Abstract
Chronic hepatitis B virus (HBV) infection is a major risk factor for hepatocellular carcinoma (HCC), and surveillance is recommended for patients without cirrhosis when risk exceeds an incidence rate (IR) of 0.2%. Populations in Asia and sub‐Saharan Africa have been associated with HCC at younger ages, but the risk after immigration to Western countries should be investigated. The aim of this study was to study HCC by age and country of origin in people with chronic HBV infection in Sweden. Through national registers, residents with chronic HBV diagnosis (1990–2015) were identified with information on country of origin, immigration/emigration, death, coinfections, antiviral therapy, and HCC. Observation time started at HBV diagnosis, and IR and hazard ratios for HCC were calculated by sex, age, and region of origin. Among 16,410 individuals (47% women), the origin and observation time (person years) were as follows: Western Europe, 2316 (25,415); Eastern Europe, 2349 (26,237); Middle East/North Africa, 4402 (47,320); sub‐Saharan Africa, 3677 (30,565); Asia, 3537 (35,358); and other, 129 (1277). There were 232 individuals with HCC (82% in men). The IR increased with age and exceeded 0.2% for Asian men from age group 40–49 years (IR, 0.63; 95% confidence interval, 0.39–1.00), for men of other origins from age group 50–59 years, and for women aged ≥60 years originating from Eastern Europe, Asia, and Middle East/North Africa. After exclusion of patients with cirrhosis or HBV treatment, the IR still exceeded 0.2% in Asian men aged 40–49 years. This study demonstrates that HBV‐infected men of Asian origin should be recommended HCC surveillance at younger ages, but there is a need for further studies of HCC incidence in African‐born men without cirrhosis living in the Western world.
This nationwide study of people with chronic HBV‐infection in Sweden found that only men of Asian origin exceeded the proposed cut‐off for HCC‐surveillance among people as young as 40‐49 years, and that HCC before age 40 years was uncommon in non‐cirrhotic patients. The study confirms the high risk for HCC in patients with chronic HBV‐infection, and points to the need for further studies of HCC‐incidence in young non‐cirrhotic African men who have migrated to the Western world.

INTRODUCTION
Worldwide, an estimated 260 million people are living with a chronic hepatitis B virus (HBV) infection.[ 1 , 2 ] Chronic hepatitis B is a major risk factor for hepatocellular carcinoma (HCC), with the highest risk in people with cirrhosis but an increased risk also in noncirrhotic disease. The highest risks for HCC have been reported from HBV‐endemic regions, predominantly Asia and sub‐Saharan Africa, where HBV infection often has been transmitted at birth or early childhood.[ 3 , 4 ] Many studies originate from Eastern Asia, but there are few from Africa. However, in the 1960–1980s, studies from Mozambique and southern Africa reported median ages around 30 years at HCC diagnosis in young adults living in rural areas.[ 5 , 6 ] It was assumed that other risk factors, such as aflatoxins and dietary iron overload, contributed to this very high risk, and studies indicated that leaving these rural areas lessened the risk of HCC.
There are only a few studies of HCC incidence in people infected with HBV of different country origins, particularly African born who have immigrated to Europe or the United States. One study from the United States found that Asian ethnic origin (including US‐born individuals) was associated with a significantly increased risk of HBV‐related HCC, but median age at diagnosis was lowest among African immigrants.[ 7 ] A study of all‐cause HCC in the US general population demonstrated that country of birth rather than race/ethnicity had the largest impact on the age of HCC diagnosis and that birth in Africa was strongly associated with early onset HCC.[ 8 ] A study of predominantly male US veterans with chronic HBV infection found that race and age were significantly associated with the risk of HCC, with the highest annual HCC incidence in Asian Pacific Islanders and the lowest among African Americans; the risk was very low in patients younger than 40 years.[ 9 ]
Patients with increased risk of HCC are recommended to undergo surveillance with ultrasound every 6 months for early detection of incident HCC. This is recommended for all HBV‐infected patients with cirrhosis but also for patients without cirrhosis with an estimated risk corresponding to an annual incidence rate (IR) of 0.2% or higher.[ 10 , 11 ] For patients with chronic HBV infection without cirrhosis, guidelines recommend HCC surveillance for men and women originating from Asia from the age of 40 and 50 years, respectively. For patients originating from Africa, surveillance was previously suggested from age 20 years, but this has now been changed to “at earlier ages.”[ 11 , 12 ] However, the risk of HCC based on age and country of origin in people who have immigrated and now live in Sweden or other European countries needs to be further investigated.
In Sweden, a country with about 10 million inhabitants, the HBV prevalence is estimated at 0.2%; about 90% of people with chronic HBV infection are immigrants from HBV‐endemic areas where they probably were infected perinatally or during infancy.[ 1 ] There are approximately as many people from each of the following four regions: East/Southeast Asia, sub‐Saharan Africa, Greater Middle East, and Europe (including Sweden). These groups of people from different geographical regions, now Swedish residents with a personal identification number (PIN), together with the public health care and the national registers with prospectively collected data make Sweden a suitable country to study HBV‐related outcomes by country of origin.
This is a population‐based register study of a national cohort of people with chronic HBV infection. The aim was to study the incidence of HCC by age and region of origin in people living in Sweden. The hypothesis was that people born in East/Southeast Asia and sub‐Saharan Africa have a higher HCC incidence at young ages than people originating from other regions.
METHODS
Study population
In Sweden, HBV, hepatitis D virus (HDV), and hepatitis C virus (HCV) infections are notifiable diseases that are reported to the National Surveillance Register at the Public Health Agency of Sweden, both by the diagnosing laboratories and the physicians (i.e., dual notifications) at first diagnosis.[ 13 ] These notifications include the PIN issued to everyone living permanently in Sweden (used in all national registers), with information on sex and age, type of infection, and the plausible route of transmission.
For this study, all people notified with chronic HBV infection in Sweden during 1990–2015 were identified from the National Surveillance Register. Additionally, notifications of HDV and/or HCV infection were linked to the HBV register to identify HDV or HCV coinfections.
There were 41,796 HBV notifications during 1990–2015. These files were linked to the national Total Population Register at Statistics Sweden[ 14 ] to check the PINs and country of residence. Information about immigration/emigration, country of birth, and date of death was added.
Among the HBV notifications, there were 15,753 with provisional identification number/duplicates (notified again when assigned a permanent PIN) and another 3041 without permanent residence in Sweden that had to be excluded, leaving 23,002 residents notified with HBV infection. Of those, 4649 had acute HBV infection and were therefore not included in this study of chronic HBV infection, and another 1943 were coinfected with HCV and therefore excluded. The final study population consisted of 16,410 individuals (Table 1).
TABLE 1.
