Abstract
While direct‐acting antivirals (DAAs) cure chronic hepatitis C virus (HCV) infection in almost all patients, some patients remain at risk of liver disease despite HCV cure. In order to identify risk factors indicating liver‐related morbidity and death after viral cure, we included 6982 patients from the national multicenter real‐world German Hepatitis C Registry with regular follow‐up visits for up to 7 years after DAA therapy. Definitions for normal liver function tests (in women/men) were alanine aminotransferase (ALT; ≤35/≤50 U/L), ALT according to American Association for the Study of Liver Diseases (AASLD; ≤19/≤30 U/L), and gamma‐glutamyltransferase (GGT; ≤40/≤60 U/L). In our cohort, 97.4% of patients achieved sustained virologic response (SVR). At 24 weeks after SVR (SVR24), elevated ALT occurred in 657/6982 (9.4%), elevated ALT (AASLD) in 2609/6982 (37.4%), and elevated GGT in 1777/6982 (25.5%) patients. Risk factors for increased ALT at SVR24 were obesity, alcohol, cirrhosis, elevated baseline ALT, and non‐SVR. Increased GGT at SVR24 was significantly (p < 0.05) and independently associated with male sex (odds ratio [OR], 2.12), higher body mass index (OR, 1.04), age >50 years (OR, 1.60), liver cirrhosis (OR, 3.97), alcohol consumption (OR, 2.99), diabetes (OR, 1.63), non‐SVR (OR, 8.00), and elevated GGT at baseline (OR, 17.12). In multivariate regression analysis, elevated GGT at SVR24, particularly in combination with cirrhosis, was the best predictor for hepatic decompensation, hepatocellular carcinoma development, and death, followed by elevated ALT (AASLD) and standard ALT, which predicted hepatic decompensation. Despite successful HCV therapy, elevated GGT at SVR24 and to a lesser extent ALT are predictive of the future clinical outcome and linked with liver‐associated comorbidities. This may highlight the relevance of nonalcoholic fatty liver disease, diabetes mellitus, alcohol, and cirrhosis for the clinical outcome in a vulnerable population, even after HCV cure.
Despite successful treatment for hepatitis C virus (HCV) infection, some patients develop liver‐related complications or die. Using a large national registry with a long, scheduled observation period, we here define risk factors for developing clinical complications after cure from HCV infection. In this context, elevated GGT after HCV treatment predict a particular risk for subsequent decompensation, liver cancer and death, likely highlighting the relevance of non‐alcoholic fatty liver disease (NAFLD), diabetes mellitus, and alcohol consumption as hepatic co‐morbidities in this patient population.
![]()
INTRODUCTION
The introduction of highly effective direct‐acting antiviral (DAA) agents against hepatitis C has resulted in viral cure in the vast majority of patients. Hepatitis C virus (HCV) infections can be effectively cured if guidelines for the optimal use of DAAs are being followed.[ 1 ] Successful HCV eradication, as determined by sustained virologic response (SVR) after DAA therapy, results in liver fibrosis regression and improves extrahepatic manifestations.[ 2 , 3 ] More importantly, in a Canadian population‐based study with 10,851 patients infected with HCV and treated with DAAs and 10,851 matched untreated individuals, DAA therapy substantially reduced all‐cause as well as liver‐related mortality.[ 4 ]
However, despite successful HCV eradication, some patients may still carry an elevated risk for progressive liver disease, including the risk of hepatic decompensation, hepatocellular carcinoma (HCC), and death.[ 5 ] Potential reasons for this liver‐associated morbidity may include liver cirrhosis at the start of DAA therapy that may not fully resolve after HCV elimination as well as (potentially unrecognized) hepatic comorbidities, such as excessive alcohol consumption, obesity, or metabolic disorders.[ 5 ] Particularly, nonalcoholic fatty liver disease (NAFLD) has been identified as a relevant cofactor in patients infected with HCV,[ 6 ] and this may simply be related to the high prevalence of NAFLD that affects around 25% of the adult population globally.[ 7 ] For patients infected with HCV[ 4 , 8 , 9 ] as well as for patients with NAFLD,[ 10 , 11 , 12 ] advanced liver fibrosis and cirrhosis have been linked to long‐term morbidity and mortality. However, it is currently not well defined how to optimally monitor patients after HCV cure.
Using the nationwide German Hepatitis C Registry (DHC‐R), we had previously identified potential risk factors for concomitant hepatic comorbidities in patients infected with HCV undergoing DAA therapy. In a comprehensive cohort of 1774 patients treated with DAAs, male sex, higher body mass index (BMI), liver cirrhosis, baseline alanine aminotransferase (ALT), HCV genotype 2, and younger age were identified as independent risk factors for elevated ALT levels despite SVR at week 12 (SVR12) after DAA therapy.[ 13 ] In a follow‐up analysis including 4946 patients treated for hepatitis C in the DHC‐R, we were able to demonstrate that baseline risk factors determine persistently elevated liver function tests after HCV cure, including obesity, diabetes, liver cirrhosis, and alcohol consumption.[ 14 ]
In order to provide guidance on which patients may benefit from continued hepatological surveillance after HCV cure, we have used the nationwide real‐world DHC‐R to identify risk factors for liver‐related morbidity and death after viral cure of hepatitis C. Based on our cohort of now 6982 patients with a follow‐up of up to 7 years, we demonstrate that elevated liver function tests at SVR24 are associated with an increased risk of hepatic decompensation, HCC, and death.
