Abstract
BACKGROUND
Coil migration during endovascular treatment for an intracranial aneurysm is rare. When it occurs intraoperatively, it often mandates prompt endovascular retrieval or, as a salvage maneuver, microsurgical extraction if it fails endovascularly.
OBSERVATIONS
The authors presented a case of immediate coil migration during embolization of a giant intracranial cavernous segment of the internal carotid aneurysm. The patient immediately underwent emergency surgical extraction after unsuccessful endovascular retrieval attempts. The migrated coil was successfully removed through the M1 segment of the middle cerebral artery. The patient had full recovery without new neurological deficits. Four years after the incident, she was living independently. Previous case reports of emergency surgical removal of immediate coil migration were provided.
LESSONS
Surgical extraction of migrated coil after unfeasible endovascular retrieval served as an alternative salvage procedure. Hybrid neurological angiography in the operating suite may prevent unnecessary transfer and provide better real-time visualization of the migrated coil.
Keywords: endovascular, embolization, coil migration, malposition, surgery, craniotomy
ABBREVIATIONS : CT = computed tomography, ICA = internal carotid artery, MCA = middle cerebral artery
Endovascular coiling of an intracranial aneurysm has been considered one of the mainstay treatments in most respectable centers worldwide because of its safety and efficacy, lower morbidity and mortality, and better clinical outcome than surgical clipping.1,2 A detachable coil induces thrombosis, which results in eventual aneurysm obliteration. Coil migration is considered a rare complication of endovascular embolization of cerebral aneurysms. Literature review has shown such incidences to be approximately 0.3% to 6%.3–12 The dislodged material travels distally along its parent vessel and eventually clogs the downstream vessel. A coil inside the vascular lumen is thrombogenic and, therefore, disrupts cerebral blood flow beyond the obstruction. Catastrophic cerebral ischemia, or infarction, will ensue unless adequate arterial flow restoration is established in time. We report our experience regarding immediate surgical extraction of migrated coil by arteriotomy of the M1 segment of the middle cerebral artery (MCA) following endovascular coiling of a giant cavernous aneurysm.
Illustrative Case
A healthy 56-year-old woman presented with well-controlled systemic hypertension and dyslipidemia. For the past 4 years, she had progressive horizontal binocular diplopia accompanied by right ptosis from oculomotor paresis. Computed tomography (CT) scans revealed a 3-cm (transverse dimension) unruptured giant saccular aneurysm at the cavernous part of the right internal carotid artery (ICA). Despite passing a balloon occlusion test, the patient declined surgical and endovascular coiling of the aneurysm. At that time, her decision was to continue following it radiographically. Unfortunately, 2 years later, she presented to our emergency department with a sudden onset of severe headache.
Her vital signs at the emergency department were as follows: blood pressure, 190/100 mm Hg; pulse rate, 75 beats per minute; and body temperature, 37°C. Despite her severe throbbing headache, she was fully awake and alert with right oculomotor nerve paresis, unchanged from previously, and newly developed right facial numbness. She had no other new motor or sensory deficit or stiff neck. CT scan of the brain revealed the previous unruptured aneurysm with notable enlargement in size (3.1 × 3.2 × 2.9 cm). The patient was offered a craniotomy for arterial bypass with subsequent aneurysm reconstruction as the preferred choice, but she opted for an endovascular coiling instead. Nevertheless, she agreed to the bypass should the endovascular attempt fail.
During the endovascular intervention, it was noted that a coil was migrating downstream toward the M1 segment of the MCA. Total occlusion of the MCA distal outflow was detected. At that time, it was determined that endovascular retrieval of the dislodged coil was impossible because of her unfavorable vascular architecture (Fig. 1). Therefore, the patient was promptly transferred to the operating room while she was still under general anesthesia.
FIG. 1.