Description of the population with chronic HBV infection diagnosis 1990–2015 in Sweden, n = 16,410 (coinfection with HCV excluded)
| Western Europe | Eastern Europe | Middle East–North Africa | Sub‐Saharan Africa | East/Southeast Asia | Other | |
|---|---|---|---|---|---|---|
| HBV population (%) | 2316 (14.1) | 2349 (14.3) | 4402 (26.8) | 3677 (22.4) | 3537 (21.6) | 129 (0.8) |
| Women (%) | 903 (39) | 1139 (48) | 1843 (42) | 1510 (41) | 2273 (64) | 56 (43) |
| Birth year a (IQR) | 1958 (1947–1977) | 1969 (1956–1981) | 1970 (1961–1979) | 1976 (1967–1985) | 1975 (1966–1982) | 1968 (1958–1978) |
| Deceased (%) | 462 (20.0) | 177 (7.5) | 203 (4.6) | 79 (2.1) | 103 (2.9) | 10 (7.7) |
| Immigration year a (IQR) | 1979 c (1970–1998) | 1994 (1991–2003) | 1995 (1988–2007) | 2005 (1995–2010) | 2000 (1990–2007) | 1996 (1988–2006) |
| Age a at immigration (IQR) | 23.6 c (17.1–29.9) | 27.0 (19.2–34.9) | 25.9 (18.6–34.2) | 26.8 (18.3–33.7) | 25.5 (15.2–32.0) | 27.7 (21.2–34.3) |
| Years b in Sweden | NA c | 19.4 | 18.6 | 11.6 | 16.8 | 18.6 |
| HBV diagnosis year a (IQR) | 2002 (1997–2007) | 2003 (1997–2009) | 2004 (1998–2010) | 2007 (2001–2012) | 2006 (1999–2010) | 2004 (1999–2009) |
| Age a in years at HBV diagnosis (IQR) | 42 (26–56) | 34 (25–45) | 33 (25–42) | 30 (22–38) | 31 (24–37) | 36 (27–45) |
| HDV coinfection d (%) | 8 (0.3) | 19 (0.8) | 84 (1.9) | 46 (1.3) | 42 (1.2) | 1 (0.8) |
| HIV coinfection d (%) | 55 (2.4) | 4 (0.2) | 7 (0.2) | 97 (2.6) | 46 (1.3) | 7 (5.4) |
| HBV treatment (%) | 228 (9.8) | 195 (8.3) | 460 (10.5) | 181 (4.9) | 530 (15.0) | 9 (7.0) |
| Serious liver disease/cirrhosis (%) | 128 (5.5) | 73 (3.1) | 118 (2.7) | 35 (1.0) | 71 (2.0) | 5 (3.9) |
| Liver transplant (%) | 25 (1.1) | 15 (0.6) | 41 (0.9) | 6 (0.2) | 10 (0.3) | 1 (0.8) |
| HCC (%) | 49 (2.1) | 45 (1.9) | 58 (1.3) | 23 (0.6) | 54 (1.5) | 3 (2.3) |
| Age in years b at HCC diagnosis | 61.3 | 61.0 | 59.2 | 44.7 | 51.0 | 54.5 |
| Total in statistical analyses | ||||||
| n = 16,352 (%) | 2293 (14.0) | 2343 (14.3) | 4390 (26.8) | 3673 (22.5) | 3525 (21.6) | 128 (0.8) |
| Observation time, all, person years | 25,415 | 26,237 | 47,320 | 30,565 | 35,358 | 1177 |
| Observation time, men, person years | 15,406 | 13,704 | 25,544 | 17,008 | 12,614 | 526 |
| Observation time, years, mean/person | 11.1 | 11.2 | 10.8 | 8.3 | 10.0 | 9.2 |
| HCC in analyses (%) | 34 (1.5) | 40 (1.7) | 47 (1.0) | 17 (0.5) | 43 (1.2) | 2 (1.6) |
Abbreviations: HBV, hepatitis B virus; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HDV, hepatitis D virus; HIV, human immunodeficiency virus; IQR, interquartile range; NA, not available.
Median.
Mean.
28% immigrants (72% from Sweden).
Eight HCC in HDV coinfected, two HCC in HIV coinfected.
Country of birth was grouped into the following regions: Western Europe, Eastern Europe, Middle East/North Africa, sub‐Saharan Africa, East/Southeast Asia, and “other” (Figure 1). More information about the origin of the study population and the countries included in the regions is presented in Table 2 and Table S1.
FIGURE 1.
Regions of origin for the study population consisting of people with chronic hepatitis B that live in Sweden, n = 16,410. 1, Western Europe (green), n = 2316; 2, Eastern Europe (turquoise), n = 2349; 3, Middle East/North Africa (yellow), n = 4402; 4, sub‐Saharan Africa (red) n = 3677; 5, East/Southeast Asia (lilac), n = 3537; 6, other (blue), n = 129
TABLE 2.
Regions of origin a and number of HCC per region for the study population with chronic hepatitis B virus infection (n = 16,410)
| Region of origin | Population, n | HCC, n |
|---|---|---|
| Western Europe, all | 2316 | 49 |
| Sweden | 1759 | 33 |
| Other Nordic countries | 154 | 2 |
| Northern Europe | 106 | 5 |
| Western Europe | 76 | 0 |
| Southern Europe | 221 | 9 |
| Eastern Europe, all | 2349 | 45 |
| Eastern Europe | 972 | 13 |
| Southeastern Europe | 1377 | 32 |
| Middle East and North Africa b | 4402 | 58 |
| Northern Africa | 264 | 6 |
| Middle East/Western Asia | 2972 | 40 |
| Southern Asia | 1166 | 12 |
| Sub‐Saharan Africa | 3677 | 23 |
| Eastern Africa | 2527 | 11 |
| Middle Africa | 238 | 4 |
| Western Africa | 897 | 8 |
| Southern Africa | 15 | 0 |
| Eastern and southeastern Asia | 3537 | 54 |
| Southeast Asia | 2296 | 36 |
| Eastern Asia | 1150 | 18 |
| Central Asia | 91 | 0 |
| Other | 129 | 3 |
| North America | 47 | 0 |
| South America | 66 | 3 |
| Oceania | 13 | 0 |
| Unknown | 3 | 0 |
Abbreviation: HCC, hepatocellular carcinoma.
For information of countries included in each region, see Table S1.
Greater Middle East.
Linkage to other registers
The files with the study population were then linked to the registers at the National Board of Health and Welfare—the Cancer Register (CR), Patient Register (PR), Cause of Death Register (DR), and the Prescription Register (PrR). These registers include prospectively collected data with high coverage.[ 15 ] All diagnoses in the registers are coded according to the International Statistical Classification of Diseases (ICD), and the prescribed drugs in the PrR are coded according to the Anatomic Therapeutic Chemical classification system.
About the registers
The CR was founded in 1958, and it is compulsory to report newly detected cancers to the register. The CR is validated to be 96% complete[ 15 ]; however, it has been demonstrated that the CR is less complete for some cancers diagnosed by imaging technologies without biopsy/histology, like HCC.[ 16 ] The CR was used to identify primary liver cancers, predominantly HCC.