MATERIALS AND METHODS
The DHC‐R is a national multicenter registry study. Patients are treated at the discretion of the physician. Data are collected by a web‐based data system and confirmed by plausibility checks as well as on‐site monitoring.[ 14 , 15 ] For this analysis, we included all patients that initiated DAA therapy between February 1, 2014, and June 30, 2020. This analysis included data through January 1, 2021. Importantly, only patients with complete data sets for ALT levels at baseline and at follow‐up (at least once and at least 24 weeks after end of treatment) were included. ALT activity and gamma‐glutamyltransferase (GGT) activity were measured (at 37°C) at the local sites participating in the registry.
Normal ALT (at 37°C) was defined as (i) ≤35 U/L for women and ≤50 U/L for men, representing the commonly used reference intervals,[ 16 ] or (ii) according to the American Association for the Study of Liver Diseases (AASLD) as ≤19 U/L for women and ≤30 U/L for men.[ 17 ] Normal GGT (at 37°C) was defined as ≤40 U/L (women) and ≤60 U/L (men).[ 16 ]
From the registry database, the following variables were selected for regression analyses: HCV genotype, sex, BMI, human immunodeficiency virus (HIV) coinfection, opioid substitution, age, liver cirrhosis, alcohol, type 2 diabetes mellitus, ethnicity, country of birth, and SVR.
Patients were identified as having liver cirrhosis if one of the following criteria was fulfilled[ 15 , 18 ]: liver biopsy showing cirrhosis (F4), transient elastography (FibroScan) ≥12.5 kPa, ultrasound confirming cirrhosis, or clinical diagnosis of cirrhosis (e.g., presence of ascites, esophageal varices). Using these criteria, 2297 (32.9%) out of 6892 individuals were identified as having liver cirrhosis according to the definition. In the entire cohort, liver fibrosis assessment either by transient elastography, liver biopsy, or by the above‐mentioned cirrhosis criteria were available for 4335/6982 (62.1%) patients. Within the total cohort (n = 6982), 1235 (17.7%) were staged fibrosis F0–F1, 535 (7.7%) F2, 268 (3.8%) F3, and 2297 (32.9%) F4/cirrhosis; no data on fibrosis at baseline were available for 2647 (37.9%) patients. Alcohol consumption was based on self‐reported intake and categorized in none, moderate (men ≤40 g ethanol per day or women ≤30 g/day), and severe (men >40 g/day or women >30 g/day).
The DHC‐R systematically records liver function tests as well as outcome events (including hepatic decompensation, HCC, or death) on a yearly basis.[ 14 ]
Statistical analysis was performed using univariate and multivariate logistic regression analyses and Kaplan‐Meier survival analysis (release 2019; IBM SPSS Statistics for Windows, version 26.0). Analyses were separately performed using ALT elevated, ALT elevated AASLD, and GGT elevated as categorical variables plus the four options of a combination of ALT normal/elevated and GGT normal/elevated and ALT AASLD normal/elevated and GGT normal/elevated, with different cut‐off values for male and female patients. Categorical data assessing the association of clinical outcomes and GGT were analyzed by chi‐square test. The present data were analyzed based on a data base extract on behalf of Leberstiftungs‐GmbH Deutschland.
RESULTS
Demographics of the nationwide patient population with DAA‐treated HCV
In total, 6982 patients that completed DAA therapy with a follow‐up of up to 7 years were included. HCV genotype distribution was genotype 1, n = 5257 (75.3%); genotype 2, n = 223 (3.2%); genotype 3, n = 1096 (15.7%); genotype 4, n = 387 (5.5%); genotype 5, n = 10 (0.1%); and genotype 6, n = 9 (0.1%). In total, 4245 (60.8%) patients were men and 2737 (39.2%) women. Mean age was 52.6 years (SD, 12.8 years), mean BMI was 26.0 kg/m2 (SD, 4.8 kg/m2), opioid substitution was present in n = 816 (11.7%), and liver cirrhosis was diagnosed in n = 2297 (32.9%). Child‐Pugh B or C cirrhosis was observed in 165 of 2297 patients with liver cirrhosis (7.1%).
Reported comorbidities were psychiatric disorders, n = 1066 (15.3%); cardiovascular diseases, n = 1991 (28.5%); diabetes mellitus, n = 691 (9.9%); chronic respiratory diseases, n = 433 (6.2%); renal insufficiency, n = 254 (3.6%); and HIV coinfection, n = 645 (9.2%). Alcohol consumption (as self‐reported by the patient) was none in n = 5418 (77.6%), ≤30 g/day (women) or ≤ 40 g/day (men) in n = 963 (13.8%), >30 g/day (women) or > 40 g/day (men) in n = 198 (2.8%), and remained unknown in n = 403 (5.8%).
Biochemical pattern before and after DAA therapy
The absolute levels of ALT and GGT over time before, during, and after HCV therapy are shown in Table 1. Levels of both enzymes showed a rapid drop after treatment initiation. The average ALT and GGT levels for the total cohort remained low at the follow‐up assessments after the end of therapy.