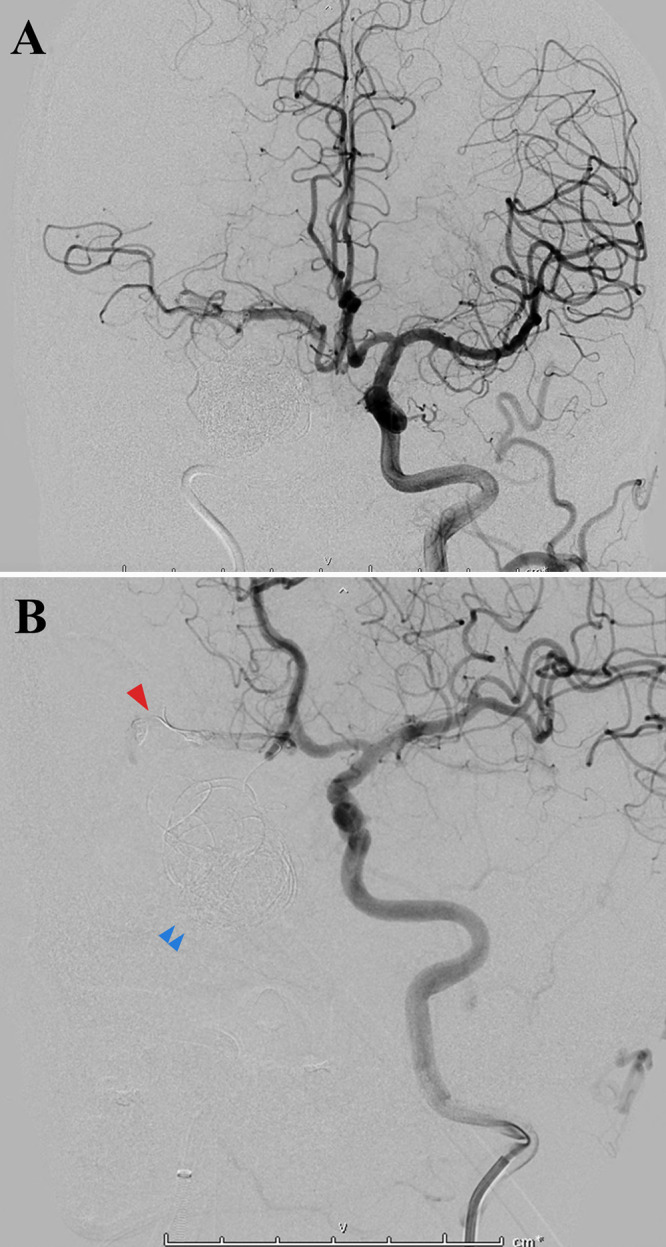
Cerebral angiograms demonstrating a migrated coil occlusion at the distal M1 segment of the right MCA (arrowhead). There was no contrast flow beyond the point of occlusion or contrast leakage into the subarachnoid space in the vicinity. The migrated coil was deemed irretrievable. It was noted that most of the coil material still occupied the giant aneurysmal dome volume (double arrowheads).
During a standard right pterional craniotomy and microscopic dissection of the Sylvian fissure, there was no sign of subarachnoid hemorrhage. The large, thrombosed aneurysm dome was seen. The dislodged coil was readily identified as causing obstruction of the distal M1 segment (Fig. 2). A small incision was made on this MCA segment for coil retrieval. Primary interrupted suture repair of the arterial incision was performed thereafter. Confirmed by micro-Doppler ultrasound, both proximal and distal MCA arterial flows were intact after removing temporary clips. At the end of surgery, right ICA ligation was performed just above the common carotid bifurcation.
FIG. 2.
