Abstract
Studies have examined nonalcoholic fatty liver disease (NAFLD) prevalence and severity in Asians; however, this is not well understood in Asian Americans (both East and South Asian Americans) as few studies have analyzed this population. We aimed to describe characteristics, prevalence of NAFLD, and its severity in Asian Americans in the National Health and Nutrition Examination Surveys (NHANES) from 2017 to 2018. Respondents 18 years and older with interview, laboratory testing, and transient elastography data were included. Other causes of liver disease were excluded. Controlled attenuation parameter (CAP) cutoff ≥ 274 dB/m, as published in the literature, defined NAFLD. Sensitivity analysis for CAP cutoffs ≥ 248 and ≥302 dB/m were performed. We found that 450 out of 3639 respondents were Asian Americans, and prevalence using CAP ≥ 274 dB/m was 43.23%. Using sensitivity analysis cutoffs of CAP ≥ 248 dB/m and CAP ≥ 302 dB/m, the prevalence was 57.38% and 28.03%, respectively. Compared with non‐Asian Americans with NAFLD, Asian Americans with NAFLD had significantly lower body mass index (BMI) and less prevalent smoking history. Comorbidities, such as prediabetes, diabetes, and hypertension, were not significantly different between Asian and non‐Asian Americans with NAFLD. Compared to non‐Asian Americans with NAFLD, Asian Americans with NAFLD exhibited higher aminotransferases and triglycerides. Fibrosis assessed by transient elastography was not significantly different between Asian and non‐Asian Americans with NAFLD. Despite decreased prevalence of BMI ≥ 30 kg/m2, Asian Americans experienced similar NAFLD prevalence with increased hepatocellular injury and triglyceridemia compared to non‐Asian Americans. Fibrosis stages were similar to non‐Asian Americans.
This study aimed to describe characteristics, prevalence of NAFLD, and its severity in Asian Americans in the National Health and Nutrition Examination Surveys (NHANES) from 2017 to 2018. Despite younger age, lower BMI cutoffs, and decreased prevalence of obesity compared to non‐Asian Americans with NAFLD, Asian Americans demonstrate similar prevalence of NAFLD with comparable severity of disease as evidenced by the FibroScan‐AST (FAST) score, prevalence of FAST score ≥ 0.67, and liver stiffness measurement on vibration controlled transient elastography.

INTRODUCTION
Nonalcoholic liver disease (NAFLD) is the leading cause of chronic liver disease worldwide.[ 1 , 2 ] Cardiometabolic risk factors associated with metabolic syndrome, such as obesity, diabetes, hypertension, and hyperlipidemia, increase the risk of NAFLD and disease progression to nonalcoholic steatohepatitis (NASH), fibrosis, and even cirrhosis.[ 3 ] Despite these cardiometabolic risk factors, NAFLD develops in 10%–20% of Americans without obesity (body mass index [BMI] < 30 kg/m2 in non‐Asians, <25 kg/m2 in Asians) or Americans who are lean (BMI < 25 kg/m2 in non‐Asians, <23 kg/m2 in Asians).[ 4 ] Compared to those with non‐lean NAFLD, those with lean NAFLD exhibit lower rates of cirrhosis, diabetes, dyslipidemia, and hypertension.[ 5 ]
In Asia, the overall prevalence of NAFLD is 29.62%, and this has increased over time.[ 6 ] Approximately 8%–19% of Asians who are not obese or are lean have NAFLD, and Asians with nonobese/lean NAFLD exhibit less severe metabolic features compared to Asians with obese NAFLD.[ 7 ] Although Asian Americans comprise one of the most rapidly growing ethnic minorities in the United States, with Asian Americans accounting for nearly half of individuals with nonobese/lean NAFLD, the prevalence of NAFLD and its severity among Asian Americans remain poorly characterized, especially in a large real‐world cohort across the United States.[ 5 , 8 ] Studies investigating NAFLD have frequently lumped Asians or Asian Americans into overall analyses of nonobese/lean NAFLD cohorts; yet, assessing NAFLD in Asian Americans separately remains important due to possibly distinct genetic signatures and lifestyle factors, including diet and cultural influences, among Asian Americans. In a cross‐sectional National Health and Examination Nutrition Survey (NHANES) study using ultrasound combined with fatty liver index from the 2011 to 2016 cycle to compare non‐Hispanic Whites with NAFLD (prevalence, 28.4%) to Asian Americans with NAFLD (prevalence, 18.3%), Asian Americans with NAFLD were found to have a lower BMI and waist circumference and lower likelihood of coexisting metabolic syndrome or cardiovascular disease.[ 9 ] Presence of type 2 diabetes was independently associated with advanced fibrosis (defined by at least one of the following noninvasive tests: aminotransferase‐to‐platelet ratio >1.0, fibrosis‐4 index >2.67, or NAFLD fibrosis score >0.676) in Asian Americans with NAFLD.[ 9 ] However, this study was limited in that various degrees of fibrosis staging were not readily available by liver biopsy or more accurate noninvasive test assessment.[ 9 ] Overall, the prevalence and severity of NAFLD is not well understood in Asian Americans as this population is usually combined with other subjects with nonobese/lean NAFLD in analyses. A recent study that used NHANES 2017–2018 and vibration controlled transient elastography (VCTE) to assess the prevalence of NAFLD did not focus on Asian Americans. Therefore, the prevalence of NAFLD in Asian Americans remains unclear.[ 10 ]