TABLE 1.
Liver function tests in patients infected with hepatitis C virus (n = 6982) at baseline and during and after DAA therapy, from the German Hepatitis C Registry
| ALT (U/L) | GGT (U/L) | |||
|---|---|---|---|---|
| Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | |
| Baseline, n = 6982 | 87 ± 76 | 64 (41–104) | 102 ± 135 | 63 (34–120) |
| DAA therapy week 4, n = 5821 | 30 ± 26 | 24 (17–34) | 49 ± 62 | 33 (22–56) |
| DAA therapy week 8, n = 4311 | 30 ± 23 | 23 (17–34) | 45 ± 65 | 29 (20–46) |
| End‐of‐treatment, n = 6238 | 28 ± 24 | 22 (16–31) | 38 ± 57 | 24 (17–40) |
| Follow‐up week 12, n = 5381 | 26 ± 25 | 21 (16–29) | 40 ± 64 | 24 (17–41) |
| Follow‐up week 24, n = 6982 | 27 ± 28 | 20 (15–29) | 42 ± 74 | 24 (16–40) |
| Follow‐up 1 year, n = 3401 | 27 ± 92 | 21 (16–29) | 44 ± 73 | 25 (17–43) |
| Follow‐up 2 years, n = 2552 | 26 ± 22 | 21 (15–29) | 42 ± 71 | 25 (17–43) |
| Follow‐up 3 years, n = 1651 | 25 ± 17 | 21 (16–29) | 41 ± 68 | 25 (17–40) |
| Follow‐up 4 years, n = 1281 | 25 ± 15 | 21 (17–29) | 39 ± 46 | 25 (17–41) |
| Follow‐up 5 years, n = 721 | 28 ± 31 | 22 (17–30) | 42 ± 60 | 25 (17–42) |
Abbreviations: ALT, alanine aminotransferase; DAA, direct acting antivirals; GGT, gamma‐glutamyltransferase; IQR, interquartile range.
At baseline, elevated ALT was found in 5157/6982 (73.9%) patients, elevated ALT (AASLD) in 6545/6982 (93.7%) patients, and elevated GGT in 4981/6982 (70.1%) patients; 97.4% patients achieved SVR12 after DAA therapy, in line with prior reports from this nationwide German registry.[ 19 ] At SVR24, elevated ALT was documented in 657/6982 (9.4%), elevated ALT (AASLD) in 2609/6982 (37.4%), and elevated GGT in 1777/6982 (25.5%).
Identifying risk factors for elevated ALT or GGT after viral cure
We next conducted multivariate regression analyses to identify the risk factors at baseline that would determine elevated ALT or GGT after DAA therapy. At the time point of SVR24 in all patients (n = 6982), elevated ALT was associated with categorized higher BMI (p < 0.05), liver cirrhosis (odds ratio [OR], 2.29; p < 0.0001), alcohol consumption (>30/40 g/day) (OR, 2.93; p < 0.001), elevated ALT at baseline (OR, 4.46; p < 0.0001), and no SVR (OR, 43.49; p < 0.0001). The same variables were identified using AASLD criteria plus additionally diabetes mellitus (OR, 1.49; p < 0.001), younger age (OR, 1.02; p < 0.05), and female sex (OR, 2.39; p < 0.001). Increased GGT at SVR24 was associated with male sex (OR, 2.12; p < 0.001), higher continuous BMI (OR, 1.04; p < 0.05), age >50 years (OR, 1.60; p < 0.01), liver cirrhosis (OR, 3.97; p < 0.0001), alcohol consumption (OR, 2.99; p < 0.001), diabetes mellitus (OR, 1.63; p < 0.001), non‐SVR (OR, 8.00; p < 0.0001), and elevated GGT at baseline (OR, 17.12; p < 0.001).
In patients who achieved SVR24 (n = 6802), elevated ALT was associated with categorized higher BMI (p < 0.01), liver cirrhosis (OR, 2.38; p < 0.0001), alcohol consumption (>30/40 g/day) (OR, 2.96; p < 0.001), and elevated ALT at baseline (OR 4.41; p < 0.0001). The same variables were identified using AASLD criteria plus younger age (OR, 1.01; p < 0.05), diabetes mellitus (OR, 1.41; p < 0.01), and female sex (OR, 2.40; p < 0.001). Increased GGT at SVR24 was associated with male sex (OR, 2.13; p < 0.001), higher BMI (OR 1.03, p < 0.05), age >70 years (OR 1.56, p < 0.01), liver cirrhosis (OR, 4.00; p < 0.0001), alcohol consumption (OR, 3.11; p < 0.001), diabetes mellitus (OR, 1.63; p < 0.001), and elevated GGT at baseline (OR, 16.76; p < 0.001).
Association of elevated ALT or GGT after viral cure with clinical outcome
We next wondered whether a persistently elevated ALT and/or GGT after DAA therapy would indicate an increased risk for liver‐related complications or for mortality. In our cohort of 6982 patients undergoing DAA therapy, n = 128 (1.8%) presented during the follow‐up period with hepatic decompensation (e.g., ascites, bleeding, hepatic encephalopathy). A total of n = 75 patients (1.1%) developed de novo HCC. Overall, n = 103 patients (1.7%) died within the observation period. Kaplan‐Meier curves were plotted to visualize the rates of decompensation (Figure 1A), HCC development (Figure 1B), and death (Figure 1C) over time in our cohort.
