Abstract
The ppk gene encodes polyphosphate kinase (PPK), the principal enzyme in many bacteria responsible for the synthesis of inorganic polyphosphate (polyP) from ATP. A null mutation in the ppk gene of six bacterial pathogens renders them greatly impaired in motility on semisolid agar plates; this defect can be corrected by the introduction of ppk gene in trans. In view of the fact that the motility of pathogens is essential to invade and establish systemic infections in host cells, this impairment in motility suggests a crucial and essential role of PPK or polyP in bacterial pathogenesis.
Inorganic polyphosphate (polyP) is a linear polymer of hundreds of orthophosphate residues linked by high-energy phosphoanhydride bonds. It is ubiquitous in nature (10), having been found in every bacterial, plant, and animal cell. Among the many functions proposed for polyP is a role in Escherichia coli in regulating the networks essential for responses to nutritional stringencies and environmental stresses and for survival in the stationary phase of growth (2, 15, 16). The regulatory role in E. coli is inferred from the behavior of null mutants of ppk, the gene that encodes polyP kinase (PPK), the enzyme responsible for the synthesis of polyP from ATP.
Inasmuch as some virulence factors are also expressed in stationary phase (11, 19), there may be a dependence on polyP for gene regulation in a pathogen (23). The relationship of polyP to virulence in pathogens is suggested by three observations: (i) massive accumulation of polyP in Helicobacter pylori during its infectious stage (S. Liu and A. Kornberg, unpublished data), (ii) sensitivity of the ppk mutant of Neisseria meningitidis to 10% human serum (22), and (iii) coregulation of capsular polysaccharide (alginate) synthesis and polyP accumulation in Pseudomonas aeruginosa (9).
Among these three pathogens and at least ten others, there is a remarkable conservation of the PPK amino acid sequence (reference 23 and C.-M. Tzeng and A. Kornberg, unpublished data). This list of pathogens includes P. aeruginosa, which causes infections in humans, particularly with cystic fibrosis patients, burn victims, and individuals with AIDS or cancer. Other pathogens in this list are Salmonella spp., the causative agents of typhoid and/or gastroenteritis; Vibrio cholerae, the cause of severe diarrheal disease; Klebsiella pneumoniae, an agent of pneumonia in humans; H. pylori, the cause of chronic gastritis and peptic ulcers; and Mycobacterium tuberculosis and Mycobacterium leprae, the agents of tuberculosis and leprosy, respectively.
To elucidate the roles of polyP in bacterial pathogenesis, we prepared ppk null mutants of P. aeruginosa PAO1, V. cholerae 92A1552, Salmonella enterica serovar Typhimurium FIRN, and Salmonella enterica serovar Dublin SVA47, in addition to the available E. coli MG1655 and K. pneumoniae ATCC9621 mutants. The ppk::tet mutation in P. aeruginosa and the ppk::kan mutations in V. cholerae and Salmonella serovars Typhimurium and Dublin were verified at the genetic level either by genomic PCR or Southern hybridization. Biochemical verifications were performed by assaying the loss of activities of PPK and exopolyphosphatase (PPX) (where appropriate) relative to the wild type and by the deficiency in polyP accumulation under defined conditions (unpublished data).
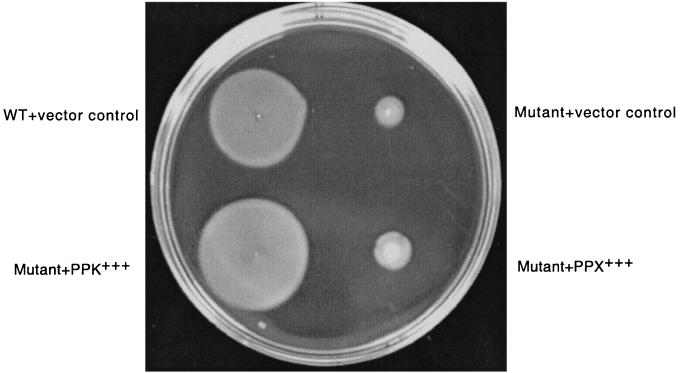
To examine whether the ppk mutation has any effect on the flagellar motility of these pathogens, the motility of these mutants was compared with that of the corresponding wild-type strains on swim plates (1% tryptone, 0.5% NaCl) containing 0.3% agar. As shown in Fig. 1 for P. aeruginosa, the mutant is severely impaired in motility. Since the P. aeruginosa ppk mutant still accumulates at least 20% as much polyP under some conditions compared to the wild type (unpublished data), the mutant was transformed with a plasmid overexpressing the yeast PPX (ScPPX1) (24) to deplete residual polyP. This strain behaved much like the mutant. When the mutant was transformed with a plasmid expressing P. aeruginosa PPK, the motility was completely restored. This clearly demonstrates the dependence of flagellar motility on PPK function. This observation has been extended to other pathogens (Table 1). Impairments of swarming in the ppk mutants were between 13 and 79% of those of the wild-type levels. As in P. aeruginosa, the motility deficiency of the mutant could be complemented in E. coli by introducing the ppk gene on a plasmid.
