Abstract
Children with progressive familial intrahepatic cholestasis, including bile salt export pump (BSEP) and familial intrahepatic cholestasis–associated protein 1 (FIC1) deficiencies, suffer debilitating cholestatic pruritus that adversely affects growth and quality of life (QoL). Reliance on surgical interventions, including liver transplantation, highlights the unmet therapeutic need. INDIGO was an open‐label, Phase 2, international, long‐term study to assess the efficacy and safety of maralixibat in children with FIC1 or BSEP deficiencies. Thirty‐three patients, ranging from 12 months to 18 years of age, were enrolled. Eight had FIC1 deficiency and 25 had BSEP deficiency. Of the latter, 6 had biallelic, protein truncating mutations (t)‐BSEP, and 19 had ≥ 1 nontruncating mutation (nt)‐BSEP. Patients received maralixibat 266 μg/kg orally, once daily, from baseline to Week 72, with twice‐daily dosing permitted from Week 72. Long‐term efficacy was determined at Week 240. Serum bile acid (sBA) response (reduction in sBAs of > 75% from baseline or concentrations <102.0 μmol/L) was achieved in 7 patients with nt‐BSEP, 6 during once‐daily dosing, and 1 after switching to twice‐daily dosing. sBA responders also demonstrated marked reductions in sBAs and pruritus, and increases in height, weight, and QoL. All sBA responders remained liver transplant–free after > 5 years. No patients with FIC1 deficiency or t‐BSEP deficiency met the sBA responder criteria during the study. Maralixibat was generally well‐tolerated throughout the study. Conclusion: Response to maralixibat was dependent on progressive familial intrahepatic cholestasis subtype, and 6 of 19 patients with nt‐BSEP experienced rapid and sustained reductions in sBA levels. The 7 responders survived with native liver and experienced clinically significant reductions in pruritus and meaningful improvements in growth and QoL. Maralixibat may represent a well‐tolerated alternative to surgical intervention.
The long‐term efficacy and safety of maralixibat (a minimally absorbed, selective inhibitor of the ileal bile acid transporter) was assessed in children with BSEP or FIC1 deficiencies. Rapid and sustained reductions in sBA levels were observed in a subset of patients with non‐truncating BSEP deficiency, leading to transplant‐free survival, as well as reductions in pruritus and meaningful improvements in growth and quality of life. Maralixibat can be considered as an effective, well‐tolerated, nonsurgical alternative to surgical biliary diversion in these patients.

INTRODUCTION
Progressive familial intrahepatic cholestasis (PFIC) is a group of rare autosomal recessive disorders caused by defects in bile formation.[ 1 , 2 ] Children typically present in the first year of life, initially with jaundice, and subsequently with other features of cholestasis (notably, pruritus and deficiency of fat‐soluble vitamins [FSV]).[ 1 , 2 , 3 , 4 , 5 ] Several subtypes of PFIC are associated with significant risk of hepatocellular carcinoma.[ 6 , 7 ] Due to failure of aggressive management in addressing symptoms, many patients undergo surgical interruption of the enterohepatic circulation of bile acids or liver transplantation.[ 1 , 2 , 8 ] There is clearly a significant, unmet need for treatments of both pruritus and the underlying liver disease.
Most forms of PFIC are caused by mutations in transporters expressed in the canalicular membrane of hepatocytes.[ 1 , 2 ] Two of the major types of PFIC, bile salt export pump (BSEP) or familial intrahepatic cholestasis‐associated protein 1 (FIC1) deficiencies, were included in the current study. BSEP deficiency (or PFIC type 2) is caused by mutations in adenosine triphosphate (ATP) binding cassette subfamily B member 11 (ABCB11).[ 1 , 9 ] BSEP is the major bile acid transporter from hepatocytes into canaliculi, and BSEP deficiency is the most common PFIC type.[ 1 ] Most patients with BSEP deficiency have at least one nonprotein truncating mutation (nt‐BSEP) with the potential for some residual BSEP function, but about 15% have two variants predicted to cause protein truncation (truncating BSEP [t‐BSEP]), resulting in an absence of BSEP function.[ 6 , 10 , 11 ] FIC1 is encoded by P‐type ATPase phospholipid transporting 8B1 (ATP8B1), a lipid transporter that is expressed in multiple epithelia, including the canalicular membrane.[ 1 ] Deficiency of FIC1 (or PFIC type 1) is associated with extrahepatic manifestations, including chronic diarrhea, pancreatic insufficiency, renal tubular dysfunction, and growth failure.[ 10 ]
While the prevention of liver disease progression is always paramount, early management of PFIC is largely through nutritional support and the treatment of pruritus.[ 4 ] Medical treatment of cholestatic pruritus is particularly unsatisfactory and includes the use of rifampicin, ursodeoxycholic acid, bile acid binding resins, inhibitors of serotonin reuptake, an opioid antagonist (naloxone), and antipruritics (notably, antihistamines).[ 1 , 2 ] The failure of medical treatment of pruritus has led to surgical interventions.[ 2 ] Short of transplantation, the mainstay of surgical management has been depletion of the bile salt pool size through surgical biliary diversion (SBD).[ 2 ] A large, retrospective analysis has recently shown that SBD extends transplant‐free survival in patients with nt‐BSEP deficiency by up to 15 years.[ 5 ] Furthermore, this study also showed that reduction of the serum bile acids (sBAs), after surgery, by 75% or to less than 102 μmol/L, is associated with long‐term native liver survival.[ 5 ] Depletion of bile salts, through exteriorization of bile or through prevention of uptake in the terminal ileum, is dependent on some residual bile salt excretion into bile. The retrospective analysis of surgical management of PFIC confirmed this hypothesis and showed that patients with t‐BSEP did not respond and progressed, requiring liver transplantation.[ 5 ]
Given the limited efficacy of currently available antipruritic medications, along with the risks and burden of surgical interventions, there remains a high unmet need for alternative treatments for patients with PFIC. Pharmacological interruption of the enterohepatic bile acid recirculation has the potential to reduce the bile salt pool size, alleviate cholestatic pruritus, prevent liver damage, and reduce the need for surgical intervention.[ 12 , 13 , 14 ] Maralixibat, a minimally absorbed, selective inhibitor of the ileal bile acid transporter (IBAT), reduces sBA levels and improves growth in patients with cholestatic liver disease, as demonstrated in previous studies in children with Alagille syndrome.[ 13 , 15 , 16 ] Maralixibat is currently Food and Drug Administration–approved for the treatment of cholestatic pruritus in patients with Alagille syndrome aged 1 year and older.[ 17 ]
Here, we present data from INDIGO (Open Label Study of the Efficacy and Long‐Term Safety of LUM001 in the Treatment of Cholestatic Liver Disease in Pediatric Patients with Progressive Familial Intrahepatic Cholestasis; LUM001‐501; NCT02057718), an open‐label, Phase 2 study of the long‐term efficacy and safety of maralixibat in children with BSEP or FIC1 deficiencies. INDIGO investigated the effect of maralixibat on sBA levels and cholestatic pruritus, alongside other biochemical markers of cholestasis and liver disease in these patients.