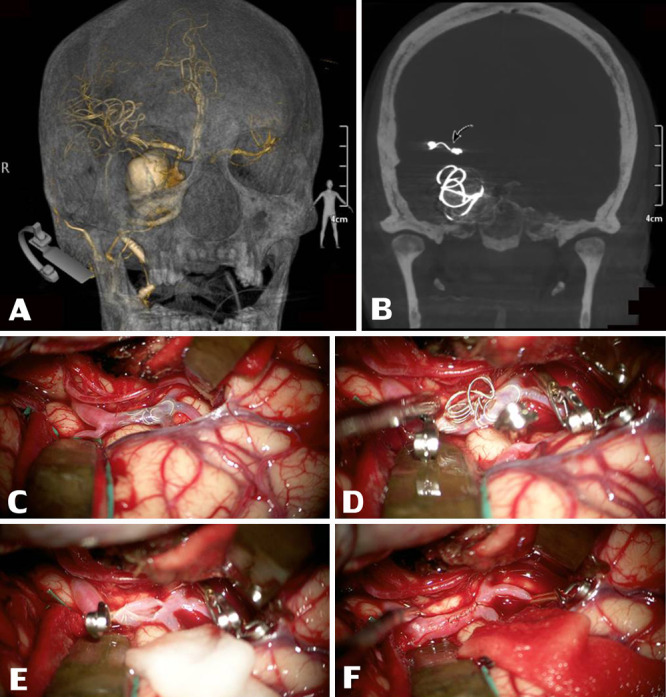
Three-dimensional reconstruction CT revealed a giant saccular intracranial aneurysm at the right cavernous part of the ICA, 3.1 × 3.2 × 2.9 cm (A) along with a migrated coil occlusion at the distal M1 segment of the right MCA (curved arrow, B). After Sylvian fissure splitting, intraoperative footage revealed an intravascular migrated coil visibly inside the right M1 segment of the MCA (C). After securing this MCA segment properly with temporary aneurysm clips at both distal and proximal ends, the coiling material was retrieved through a small linear incision on the MCA (D and E). This MCA segment was later repaired by an interrupting simple suture using nylon suture material under a microscope (F).
With a low daily dose of aspirin (81 mg), the patient’s postoperative course was uneventful. She had no evidence of vasospasm or new neurological difficulty before hospital discharge 10 days later. At the time of this manuscript preparation, 4 years after surgery, the patient has lived independently with almost complete recovery of oculomotor paresis.
Discussion
Observations
Coil migration is a tragic intraprocedural complication during aneurysm embolization. The dislodged piece may result in large territorial cerebral infarction. The reported rates of coil migration range from 0.3% to 6%.3–12 Possible risk factors for coil migration are low dome-height-to-neck ratio, wide-necked aneurysm, and communicating segment aneurysm.3
From a literature review by Abdalkader et al., coil migration can be categorized into two groups based on the timing of its occurrence: immediate and delayed.3 The endovascular team typically recognizes dislodged coil during the coiling procedure, which would be defined as immediate. More frequently occurring than the immediate category, the delayed coil migration is detected after an endovascular procedure.1,3,6,7,9 The timing varies between hours and several months, either by incidental finding on follow-up imaging or presentation with neurological symptoms resulting from coil occlusion.
To our knowledge, there is no standard management or well-established guideline for migrated coil retrieval because of the scarcity of reports. Its extraction is necessary when observed in the immediate intraprocedural occurrence and if it is accessible. In contrast, delayed migrated coil retrieval decision is based on a patient’s clinical symptoms and the importance of occluded vessels and its collateral supply. With endovascular extraction often being the choice for the initial attempt, the decision regarding the retrieval technique is also at the surgeon’s discretion.3,9
Endovascular Treatment
The techniques of coil retrieval depend on the interventionist’s preference, experiences, and device availability. Several techniques and devices have been used since the invention of the endovascular aneurysm treatment. We can categorize retrievable endovascular techniques into two main categories. First is the standard method using specific foreign body removal devices available on the market (e.g., Alligator Retrieval Device, Chestnut Medical Technologies;6 Gooseneck micro snare).13 They are primarily used in peripheral interventional radiology. However, these devices have limited applicability in the brain. The critical differences are neurovascular complexity and its fragile anatomy. The brain arteries are often more tortuous, typically smaller than the peripheral arteries, and more delicate due to the lack of the surrounding connective tissue and thick adventitia.14 These facts limit the use of several peripheral devices intracranially or increase the potential risk of vascular dissection or perforation. Second is the modification/adapted device method using retrievable neurovascular devices, specifically the thrombectomy stents (e.g., Solitaire stent or Catch Plus, Balt Extrusion;7 Solitaire stent, Medtronic; Trevo, Stryker; and Embotrap II, Cerenovus, Johnson & Johnson).14–16 The design of these devices has advantages over the snare devices in the tortuous neurovascular and smaller vessels. The stentriever method has been reported with a varying degree of clinical outcomes despite the tremendous success of the coil removal, which is probably due to the challenges of the procedure