FIGURE 1.
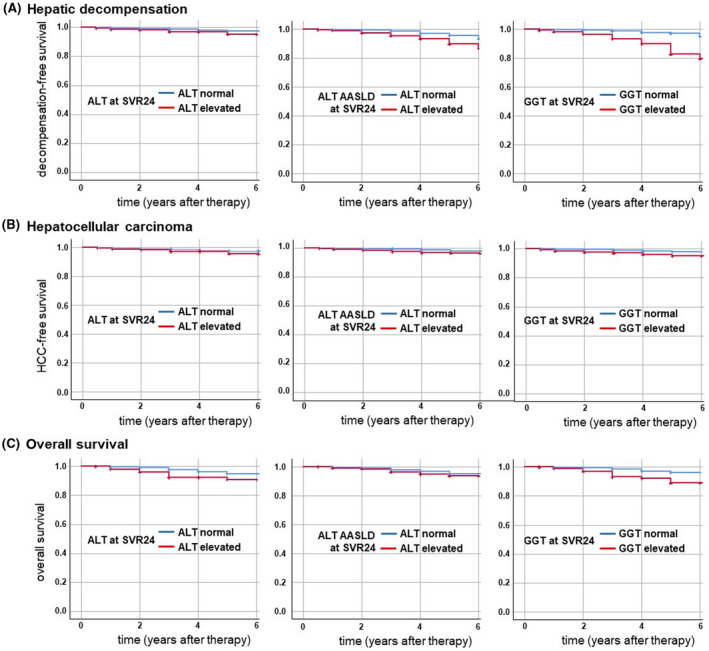
Clinical endpoints in the German Hepatitis C Registry. Elevated liver enzymes (ALT, ALT according to AASLD criteria, and GGT) at 24 weeks after HCV therapy were tested for their predictive value regarding clinical endpoints. Kaplan‐Meier curves demonstrating the rate of (A) hepatic decompensation, (B) de novo hepatocellular carcinoma, and (C) death in patients infected with HCV (n = 6982) that had undergone direct‐acting antiviral therapy. AASLD, American Association for the Study of Liver Diseases; ALT, alanine aminotransferase; GGT, gamma‐glutamyltransferase; HCV, hepatitis C virus; SVR24, sustained virologic response at 24 weeks.
Hepatic decompensation was associated with elevated ALT at SVR24 (p < 0.05), elevated ALT AASLD at SVR24 (p < 0.01), and elevated GGT at SVR24 (p < 0.001) (Figure 1A).
For HCC, elevated ALT at SVR24 (p < 0.05), elevated ALT AASLD at SVR24 (p = 0.001), as well as elevated GGT at SVR24 (p < 0.001) were associated with de novo liver cancer (Figure 1B). For overall mortality, elevated ALT at SVR24 (p < 0.001), elevated ALT AASLD at SVR12 (p = 0.01), and elevated GGT at SVR12 (p < 0.001) were associated with mortality during follow‐up (Figure 1C).
In a second step, multiple logistic regression analysis was performed. The results of the univariate and multivariate analyses are shown in Table 2. By multivariate analysis, only GGT was consistently and independently associated with HCC development, hepatic decompensation, and death.
TABLE 2.
Regression analysis for predicting liver‐related clinical events and death by elevated liver enzymes after SVR24
| Hepatocellular carcinoma | Liver decompensation | Death | ||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |
| Elevated ALT at SVR24 | 1.98 (1.03–3.80) | 0.81 (0.36–1.66) | 1.70 (1.04–2.78) | 0.58 (0.34–0.99) | 2.36 (1.44–3.87) | 1.29 (0.72–2.39) |
| Elevated ALT (AASLD) at SVR24 | 2.54 (1.54–4.18) | 1.83 (1.05–3.19) | 2.76 (1.92–3.95) | 1.87 (1.25–2.78) a | 1.72 (1.67–2.54) | 1.04 (0.66–1.66) |
| Elevated GGT at SVR24 | 3.69 (1.82–5.33) | 3.12 (1.82–5.33) a | 5.43 (3.77–7.82) | 4.88 (3.29–7.21) a | 3.57 (2.41–5.28) | 3.30 (2.14–5.09) a |
Note: Odds ratio is given, with 95% confidence interval in parenthesis; only statistically significant univariate factors were included in the multivariate analysis.
Abbreviations: AASLD, American Association for the Study of Liver Diseases; ALT, alanine aminotransferase; DAA, direct acting antivirals; GGT, gamma‐glutamyltransferase; IQR, interquartile range; SVR24, sustained virologic response at 24 weeks.
Statistically significant results in multivariate analysis.