The PR started to record hospital admissions in 1964, and all inpatient care in Sweden has been included since 1987. Since 2001, the PR has also included outpatient visits in specialist care. The register includes dates and diagnoses at discharge/visit. The validity is reported to be high.[ 17 ] The PR was used to identify individuals with diagnosis of liver cancer, liver cirrhosis, decompensated liver cirrhosis, liver transplantation, or human immunodeficiency virus (HIV) infection (for ICD codes used in PR, see Table S2).
The DR includes all deaths among Swedish residents, with date and cause of death and the basis for diagnosis of the causes. The information relies on information from death certificates, missing in only 1%–2% of deaths.[ 18 ] The DR was used to identify deaths from liver cancer.
All prescribed drugs have been recorded for individuals in the PrR since July 2005. We used the PrR to identify patients who had received or still were on HBV treatment with the nucleos(t)ide analogues tenofovir, entecavir, telbivudine, lamivudine, or adefovir.
The outcome of interest is HCC; however, the ICD codes for primary liver cancer (predominantly HCC) were used because misclassification and underreporting may be a problem when restricting to ICD codes for HCC and it was important not to underestimate the incidence.[ 16 , 19 ] The ICD codes were ICD‐7 155.0 and 156 in the CR, and ICD‐9 (1987–1996) 155.0, 155.1, 155.2 and ICD‐10 (from 1997) C22.0, C22.1, and C22.9 in the PR and DR. This may include a minority of intrahepatic cholangiocarcinoma (ICC) and unspecified liver cancers, but in a cohort with chronic hepatitis >90%, these are presumed to be HCC. In a previous study of the national HCV cohort, most liver cancers were HCC and only 3% were ICC.[ 20 ]
Patients with an HCC diagnosis or liver transplantation before HBV notification were not included in the analyses.
Statistical analyses
The observation time started at date of reported HBV diagnosis (date of HBV notification) and ended at outcome (date of HCC diagnosis) or at liver transplantation, emigration (first), death (other than HCC), or December 31, 2015, whichever occurred first. The IR for HCC was calculated by dividing the number of HCC cases with the observation time in years for each age group (<20, 20–39, 30–39, 40–49, 50–59, and ≥60 years) and region of birth. This was done for each entire group by origin, irrespective of stage of liver fibrosis, in order not to underestimate the risk. Additionally, the IR was calculated after exclusion of people with diagnosis of serious liver disease/cirrhosis and/or HBV treatment with nucleos(t)ide analogues to exclude those who already were identified as patients at higher risk and to study those with milder liver disease separately. The results are presented as IR (HCC/100 person years) with 95% confidence intervals (CIs) together with number of HCCs and observation time in person years by age group and region of birth for men and women separately.
Additionally, the risk of HCC in the “region of birth” groups was compared by using Cox regression with age as the underlying timescale and Western Europe as reference and with adjustment for sex and HDV coinfection. A separate analysis compared people younger than age 50 years. Further, we used Cox regression to compare men and women and to compare those with and without HDV coinfection, irrespective of origin. The results are presented as hazard ratios (HRs) with 95% CIs and p values. A minor violation of the proportional hazards assumption was detected for region of birth, using Schoenfeld residuals. However, the degree of violation was borderline, so the results assume proportional hazards.
Observation time for the main analyses started at HBV notification as described above. Supplemental analyses starting at observation time 6 months (lag time) after HBV notification to reduce eventual selection/surveillance bias were performed and are presented in Table S3. The analyses were performed using STATA 14.
RESULTS
Characteristics
In total, there were 16,410 persons with chronic HBV infection and 200 (1.2%) were coinfected with HDV (Table 1). In this cohort, 90% were immigrants; the regions of origin are presented in Figure 1 and Table 2. In total, 47% were women, but in the Asian group there were more women (64%) than men. The group from sub‐Saharan Africa was youngest (median birth year, 1976) and had the shortest mean time in Sweden (11.6 years), with median immigration year 2005. Besides the Western European group (72% from Sweden), the groups from Eastern Europe and Middle East/North Africa had the longest mean time in Sweden, 19.4 and 18.6 years, respectively. However, starting at date of reported HBV diagnosis, the mean observation time by group (in person years) was similar, varying from 8.3 (sub‐Saharan Africa) to 11.2 (Eastern Europe) (Table 1).
In the whole cohort, there were 1603 (9.8%) that were prescribed treatment with nucleos(t)ide analogues, with the highest proportion in the group from Asia (15%). In total, 430 (2.6%) had cirrhosis or cirrhosis‐related diagnoses and 98 (0.6%) had liver transplantation (Table 1).
There were 58 with an outcome or censoring event before HBV notification, leaving 16,352 individuals and 183 HCC cases for the statistical analyses. The number of persons and the observation time (in parenthesis) in person years by origin were Western Europe, 2293 (25,415); Eastern Europe, 2343 (26,237); Middle East/North Africa, 4390 (47,320); sub‐Saharan Africa, 3673 (30,565); East/Southeast Asia, 3525 (35,358); and other, 128 (1177).
HCC incidence rates
There were in total 232 individuals with incident HCC (82% men); 23, 54, and 58 were from sub‐Saharan Africa, East/Southeast Asia, and Middle East/North Africa, respectively, and the corresponding mean ages at HCC diagnoses were 45, 51, and 59 years (Table 1). Most HCCs (175, 75.5%) were identified from the CR, another 40 (17.2%) were found in the DR (missing in the CR), and additionally 17 (7.3%) were from the PR (missing in the CR and DR).
Among the 16,352 individuals included in the statistical analyses, 183 had an HCC diagnosis. The number of HCC diagnoses, observation time, and IR by sex, origin, and age group are presented in Figure 2 and Tables 3 and 4. The IR was higher among men than women and increased with age. The IR exceeded 0.2/100 person years (0.2%) for men from Asia aged 40–49 years (IR, 0.63; 95% CI, 0.39–1.00) and for men in all other groups of origin from age group ≥50–59 years. Among sub‐Saharan African men younger than 40 years, there were seven HCC diagnoses, with IRs of 0.05 (95% CI, 0.01–0.21) in age group 20–29 and 0.11 (95% CI, 0.04–0.26) in age group 30–39 years, per 100 person years. In other groups, there were only occasional HCCs before age 40 years. In women, HCC was rare but exceeded 0.2% among those aged ≥60 years with origins from East Europe, Asia, and the Middle East/North Africa. These analyses included 1044 men and 672 women with diagnosis of serious liver disease/cirrhosis and/or HBV medication, corresponding to 9642 (11.4%) and 6539 (8%) of observation time in person years, with 72 (47%) and seven (23%) HCC diagnoses for men and women, respectively.
FIGURE 2.
