A, Ali A, Lewis CA, et al. Increased demand for NAD(+) relative to ATP drives aerobic glycolysis. Mol Cell. 2021;81:691–707.e696. doi: 10.1016/j.molcel.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fernie AR, Zhang Y, Sampathkumar A. Cytoskeleton architecture regulates glycolysis coupling cellular metabolism to mechanical cues. Trends Biochem Sci. 2020;45:637–638. doi: 10.1016/j.tibs.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Wang C, Silverman RM, Shen J, O'Keefe RJ. Distinct metabolic programs induced by TGF-β1 and BMP2 in human articular chondrocytes with osteoarthritis. J Orthop Translat. 2018;12:66–73. doi: 10.1016/j.jot.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhou KL, Zhu ZH, Zhou JP, Zhao JJ, Zhang Y, Jiang B. Increased hexokinase-2 as a novel biomarker for the diagnosis and correlating with disease severity in rheumatoid arthritis. Medicine (Baltimore) 2021;100:e26504. doi: 10.1097/MD.0000000000026504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang B, Chen H, Ouyang J, Xie Y, Chen L, Tan Q, Du X, Su N, Ni Z, Chen L. SQSTM1-dependent autophagic degradation of PKM2 inhibits the production of mature IL1B/IL-1β and contributes to LIPUS-mediated anti-inflammatory effect. Autophagy. 2020;16:1262–1278. doi: 10.1080/15548627.2019.1664705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mobasheri A, Rayman MP, Gualillo O, Sellam J, van der Kraan P, Fearon U. The role of metabolism in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2017;13:302–311. doi: 10.1038/nrrheum.2017.50. [DOI] [PubMed] [Google Scholar]
- 59.Gao N, Ding L, Pang J, Zheng Y, Cao Y, Zhan H, Shi Y. Metabonomic-transcriptome integration analysis on osteoarthritis and rheumatoid arthritis. Int J Genomics. 2020;2020:5925126. doi: 10.1155/2020/5925126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Damyanovich AZ, Staples JR, Chan AD, Marshall KW. Comparative study of normal and osteoarthritic canine synovial fluid using 500 MHz 1H magnetic resonance spectroscopy. J Orthop Res. 1999;17:223–231. doi: 10.1002/jor.1100170211. [DOI] [PubMed] [Google Scholar]
- 61.Showiheen SAA, Sun AR, Wu X, Crawford R, Xiao Y, Wellard RM, Prasadam I. Application of metabolomics to osteoarthritis: from basic science to the clinical approach. Curr Rheumatol Rep. 2019;21:26. doi: 10.1007/s11926-019-0827-8. [DOI] [PubMed] [Google Scholar]
- 62.Windhaber RA, Wilkins RJ, Meredith D. Functional characterisation of glucose transport in bovine articular chondrocytes. Pflugers Arch. 2003;446:572–577. doi: 10.1007/s00424-003-1080-5. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Carbonell R, Divakaruni AS, Lodi A, Vicente-Suarez I, Saha A, Cheroutre H, Boss GR, Tiziani S, Murphy AN, Guma M. Critical role of glucose metabolism in rheumatoid arthritis fibroblast-like synoviocytes. Arthritis Rheumatol. 2016;68:1614–1626. doi: 10.1002/art.39608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kierans SJ, Taylor CT. Regulation of glycolysis by the hypoxia-inducible factor (HIF): implications for cellular physiology. J Physiol. 2021;599:23–37. doi: 10.1113/JP280572. [DOI] [PubMed] [Google Scholar]
- 65.Zhou L, Wang Y, Zhou M, Zhang Y, Wang P, Li X, Yang J, Wang H, Ding Z. HOXA9 inhibits HIF-1α-mediated glycolysis through interacting with CRIP2 to repress cutaneous squamous cell carcinoma development. Nat Commun. 2018;9:1480. doi: 10.1038/s41467-018-03914-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Contreras-Lopez R, Elizondo-Vega R, Paredes MJ, Luque-Campos N, Torres MJ, Tejedor G, Vega-Letter AM, Figueroa-Valdés A, Pradenas C, Oyarce K, et al. HIF1α-dependent metabolic reprogramming governs mesenchymal stem/stromal cell immunoregulatory functions. Faseb J. 2020;34:8250–8264. doi: 10.1096/fj.201902232R. [DOI] [PubMed] [Google Scholar]