itself, time constraint, and tolerability of the individual neural tissue in occluded vessels. The stentriever technique has some limitations. The vessel caliber must be sufficient for the microcatheter to pass alongside the dislodged devices and the stent to be deployed. The procedure in an inappropriate vessel can push the migrated coil mass more distally or it may trap the coils with rescue devices in those smaller vessels, which can result in devastating consequences. In some cases, the standard coil-assisted stent or deployable stent can trap the coil mass along the wall of the vessels. This technique may be helpful in minor coil protrusion or small fragment of the device. However, it necessitates anticoagulation, which increases hemorrhagic risk and potentially immediate or delayed occlusion. Modification of devices, such as shaping the tip of micro guidewires to become the pigtail (Merci retriever), has been reported.17,18
When standard approaches to coil retrieval fail by any attempted endovascular means or salvage methods are potentially unsuccessful due to tortuosity and unfavorable vascular architecture, microsurgery for coil retrieval and revascularization must be done immediately to minimize morbidity and mortality.
Open Surgical Treatment
Open microsurgery still has a role when endovascular retrieval is complicated or unsuccessful.19–21 The microsurgical approach permits surgeons to retrieve the migrated coil and apply clip(s) to the aneurysm dome at the same time. This microsurgical maneuver depends on the exact location of the dislodged coil itself. An incision to open the dome of an aneurysm has proven safe and effective for the migrated coil, located partly within the aneurysm dome.22 Direct arteriotomy had been suggested by several reports.9,19,22,23 Mariak et al. considered doing so via an adjacent downstream, less important artery to keep the main vessel intact.21
Timing of retrieval is also paramount. The literature review of immediate surgical coil extraction consistently described immediate transfer of patients from the angiography suite to the operating theater. However, there was no information regarding the amount of time between the two procedures.3,9,19,22,24–27 The time from dislodged coil detection to the actual operation should be shortened as much as possible to restore blood supply to downstream brain tissues, the same logic applied to ischemic stroke caused by embolism. When general anesthesia is used during endovascular intervention, it can be helpful during patient transfer to the operating theater with regards to cerebral protection by means of cerebral metabolic requirement. Deshmukh et al. used barbiturate and mannitol administration during transfer to further enhance cerebral protection.19 Having a hybrid angiography facility within an operating theater may be a critical factor in omitting the need for time-consuming patient transfer and, perhaps, better real-time visualization of the migrated coil during surgical extraction.
Obtaining proximal and distal control of the artery is mandatory. After arteriotomy, simple repair with monofilament suture material is sufficient. Chen et al. and Turek et al. preferred the less important more negligible adjacent downstream vessel (i.e., the A1 segment of the anterior cerebral artery for the distal ICA, anterior temporal artery branch of the MCA for the proximal segment of the MCA occlusion). In contrast, others preferred direct arteriotomy for dislodged coil removal. Despite the difference in arteriotomy location, successful extraction can be achieved in all reported cases, including ours, and a good outcome was typically expected and influenced by prompt management at the time. In addition, Doppler ultrasonography can be used to confirm the downstream arterial flow within the vessel. An antiplatelet should be considered as prophylaxis given the potential for endothelial injury from the arteriotomy and possible reaction from the suture material, which could result in secondary stenosis. This effect may be drastic, particularly in patients with extensive subarachnoid hemorrhage, and subsequent vasospasm is anticipated. Permissive systemic hypertension and controlled euvolemic status are often continued as preventive measures for vasospasm.