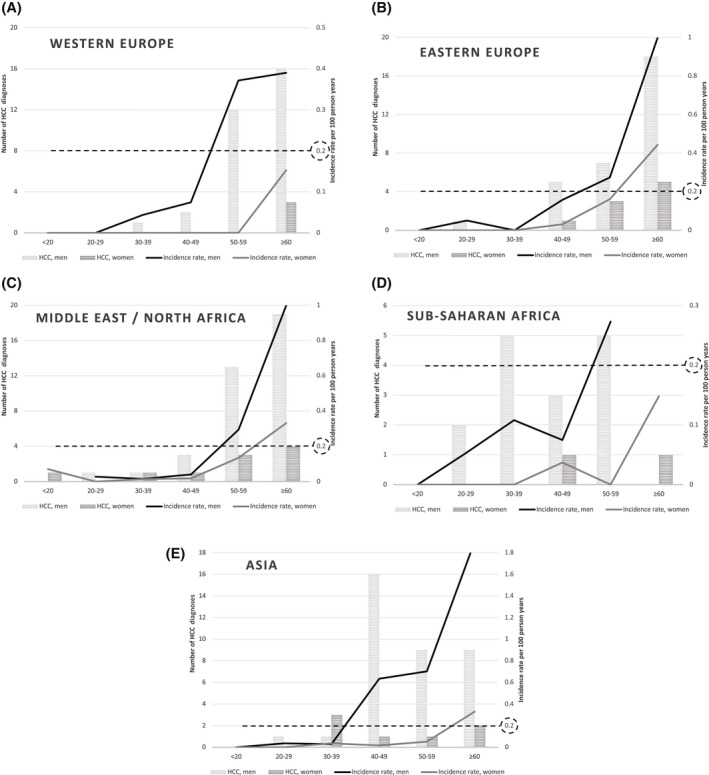
HCC incidence for the entire cohort by age group (years), sex, and origin. The proposed cutoff for surveillance (incidence rate, 0.2%) is marked with a dashed line. (A) Western Europe, (B) Eastern Europe, (C) Middle East/North Africa, (D) sub‐Saharan Africa, (E) Asia. HCC, hepatocellular carcinoma.
TABLE 3.
Incidence rate of HCC in men by region of origin and age group, calculated for all men in the cohort (n = 8643) and for men with milder disease by excluding 1044 men with cirrhosis and/or HBV treatment
| Men without cirrhosis or HBV treatment (n = 7599) a | All men in cohort (n = 8643) b | ||||||
|---|---|---|---|---|---|---|---|
| Region of origin, age group (years) | Observation time (person years) | HCC (n) | IR/100 person years | 95% CI | HCC (n) | IR/100 person years | 95% CI |
| Sub‐Saharan Africa | |||||||
| <20 | 2167 | 0 | – | – | 0 | – | – |
| 20–29 | 3494 | 1 | 0.03 | 0.004–0.20 | 2 | 0.05 | 0.01–0.21 |
| 30–39 | 4317 | 1 | 0.02 | 0.003–0.16 | 5 | 0.11 | 0.04–0.26 |
| 40–49 | 3758 | 1 | 0.03 | 0.004–0.19 | 3 | 0.07 | 0.02–0.23 |
| 50–59 | 1639 | 1 | 0.06 | 0.009–0.43 | 5 | 0.27 c | 0.12–0.67 |
| ≥60 | 464 | 0 | – | – | 0 | – | – |
| East/Southeast Asia | |||||||
| <20 | 2052 | 0 | – | – | 0 | – | – |
| 20–29 | 2421 | 1 | 0.04 | 0.006–0.29 | 1 | 0.04 | 0.005–0.26 |
| 30–39 | 2776 | 1 | 0.04 | 0.005–0.26 | 1 | 0.03 | 0.004–0.21 |
| 40–49 | 1997 | 12 | 0.60 c | 0.34–1.10 | 16 | 0.63 c | 0.39–1.00 |
| 50–59 | 953 | 5 | 0.52 c | 0.22–1.30 | 9 | 0.70 c | 0.37–1.30 |
| ≥60 | 397 | 5 | 1.30 c | 0.52–3.00 | 9 | 1.90 c | 0.97–3.60 |
| Western Europe | |||||||
| <20 | 1424 | 0 | – | – | 0 | – | – |
| 20–29 | 1573 | 0 | – | – | 0 | – | – |
| 30–39 | 2070 | 1 | 0.05 | 0.007–0.34 | 1 | 0.04 | 0.006–0.31 |
| 40–49 | 2291 | 2 | 0.09 | 0.02–0.35 | 2 | 0.07 | 0.02–0.30 |
| 50–59 | 2772 | 4 | 0.14 | 0.05–0.38 | 12 | 0.37 c | 0.21–0.65 |
| ≥60 | 3507 | 7 | 0.20 | 0.10–0.41 | 16 | 0.39 c | 0.24–0.64 |
| Eastern Europe | |||||||
| <20 | 1520 | 0 | – | – | 0 | – | – |
| 20–29 | 1871 | 1 | 0.05 | 0.008–0.38 | 1 | 0.05 | 0.007–0.36 |
| 30–39 | 2334 | 0 | – | – | 0 | – | – |
| 40–49 | 2818 | 2 | 0.07 | 0.02–0.28 | 5 | 0.16 | 0.07–0.38 |
| 50–59 | 2204 | 2 | 0.09 | 0.02–0.36 | 7 | 0.27 c | 0.13–0.57 |
| ≥60 | 1553 | 13 | 0.84 c | 0.49–1.40 | 18 | 1.00 c | 0.63–1.60 |
| Middle East/North Africa | |||||||
| <20 | 1555 | 0 | – | – | 0 | – | – |
| 20–29 | 3214 | 1 | 0.03 | 0.004–0.22 | 1 | 0.03 | 0.004–0.20 |
| 30–39 | 5827 | 1 | 0.02 | 0.002–0.12 | 1 | 0.02 | 0.002–0.11 |
| 40–49 | 6478 | 1 | 0.02 | 0.002–0.11 | 3 | 0.04 | 0.01–0.12 |
| 50–59 | 3694 | 6 | 0.16 | 0.07–0.36 | 13 | 0.29 c | 0.17–0.51 |
| ≥60 | 1541 | 10 | 0.65 c | 0.35–1.20 | 19 | 1.00 c | 0.67–1.60 |
| Other | |||||||
| All ages | 479 | 1 | NA | NA | 2 | NA | NA |
Abbreviations: CI, confidence interval; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; IR, incidence rate; NA, not available.
Total observation time, 75,160 person years; HCC diagnoses, 80.
Total observation time, 84,802; HCC diagnoses, 152.
Surveillance recommended for patients without cirrhosis when risk exceeds IR of 0.2%.
TABLE 4.