FIG. 1.
Swimming motility of P. aeruginosa PAO1 wild-type (WT) and derivative strains. The flagellum-mediated motility of the strains was assessed on tryptone swim plates (1% tryptone, 0.5% NaCl, 0.3% agar) with carbenicillin (300 μg/ml) and IPTG (isopropyl-β-d-thiogalactopyranoside) (1 mM) after 12 h of growth at 30°C. Migration of the cells from the point of inoculation (observed as a turbid zone) indicates that a strain is proficient for flagellar-mediated motility. The strains are (clockwise from upper left) PAO1/p66HE (WT plus vector control), PAOM-5/p66HE (Δppk plus vector control), PAOM-5/pSCPPX (Δppk plus PPX+++), and PAOM-5/pPAPPK (Δppk plus PPK+++).
TABLE 1.
Flagellum-mediated motility of pathogens on swim plates
| Strain | Relevant genotypea | Swim area (% WT ± SEM)b |
|---|---|---|
| E. coli MG1655 | WT | 100 ± 7.0 |
| Δppk-ppx | 46 ± 3.5 | |
| WT + vector | 100 ± 7.3 | |
| Δppk-ppx + vector | 33 ± 4.7 | |
| Δppk-ppx + ppk+++ | 91 ± 6.6 | |
| P. aeruginosa PAO1 | WT | 100 ± 12.7 |
| Δppk | 31 ± 1.8 | |
| WT + vector | 100 ± 8.9 | |
| Δppk + vector | 13 ± 1.7 | |
| Δppk + ppx+++ | 13 ± 1.2 | |
| Δppk + ppk+++ | 92 ± 14.7 | |
| K. pneumoniae ATCC9621 | WT | 100 ± 5.0 |
| Δppk-ppx | 33 ± 0.7 | |
| V. cholerae 92A1552 | WT | 100 ± 4.5 |
| Δppk | 57 ± 4.8 | |
| Salmonella serovar Dublin SVA47 | WT | 100 ± 3.6 |
| Δppk-ppx | 58 ± 3.8 | |
| Salmonella serovar Typhimurium FIRN | WT | 100 ± 6.4 |
| Δppk-ppx | 79 ± 8.6 |
WT, wild type.
Swim area was measured after 10 to 12 h of incubation at 37°C on tryptone swim plates. The standard error of the mean equals ςn-1/√n (17), where n = 10, except for P. aeruginosa, where incubation was at 30°C and n = 4. WT, wild type.
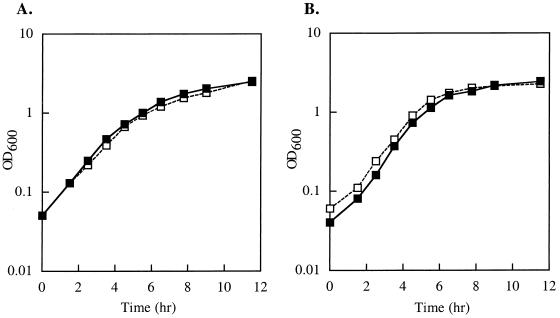
To determine whether the impairment in swimming motility of the ppk mutants on semisolid agar plates is due to a growth impairment, the growth of E. coli and P. aeruginosa wild-type and ppk mutants was monitored in shaking cultures at 30°C in tryptone broth. No growth defect could be observed (Fig. 2) to account for the reduced swimming motility of the ppk mutants on semisolid tryptone plates. Electron microscopy revealed that the ppk mutants of E. coli, P. aeruginosa, K. pneumoniae, V. cholerae, and Salmonella serovar Dublin all possessed apparently intact flagella indistinguishable from those of the wild-type strains (data not shown). Thus, the effect of polyP on swimming motility is likely due to altered functioning of the flagella.