In summary, only four case reports, totaling six patients, of emergency microsurgical coil extraction after dislodgement during the endovascular procedure were discovered in an English language literature search via PubMed, Google Scholar, Web of Science, and Scopus.9,19,23,25 Table 1 summarizes the patients, including this report. The list consists of either ruptured or unruptured intracranial aneurysm cases. It is worth mentioning that all aneurysms undergoing embolization procedures were located on either the ICA or the proximal segment of the MCA. This high arterial flow makes the embolization procedure more challenging, and wide-necked aneurysm features often complicate the situation and can cause significant morbidity if complications occur.
TABLE 1.
Summary of clinical features of the immediate migrated coil in patients who underwent salvage surgical extraction
| Authors & Year | Age (yrs)/Sex | Initial Presenting Sxs | Diagnosis | Location of Coil Migration | Partial or Total Coil Migration | Time Lag* | Mode of Transfer | Surgical Extraction Technique | Coil Retrieval | Immediate Postoperative Status | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deshmukh et al., 200619 |
34/F |
Stupor |
Ruptured lt saccular 3-mm ophthalmic ICA aneurysm |
Lt M3 occlusion |
Total |
NS |
GA, barbiturate, mannitol |
Lt M3 arteriotomy |
Success |
Neurologically intact |
Neurologically intact |
| Chen et al., 200923 |
61/F |
Stupor |
Ruptured lt wide-neck saccular PComA aneurysm |
Lt distal ICA occlusion |
Total |
NS |
NS |
Lt A1 arteriotomy |
Success |
Transient hemiparesis |
Neurologically intact |
| Kim et al., 201425 |
63/M |
NS |
Unruptured lt wide-neck saccular 9-mm MCA bifurcation aneurysm |
Lt inferior M2 branch occlusion |
Total |
>2 hrs |
Awake |
Lt M2 arteriotomy |
Success |
Transient hemiparesis |
Neurologically intact |
| Turek et al., 20159 | 54/F |
Rt 3rd cranial nerve palsy |
Unruptured rt saccular 16-mm cavernous segment ICA aneurysm |
Rt M1 occlusion |
Total |
NS |
GA |
Rt ATA arteriotomy |
Success |
Transient hemiparesis |
Neurologically intact |
| 58/F |
Alert, intact |
Unruptured residual rt saccular distal ICA aneurysm |
Rt M1 occlusion |
Total |
NS |
GA |
Rt ATA arteriotomy |
Success |
Moderate hemiparesis |
Mild hemiparesis |
|
| 39/M |
Headache |
Unruptured rt fusiform distal ICA & M1 segment aneurysm |
Rt M1 occlusion |
Total |
NS |
GA |
Rt M1 arteriotomy |
Success |
Moderate hemiparesis |
Neurologically intact |
|
| Present case | 56/F | Rt 3rd cranial nerve palsy | Unruptured rt giant 32-mm saccular cavernous segment ICA aneurysm | Rt M1 occlusion | Total | 3 hrs | GA | Rt M1 arteriotomy | Success | Neurologically intact | Neurologically intact |
A1 = A1 segment of anterior cerebral artery; ATA = anterior temporal artery; GA = general anesthesia; M1 = M1 segment of MCA; M2 = M2 segment of MCA; M3 = M3 segment of MCA; NS = not specified; PComA = posterior communicating artery; Sxs = symptoms.
Time lag between occlusion and extraction.
Lessons
Migrated coil after endovascular coiling of intracranial aneurysm is a rare complication. Surgical extraction of migrated coil after unfeasible endovascular retrieval serves as an alternative salvage procedure. Early recognition, well-established protocol, and prompt treatment are paramount to prevent an undesirable outcome. Hybrid neurological angiography in the operating suite may prevent unnecessary transfer and provide better real-time visualization of the migrated coil.
Acknowledgments
We thank Miss Wijittra Matang, BSc, BPH, for her assistance in the preparation of table and figures.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: all authors. Acquisition of data: all authors. Analysis and interpretation of data: all authors. Drafting the article: Muninthorn, Kobkitsuksakul. Critically revising the article: Muninthorn, Kobkitsuksakul. Reviewed submitted version of manuscript: Muninthorn, Kobkitsuksakul. Approved the final version of the manuscript on behalf of all authors: Boongird. Administrative/technical/material support: Muninthorn.