Incidence rate of HCC in women by region of origin and age group, calculated for all women in cohort (n = 7709) and for those with milder disease by excluding 672 women with cirrhosis and/or HBV treatment
| Women without cirrhosis or HBV treatment (n = 7037) a | Women, all in cohort (n = 7709) b | ||||||
|---|---|---|---|---|---|---|---|
| Region of origin, age group (years) | Observation time (person years) | HCC (n) | IR/100 person years | 95% CI | HCC (n) | IR/100 person years | 95% CI |
| Sub‐Saharan Africa | |||||||
| <20 | 1229 | 0 | – | – | 0 | – | – |
| 20–29 | 3219 | 0 | – | – | 0 | – | – |
| 30–39 | 4665 | 0 | – | – | 0 | – | – |
| 40–49 | 2540 | 1 | 0.04 | 0.006–0.28 | 1 | 0.04 | 0.005–0.26 |
| 50–59 | 733 | 0 | – | – | 0 | – | – |
| ≥60 | 663 | 1 | 0.15 | 0.02–1.10 | 1 | 0.15 | 0.02–1.10 |
| East/Southeast Asia | |||||||
| <20 | 1911 | 0 | – | – | 0 | – | – |
| 20–29 | 3518 | 0 | – | – | 0 | – | – |
| 30–39 | 7231 | 2 | 0.03 | 0.007–0.11 | 3 | 0.04 | 0.01–0.11 |
| 40–49 | 4882 | 1 | 0.02 | 0.003–0.15 | 1 | 0.02 | 0.003–0.13 |
| 50–59 | 1624 | 1 | 0.06 | 0.009–0.43 | 1 | 0.05 | 0.007–0.37 |
| ≥60 | 553 | 2 | 0.36 c | 0.09–1.40 | 2 | 0.33 c | 0.08–1.30 |
| Western Europe | |||||||
| <20 | 1091 | 0 | – | – | 0 | – | – |
| 20–29 | 1745 | 0 | – | – | 0 | – | – |
| 30–39 | 1810 | 0 | – | – | 0 | – | – |
| 40–49 | 1441 | 0 | – | – | 0 | – | – |
| 50–59 | 1423 | 0 | – | – | 0 | – | – |
| ≥60 | 1771 | 2 | 0.11 | 0.03–0.45 | 3 | 0.15 | 0.05–0.47 |
| Eastern Europe | |||||||
| <20 | 722 | 0 | – | – | 0 | – | – |
| 20–29 | 2018 | 0 | – | – | 0 | – | – |
| 30–39 | 3357 | 0 | – | – | 0 | – | – |
| 40–49 | 2982 | 1 | 0.03 | 0.005–0.24 | 1 | 0.03 | 0.005–0.23 |
| 50–59 | 1720 | 2 | 0.12 | 0.03–0.46 | 3 | 0.16 | 0.05–0.50 |
| ≥60 | 1025 | 4 | 0.39 c | 0.15–1.00 | 5 | 0.44 c | 0.18–1.10 |
| Middle East/North Africa | |||||||
| <20 | 1333 | 1 | 0.07 | 0.01–0.53 | 1 | 0.07 | 0.01–0.50 |
| 20–29 | 4257 | 0 | – | – | 0 | – | – |
| 30–39 | 6578 | 1 | 0.01 | 0.002–0.11 | 1 | 0.01 | 0.002–0.10 |
| 40–49 | 4966 | 0 | 0.02 | – | 1 | 0.02 | 0.003–0.13 |
| 50–59 | 1979 | 2 | 0.14 | 0.03–0.40 | 3 | 0.14 | 0.04–0.42 |
| ≥60 | 1109 | 3 | 0.33 c | 0.08–0.84 | 4 | 0.33 c | 0.12–0.88 |
| Other | |||||||
| All ages | 637 | 0 | NA | NA | 0 | NA | NA |
Abbreviations: CI, confidence interval; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; IR, incidence rate; NA, not available.
Total observation time, 74,732 person years; HCC diagnoses, 24.
Total observation time, 81,271; HCC diagnoses, 31.
Surveillance recommended for patients without cirrhosis when risk exceeds IR of 0.2%.
In the HBV‐infected population without a diagnosis of serious liver disease/cirrhosis and/or HBV medication (n = 14,636) who are supposed to be at lower risk of HCC, there were 104 HCC diagnoses. The IR was lower but still exceeded 0.2% for men of Asian origin from age group 40–49 (IR, 0.60; 95% CI, 0.34–1.10) and from age group ≥60 years for men from Eastern Europe (IR, 0.84; 95% CI, 0.49–1.4) and Middle East/North Africa (IR, 0.65; 95% CI, 0.35–1.2) (Tables 3 and 4). Among men from sub‐Saharan Africa, there were very few HCC diagnoses in all age groups.
The IR analysis with a 6‐month lag time from HBV notification demonstrated a slightly lower IR with wider 95% CI but confirmed the high IR (>0.2%) at younger ages in men of Asian origin (Table S3).
Excluding the 215 patients with HIV coinfection (two with HCC: a Western European man >60 years and an Asian woman 30–39 years) did not significantly change the results (data not shown).
Comparison of risk for HCC using Cox regression
With Western Europe as reference, Cox analyses demonstrated that the HCC risk was higher in all groups of other origins but without statistical significance for sub‐Saharan Africans (Table 5). The group from Asia had the highest risk, with HR of 3.85 (95% CI, 2.39–6.20; p < 0.001) after adjustment for sex and HDV coinfection. This did not change significantly with the introduction of a 6‐month lag time (data not shown). Likewise, among people younger than 50 years, the risk was highest among those from Asia (HR, 5.50; 95% CI, 1.63–18.52; p = 0.006) (Table 6).
TABLE 5.
Hazard ratio with 95% CI for risk of HCC (age as underlying timescale) comparing the groups of different origin with Western Europe as reference, comparing men and women, and comparing those with or without HDV coinfection
| Unadjusted HR (95% CI) | p value | Model 1 HR (95% CI) | p value | Model 2 HR (95% CI) | p value | |
|---|---|---|---|---|---|---|
| Region of origin | ||||||
| Western Europe | 1 (ref) | 1 (ref) | 1 (ref) | |||
| Eastern Europe | 1.77 (1.11–2.80) | 0.016 | 1.94 (1.22–3.08) | 0.005 | 1.91 (1.21–3.04) | 0.006 |
| Middle East/North Africa | 1.54 (0.98–2.42) | 0.063 | 1.63 (1.03–2.56) | 0.036 | 1.51 (0.95–2.39) | 0.078 |
| Sub‐Saharan Africa | 1.24 (0.68–2.24) | 0.485 | 1.37 (0.75–2.50) | 0.303 | 1.27 (0.69–2.32) | 0.442 |
| East/Southeast Asia | 2.80 (1.75–4.49) | <0.001 | 3.90 (2.42–6.28) | <0.001 | 3.85 (2.39–6.20) | <0.001 |
| Sex | ||||||
| Men | 3.63 (2.46–5.35) | <0.001 | 4.21 (2.84–6.24) | <0.001 | 4.23 (2.85–6.27) | <0.001 |
| Women | 1 (ref) | 1 (ref) | 1 (ref) | |||
| Coinfection with HDV | ||||||
| Yes | 5.84 (2.87–11.91) | <0.001 | 6.32 (3.07–13.02) | <0.001 | ||
| No | 1 (ref) | 1 (ref) |
Note: Model 1 is adjusted for sex. Model 2 is adjusted for sex and coinfection with HDV.