METHODS
Study design
INDIGO was an open‐label, Phase 2, international, long‐term, multicenter study designed to assess the efficacy and safety of maralixibat (previously known as LUM001‐501 or SHP625) in children with FIC1 deficiency or BSEP deficiency. The study was conducted at 12 hospitals in France, Poland, the United Kingdom, and the United States.
Screening evaluations were performed in the 6 weeks before the start of the study. In patients without documented ATP8B1 or ABCB11 mutations, genetic testing was performed. The study consisted of an initial maralixibat dose escalation period, followed by a long‐term stable dosing period (up to maralixibat 266 μg/kg given orally, once daily [equivalent to maralixibat chloride 280 μg/kg, and hereafter referred to as “266 μg/kg”]; Figure 1). An amendment to the study protocol permitted subsequent dose increases up to 266 μg/kg twice daily if predefined sBAs and pruritus benefits were not achieved by Week 72, as well as entry into the long‐term extension period of the study.
FIGURE 1.
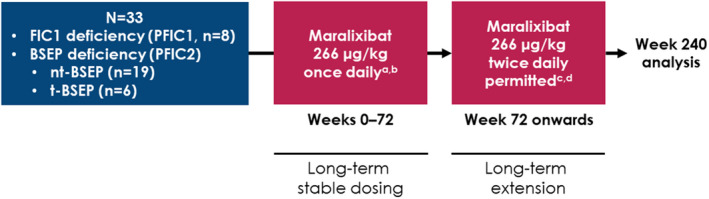
Study design. (A) Equivalent to maralixibat chloride 280 μg/kg. (B) Included a 4‐week dose escalation period. (C) Equivalent to maralixibat chloride 560 μg/kg. (D) Included a 4‐week dose escalation period for patients who had gone ≥ 7 days without receiving maralixibat. BSEP, bile salt export pump; FIC, familial intrahepatic cholestasis; nt‐BSEP, nontruncating BSEP; t‐BSEP, truncating BSEP
This study was approved by local institutional review boards, complied with the Declaration of Helsinki and Good Clinical Practice Guidelines, and was registered at ClinicalTrials.gov (NCT02057718).
Study patients
The study enrolled male and female patients after receiving written informed consent (and assent, if applicable) and meeting the following key inclusion criteria:
12 months to 18 years of age;
A diagnosis of PFIC (as defined by the presence of intrahepatic cholestasis [sBA levels > 3 times the upper limit of normal (ULN) for age] and two documented mutated alleles in either ATP8B1 or ABCB11, or evidence of chronic liver disease, with one or more of the following criteria: >6 months' duration of biochemical or clinical abnormalities, pathologic evidence of progressive liver disease, or is a sibling of a known individual affected by PFIC); and
Gamma glutamyltransferase levels < 100 IU/L at the time of screening.
Key exclusion criteria included severe diarrhea, surgical disruption of the enterohepatic circulation, liver transplantation, decompensated liver cirrhosis, alanine aminotransaminase (ALT) levels > 15 times ULN at time of screening, and history or presence of other concomitant liver disease. Patients could continue their antipruritic medication if the dose remained stable throughout the study.
Study endpoints, subgroup analyses, and long‐term analyses
The primary efficacy endpoint was the change in mean fasting sBA levels from baseline to Week 13 in the overall intent‐to‐treat (ITT) study population.
The key secondary efficacy endpoint was the mean change in observer‐rated pruritus (Itch Reported Outcome Observer [ItchRO(Obs)][ 18 ]) score from baseline to Week 13 in the overall ITT study population. Other secondary efficacy endpoints included the mean changes from baseline to Week 13 in total cholesterol, low‐density and high‐density lipoprotein cholesterol (LDL‐C and HDL‐C, respectively), and serum triglyceride levels in the overall ITT study population. Additional efficacy and safety analyses included the change from baseline to Week 72 and Week 240 in sBA levels, patient height and weight, bile acid synthesis (as determined by the ratio between sBA levels and 7α‐hydroxy‐4‐cholesten‐3‐one [7α‐C4] levels), quality of life as measured by the Pediatric Quality of Life Inventory (PedsQL), mean changes in ALT aspartate aminotransferase (AST), and bilirubin (total and direct), lipid profiles, and FSV levels. The ItchRO assessment was not performed at Week 72, so data from Week 48 were used instead.
Efficacy and safety analyses were performed for the overall ITT study population as well as the two PFIC subtypes (FIC1 deficiency and BSEP deficiency) separately. Patients with BSEP deficiency were analyzed overall and according to their mutation type (t‐BSEP and nt‐BSEP). Long‐term changes in pruritus scores, patient height, blood lipids, liver parameters, and transplant‐free survival were assessed according to a responder analysis (see “Analyses” for the predefined definition of response). Analyses were made at Week 72 (due to a natural delineation between the two main periods of the study) and Week 240 (chosen to provide long‐term data while minimizing the effects of low patient numbers and missing data observed at later time points).
Analyses
Quantitative analysis of the 15 major sBAs and serum 7α‐C4 was performed at a single site (Cincinnati) by stable isotope dilution electrospray ionization liquid chromatography‐mass spectrometry using a fully validated proprietary assay that complies with College of American Pathologists/Clinical Laboratory Improvement Amendments certification.