FIG. 2.
Growth curves of E. coli MG1655 (A) and P. aeruginosa PAO1 (B) wild-type and ppk mutants in tryptone broth at 30°C. Growth was monitored at an optical density at 600 nm (OD600). Symbols: ■, wild type; □, mutant (ppk).
Direct microscopic observations revealed that the E. coli and P. aeruginosa ppk mutants were motile in liquid culture. Cells were in exponential phase (0.4 to 0.7 optical density at 600 nm) grown in tryptone broth (1% tryptone, 0.5% NaCl) at 30°C. Peritrichous E. coli and monotrichous P. aeruginosa change direction of movement by similar mechanisms, a reversal of flagellar rotation (21); in E. coli, reversal causes tumbling, and in P. aeruginosa, reversal causes the bacteria to back up. The ppk mutants of E. coli and P. aeruginosa along with their respective wild-types examined under phase-contrast microscopy (magnification, ×800) revealed no striking differences in changes of movement direction between wild-type and mutant cells. Possibly, more refined techniques will disclose the role of polyP in flagellar swimming.
Flagella are highly complex and conserved bacterial organelles requiring coordinated and ordered expression of about 50 genes for their synthesis and function (18). The roles of flagella in chemotaxis and motility are important for the survival of many organisms. A connection between virulence and flagellum-based motility has long been observed in many pathogens, some of which require functional flagella for virulence (7, 8, 12) and others in which motility must be suppressed for virulence (1).
The roles of flagella and flagellum-mediated motility in P. aeruginosa pulmonary and burn infections have been studied in detail (4–6, 13, 20). In the pathogenesis of respiratory tract infection, it has been shown that flagella and/or flagellar motility are necessary at three distinct stages of infection: (i) acquisition of motile organisms, (ii) immunostimulation, and (iii) adaptation (6). Flagellar motility and type IV pili-based twitching motility have been found necessary for the development of a P. aeruginosa biofilm (14). Bacterial biofilms are troublesome when they form on tissues, on catheters, or on medical implants because of their innate resistance to antibiotics and other biocides (for a review, see reference 3). We have found that the ppk mutant of P. aeruginosa is also defective in twitching motility and biofilm formation on abiotic surfaces (data not shown). Taken together, these several lines of evidence suggest that PPK or polyP might be a virulence determinant of pathogens, like P. aeruginosa.
Acknowledgments
We are grateful to the NIH for support of this research.
We thank A. Kuroda, K.-S. Kim, C. Fraley, and N. Ogawa, respectively, for strains of K. pneumoniae, Salmonella serovar Typhimurium, Salmonella serovar Dublin, and V. cholerae. We thank E. Peter Greenberg (University of Iowa) for helpful suggestions.
REFERENCES
- 1.Akerley B J, Cotter P A, Miller J F. Ectopic expression of the flagellar regulon alters development of Bordetella-host interaction. Cell. 1995;80:611–620. doi: 10.1016/0092-8674(95)90515-4. [DOI] [PubMed] [Google Scholar]
- 2.Ault-Riché D, Fraley C F, Tzeng C-M, Kornberg A. Novel assay reveals multiple pathways regulating stress-induced accumulations of inorganic polyphosphate in Escherichia coli. J Bacteriol. 1998;180:1841–1847. doi: 10.1128/jb.180.7.1841-1847.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costerton J W, Stewart P S, Greenberg E P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 4.Craven R C, Montie T C. Motility and chemotaxis of three strains of Pseudomonas aeruginosa used for virulence studies. Can J Microbiol. 1981;27:458–460. doi: 10.1139/m81-070. [DOI] [PubMed] [Google Scholar]
- 5.Drake D, Montie T C. Flagella, motility and invasive virulence of Pseudomonas aeruginosa. J Gen Microbiol. 1988;134:43–52. doi: 10.1099/00221287-134-1-43. [DOI] [PubMed] [Google Scholar]