Abbreviations: CI, confidence interval; HCC, hepatocellular carcinoma; HDV, hepatitis D virus; HR, hazard ratio; ref, reference.
TABLE 6.
Hazard ratio with 95% CI for risk of HCC before age 50 years (age as underlying timescale) comparing groups of different origin with Western Europe as reference, comparing men and women, and comparing those with or without HDV coinfection
| Unadjusted HR (95% CI) | p value | Model 1 HR (95% CI) | p value | Model 2 HR (95% CI) | p value | |
|---|---|---|---|---|---|---|
| Region of origin | ||||||
| Western Europe | 1 (ref) | 1 (ref) | 1 (ref) | |||
| Eastern Europe | 1.63 (0.42–6.30) | 0.480 | 1.89 (0.49–7.33) | 0.355 | 1.89 (0.49–7.32) | 0.356 |
| Middle East/North Africa | 0.90 (0.24–3.40) | 0.877 | 0.95 (0.25–3.60) | 0.944 | 0.94 (0.25–3.54) | 0.924 |
| Sub‐Saharan Africa | 2.13 (0.59–7.65) | 0.246 | 2.20 (0.61–7.90) | 0.227 | 2.17 (0.60–7.80) | 0.235 |
| East/Southeast Asia | 3.58 (1.07–11.97) | 0.039 | 5.55 (1.65–18.70) | 0.006 | 5.50 (1.63–18.52) | 0.006 |
| Sex | ||||||
| Men | 4.79 (2.32–9.86) | <0.001 | 6.36 (3.05–13.26) | <0.001 | 6.35 (3.05–13.23) | <0.001 |
| Women | 1 (ref) | 1 (ref) | 1 (ref) | |||
| Coinfection with HDV | ||||||
| Yes | 2.13 (0.29–15.43) | 0.454 | 2.38 (0.33–17.26) | 0.393 | ||
| No | 1 (ref) | 1 (ref) |
Note: Model 1 is adjusted for sex. Model 2 is adjusted for sex and coinfection with HDV.
Abbreviations: CI, confidence interval; HCC, hepatocellular carcinoma; HDV, hepatitis D virus; HR, hazard ratio; ref, reference.
For the study population irrespective of region of origin, Cox analyses demonstrated a higher risk in men than in women (adjusted HR, 4.23; 95% CI, 2.85–6.27; p < 0.001); also, HDV coinfection was associated with an increased risk (Tables 5 and 6).
DISCUSSION
This study demonstrates that most people with chronic HBV infection in Sweden originate from countries in Eastern Europe, Africa, the Middle East, or East/Southeast Asia and that the risk of HCC is highest among immigrants from East/Southeast Asia. The HCC incidence increases with age, and only men of Asian origin exceeded the proposed 0.2% cutoff for HCC surveillance in age group 40–49 years. Among men from sub‐Saharan Africa, there were incident HCCs in younger age groups, but the IR did not exceed 0.2% until the age of 50–59 years; after exclusion of patients with a diagnosis of liver disease/cirrhosis or HBV treatment, there were few HCCs and the IR was low. Among women, there were only a few with HCC; the IR exceeded 0.2% in women originating from Asia, Eastern Europe, and Middle East/North Africa in age groups ≥60 years.
The highest risk for HCC was found in men with HBV infection originating from East/Southeast Asia, and the risk was also high when restricting analysis to those supposed to have milder disease. This is consistent with earlier findings and with the American Association for the Study of Liver Diseases recommendation of HCC surveillance from age 40 years for Asian men without cirrhosis.[ 11 ] However, the results indicate that for people of other origins, it may not be necessary to recommend HCC surveillance for patients without cirrhosis before age 50 or even 60 years, based on sex, age, and origin only. Yet, for age group 50–59 years, the study demonstrates an IR exceeding 0.2% for men irrespective of origin when including all stages of disease. Additionally, the analyses indicate that the Western European group has a lower HCC risk than the other groups.
Transmission of HBV at birth or in early childhood is uncommon in Sweden, and most people with chronic HBV infection have been infected in another country and migrated to Sweden. The majority of individuals with chronic HBV infection originate from HBV‐endemic regions, and the annual number of detected infections in Sweden varies with immigrant flows. This study found that there were approximately as many people from the Greater Middle East, East/Southeast Asia, and sub‐Saharan Africa and that the median immigration year was 1995, 2000, and 2005, respectively, often related to the conflict hotspots of the world. In the sub‐Saharan group, 69% originated from East Africa and 24% from West Africa (Table 2; Table S1), including countries with reported high HCC incidence at young ages.[ 21 ] In our study population, the African‐ and Asian‐born individuals were the youngest, with less observation time in ages ≥60 years than the other regions of origin. This limits the robustness of the analyses for those aged ≥60 years and could explain why there was only one HCC diagnosis among sub‐Saharan Africans in this age group; however, despite an almost equally short observation time, there were 11 HCC diagnoses among Asian‐born individuals in the same age group.
Some of the highest HCC incidences in the world have been reported from countries in sub‐Saharan Africa,[ 21 ] and median age for HBV‐related HCC was recently reported to be younger than 45 years in most sub‐Saharan African countries.[ 22 ] Some African studies have reported a median age for HCC around 30 years.[ 5 , 6 ] This was associated with chronic HBV infection, exposure to aflatoxins, and possibly dietary iron overload,[ 23 ] and a study from southern Africa demonstrated that the risk of early onset HCC was higher in rural than in urban areas and that leaving rural areas may have lessened the risk.[ 5 ] In our study, the sub‐Saharan African group had the shortest time in Sweden (median, 11.6 years); this could possibly be of importance if the risk decreases with time after leaving a high‐risk area. The mean age at HCC diagnosis was 44.7 years in sub‐Saharan Africa‐born individuals; this was lower than for the other groups but may be explained by a younger population with more observation time in the younger age groups and less observation time (and few HCC cases) in older age groups. HCC was rare before age 40 years (irrespective of origin), but more common among sub‐Saharan African‐born men with a diagnosis of liver disease/cirrhosis and/or HBV treatment (i.e., among those identified to be at raised risk). However, when comparing the risk before age 50 years using Cox regression, only the group from Asia had a statistically significant higher risk than the others (Table 6).
There are few other studies of the HCC risk in people infected with HBV from different countries of origin who have immigrated to Europe or the United States. A case–control study of 278 patients with chronic hepatitis B and HCC in the United States found that Asian ethnic origin (mainly born in the United States) was associated with a significantly increased risk of HCC; however, African immigrants did not have excess risk but were diagnosed with HCC at younger ages than other groups.[ 7 ] Another study investigated the impact of country of birth on the age of HCC diagnosis in the US general population. Among 59,907 patients with HCC diagnosis (regardless of underlying cause), 75% had country of birth information, of whom 29% were foreign born. Country of birth rather than race/ethnicity was associated with age at HCC diagnosis, and birth in Africa (especially West Africa) and Oceania was notably associated with early onset; however, information on HBV infection, duration spent in the United States, and IR were missing in this study.[ 8 ] A study of predominantly male US veterans with HBV infection demonstrated that race and age were significantly associated with the risk of HCC, with the highest annual HCC incidence (0.65%) in Asian Pacific Islanders followed by whites (0.57%) and the lowest among African Americans (0.40%). In adjusted analysis, there was no difference between whites and African Americans, and the risk was very low in patients younger than 40 years in all racial/ethnic groups,[ 9 ] which is in accordance with our findings.