The study protocol defined a composite response to maralixibat as achieving a ≥ 1.0‐point reduction in ItchRO(Obs) score and a ≥ 70% reduction or normalization of sBA levels from baseline. Following the publication reporting improved transplant‐free survival in individuals who achieved a reduction of sBAs > 75% from baseline or concentrations of <102.0 μmol/L after surgical interruption of the enterohepatic circulation,[ 5 ] thresholds were changed to use this new definition in the post hoc responder analyses for this study. Regardless of definition, the number of responders remained the same.
Pruritus was measured using the ItchRO tool, which was specifically developed and validated to evaluate pruritus in children with cholestatic liver diseases, based on a 5‐point scale where 0 = “none” and 4 = “very severe.”[ 18 , 19 ] This tool has been shown to detect clinically relevant changes in cholestatic pruritus in children across all ages, with changes ≥ 1.0 point being clinically meaningful.[ 19 , 20 ] The ItchRO measure was completed twice daily via an eDiary. Parents/caregivers completed the ItchRO(Obs) assessment for all patients. Additionally, children ≥ 9 years of age completed the ItchRO(Pt) assessment.
Levels of ALT, AST, bilirubin (total and direct), lipids, and FSV were assessed using standard clinical laboratory techniques. Quality of life was assessed using the PedsQL questionnaire (see Supporting Materials). The minimum clinically important difference for child‐reported and parent/caregiver‐reported scores was a change of 4.4 or 4.5 points in total scale score, respectively.[ 21 ]
The occurrence of treatment‐emergent adverse events (TEAEs) and serious adverse events (SAEs) was assessed throughout the study.
Statistical methods
An enrollment target of 33 patients was based on operational and practical considerations. The ITT and safety populations included all patients who had received at least one dose of maralixibat. Two‐tailed tests were used to assess statistical significance, with a p value of < 0.05 indicating statistical significance. Kruskal–Wallis tests were used to assess statistical significance between patient groups. The Week 240 analyses used data up to and including May 20, 2020, and the native liver survival assessment included data up to July 30, 2020.
Logistic regression models using stepwise selection were used to identify potential predictors of response. Age, sex, and the following baseline parameters were considered: sBAs, weekly morning average ItchRO(Obs) score, ALT, AST, total bilirubin, direct bilirubin, total cholesterol, triglycerides, C4, vitamin A, vitamin D, vitamin E, PedsQL total score, and C4/sBA ratio.
RESULTS
Patient disposition and characteristics
Thirty‐seven patients were screened between February 2014 and July 2015. Three patients had low sBAs (sBAs < 3 ULN of 8.5 μmol/L) and 1 participant had elevated international normalized ratio, and these were not included in the study. Thirty‐three patients were enrolled; a total of 8 (24%) had FIC1 deficiency and 25 (76%) had BSEP deficiency (6 with t‐BSEP and 19 with nt‐BSEP; Figure S1). Patient demographics and characteristics at baseline are described in Table 1. Of the 33 patients enrolled, all completed study treatment up to Week 13; 22 (67%) completed study treatment up to Week 72 (6 with FIC1 deficiency and 16 with BSEP deficiency); and 11 (33%) discontinued. Eighteen patients consented to remain on study treatment following Week 72; and 6 patients discontinued during this period (Figure S1). The mean study week at which dose escalation to 266 μg/kg twice daily occurred was Week 110 (range: Weeks 94–152). At end of study (Week 240), a total of 12 patients had each received > 4 years of maralixibat treatment.
TABLE 1.
Patient demographics and characteristics at baseline
| FIC1 deficiency (n = 8) | t‐BSEP (n = 6) | nt‐BSEP (n = 19) | All BSEP deficiency a (n = 25) | Overall (n = 33) | |
|---|---|---|---|---|---|
| Median age, years (range) | 2.0 (1.0, 7.0) | 7.0 (1.0, 10.0) | 3.0 (1.0, 13.0) | 4.0 (1.0, 13.0) | 3.0 (1.0, 13.0) |
| Male, n (%) | 6 (75.0) | 2 (33.3) | 6 (31.6) | 8 (32.0) | 14 (42.4) |
| Mean sBAs, μmol/L (SD) | 261.8 (99.57) | 404.9 (112.40) | 373.4 (161.95) | 380.9 (149.97) | 352.1 (147.40) |
| Mean ItchRO(Obs) score (SD) | 2.1 (0.75) | 2.9 (0.68) | 2.1 (0.85) | 2.3 (0.86) | 2.3 (0.83) |
| PedsQL score (SD) | 57 (18.7) | 65 (17.7) | 62 (12.8) | 63 (13.8) | 61 (15.1) |
| Mean ALT, U/L (SD) | 56 (29.7) | 152 (151.0) | 116 (109.2) | 125 (118.0) | 108 (107.4) |
| Mean AST, U/L (SD) | 77 (23.8) | 206 (167.3) | 148 (165.1) | 162 (164.0) | 141 (147.2) |
| Mean total bilirubin, mg/dl (SD) | 5.5 (5.13) | 2.9 (1.50) | 1.8 (1.78) | 2.1 (1.75) | 2.9 (3.20) |
| Mean direct bilirubin, mg/dl (SD) | 4.0 (3.67) | 2.3 (1.30) | 1.4 (1.33) | 1.6 (1.36) | 2.2 (2.33) |
| Mean total cholesterol, mg/dl (SD) | 112.9 (23.22) | 233.5 (46.47) | 193.3 (52.40) | 202.9 (53.08) | 181.1 (61.37) |
| Mean serum triglycerides, mg/dl (SD) | 124.5 (31.27) | 200.7 (64.91) | 175.2 (81.56) | 181.3 (77.39) | 167.5 (79.92) |
| Mean serum 7α‐C4, ng/mL (SD) | 2.71 (2.190) | 10.50 (18.228) | 2.76 (1.988) | 4.62 (9.141) | 4.16 (8.025) |
| Mean retinol, μg/dl (SD) | 73.45 (35.984) | 39.60 (23.701) | 51.41(21.548) | 48.58 (22.176) | 54.61 (27.735) |
| Mean serum 25‐hydroxyvitamin D, ng/mL (SD) | 37.88 (17.334) | 23.48 (13.399) | 28.58 (10.435) | 27.47 (11.018) | 30.16 (13.437) |
| Mean alpha‐tocopherol, mg/dl (SD) | 0.34 (0.167) | 0.70 (0.489) | 0.42 (0.277) | 0.49 (0.351) | 0.45 (0.320) |
| Mean 7α‐C4/sBA ratio (log10 scale) | −4.77 (0.640) | −4.75 (0.818) | −4.79 (0.585) | −4.78 (0.629) | −4.78 (0.622) |
Abbreviations: 7α‐C4, 7α‐hydroxy‐4‐cholesten‐3‐one; ALT, alanine aminotransaminase; AST, aspartate aminotransaminase; ItchRO(Obs), Itch Reported Outcome Observer; NA, not applicable; PedsQL, Pediatric Quality of Life Inventory.