References
- 1. Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–817. doi: 10.1016/S0140-6736(05)67214-5. [DOI] [PubMed] [Google Scholar]
- 2. Eskey CJ, Meyers PM, Nguyen TN, et al. Indications for the performance of intracranial endovascular neurointerventional procedures: a scientific statement from the American Heart Association. Circulation. 2018;137(21):e661–e689. doi: 10.1161/CIR.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 3. Abdalkader M, Piotin M, Chen M, et al. Coil migration during or after endovascular coiling of cerebral aneurysms. J Neurointerv Surg. 2020;12(5):505–511. doi: 10.1136/neurintsurg-2019-015278. [DOI] [PubMed] [Google Scholar]
- 4. Casasco AE, Aymard A, Gobin YP, et al. Selective endovascular treatment of 71 intracranial aneurysms with platinum coils. J Neurosurg. 1993;79(1):3–10. doi: 10.3171/jns.1993.79.1.0003. [DOI] [PubMed] [Google Scholar]
- 5. Guglielmi G, Viñuela F, Duckwiler G, et al. Endovascular treatment of posterior circulation aneurysms by electrothrombosis using electrically detachable coils. J Neurosurg. 1992;77(4):515–524. doi: 10.3171/jns.1992.77.4.0515. [DOI] [PubMed] [Google Scholar]
- 6. Henkes H, Fischer S, Weber W, et al. Endovascular coil occlusion of 1811 intracranial aneurysms: early angiographic and clinical results. Neurosurgery. 2004;54(2):268–285. doi: 10.1227/01.neu.0000103221.16671.f0. [DOI] [PubMed] [Google Scholar]
- 7. Leslie-Mazwi TM, Heddier M, Nordmeyer H, et al. Stent retriever use for retrieval of displaced microcoils: a consecutive case series. AJNR Am J Neuroradiol. 2013;34(10):1996–1999. doi: 10.3174/ajnr.A3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Phatouros CC, McConachie NS, Jaspan T. Post-procedure migration of Guglielmi detachable coils and mechanical detachable spirals. Neuroradiology. 1999;41(5):324–327. doi: 10.1007/s002340050757. [DOI] [PubMed] [Google Scholar]
- 9. Turek G, Kochanowicz J, Lewszuk A, et al. Early surgical removal of migrated coil/stent after failed embolization of intracranial aneurysm. J Neurosurg. 2015;123(4):841–847. doi: 10.3171/2015.1.JNS132788. [DOI] [PubMed] [Google Scholar]
- 10. Viñuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 2008;108(4):832–839. doi: 10.3171/JNS/2008/108/4/0832. [DOI] [PubMed] [Google Scholar]
- 11. White PM, Lewis SC, Nahser H, Sellar RJ, Goddard T, Gholkar A. HydroCoil Endovascular Aneurysm Occlusion and Packing Study (HELPS trial): procedural safety and operator-assessed efficacy results. AJNR Am J Neuroradiol. 2008;29(2):217–223. doi: 10.3174/ajnr.A0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ding D, Liu KC. Management strategies for intraprocedural coil migration during endovascular treatment of intracranial aneurysms. J Neurointerv Surg. 2014;6(6):428–431. doi: 10.1136/neurintsurg-2013-010872. [DOI] [PubMed] [Google Scholar]
- 13. Zoarski GH, Bear HM, Clouston JC, Ragheb J. Endovascular extraction of malpositioned fibered platinum microcoils from the aneurysm sac during endovascular therapy. AJNR Am J Neuroradiol. 1997;18(4):691–695. [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou KZ, Maingard J, Kok HK, et al. Endovascular retrieval of dislodged neurovascular devices with a Stentriever: case series and technical review. World Neurosurg. 2019;123:e661–e669. doi: 10.1016/j.wneu.2018.11.248. [DOI] [PubMed] [Google Scholar]