Globally, the highest HCC incidence today is reported from East/Southeast Asia,[ 21 ] driven primarily by HBV infection but also HCV and aflatoxins. In this study of people with chronic HBV infection in Sweden, the Asian‐born population stands out as having the highest HCC incidence. In other parts of the world, HCC is related to HCV, alcohol, and nonalcoholic fatty liver disease (NAFLD). In Sweden, a large part of HCC diagnoses have been related to HCV, and the median age for HCC diagnosis in the mainly Swedish‐born HCV population has been 60 years.[ 20 ] In a study from a region of Sweden, an increase in HCC incidence related to immigration was found in people younger than 50 years.[ 24 ]
The main analyses in our study included all patients with chronic HBV infection, irrespective of stage of fibrosis, in order to not underestimate the risk and the need for HCC surveillance. However, 2.6% had a liver cirrhosis diagnosis, and 9.8% in total had received HBV treatment (15% in the Asian group), which is recommended for those with progressive liver disease. This indicates that at least 10% of the study population could have more serious chronic hepatitis B with a higher risk of HCC. The proposed 0.2% cutoff for HCC surveillance is recommended for people without cirrhosis.[ 10 , 11 ] Therefore, analyses excluding people with cirrhosis and/or HBV treatment were performed. As expected, these IRs were lower but still exceeded 0.2% for Asian men in the relatively young 40–49‐year age group. A meta‐analysis including studies from East Asia, North America, or Europe demonstrated that HCC incidence in untreated subjects with chronic HBV infection was strongly related to age and liver disease status but found no statistically significant difference by geographic area after adjusting for age.[ 25 ] In our study, East/Southeast Asian origin was associated with a higher IR from age 40 and a higher HR when taking age into account.
A contributing factor to the difference in HCC risk between regions could be related to the global distribution of HBV genotypes, with a dominance of genotypes C and B in East Asia/Southeast Asia; genotype D in the Greater Middle East; genotype A in East Africa; and genotype E in West Africa. Genotype C has been associated with a higher risk of HCC than other genotypes.[ 26 , 27 ] Other viral factors, host genetic differences, lifestyle factors, and access to diagnostics and treatment could add to differences in risk by origin.[ 28 ] Risk factors, like aflatoxins, have been mentioned in both sub‐Saharan Africa and Asia, and today, obesity and NAFLD are of increasing importance for liver disease.
Several risk scores to estimate the risk for HCC in patients with HBV infection have been proposed for Asian patients and some for treated Caucasians (but not for Africans). These scores could be indicative for patients with chronic hepatitis B and fibrosis/cirrhosis.[ 29 ] However, one challenge is to estimate the risk for HCC and the need for surveillance in patients infected with HBV without progressive fibrosis and treatment, where these risk scores give little guidance. In patients without cirrhosis, an increased risk has been associated with sex, age, family history of HCC and active viral replication with persistent hepatitis B e antigen and high levels of HBV DNA.[ 10 , 11 ] Also, African and Asian origin have been considered risk factors. Our study indicates that Asian‐born individuals have a higher risk than the other groups. More studies of HBV infection and its complications in the African and Middle East populations are needed.
The major strengths of this study are the well‐characterized nationwide study population and the registers with comprehensive data, including date of immigration, origin, dates of HBV diagnosis, and incident HCC. The nationwide approach enables a large number of people and observation time in each group of origin. An almost equal size of the groups from the HBV‐endemic regions of the world and the possibility to use PINs to link with prospectively collected data from several national health care registers of high quality made it possible to compare people of different origins living in Sweden in one study.
Some possible limitations are the lack of some information that is not recorded or underidentified in the national registers used here, such as results from blood tests, detailed virology (like HBV genotypes and viral load), information on stage of liver fibrosis other than a cirrhosis diagnosis, weight, and alcohol consumption. Also, family history of HCC is not included because this is a study of migrant populations and details of disease in relatives are disproportionally unavailable in people with family members in other countries. Furthermore, for the reason of patient secrecy, we could not use information on specific country of origin, such that broader regional categories were used; however, studying by region ensures there are more people in each group. The recommendations of HCC surveillance have so far differed by continent only. Despite the nationwide approach and total observation time of more than 166,000 person years, there are few incident HCC diagnoses in each subgroup, especially at younger ages with lower IR, and the number of persons at risk and the observation time are small for some age groups, which limits the robustness of some analyses and limits the possibility of further stratified analysis. Therefore, more studies are needed to confirm our findings, especially for the migrant population from sub‐Saharan Africa.
In conclusion, this nationwide study of people with chronic HBV infection in Sweden found that only men of Asian origin exceeded the proposed cutoff for HCC surveillance among people as young as 40–49 years and that HCC before age 40 years was uncommon in patients without cirrhosis. The study confirms the high risk for HCC in patients with chronic HBV infection and points to the need for further studies of HCC incidence in young African men without cirrhosis who have migrated to the Western world. In general, more studies of HBV infection and the risk of HCC in people from Africa or the Middle East are warranted.
CONFLICT OF INTEREST
Soo Aleman is on the speaker's bureau of and received grants from Gilead and AbbVie; she is on the speaker's bureau of MSD/Merck. Ann‐Sofi Duberg is on the speaker's bureau of Gilead, MSD, and AbbVie. The other authors have nothing to report.
ETHICAL APPROVAL
The linkage of the register files was undertaken by the Public Health Agency, Statistics Sweden and National Board of Health and Welfare. All data were anonymized before they were seen by the research team. This study was approved by the Regional Ethical Review Board in Stockholm, Sweden.
Supporting information
Supplemental Table S1 Country key for the Regions of origin (see unstats.un.org)
Supplemental Table S2 The diagnosis codes used to identify patients with serious liver disease/cirrhosis, liver cancer, liver transplantation or HIV‐infection recorded in the Patient register. The ICD‐9 codes were used in the years 1987–1996 and ICD‐10 from 1997.
Supplemental Table S3 Incidence rate (IR) of HCC in men by region of origin and age group, observation time starting 6 months after HBV notification (6 months lag‐time). The IR is presented by 100 person years (with 95% CI) together with the observation time in person years and the number of HCC.