Includes patients with nt‐BSEP and t‐BSEP.
Efficacy
Efficacy results for both short term (13 weeks) and long term (up to 72 weeks) are described subsequently. Notable differences in short‐term and long‐term efficacy results were observed between the disease subtypes, with patients with nt‐BSEP deficiency demonstrating significant improvements in various parameters (shown subsequently).
Short‐term efficacy analyses
Primary efficacy endpoint
Within the overall ITT study population (all 33 enrolled patients; PFIC1 and PFIC2 combined), there was no significant decrease in sBA levels from baseline to Week 13 (−16.9 μmol/L [95% confidence interval (CI) 74.830, 41.070; p = 0.884]).
Key secondary efficacy endpoint
Pruritus significantly improved by Week 13 in the ITT population, with a mean reduction from baseline in ItchRO(Obs) score of −0.8 (95% CI −1.04, −0.53; p < 0.001) and ItchRO(Pt) score of −1.0 (95% CI −1.55, −0.47; p = 0.002).
Additional secondary efficacy endpoints
There were trends toward a reduction in mean serum total cholesterol, LDL‐C, HDL‐C, and triglyceride levels from baseline to Week 13; none of these parameters reached statistical significance.
BSEP deficiency: long‐term response to treatment and transplant‐free survival
Patients with BSEP deficiency demonstrated predictably varied responses to treatment according to disease type, with patients with nt‐BSEP (n = 19) showing the greatest response. During the study, sBA response (reduction in sBAs of > 75% from baseline or concentrations < 102.0 μmol/L) was achieved by a subset of 7 patients (37%) with nt‐BSEP (hereafter referred to as “sBA responders”). These sBA responders demonstrated profound and sustained sBA reductions, as well as reductions in pruritus and other parameters. Six achieved response while receiving 266 μg/kg once‐daily dosing and 1 achieved response while receiving 266 μg/kg twice‐daily dosing after Week 97. No patient with t‐BSEP met the sBA responder criteria at any point in the study, and all patients with t‐BSEP discontinued before Week 240. The following results summarize the long‐term efficacy for all patients with BSEP deficiency (Table 2 and Table S1) and summarize data for sBA responders versus sBA nonresponders. Baseline characteristics comparing sBA responders and sBA nonresponders are found in Table S2. Baseline serum ALT, AST, total and direct bilirubin, cholesterol, and triglycerides were all significantly lower in responders than in nonresponders.
TABLE 2.
Mean change in efficacy variables from baseline to Week 72 and Week 240
| Mean (95% CI) | Week 72 | Week 240 | ||||||
|---|---|---|---|---|---|---|---|---|
| FIC1 deficiency | t‐BSEP | nt‐BSEP | All BSEP deficiency a | Overall | FIC1 deficiency | nt‐BSEP b | Overall | |
| sBAs, μmol/L | +22.5 (−82.34, 127.28) | −3.3 (−135.55, 128.88) | −38.1 (−151.49, 75.23) | −30.4 (−117.84, 57.04) | −17.2 (−84.70, 50.34) | −17.6 (−106.10, 70.97) | −220.3 (−368.74, −71.84) | −152.7 (−264.35, −41.08) |
| n = 6 | n = 4 | n = 14 | n = 18 | n = 24 | n = 4 | n = 8 | n = 12 | |
| p = 0.605 | p = 0.941 | p = 0.480 | p = 0.473 | p = 0.604 | p = 0.573 | p = 0.010 | p = 0.012 | |
| ItchRO(Obs) score c | −0.7 (−1.75, 0.34) | −1.4 (−2.68, −0.17) | −1.0 (−1.42, −0.54) | −1.1 (−1.44, −0.67) | −1.0 (−1.33, −0.64) | −0.9 (−3.65, 1.79) | −1.7 (−2.60, −0.86) | −1.6 (−2.27, −0.87) |
| n = 6 | n = 4 | n = 18 | n = 22 | n = 28 | n = 2 | n = 8 | n = 10 | |
| p = 0.142 | p = 0.037 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.144 | p = 0.002 | p < 0.007 | |
| ItchRO(Pt) score c | −0.9 (−3.65, 1.79) | −1.6 (−11.77, 8.50) | −1.0 (−2.01, −0.07) | −1.2 (−1.98, −0.43) | −1.1 (−1.72, −0.57) | −1.1 (NA, NA) | −1.8 (−4.51, 0.94) | −1.6 (−2.69, −0.42) |
| n = 2 | n = 2 | n = 5 | n = 7 | n = 9 | n = 1 | n = 2 | n = 3 | |
| p = 0.144 | p = 0.290 | p = 0.041 | p = 0.009 | p = 0.002 | p = NA | p = 0.076 | p = 0.028 | |
| Height z score | −0.14 (−0.602, 0.327) | −0.16 (−0.840, 0.518) | −0.01 (−0.523, 0.510) | −0.04 (−0.441, 0.360) | −0.06 (−0.371, 0.241) | −0.52 (−2.338, 1.289) | +0.33 (−0.346, 1.010) | +0.05 (−0.573, 0.666) |
| n = 6 | n = 4 | n = 14 | n = 18 | n = 24 | n = 4 | n = 8 | n = 12 | |
| p = 0.481 | p = 0.506 | p = 0.979 | p = 0.832 | p = 0.664 | p = 0.425 | p = 0.285 | p = 0.871 | |
| Weight z score | +0.06 (−0.905, 1.020) | −0.11 (−0.657, 0.432) | −0.05 (−0.368, 0.266) | −0.06 (−0.314, 0.185) | −0.03 (−0.292, 0.224) | +0.29 (−2.195, 2.772) | 0.00 (−0.339, 0.347) | +0.10 (−0.466, 0.664) |
| n = 6 | n = 4 | n = 14 | n = 18 | n = 24 | n = 4 | n = 8 | n = 12 | |
| p = 0.884 | p = 0.558 | p = 0.734 | p = 0.592 | p = 0.787 | p = 0.736 | p = 0.977 | p = 0.707 | |
| PedsQL score | +16 (−0.7, 33.1) | −5 (−37.7, 27.7) | +11 (0.2, 21.2) | +7 (−2.4, 16.8) | +9 (1.2, 17.1) | +19 (−24.3, 62.3) | +22 (7.4, 36.7) | +21 (8.4, 33.8) |
| n = 5 | n = 4 | n = 14 | n = 18 | n = 23 | n = 4 | n = 8 | n = 12 | |
| p = 0.056 | p = 0.662 | p = 0.047 | p = 0.131 | p = 0.025 | p = 0.256 | p = 0.009 | p = 0.004 | |
Note: Shaded cells indicate statistical significance.