We next analyzed the impact of liver cirrhosis for outcome measures in patients with either normal or elevated GGT at SVR24. A total of 5142 patients had normal GGT at SVR24, comprising 3925 patients without cirrhosis (77%) and 1217 (23%) patients with cirrhosis. In these patients, hepatic decompensation (n = 3, 0.1% vs. n = 42, 3.5%; p < 0.001), de novo HCC (n = 5, 0.1% vs. n = 24, 2.0%; p < 0.001), and death from any cause (n = 24, 0.6% vs. n = 22, 1.8%; p < 0.001) occurred less frequently in patients without compared to those with cirrhosis.
A total of 1660 patients had elevated GGT at SVR24, comprising 651 patients without (39%) and 1009 (61%) patients with cirrhosis. Again, in these patients with elevated GGT at SVR24, hepatic decompensation (n = 2, 0.3% vs. n = 72, 7.1%; p < 0.001), de novo HCC (n = 1, 0.2% vs. n = 32, 3.2%; p < 0.001), and death from any cause (n = 7, 1.1% vs. n = 43, 4.3%; p < 0.001) occurred less frequently in patients without compared to those with cirrhosis. In patients with cirrhosis, an elevated GGT at SVR24 increased the risk of having adverse clinical outcome measures compared to normal GGT at SVR24. This difference was statistically significant for hepatic decompensation (p < 0.001) and death (p < 0.01) but not for de novo HCC.
Prognostic value of elevated ALT or GGT after viral cure for clinical outcome
Subsequently, we performed receiver operating characteristic (ROC) curve analyses to assess the predictive value of liver enzymes after DAA therapy for clinical endpoints. For hepatic decompensation (Figure 2A), GGT at SVR24 reached an area under the ROC curve (AUROC) of 0.692 (95% confidence interval [CI], 0.641–0.743) compared to lower values for ALT (AUROC, 0.512; 95% CI, 0.459–0.565) and ALT AASLD (AUROC, 0.616; 95% CI, 0.565–0.668) at SVR24. When HCC during follow‐up was chosen as the endpoint (Figure 2B), GGT at SVR24 achieved an AUROC of 0.645 (95% CI, 0.572–0.719), while ALT (AUROC, 0.534; 95% CI, 0.459–0.610) and ALT AASLD (AUROC, 0.612; 95% CI, 0.540–0.683) at SVR24 showed lower power. For overall mortality (Figure 2C), the highest AUROC of 0.640 (95% CI, 0.581–0.700) was found for GGT at SVR24, followed by ALT AASLD (AUROC, 0.561; 95% CI, 0.502–0.619) and ALT (AUROC, 0.551; 95% CI, 0.489–0.613) at SVR24.
FIGURE 2.
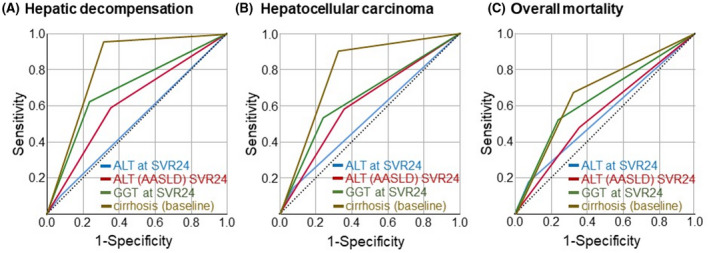
ROC curve analyses of liver enzymes after direct‐acting antiviral treatment for clinical endpoints. ROC curves were plotted to assess the predictive power of posttreatment (SVR24) liver enzymes in patients infected with HCV from the German hepatitis C Registry for (A) hepatic decompensation, (B) hepatocellular carcinoma, and (C) overall mortality. Blue, ALT at SVR24; red, ALT (AASLD criteria) at SVR24; green, GGT at SVR24; brown, cirrhosis (at baseline); dashed line, reference line (0.5). AASLD, American Association for the Study of Liver Diseases; ALT, alanine aminotransferase; GGT, gamma‐glutamyltransferase; HCV, hepatitis C virus; ROC, receiver operating characteristic; SVR24, sustained virologic response at 24 weeks.
Adding liver cirrhosis as a variable to the ROC curve analysis resulted in a high predictive power of cirrhosis (as assessed at baseline) for hepatic decompensation (AUROC, 0.821; 95% CI, 0.795–0.847), for de novo HCC (AUROC, 0.791; 95% CI, 0.745–0.837), and for overall mortality (AUROC, 0.677; 95% CI, 0.623–0.732). Thus, liver cirrhosis was the numerically best predictor for hepatic decompensation (Figure 2A), HCC development (Figure 2B), and death (Figure 2C), followed by elevated GGT at SVR24, elevated ALT (AASLD), and standard ALT. No prognostic improvement was achieved by combining ALT and GGT (detailed data not shown).
Performing ROC analyses in the patient subset without cirrhosis showed the following results: for hepatic decompensation, elevated GGT (AUROC, 0.629; 95% CI, 0.355–0.903), ALT (AUROC, 0.575; 95% CI, 0.298–0.853), and ALT AASLD (AUROC, 0.661; 95% CI, 0.411–0.910); for de novo HCC, elevated GGT (AUROC, 0.512; 95% CI, 0.277–0.747), ALT (AUROC, 0.559; 95% CI, 0.308–0.809), and ALT AASLD (AUROC, 0.611; 95% CI, 0.374–0.847); and for death, elevated GGT (AUROC, 0.542; 95% CI, 0.435–0.649), ALT (AUROC, 0.524; 95% CI, 0.418–0.630), and ALT AASLD (AUROC, 0.506; 95% CI, 0.403–0.608; detailed data not shown).