- 67.Du Y, Wei N, Ma R, Jiang S, Song D. A miR-210-3p regulon that controls the Warburg effect by modulating HIF-1α and p53 activity in triple-negative breast cancer. Cell Death Dis. 2020;11:731. doi: 10.1038/s41419-020-02952-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stegen S, Laperre K, Eelen G, Rinaldi G, Fraisl P, Torrekens S, Van Looveren R, Loopmans S, Bultynck G, Vinckier S, et al. HIF-1α metabolically controls collagen synthesis and modification in chondrocytes. Nature. 2019;565:511–515. doi: 10.1038/s41586-019-0874-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu S, Zhang C, Ni L, Huang C, Chen D, Shi K, Jin H, Zhang K, Li Y, Xie L, et al. Stabilization of HIF-1α alleviates osteoarthritis via enhancing mitophagy. Cell Death Dis. 2020;11:481. doi: 10.1038/s41419-020-2680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu WJ, Chang BY, Wang XF, Zang YF, Zheng ZX, Zhao HJ, Cui QD. FBW7 regulates HIF-1α/VEGF pathway in the IL-1β induced chondrocytes degeneration. Eur Rev Med Pharmacol Sci. 2020;24:5914–5924. doi: 10.26355/eurrev_202006_21484. [DOI] [PubMed] [Google Scholar]
- 71.Kong P, Chen R, Zou FQ, Wang Y, Liu MC, Wang WG. HIF-1α repairs degenerative chondrocyte glycolytic metabolism by the transcriptional regulation of Runx2. Eur Rev Med Pharmacol Sci. 2021;25:1206–1214. doi: 10.26355/eurrev_202102_24823. [DOI] [PubMed] [Google Scholar]
- 72.Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nat Rev Mol Cell Biol. 2018;19:121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lin SC, Hardie DG. AMPK: sensing glucose as well as cellular energy status. Cell Metab. 2018;27:299–313. doi: 10.1016/j.cmet.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 74.Zhou S, Lu W, Chen L, Ge Q, Chen D, Xu Z, Shi D, Dai J, Li J, Ju H, et al. AMPK deficiency in chondrocytes accelerated the progression of instability-induced and ageing-associated osteoarthritis in adult mice. Sci Rep. 2017;7:43245. doi: 10.1038/srep43245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li J, Zhang B, Liu WX, Lu K, Pan H, Wang T, Oh CD, Yi D, Huang J, Zhao L, et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann Rheum Dis. 2020;79:635–645. doi: 10.1136/annrheumdis-2019-216713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Zhao X, Lotz M, Terkeltaub R, Liu-Bryan R. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator-activated receptor γ coactivator 1α. Arthritis Rheumatol. 2015;67:2141–2153. doi: 10.1002/art.39182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen LY, Wang Y, Terkeltaub R, Liu-Bryan R. Activation of AMPK-SIRT3 signaling is chondroprotective by preserving mitochondrial DNA integrity and function. Osteoarthritis Cartilage. 2018;26:1539–1550. doi: 10.1016/j.joca.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohashi Y, Takahashi N, Terabe K, Tsuchiya S, Kojima T, Knudson CB, Knudson W, Imagama S. Metabolic reprogramming in chondrocytes to promote mitochondrial respiration reduces downstream features of osteoarthritis. Sci Rep. 2021;11:15131. doi: 10.1038/s41598-021-94611-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y, Alexander PB, Wang XF. TGF-β family signaling in the control of cell proliferation and survival. Cold Spring Harb Perspect Biol. 2017;9:a022145. doi: 10.1101/cshperspect.a022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang J, Xiang H, Lu Y, Wu T. Role and clinical significance of TGF-β1 and TGF-βR1 in malignant tumors (review) Int J Mol Med. 2021;47:1. doi: 10.3892/ijmm.2021.