Duberg A‐S, Lybeck C, Fält A, Montgomery S, Aleman S. Chronic hepatitis B virus infection and the risk of hepatocellular carcinoma by age and country of origin in people living in Sweden: A national register study. Hepatol Commun. 2022;6:2418–2430. 10.1002/hep4.1974
Funding information Region Örebro County, Medical Training and Research Agreement (ALF) Grant: OLL‐880331. Region Stockholm County ALF grant. Region Örebro County, Nyckelfonden, Grant: OLL‐507391. Region Stockholm County, Research Grant: 21080655. The Swedish Cancer Society, Grant: CAN 2017/434.
REFERENCES
- 1. Polaris Observatory Collaborators . Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3:383–403. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global hepatitis report, 2017. https://www.who.int/hepatitis/publications/global‐hepatitis‐report2017/en/ (2017). Accessed Feb 21, 2022.
- 3. Kew MC. Hepatocellular carcinoma: epidemiology and risk factors. J Hepatocell Carcinoma. 2014;1:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McGlynn KA, Petrick JL, El‐Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kew MC, Rossouw E, Hodkinson J, Paterson A, Dusheiko GM, Whitcutt JM. Hepatitis B virus status of southern African Blacks with hepatocellular carcinoma: comparison between rural and urban patients. Hepatology. 1983;3:65–8. [DOI] [PubMed] [Google Scholar]
- 6. Prates MD, Torres FO. A cancer survey in Lourenço Marques, Portuguese East Africa. J Natl Cancer Inst. 1965;35:729–57. [PubMed] [Google Scholar]
- 7. Kennedy K, Graham SM, Arora N, Shuhart MC, Kim HN. Hepatocellular carcinoma among US and non‐US‐born patients with chronic hepatitis B: risk factors and age at diagnosis. PLoS One. 2018;13:e0204031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yang JD, Altekruse SF, Nguyen MH, Gores GJ, Roberts LR. Impact of country of birth on age at the time of diagnosis of hepatocellular carcinoma in the United States. Cancer. 2017;123:81–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mittal S, Kramer JR, Omino R, Chayanupatkul M, Richardson PA, El‐Serag HB, et al. Role of age and race in the risk of hepatocellular carcinoma in veterans with hepatitis B virus infection. Clin Gastroenterol Hepatol. 2018;16:252–9. [DOI] [PubMed] [Google Scholar]
- 10. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. Erratum in: J Hepatol. 2019;70:817. [DOI] [PubMed] [Google Scholar]
- 11. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–50. [DOI] [PubMed] [Google Scholar]
- 12. Bruix J, Sherman M, Practice Guidelines Committee, American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–36. [DOI] [PubMed] [Google Scholar]
- 13. Folkhälsomyndigheten [Public Health Agency of Sweden]. Statistik A‐Ö om smittsamma sjukdomar. https://www.folkhalsomyndigheten.se/folkhalsorapportering‐statistik/statistik‐a‐o/sjukdomsstatistik/ (2021). Accessed Feb 21, 2022.
- 14. Statistics Sweden . Statistics Sweden provides society with useful and trusted statistics. https://www.scb.se/en/ (2021). Accessed Feb 21, 2022.
- 15. The National Board of Health and Welfare . Statistics and data. https://www.socialstyrelsen.se/en/statistics‐and‐data/ (2021). Accessed Feb 21, 2022.
- 16. Törner A, Stokkeland K, Svensson A, Dickman PW, Hultcrantz R, Montgomery S, et al. The underreporting of hepatocellular carcinoma to the cancer register and a log‐linear model to estimate a more correct incidence. Hepatology. 2017;65:885–92. [DOI] [PubMed] [Google Scholar]
- 17. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brooke HL, Talbäck M, Hörnblad J, Johansson LA, Ludvigsson JF, Druid H, et al. The Swedish cause of death register. Eur J Epidemiol. 2017;32:765–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hong TP, Gow P, Fink M, Dev A, Roberts S, Nicoll A, et al. Novel population‐based study finding higher than reported hepatocellular carcinoma incidence suggests an updated approach is needed. Hepatology. 2016;63:1205–12. [DOI] [PubMed] [Google Scholar]
- 20. Batyrbekova N, Aleman S, Lybeck C, Montgomery S, Duberg AS. Hepatitis C virus infection and the temporal trends in the risk of liver cancer: a national register‐based cohort study in Sweden. Cancer Epidemiol Biomarkers Prev. 2020;29:63–70. [DOI] [PubMed] [Google Scholar]
- 21. International Agency for Research on Cancer; World Health Organization . The Global Cancer Observatory. https://gco.iarc.fr/ (2020). Accessed Feb 21, 2022.
- 22. Yang JD, Mohamed EA, Aziz AO, Shousha HI, Hashem MB, Nabeel MM, et al. Characteristics, management, and outcomes of patients with hepatocellular carcinoma in Africa: a multicountry observational study from the Africa Liver Cancer Consortium. Lancet Gastroenterol Hepatol. 2017;2:103–11. [DOI] [PubMed] [Google Scholar]
- 23. Kew MC. Hepatocellular carcinoma in African Blacks: recent progress in etiology and pathogenesis. World J Hepatol. 2010;2:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taflin H, Hafström L, Holmberg E, Castedal M, Lindnér P. The impact of increased immigration to Sweden on the incidence and treatment of patients with HCC and underlying liver disease. Scand J Gastroenterol. 2019;54:746–52. [DOI] [PubMed] [Google Scholar]
- 25. Raffetti E, Fattovich G, Donato F. Incidence of hepatocellular carcinoma in untreated subjects with chronic hepatitis B: a systematic review and meta‐analysis. Liver Int. 2016;36:1239–51. [DOI] [PubMed] [Google Scholar]
- 26. An P, Xu J, Yu Y, Winkler CA. Host and viral genetic variation in HBV‐related hepatocellular carcinoma. Front Genet. 2018;9:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wongjarupong N, Yonli AT, Nagalo BM, Djigma FW, Somda SK, Hassan MA, et al. Characteristics of patients with chronic hepatitis B virus infection with genotype E predominance in Burkina Faso. Hepatol Commun. 2020;4:1781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li W, Deng R, Liu S, Wang K, Sun J. Hepatitis B virus‐related hepatocellular carcinoma in the era of antiviral therapy: the emerging role of non‐viral risk factors. Liver Int. 2020;40:2316–25. [DOI] [PubMed] [Google Scholar]
- 29. Voulgaris T, Papatheodoridi M, Lampertico P, Papatheodoridis GV. Clinical utility of hepatocellular carcinoma risk scores in chronic hepatitis B. Liver Int. 2020;40:484–95. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1 Country key for the Regions of origin (see unstats.un.org)
Supplemental Table S2 The diagnosis codes used to identify patients with serious liver disease/cirrhosis, liver cancer, liver transplantation or HIV‐infection recorded in the Patient register. The ICD‐9 codes were used in the years 1987–1996 and ICD‐10 from 1997.
Supplemental Table S3 Incidence rate (IR) of HCC in men by region of origin and age group, observation time starting 6 months after HBV notification (6 months lag‐time). The IR is presented by 100 person years (with 95% CI) together with the observation time in person years and the number of HCC.