DISCUSSION
Modern DAA therapy allows the successful elimination of HCV in the vast majority of patients, thereby substantially reducing liver‐related as well as overall morbidity and mortality.[ 1 , 3 ] In our real‐world cohort of 6982 patients with available follow‐up data, 97.4% of patients achieved SVR12, in line with prior reports from this nationwide German registry.[ 19 ] Despite a substantial fraction of patients with liver cirrhosis (32.9%) undergoing HCV therapy, the rates of hepatic decompensation (e.g., ascites, bleeding, hepatic encephalopathy), de novo HCC, or death were only 1.8%, 1.1%, and 1.7%, respectively, during the observation period. These findings emphasize the efficacy and benefit of HCV therapy but also underscore the need to clearly identify the risk factors for the remaining risk of liver‐related complications and death.
In a prior analysis of this cohort, including 1774 patients treated with DAA at that time, several patient characteristics were associated with an elevated ALT after SVR12, including male sex, higher BMI, liver cirrhosis, baseline ALT, HCV genotype 2, and younger age.[ 13 ] A follow‐up analysis with 4946 patients revealed that high BMI, age <70 years, liver cirrhosis, diabetes, alcohol consumption, and not achieving SVR12 were associated with persistently elevated ALT after DAA therapy.[ 14 ] Our current analysis comprising 6982 patients confirmed three major risk factors for persistently elevated liver function tests after DAA therapy: liver cirrhosis, alcohol consumption (even if deemed moderate), and obesity/metabolic disorders (diabetes). These data demonstrate that hepatic comorbidities can exist and will persist even if DAA therapy effectively eradicates the virus.[ 5 ] Among these, NAFLD is the most prevalent chronic liver disease worldwide, and NAFLD has been linked to liver‐related as well as extrahepatic (e.g., cardiovascular) morbidity and mortality.[ 20 ] In addition, HCV is known to contribute to steatosis development and metabolic liver disease[ 21 ] by inducing hepatic lipogenesis (which favors indirectly HCV lipoviroparticle assembly),[ 22 ] affecting lipoprotein synthesis[ 23 ] and fatty acid oxidation in mitochondria.[ 24 ]
Our large real‐world registry with the long follow‐up period allowed us to analyze to what extent routine liver function tests can predict liver decompensation, HCC, and death. We could demonstrate that both GGT and ALT elevation at SVR24 is predictive of future clinical outcome as surrogate markers for persisting liver disease despite viral cure of HCV. Interestingly, GGT appears to be a more sensitive predictor for hepatic decompensation, HCC, and death than ALT. This observation was made in principle for patients with and without liver cirrhosis as GGT indicated an increasing risk for clinical events in both groups. However, cirrhosis itself was the strongest predictor of adverse outcome, in line with available literature.[ 8 , 9 ] Combining ALT and GGT did not improve the association between liver enzyme elevation and clinical outcome. The association of persisting ALT or GGT elevations at SVR24 with higher BMI, diabetes mellitus, alcohol consumption, and liver cirrhosis confirms the relevance of comorbidities for ongoing liver damage and the occurrence of liver cancer.
Collectively, our data indicate that assessing liver function tests after DAA therapy may be helpful in patients with hepatitis C to identify remaining liver disease. In the majority of patients, ALT and GGT will normalize after HCV cure.[ 14 ] Abnormal liver enzymes, particularly GGT, should prompt the search for (additional) hepatic diseases.[ 25 , 26 ] The association of elevated GGT with hepatic decompensation, HCC, and mortality indicates that patients with increased GGT after HCV cure may benefit from additional hepatological care and treatment of concomitant hepatic conditions.
CONFLICTS OF INTEREST
Stefan Mauss has received sponsorship for lectures (national or international) from Gilead; he has served on an advisory committee or review panel for Gilead, Janssen, MSD, ViiV. Frank Tacke has received research grants (to the institution) from Allergan, BMS, Gilead, Inventiva; he has been a consultant for Allergan, Bayer, Gilead, BMS, Boehringer, Intercept, Ionis, Inventiva, Merz, Pfizer, Alnylam, NGM, CSL Behring, Novo Nordisk, Novartis; he has received sponsorship for lectures (national or international) from Gilead, AbbVie, Falk, Merz, Intercept. Hartwig Klinker; he has served on an advisory committee or review panel for AbbVie, BMS, Gilead, Janssen, MSD; he has received fees for speaking and teaching from AbbVie, BMS, Gilead, Janssen, MSD. Klaus Boeker has received sponsorship for lectures (national/international) and consultant fees from AbbVie and Gilead. Uta Merle has served on an advisory committee or review panel for CSL‐Behring, Gilead, Takeda; she has received speaking and teaching fees from CSL‐Behring, FALK, MSD. Peter Buggisch has received sponsorship for lectures (national/international) from AbbVie, Gilead, MSD; he has served on an advisory committee or review panel for AbbVie, Gilead, MSD. Dietrich Hüppe has received speaking and teaching fees from AbbVie GmbH, Falk Pharma, Ferring Arzneimittel GmbH. Markus Cornberg has received speaking and teaching fees and has served on an advisory or review panel for Abbvie, Bristol‐Myers Squibb, Gilead Sciences, Janssen‐Cilag, Roche, Merck, MSD, Biogen, Falk Foundation, Boehringer Ingelheim, Siemens, Spring Bank; he has received grants from Roche. Christoph Sarrazin has served on an advisory committee or review panel for AbbVie, Gilead, Merck/MSD; he has received grant/research support from AbbVie, Gilead; he has received speaking and teaching fees from AbbVie, Gilead, Merck/MSD. Heiner Wedemeyer has received sponsorship for lectures (national or international) from, has received grants from, and/or has served as a consultant for Abbott, AbbVie, Altimmune, Biotest, BMS, BTG, Dicerna, Gilead, Janssen, Merck/MSD, MYR GmbH, Novartis, Roche, Siemens, Transgene. Thomas Berg has served as a consultant/advisory board member/investigator/speaker for AbbVie, BMS, Boehringer, Gilead, Janssen, Merck, Novartis, Roche, Vertex Pharmaceuticals; he has received grants from Gilead, Janssen, Novartis, Roche. The other authors have nothing to disclose.