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jeong JH, Jang HJ, Kwak S, Sung GJ, Park SH, Song JH, Kim H, Na Y, Choi KC. Novel TGF-β1 inhibitor antagonizes TGF-β1-induced epithelial-mesenchymal transition in human A549 lung cancer cells. J Cell Biochem. 2019;120:977–987. doi: 10.1002/jcb.27460. [DOI] [PubMed] [Google Scholar]
- 82.Henderson J, Duffy L, Stratton R, Ford D, O'Reilly S. Metabolic reprogramming of glycolysis and glutamine metabolism are key events in myofibroblast transition in systemic sclerosis pathogenesis. J Cell Mol Med. 2020;24:14026–14038. doi: 10.1111/jcmm.16013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shen J, Li S, Chen D. TGF-β signaling and the development of osteoarthritis. Bone Res. 2014;2:14002. doi: 10.1038/boneres.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ni Z, Deng J, Potter CMF, Nowak WN, Gu W, Zhang Z, Chen T, Chen Q, Hu Y, Zhou B, et al. Recipient c-kit lineage cells repopulate smooth muscle cells of transplant arteriosclerosis in mouse models. Circ Res. 2019;125:223–241. doi: 10.1161/CIRCRESAHA.119.314855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20:74–88. doi: 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vasan N, Toska E, Scaltriti M. Overview of the relevance of PI3K pathway in HR-positive breast cancer. Ann Oncol. 2019;30(Suppl 10):x3–x11. doi: 10.1093/annonc/mdz281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Panasyuk G, Espeillac C, Chauvin C, Pradelli LA, Horie Y, Suzuki A, Annicotte JS, Fajas L, Foretz M, Verdeguer F, et al. PPARγ contributes to PKM2 and HK2 expression in fatty liver. Nat Commun. 2012;3:672. doi: 10.1038/ncomms1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wang P, Cong M, Liu T, Li Y, Liu L, Sun S, Sun L, Zhu Z, Ma H, You H, et al. FoxA2 inhibits the proliferation of hepatic progenitor cells by reducing PI3K/Akt/HK2-mediated glycolysis. J Cell Physiol. 2020;235:9524–9537. doi: 10.1002/jcp.29759. [DOI] [PubMed] [Google Scholar]
- 90.Liu S, Chen Q, Wang Y. MiR-125b-5p suppresses the bladder cancer progression via targeting HK2 and suppressing PI3K/AKT pathway. Hum Cell. 2020;33:185–194. doi: 10.1007/s13577-019-00285-x. [DOI] [PubMed] [Google Scholar]
- 91.Tian C, Yuan Z, Xu D, Ding P, Wang T, Zhang L, Jiang Z. Inhibition of glycolysis by a novel EGFR/HER2 inhibitor KU004 suppresses the growth of HER2+ cancer. Exp Cell Res. 2017;357:211–221. doi: 10.1016/j.yexcr.2017.05.019. [DOI] [PubMed] [Google Scholar]
- 92.Lu C, Li Y, Hu S, Cai Y, Yang Z, Peng K. Scoparone prevents IL-1β-induced inflammatory response in human osteoarthritis chondrocytes through the PI3K/Akt/NF-κB pathway. Biomed Pharmacother. 2018;106:1169–1174. doi: 10.1016/j.biopha.2018.07.062. [DOI] [PubMed] [Google Scholar]