- 15. Hopf-Jensen S, Hensler HM, Preiß M, Müller-Hülsbeck S. Solitaire stent for endovascular coil retrieval. J Clin Neurosci. 2013;20(6):884–886. doi: 10.1016/j.jocn.2012.06.012. [DOI] [PubMed] [Google Scholar]
- 16. Kabbani MR, Smith A, Leider M. Endovascular coil retrieval using a TrevoProVue stentriever. BMJ Case Rep. 2015;7:e19. doi: 10.1136/bcr-2014-011181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee CY. Use of wire as a snare for endovascular retrieval of displaced or stretched coils: rescue from a technical complication. Neuroradiology. 2011;53(1):31–35. doi: 10.1007/s00234-010-0679-4. [DOI] [PubMed] [Google Scholar]
- 18. O’Hare A, Brennan P, Thornton J. Retrieval of a migrated coil using an X6 MERCI device. Interv Neuroradiol. 2009;15(1):99–102. doi: 10.1177/159101990901500116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Deshmukh VR, Klopfenstein J, Albuquerque FC, Kim LJ, Spetzler RF. Surgical management of distal coil migration and arterial perforation after attempted coil embolization of a ruptured ophthalmic artery aneurysm: technical case report. Neurosurgery. 2006;58(4 suppl 2):ONS-E379. doi: 10.1227/01.NEU.0000205317.27820.35. [DOI] [PubMed] [Google Scholar]
- 20. Heuer GG, Zaghloul KA, Roberts R, Stiefel MF, Storm PB. Successful microsurgical extraction of a migrated coil in a pediatric patient after failed endovascular closure of a Blalock-Taussig shunt. Case report. J Neurosurg. 2007;106(suppl 2):136–138. doi: 10.3171/ped.2007.106.2.136. [DOI] [PubMed] [Google Scholar]
- 21. Mariak Z, Kochanowicz J, Kordecki K, Jadeszko M, Łysoń T, Lewko J. Surgical evacuation of an embolization coil from the middle cerebral artery. Article in Polish. Neurol Neurochir Pol. 2004;38(6):533–537. [PubMed] [Google Scholar]
- 22. Thornton J, Dovey Z, Alazzaz A, et al. Surgery following endovascular coiling of intracranial aneurysms. Surg Neurol. 2000;54(5):352–360. doi: 10.1016/s0090-3019(00)00337-2. [DOI] [PubMed] [Google Scholar]
- 23. Chen Z, Tang W, Feng H, Zhu G. Surgical extraction of migrated coils via proximal segment of the anterior cerebral artery: an emergency alternative. Neurol India. 2009;57(3):327–330. doi: 10.4103/0028-3886.53286. [DOI] [PubMed] [Google Scholar]
- 24. Banerjee AD, Guimaraens L, Cuellar H. Asymptomatic delayed coil migration from an intracranial aneurysm: a case report. Case Rep Vasc Med. 2011;2011:901925. doi: 10.1155/2011/901925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim HS, Lee JM, Koh EJ, Choi HY. Surgical recanalization of distal middle cerebral artery occlusion due to a coil migration during endovascular coil embolization: a case report. J Cerebrovasc Endovasc Neurosurg. 2014;16(3):287–292. doi: 10.7461/jcen.2014.16.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Motegi H, Isobe M, Isu T, Kamiyama H. A surgical case of delayed coil migration after balloon-assisted embolization of an intracranial broad-neck aneurysm: case report. Neurosurgery. 2010;67(suppl 2):516–521. doi: 10.1227/NEU.0b013e3181f82588. [DOI] [PubMed] [Google Scholar]
- 27. Wada H, Tokumitsu N, Shirai W, Sako K, Kamada K. Ruptured aneurysm with delayed distal coil migration requiring surgical treatment. Case report. Neurol Med Chir (Tokyo) 2012;52(6):439–442. doi: 10.2176/nmc.52.439. [DOI] [PubMed] [Google Scholar]