DHC‐R: Peter Schirmacher, Michael P. Manns, Stefan Mauss, Heinz Hartmann, Michael R. Kraus, Pavel Khaykin, Carsten Zamani, Stefan Zeuzem, Maria‐Christina Jung, Christiane Cordes, Willidbold Schiffelholz, Gerd Klausen, Holger Hinrichsen, Axel Baumgarten, Katharina Willuweit, Christoph Antoni, Heribert Knechten, Renate Heyne, Nikolaus Kordecki, Hjordis Möller, Tobias Müller, Michael Priller, Ansgar Rieke, Thomas Lutz, Stefan Christensen, Rainer Günther, Uwe Naumann, Rainer Ullrich, Gerlinde Teuber, Albrecht Stoehr, Christine John, Karl‐Georg Simon.
ACKNOWLEDGMENTS
Data were derived from the DHC‐R, a project of Deutsche Leberstiftung (German Liver Foundation), managed by Leberstiftungs‐GmbH Deutschland in cooperation with the Association of German gastroenterologists in private practice with financial support from the German Center for Infection Research and the companies AbbVie Deutschland GmbH, Gilead Sciences GmbH, MSD Sharp & Dohme GmbH, and Bristol‐Myers Squibb GmbH. KGaA and Janssen‐Cilag GmbH (each until July 14, 2020) and Roche Pharma AG (until July 14, 2017) also provided financial support to the DHC‐R. Open Access funding enabled and organized by Projekt DEAL.
Tacke F, Klinker H, Boeker KHW, Merle U, Link R, Buggisch P, et al. Elevated liver enzymes predict morbidity and mortality despite antiviral cure in patients with chronic hepatitis C: Data from the German Hepatitis C‐Registry. Hepatol Commun. 2022;6:2488–2495. 10.1002/hep4.2015
Contributor Information
Frank Tacke, Email: frank.tacke@charite.de.
DHC‐R:
Karl‐Georg Simon, Christine John, Albrecht Stoehr, Gerlinde Teuber, Rainer Ullrich, Uwe Naumann, Rainer Günther, Stefan Christensen, Thomas Lutz, Ansgar Rieke, Michael Priller, Tobias Müller, Hjordis Möller, Nikolaus Kordecki, Renate Heyne, Heribert Knechten, Christoph Antoni, Katharina Willuweit, Axel Baumgarten, Holger Hinrichsen, Gerd Klausen, Willidbold Schiffelholz, Christiane Cordes, Maria‐Christina Jung, Stefan Zeuzem, Carsten Zamani, Pavel Khaykin, Michael R. Kraus, Heinz Hartmann, Stefan Mauss, Michael P. Manns, and Peter Schirmacher
REFERENCES
- 1. European Association for the Study of the Liver . EASL recommendations on treatment of hepatitis C: final update of the series. J Hepatol. 2020;73:1170–218. [DOI] [PubMed] [Google Scholar]
- 2. Persico M, Rosato V, Aglitti A, Precone D, Corrado M, De Luna A, et al. Sustained virological response by direct antiviral agents in HCV leads to an early and significant improvement of liver fibrosis. Antivir Ther. 2018;23:129–38. [DOI] [PubMed] [Google Scholar]
- 3. Calvaruso V, Craxi A. Hepatic benefits of HCV cure. J Hepatol. 2020;73:1548–56. [DOI] [PubMed] [Google Scholar]
- 4. Janjua NZ, Wong S, Abdia Y, Jeong D, Buller‐Taylor T, Adu PA, et al. Impact of direct‐acting antivirals for HCV on mortality in a large population‐based cohort study. J Hepatol. 2021;75:1049–57. [DOI] [PubMed] [Google Scholar]
- 5. Negro F. Residual risk of liver disease after hepatitis C virus eradication. J Hepatol. 2021;74:952–63. [DOI] [PubMed] [Google Scholar]
- 6. Noureddin M, Wong MM, Todo T, Lu SC, Sanyal AJ, Mena EA. Fatty liver in hepatitis C patients post‐sustained virological response with direct‐acting antivirals. World J Gastroenterol. 2018;24:1269–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69:2672–82. [DOI] [PubMed] [Google Scholar]
- 8. van der Meer AJ, Feld JJ, Hofer H, Almasio PL, Calvaruso V, Fernandez‐Rodriguez CM, et al. Risk of cirrhosis‐related complications in patients with advanced fibrosis following hepatitis C virus eradication. J Hepatol. 2017;66:485–93. [DOI] [PubMed] [Google Scholar]
- 9. Krassenburg LAP, Maan R, Ramji A, Manns MP, Cornberg M, Wedemeyer H, et al. Clinical outcomes following DAA therapy in patients with HCV‐related cirrhosis depend on disease severity. J Hepatol. 2021;74:1053–63. [DOI] [PubMed] [Google Scholar]