Abbreviations: CI, confidence interval; ItchRO(Pt), Itch Reported Outcome Patient.
Includes patients with nt‐BSEP and t‐BSEP.
No patients with t‐BSEP remained in the study at Week 240.
As per study protocol, ItchRO(Obs) data were acquired at Weeks 48 and 242.
Serum bile acid levels
Changes in mean sBA levels are given in Table 2. Compared with baseline, sBA levels within sBA responders decreased rapidly following treatment initiation (within Weeks 2–4), with mostly durable responses maintained long‐term throughout the study (Figure 2A). This pattern of response was not observed in sBA nonresponders (Figure 2B).
FIGURE 2.
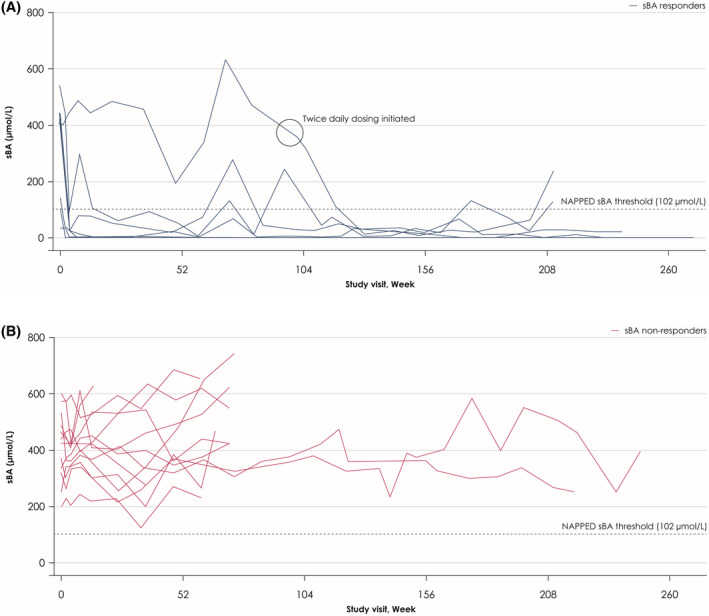
Individual changes from baseline to Week 240 in serum bile acid (sBA) levels in sBA responders (A) and sBA nonresponders (B). The black circle in Figure 2A indicates when the seventh responder initiated twice‐daily dosing at Week 97
Pruritus
Changes in mean ItchRO(Obs) scores are found in Table 2. All 7 sBA responders experienced a > 1.0‐point clinically meaningful reduction in ItchRO(Obs) scores after achieving sBA response (Figure S2A), and an additional 3 sBA nonresponders achieved clinically meaningful reductions in ItchRO(Obs) scores[ 19 , 20 ] (Figure S2B).
Growth
Changes in mean height and weight z scores are given in Table 2. sBA responders experienced a gradual increase from baseline in mean height and weight z scores to Week 72 (0.67, 95% CI 0.369, 0.976 [p = 0.0016] and 0.30, 95% CI −0.001, 0.603 [p = 0.051], respectively, which was maintained to Week 240) (Figure 3A,B). This growth benefit contrasted with a decrease in height and weight z scores in sBA nonresponders when assessed at Week 72 (0.49, 95% CI −0.948, −0.041 [p < 0.001] vs. sBA responders and −0.30, 95% CI −0.606, −0.012 [p = 0.013] vs. sBA responders, respectively).
FIGURE 3.
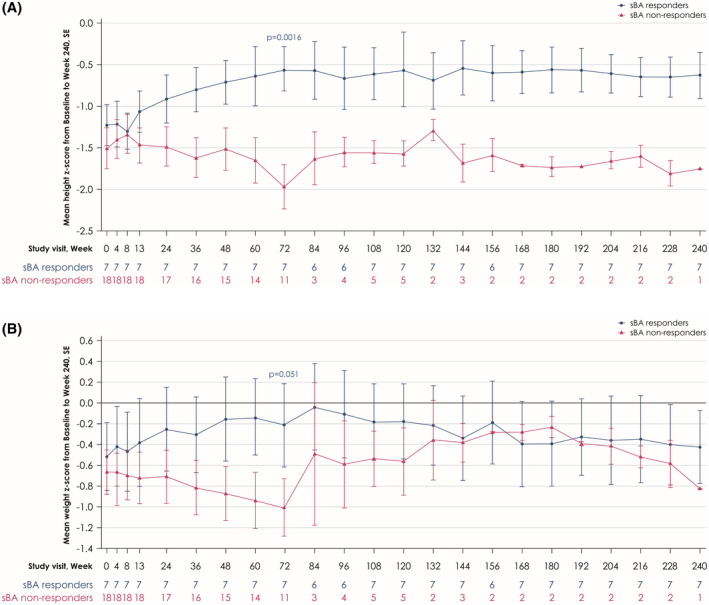
Mean changes in height z scores (A) and weight z scores (B) from baseline to Week 240 in sBA responders and sBA nonresponders
Quality of life
Changes in mean PedsQL scores are found in Table 2. Clinically meaningful improvements in mean PedsQL score were observed within sBA responders following treatment initiation and remained consistent throughout the study (a change from baseline to Week 240 of 24 points, 95% CI 6.7, 40.6 [p = 0.014], respectively) (Figure S3).