- 6.Feldman M, Bryan R, Rajan S, Scheffler L, Brunnert S, Tang H, Prince A. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect Immun. 1998;66:43–51. doi: 10.1128/iai.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harshey R M. Bees aren't the only ones: swarming in gram-negative bacteria. Mol Microbiol. 1994;13:389–394. doi: 10.1111/j.1365-2958.1994.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 8.Harshey R M, Toguchi A. Spinning tails: homologies among bacterial flagellar systems. Trends Microbiol. 1996;4:226–231. doi: 10.1016/0966-842X(96)10037-8. [DOI] [PubMed] [Google Scholar]
- 9.Kim H-Y, Schlitman D, Shankar S, Xie Z, Chakrabarty A M, Kornberg A. Alginate, inorganic polyphosphate, GTP and ppGpp synthesis co-regulated in Pseudomonas aeruginosa: implications for stationary phase survival and synthesis of RNA/DNA precursors. Mol Microbiol. 1998;27:717–725. doi: 10.1046/j.1365-2958.1998.00702.x. [DOI] [PubMed] [Google Scholar]
- 10.Kulaev I S. The biochemistry of inorganic polyphosphates. New York, N.Y: John Wiley & Sons Inc.; 1979. [Google Scholar]
- 11.Lowen P C, Henge-Aronis R. The role of the sigma factor S (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 12.Moens S, Vanderleyden J. Functions of bacterial flagella. Crit Rev Microbiol. 1996;22:67–100. doi: 10.3109/10408419609106456. [DOI] [PubMed] [Google Scholar]
- 13.Montie T C, Doyle-Huntzinger D, Craven R C, Holder I A. Loss of virulence associated with absence of flagellum in an isogenic mutant of Pseudomonas aeruginosa in the burned-mouse model. Infect Immun. 1982;38:1296–1298. doi: 10.1128/iai.38.3.1296-1298.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 15.Rao N N, Liu S, Kornberg A. Inorganic polyphosphate in Escherichia coli: the phosphate regulon and the stringent response. J Bacteriol. 1998;180:2186–2193. doi: 10.1128/jb.180.8.2186-2193.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao N N, Kornberg A. Inorganic polyphosphate supports resistence and survival of stationary-phase Escherichia coli. J Bacteriol. 1996;178:1394–1400. doi: 10.1128/jb.178.5.1394-1400.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rashid M H, Mori M, Sekiguchi J. Glucosaminidase of Bacillus subtilis: cloning, regulation, primary structure and biochemical characterization. Microbiology. 1995;141:2391–2404. doi: 10.1099/13500872-141-10-2391. [DOI] [PubMed] [Google Scholar]
- 18.Shapiro L. The bacterial flagellum: from genetic network to complex architecture. Cell. 1995;80:525–527. doi: 10.1016/0092-8674(95)90505-7. [DOI] [PubMed] [Google Scholar]
- 19.Spector M P. The starvation-stress response (SSR) of Salmonella. Adv Microb Physiol. 1998;40:233–279. doi: 10.1016/s0065-2911(08)60133-2. [DOI] [PubMed] [Google Scholar]
- 20.Tang H B, DiMango E, Bryan R, Gambello M, Iglewski B H, Goldberg J B, Prince A. Contribution of specific Pseudomonas aeruginosa virulence factors to pathogenesis of pneumonia in a neonatal mouse model of infection. Infect Immun. 1996;64:37–43. doi: 10.1128/iai.64.1.37-43.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor B L, Koshland D E. Reversal of flagellar rotation in montrichous and peritrichous bacteria: generation of changes in direction. J Bacteriol. 1974;119:640–642. doi: 10.1128/jb.119.2.640-642.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tinsley C R, Gotschlich E C. Cloning and characterization of the meningococcal polyphosphate kinase gene: production of polyphosphate synthesis mutants. Infect Immun. 1995;63:1624–1630. doi: 10.1128/iai.63.5.1624-1630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tzeng C-M, Kornberg A. Polyphosphate kinase is highly conserved in many bacterial pathogens. Mol Microbiol. 1998;29:381–382. doi: 10.1046/j.1365-2958.1998.00887.x. [DOI] [PubMed] [Google Scholar]
- 24.Wurst H, Shiba T, Kornberg A. The gene for a major exopolyphosphatase of Saccharomyces cerevisiae. J Bacteriol. 1995;177:898–906. doi: 10.1128/jb.177.4.898-906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]