- 10. Boursier J, Hagstrom H, Ekstedt M, Moreau C, Bonacci M, Cure S, et al. Non‐invasive tests accurately stratify patients with NAFLD based on their risk of liver‐related events. J Hepatol. 2022;76:1013–20. [DOI] [PubMed] [Google Scholar]
- 11. Hagstrom H, Kechagias S, Ekstedt M. Risk for hepatic and extra‐hepatic outcomes in nonalcoholic fatty liver disease. J Intern Med. 2021. 10.1111/joim.13343Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 12. Simon TG, Roelstraete B, Khalili H, Hagstrom H, Ludvigsson JF. Mortality in biopsy‐confirmed nonalcoholic fatty liver disease: results from a nationwide cohort. Gut. 2021;70:1375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mauss S, Buendgens L, Christensen S, Ingiliz P, Berger F, Huppe D, et al. Risk factors for remaining liver injury in patients with virological elimination of chronic hepatitis C. Z Gastroenterol. 2019;57:139–47. [DOI] [PubMed] [Google Scholar]
- 14. Tacke F, Boeker KHW, Klinker H, Heyne R, Buggisch P, Pathil A, et al. Baseline risk factors determine lack of biochemical response after SVR in chronic hepatitis C patients treated with DAAs. Liver Int. 2020;40:539–48. [DOI] [PubMed] [Google Scholar]
- 15. Tacke F, Gunther R, Buggisch P, Klinker H, Schober A, John C, et al. Treatment of HCV genotype 2 with sofosbuvir and ribavirin results in lower sustained virological response rates in real life than expected from clinical trials. Liver Int. 2017;37:205–11. [DOI] [PubMed] [Google Scholar]
- 16. Kwo PY, Cohen SM, Lim JK. ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 2017;112:18–35. [DOI] [PubMed] [Google Scholar]
- 17. Terrault NA, Bzowej NH, Chang KM, Hwang JP, Jonas MM, Murad MH, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology. 2016;63:261–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Honer Zu Siederdissen C, Buggisch P, Boker K, Schott E, Klinker H, Pathil A, et al. Treatment of hepatitis C genotype 1 infection in Germany: effectiveness and safety of antiviral treatment in a real‐world setting. United European Gastroenterol J. 2018;6:213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huppe D, Serfert Y, Buggisch P, Mauss S, Boker KHW, Muller T, et al. 4 years of direct‐acting antivirals (DAAs) in the German Hepatitis C‐Registry (DHC‐R) [in German]. Z Gastroenterol. 2019;57:27–36. [DOI] [PubMed] [Google Scholar]
- 20. Sanyal AJ, Van Natta ML, Clark J, Neuschwander‐Tetri BA, Diehl A, Dasarathy S, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med. 2021;385:1559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Negro F. Abnormalities of lipid metabolism in hepatitis C virus infection. Gut. 2010;59:1279–87. [DOI] [PubMed] [Google Scholar]
- 22. Lerat H, Kammoun HL, Hainault I, Merour E, Higgs MR, Callens C, et al. Hepatitis C virus proteins induce lipogenesis and defective triglyceride secretion in transgenic mice. J Biol Chem. 2009;284:33466–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Serfaty L, Andreani T, Giral P, Carbonell N, Chazouilleres O, Poupon R. Hepatitis C virus induced hypobetalipoproteinemia: a possible mechanism for steatosis in chronic hepatitis C. J Hepatol. 2001;34:428–34. [DOI] [PubMed] [Google Scholar]
- 24. Yamaguchi A, Tazuma S, Nishioka T, Ohishi W, Hyogo H, Nomura S, et al. Hepatitis C virus core protein modulates fatty acid metabolism and thereby causes lipid accumulation in the liver. Dig Dis Sci. 2005;50:1361–71. [DOI] [PubMed] [Google Scholar]
- 25. Newsome PN, Cramb R, Davison SM, Dillon JF, Foulerton M, Godfrey EM, et al. Guidelines on the management of abnormal liver blood tests. Gut. 2018;67:6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. European Association for the Study of the Liver . EASL clinical practice guidelines on non‐invasive tests for evaluation of liver disease severity and prognosis ‐ 2021 update. J Hepatol. 2021;75:659–89. [DOI] [PubMed] [Google Scholar]