Biochemical assessments
Biochemical assessments are provided in Table S1. sBA responders experienced improvements and/or normalization of serum ALT, AST, and bilirubin levels (if elevated at baseline; Figure S4). After treatment with maralixibat, the mean level of serum 7α‐C4 increased in patients with nt‐BSEP, gradually increasing throughout the study, and reaching statistical significance at Week 240 (+26.29 ng/ml, 95% CI 4.07, 48.50; p = 0.027).
7α‐C4/sBA ratio
Evidence of biological response following treatment can be assessed by looking for increased bile acid synthesis, reflected by increases in serum 7α‐C4. When combined with a reduction in sBAs, the 7α‐C4/sBA ratio, compared with baseline, becomes a sensitive marker of biological response. The 7α‐C4/sBA ratio in patients with BSEP deficiency was generally increased, compared with baseline, in sBA responders as opposed to sBA nonresponders (Figure S5). Of note, the 7α‐C4/sBA ratio in the seventh sBA responder fluctuated during once‐daily dosing but showed a clear increase following initiation of twice‐daily dosing.
Transplant‐free survival
All sBA responders demonstrated transplant‐free survival after > 5 years of receiving maralixibat compared with none of the sBA nonresponders (p < 0.001) (Figure 4). Two sBA responders, who were listed for liver transplantation (for the indication of refractory pruritus) at the beginning of the study, improved sufficiently to be removed from the transplant wait list.
FIGURE 4.
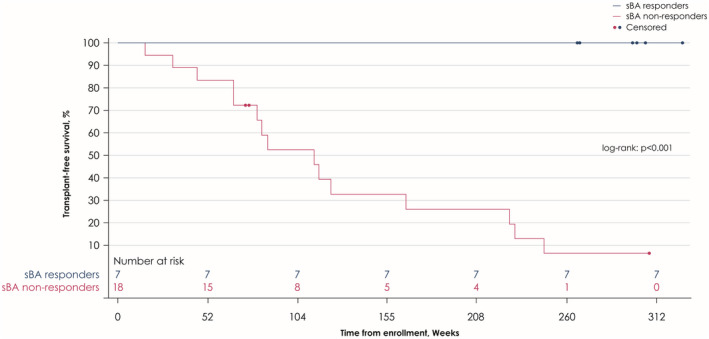
Transplant‐free survival in sBA responders and sBA nonresponders
Patients with FIC1 deficiency
Data describing the additional long‐term efficacy analyses within the patients with FIC1 deficiency are found in Table 2 and Table S1. Mean levels of sBAs, ItchRO(Obs) scores, and PedsQL scores did not change significantly from baseline. No patient with FIC1 deficiency met the sBA responder criteria over the course of the study. Four patients with FIC1 deficiency remain on the study drug, possibly due to receiving some pruritus benefit.
Safety and tolerability
Throughout the study, maralixibat was generally safe and well tolerated (Table 3 and Figure S1). All patients experienced ≥ 1 TEAE, with most being mild or moderate in severity (58%) and transient in nature. The most common TEAEs were pyrexia (20 [61%] patients), diarrhea (19 [58%] patients), and cough (18 [55%] patients). Eight patients (1 with FIC1 deficiency, 7 with BSEP deficiency) discontinued maralixibat due to an adverse event during the study (4 due to nonserious events of increases in serum bilirubin; 1 due to a nonserious event of pancreatitis; 1 due to a nonserious event of decreased vitamin E; 1 due to a nonserious event of pruritus; and 1 due to a nonserious event of hepatic mass [reported as a Grade 1 event of liver nodules, resulting in the patient subsequently undergoing liver transplantation]). The patient with pancreatitis was reported to have a history of pancreatitis (three prior episodes), which occurred within a 2‐year period before starting maralixibat. A total of 15 patients had serious TEAEs reported during the study, of which abdominal pain, diarrhea, and gastroenteritis were the only SAEs reported in at least 1 patient (2 patients each). No deaths were reported during the study.
TABLE 3.
Safety data throughout the study (to Week 240)
| n (%) | FIC1 deficiency (n = 8) | BSEP deficiency a (n = 25) | Overall (n = 33) |
|---|---|---|---|
| ≥ 1 TEAE | 8 (100.0) | 25 (100.0) | 33 (100.0) |
| Grade 3/4 TEAE | 6 (75.0) | 8 (32.0) | 14 (42.4) |
| Serious TEAE | 4 (50.0) | 11 (44.0) | 15 (45.5) |
| TEAE leading to discontinuation | 1 (12.5) | 9 (36.0) | 10 (30.3) |
| TEAE leading to death | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| TEAE potentially related to study drug | 6 (75.0) | 20 (80.0) | 26 (78.8) |
| GI events | 6 (75.0) | 22 (88.0) | 28 (84.8) |
| Hypoglycemia | 1 (12.5) | 1 (4.0) | 2 (6.1) |
Abbreviations: GI, gastrointestinal; TEAE, treatment emergent adverse event.
Includes patients with nt‐BSEP and t‐BSEP.
Other biochemical parameters
Data describing the changes in biochemical parameters (including FSV and liver transaminases) are described in Table S1. Within the overall ITT study population, mean levels of 25‐hydroxyvitamin D did not change significantly from baseline to Week 72, with the FIC1 deficiency and nt‐BSEP cohorts experiencing small increases during this period. However, in patients with t‐BSEP, there was a small reduction in the mean level of 25‐hydroxyvitamin D from baseline to Week 72. Levels of alpha‐tocopherol remained relatively stable throughout the study, apart from statistically significant increases observed in patients with nt‐BSEP at Week 240 compared with baseline. The FIC1 deficiency, nt‐BSEP, and t‐BSEP cohorts all experienced small reductions in mean vitamin A levels from baseline to Weeks 72 and 240. Reductions in the mean retinol/retinol‐binding protein ratio were observed in all cohorts throughout the study, with statistically significant reductions observed in patients with nt‐BSEP at Week 24 compared with baseline.
Patients with FIC1 deficiency experienced an increase in mean ALT levels from baseline to Week 72, whereas patients with nt‐BSEP or t‐BSEP experienced reductions in mean ALT and AST levels during the same period. By Week 240, all patients with FIC1 deficiency or nt‐BSEP experienced reductions in mean levels of ALT and AST, with the nt‐BSEP cohort reaching statistical significance. Lipid‐related TEAEs are described in the Supporting Materials.