- 93.Sun K, Luo J, Guo J, Yao X, Jing X, Guo F. The PI3K/AKT/mTOR signaling pathway in osteoarthritis: a narrative review. Osteoarthritis Cartilage. 2020;28:400–409. doi: 10.1016/j.joca.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 94.Mitchell JP, Carmody RJ. NF-κB and the transcriptional control of inflammation. Int Rev Cell Mol Biol. 2018;335:41–84. doi: 10.1016/bs.ircmb.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 95.Caron MM, Emans PJ, Surtel DA, Cremers A, Voncken JW, Welting TJ, van Rhijn LW. Activation of NF-κB/p65 facilitates early chondrogenic differentiation during endochondral ossification. PLOS ONE. 2012;7:e33467. doi: 10.1371/journal.pone.0033467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Murahashi Y, Yano F, Kobayashi H, Makii Y, Iba K, Yamashita T, Tanaka S, Saito T. Intra-articular administration of IκBα kinase inhibitor suppresses mouse knee osteoarthritis via downregulation of the NF-κB/HIF-2α axis. Sci Rep. 2018;8:16475. doi: 10.1038/s41598-018-34830-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sang LJ, Ju HQ, Liu GP, Tian T, Ma GL, Lu YX, Liu ZX, Pan RL, Li RH, Piao HL, et al. LncRNA CamK-A regulates Ca(2+)-signaling-mediated tumor microenvironment remodeling. Mol Cell. 2018;72:71–83.e77. doi: 10.1016/j.molcel.2018.08.014. [DOI] [PubMed] [Google Scholar]
- 98.Wang Y, Xian H, Qi J, Wei F, Cheng X, Li S, Wang Q, Liu Z, Yu Y, Zhou J, et al. Inhibition of glycolysis ameliorate arthritis in adjuvant arthritis rats by inhibiting synoviocyte activation through AMPK/NF-кB pathway. Inflamm Res. 2020;69:569–578. doi: 10.1007/s00011-020-01332-2. [DOI] [PubMed] [Google Scholar]
- 99.Osama A, Zhang J, Yao J, Yao X, Fang J. Nrf2: a dark horse in Alzheimer's disease treatment. Ageing Res Rev. 2020;64:101206. doi: 10.1016/j.arr.2020.101206. [DOI] [PubMed] [Google Scholar]
- 100.Hu X, Ma R, Cao J, Du X, Cai X, Fan Y. PTPN2 negatively regulates macrophage inflammation in atherosclerosis. Aging (Albany NY) 2020;13:2768–2779. doi: 10.18632/aging.202326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Theeuwes WF, van den Bosch MHJ, Thurlings RM, Blom AB, van Lent P. The role of inflammation in mesenchymal stromal cell therapy in osteoarthritis, perspectives for post-traumatic osteoarthritis: a review. Rheumatology (Oxford) 2021;60:1042–1053. doi: 10.1093/rheumatology/keaa910. [DOI] [PubMed] [Google Scholar]
- 102.Hughes MM, O'Neill LAJ. Metabolic regulation of NLRP3. Immunol Rev. 2018;281:88–98. doi: 10.1111/imr.12608. [DOI] [PubMed] [Google Scholar]
- 103.Hampton H, Hutcheon C, Subramanian N. NAGging hexokinase PEPs up NLRP3. Cell Host Microbe. 2016;20:130–132. doi: 10.1016/j.chom.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 104.Wolf AJ, Reyes CN, Liang W, Becker C, Shimada K, Wheeler ML, Cho HC, Popescu NI, Coggeshall KM, Arditi M, Underhill DM. Hexokinase is an innate immune receptor for the detection of bacterial peptidoglycan. Cell. 2016;166:624–636. doi: 10.1016/j.cell.2016.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alatshan A, Kovács GE, Aladdin A, Czimmerer Z, Tar K, Benkő S. All-trans retinoic acid enhances both the signaling for priming and the glycolysis for activation of NLRP3 inflammasome in human macrophage. Cells. 2020;9:1591. doi: 10.3390/cells9071591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chen Z, Zhong H, Wei J, Lin S, Zong Z, Gong F, Huang X, Sun J, Li P, Lin H, et al. Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res Ther. 2019;21:300. doi: 10.1186/s13075-019-2085-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McAllister MJ, Chemaly M, Eakin AJ, Gibson DS, McGilligan VE. NLRP3 as a potentially novel biomarker for the management of osteoarthritis. Osteoarthritis Cartilage. 2018;26:612–619. doi: 10.1016/j.joca.2018.02.901. [DOI] [PubMed] [Google Scholar]