DISCUSSION
Here, we report the results of up to 5 years of treatment with maralixibat, a minimally absorbed IBAT inhibitor with the potential to mimic the effects of surgical biliary diversion, in children with FIC1 deficiency or BSEP deficiency. In this study, the primary endpoint of sBA reduction in the overall ITT study population over the first 13 weeks of treatment was not met; however, importantly, response to treatment was found to be dependent on the different PFIC subtypes. A group of 7 patients (37%; 7 of 19) with nt‐BSEP (deemed treatment sBA responders) experienced a sustained, clinically relevant, and statistically significant response with improvement in multiple parameters. In addition to reductions in sBA levels, this response included a ≥ 1.0‐point reduction in ItchRO(Obs) score, improvements of serum transaminases and bilirubin, and an improvement in quality of life and growth parameters. Notably, these patients have remained transplant‐free and without surgical biliary diversion for over 5 years. These findings are consistent with the NAPPED consortium findings on SBD, which demonstrated that a threshold sBA reduction following SBD led to similar biochemical and long‐term outcomes, improving the natural history benefits.[ 5 ]
In contrast to all sBA responders who had at least one nonprotein truncating mutation within ABCB11, none of the 6 patients with t‐BSEP achieved an sBA response. These observations strongly suggest that residual BSEP function is necessary for a maralixibat response. This contention is strongly supported by the differences seen in baseline biochemistry (Table S2), in which lower levels of bilirubin, liver enzymes, and lipids were seen in the subsequent responders. These parameters all suggest milder cholestasis and are consistent with greater retained BSEP function. In patients with nt‐BSEP, there were sBA nonresponders. Although these patients may have had insufficient BSEP function, other factors may have determined their lack of response. These factors may include the degree to which farnesoid X receptor (FXR) regulated bile acid synthesis, levels of IBAT expression, dose of maralixibat, and even small bowel fluid volumes. It is also not immediately obvious why patients with FIC1 deficiency in this study failed to respond to maralixibat. It is possible that these factors also play a role. Furthermore, the reduced FXR expression that has been observed in these patients[ 22 ] might lead to increased IBAT expression, which would consequently necessitate higher doses of maralixibat in this subgroup.
The 7α‐C4/sBA ratio increased rapidly following initiation of treatment in sBA responders and was sustained throughout the duration of the study, suggesting that this ratio may be a sensitive predictor of response to treatment with maralixibat, better than changes in serum 7α‐C4 alone. The seventh sBA responder had fluctuations in their 7α‐C4/sBA ratio during once‐daily dosing, which clearly increased after twice‐daily dosing, thus becoming an sBA responder. Concordantly, a few patients who discontinued at this stage had a 7α‐C4/sBA ratio similar to those of sBA responders, suggesting that these patients may have demonstrated a similar response had they received twice‐daily dosing. Therefore, 7α‐C4/sBA ratio could be used to identify sBA nonresponders who would benefit from dose escalation.
Maralixibat was generally well tolerated in patients with PFIC after receiving up to 5 years of treatment, even in patients receiving twice‐daily dosing. Although 12 patients discontinued treatment with maralixibat due to adverse events or liver transplantation following disease progression, this was not unexpected, due the progressive nature of PFIC. Most TEAEs reported during the study were mild‐to‐moderate, transient, gastrointestinal TEAEs, possibly due to an increased colonic bile acid flux following IBAT inhibition and did not result in treatment discontinuations. These results were in line with the safety profile observed in previous studies of maralixibat.[ 13 ] Treatment with maralixibat did not have any significant detrimental effects on the levels of FSV or liver transaminases.
Limitations of this study include the lack of a placebo‐controlled element and the relatively small sample size. Considering the rare nature of PFIC,[ 4 ] and the dramatic treatment effect on sBA responders who likely avoided life‐altering surgery, the findings can be considered as highly relevant and clinically significant. The primary endpoint was based on the response in the whole cohort. However, it is now clear that there was a dichotomous response to treatment, and, as such, a responder analysis would have been more appropriate. Finally, the open‐label design limits some conclusions that can be drawn from this study in the absence of a control group, although it would not have been feasible to generate > 5 years of placebo‐controlled data. The absence of a control group is mitigated by the dramatic and sustained treatment effects seen in the sBA responders, relative to baseline.
Although baseline total cholesterol was a significant predictor of treatment response, due to the small number of patients enrolled in this study, the importance of this finding is unclear (Table S2).
In conclusion, this study showed that maralixibat led to rapid and sustained reductions in sBA levels in patients with nt‐BSEP leading to transplant‐free survival, as well as reductions in pruritus and meaningful improvements in growth and quality of life. These data support the NAPPED findings on use of therapeutic bile acid depletion in nt‐BSEP, and the observation that reductions in sBAs are a prognostic marker of native liver survival. Therefore, on the basis of this study, maralixibat appears to be a realistic and effective treatment strategy, which benefited the lives of patients and caregivers by relieving disease symptoms, increasing transplant‐free survival, and providing a well‐tolerated, nonsurgical alternative to surgical biliary diversion.
AUTHOR CONTRIBUTIONS
All authors contributed to the data collection, analysis, interpretation, and the writing and review of the manuscript.
CONFLICT OF INTEREST
K.L. has received grants and consultancy fees from Mirum Pharmaceuticals and Albireo, and consultancy fees from Travere Therapeutics (formerly known as Retrophin). R.S. was a consultant for Mirum Pharmaceuticals. D.K has served as an advisor for Mirum Pharmaceuticals, Albireo, Astellas, and Intercept. N.S. has received grants from Mirum Pharmaceuticals and Albireo. C.M. has received consultancy fees from Albireo. K.S. has received consultancy fees from Mirum Pharmaceuticals and Retrophin, and is a stockholder in Asklepion Pharmaceuticals and Aliveris. A.D. and C.K. are shareholders in Mirum Pharmaceuticals. N.D is an employee and shareholder in Takeda Pharmaceuticals. P.V. and W.G. are stockholders in and employees of Mirum Pharmaceuticals. T.J. is a stockholder in and past employee of Mirum Pharmaceuticals, and is a stockholder of Vifor and Novartis Pharma. A.M. has received grants from the National Institutes of Health and has received consultancy fees from Mirum Pharmaceuticals and Metacrine. R.T. has received consultancy fees from Mirum Pharmaceuticals, Albireo, GenerationBio, Qing Bile Therapeutics, Alnylam, EVOX Therapeutics, Horizon Pharma, Rectify Therapeutics, and Sana Biotechnologies, and has stock options in Qing Bile Therapeutics, Rectify Therapeutics, and GenerationBio. All other authors declared no conflicts of interest.
Supporting information
Appendix S1 Supporting Information
ACKNOWLEDGMENT
Editorial assistance was provided by Edward Johnson, Alpharmaxim Healthcare Communications, and funded by Mirum Pharmaceuticals.
Loomes KM, Squires RH, Kelly D, Rajwal S, Soufi N, Lachaux A, Maralixibat for the treatment of PFIC: Long‐term, IBAT inhibition in an open‐label, Phase 2 study. Hepatol Commun. 2022;6:2379–2390. 10.1002/hep4.1980
Robert H. Squires and Deirdre Kelly contributed equally to this work.
ClinicalTrials.gov registration number: NCT02057718.
Funding informationSupported by Mirum Pharmaceuticals.
REFERENCES
- 1. Baker A, Kerkar N, Todorova L, Kamath BM, Houwen RHJ. Systematic review of progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. 2019;43:20–36. [DOI] [PubMed] [Google Scholar]
- 2. Gunaydin M, Bozkurter Cil AT. Progressive familial intrahepatic cholestasis: diagnosis, management, and treatment. Hepat Med. 2018;10:95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Malatack JJ, Doyle D. A drug regimen for progressive familial cholestasis type 2. Pediatrics. 2018;141:e20163877. [DOI] [PubMed] [Google Scholar]
- 4. Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol. 2014;4:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Wessel DBE, Thompson RJ, Gonzales E, Jankowska I, Sokal E, Grammatikopoulos T, et al. Genotype correlates with the natural history of severe bile salt export pump deficiency. J Hepatol. 2020;73:84–93. [DOI] [PubMed] [Google Scholar]
- 6. Davit‐Spraul A, Fabre M, Branchereau S, Baussan C, Gonzales E, Stieger B, et al. ATP8B1 and ABCB11 analysis in 62 children with normal gamma‐glutamyl transferase progressive familial intrahepatic cholestasis (PFIC): phenotypic differences between PFIC1 and PFIC2 and natural history. Hepatology. 2010;51:1645–55. [DOI] [PubMed] [Google Scholar]
- 7. Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44:478–86. [DOI] [PubMed] [Google Scholar]
- 8. Bergasa NV. Pruritus of cholestasis. In: Carstens E, Akiyama T, editors. Itch: Mechanisms and Treatment. Boca Raton, Forida: CRC Press/Taylor & Francis; 2014. [Google Scholar]
- 9. Vitale G, Pirillio M, Mantovani V, Marasco E, Aquilano A, Gamal N, et al. Bile salt export pump deficiency disease: two novel, late onset, ABCB11 mutations identified by next generation sequencing. Ann Hepatol. 2016;15:795–800. [DOI] [PubMed] [Google Scholar]
- 10. Bull LN, Thompson RJ. Progressive familial intrahepatic cholestasis. Clin Liver Dis. 2018;22:657–69. [DOI] [PubMed] [Google Scholar]
- 11. Davit‐Spraul A, Gonzales E, Baussan C, Jacquemin E. Progressive familial intrahepatic cholestasis. Orphanet J Rare Dis. 2009;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dawson PA. Role of the intestinal bile acid transporters in bile acid and drug disposition. Handb Exp Pharmacol. 2011;201:169–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shneider BL, Spino C, Kamath BM, Magee JC, Bass LM, Setchell KD, et al. Placebo‐controlled randomized trial of an intestinal bile salt transport inhibitor for pruritus in Alagille syndrome. Hepatol Commun. 2018;2:1184–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiao L, Pan G. An important intestinal transporter that regulates the enterohepatic circulation of bile acids and cholesterol homeostasis: the apical sodium‐dependent bile acid transporter (SLC10A2/ASBT). Clin Res Hepatol Gastroenterol. 2017;41:509–15. [DOI] [PubMed] [Google Scholar]
- 15. Gonzales E, Sturm E, Stormon M, Sokal E, Hardikar W, Lacaille F, et al. Phase 2 placebo‐controlled withdrawal study of the ASBT inhibitor maralixibat in children with Alagille syndrome. Presented at International Liver Congress, 2019, Vienna, Austria.
- 16. Gonzales EM, Sturm E, Stormon M, Sokal EM, Hardikar W, Lacaille F, et al. Durability of treatment effect with long‐term maralixibat in children with Alagille syndrome: 4‐year safety and efficacy results from the ICONIC study. Presented at American Association for the Study of Liver Diseases Annual Meeting, 2019, Boston, MA, USA.
- 17. Mirum Pharmaceuticals . Livmarli™ (maralixibat) oral solution. Prescribing Information, revised September, 2021.
- 18. Kamath BM, Abetz‐Webb L, Kennedy C, Hepburn B, Gauthier M, Johnson N, et al. Development of a novel tool to assess the impact of itching in pediatric cholestasis. Patient. 2018;11:69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foster B, Gauthier M, Vig P, Jaecklin T, Kamath BM, Andrae DA. Itch Reported Outcome tool for caregivers of pediatric patients with cholestatic liver disease: an analysis of validation and scoring from the ICONIC maralixibat. Presented at Virtual ISPOR, 2020.
- 20. Thompson RJ, Jaecklin TJ, Vig P. Genotype and dose‐dependent response to maralixibat in patients with bile salt export pump deficiency. Presented at 6th World Congress of Pediatric Gastroenterology, Hepatology and Nutrition, 2020, Copenhagen, Denmark.
- 21. Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 2003;3:329–41. [DOI] [PubMed] [Google Scholar]
- 22. Chen F, Ananthanarayanan M, Emre S, Neimark E, Bull LN, Knisely AS, et al. Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid X receptor activity. Gastroenterology. 2004;126:756–64. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting Information