In this study, we aimed to determine the prevalence and characterization of NAFLD, its severity, and its comorbidities among Asian Americans by using controlled attenuation parameter (CAP) and liver stiffness measurements (LSMs) on VCTE in the most recent cycle of the NHANES data from 2017 to 2018 and to compare these characteristics to those among non‐Asian Americans.[ 10 ] As transient elastography is more accurate than ultrasound used in prior studies in assessing steatosis and degree of fibrosis, we developed highly accurate estimations of NAFLD disease prevalence and severity. To our knowledge, this cross‐sectional study comprises the largest nationally representative population of Asian Americans to date with access to VCTE assessments that empower accurate classification of steatosis and fibrosis by CAP and LSM, respectively. We hypothesized that risk factors, prevalence of NAFLD, severity of NAFLD, and comorbidities varied among Asian Americans compared to non‐Asian Americans, independent of BMI.
MATERIALS AND METHODS
Data source
The National Center for Health Statistics at the Centers for Disease Control and Prevention conducts NHANES, a nationally representative, cross‐sectional, multistage study of the nonmilitary and noninstitutionalized population of the United States and releases the NHANES data once every 2 years. NHANES data incorporate self‐reported interviews and clinical examinations from survey subjects. Each survey subject chosen at random undergoes interviews on demographics, socioeconomic background, diet, and health‐related information followed by physical examination at a mobile examination center that includes laboratory blood testing and VCTE (FibroScan) assessments.[ 11 ]
Patient selection
We analyzed pooled data from the NHANES cycle conducted from 2017 to 2018. Subjects ≥18 years old were included, whereas subjects with excessive alcohol use (seven or more drinks per week for women, 14 or more drinks per week for men), as determined using alcohol questionnaires, or with human immunodeficiency virus (HIV) or viral hepatitis B, C, D, or E infection were excluded. Those who did not undergo FibroScan were also excluded. The demographic and clinical characteristics among participants and nonparticipants of VCTE examinations are detailed in Table S1. Asian Americans included those who considered themselves to be Asian Indian, Bangladeshi, Bengalese, Bharat, Bhutanese, Burmese, Cambodian, Cantonese, Chinese, Dravidian, East Indian, Filipino, Goanese, Hmong, Indochinese, Indonesian, Iwo Jiman, Japanese, Korean, Laohmong, Laotian, Madagascar/Malagasy, Malaysian, Maldivian, Mong, Nepalese, Nipponese, Okinawan, Pakistani, Siamese, Singaporean, Sri Lankan, Taiwanese, Thai, or Vietnamese. Non‐Asian Americans included those who considered themselves to be Hispanic, American Indian or Alaska Native, African American, Native Hawaiian or Pacific Islander, White, or other.
Definition
VCTE (FibroScan) assessments were included, and CAP and LSM data were collected. Subjects were determined to have NAFLD based on CAP ≥ 274 dB/m (based on a 90% sensitivity cutoff),[ 12 ] and sensitivity analyses were performed for additional cutoffs of CAP ≥ 248 dB/m (based on a meta‐analysis from 19 studies[ 13 ]) and CAP ≥ 302 dB/m (based on the Youden's cutoff from Eddowe et al.'s study[ 12 ]). Subjects were also divided into fibrosis stages based on LSM as follows: stage 1 fibrosis or less (LSM < 8.2 kPa), stage 2 fibrosis and above (LSM ≥ 8.2 kPa), stage 3 fibrosis and above (LSM ≥ 9.7 kPa), and stage 4 fibrosis (LSM ≥ 13.6 kPa) based on Youden's index published by Eddowes et al.[ 12 ] Using a combination of CAP and LSM by VCTE with aspartate aminotransferase (AST), the FibroScan AST (FAST) score identifies those with fibrotic nonalcoholic steatohepatitis (NASH) with a NAFLD activity score ≥ 4 and significant fibrosis (F2 or higher).[ 14 ]
To assess disease severity, we assessed the proportion of patients with clinically meaningful fibrosis stages (F2 or higher, F3 or higher, F4 or higher) and with NASH + NAS ≥ 4 + ≥ F2, assessed by the FAST score using the rule‐in cutoff ≥ 0.67, as published by Newsome et al.[ 14 ]
Covariates, such as age, sex, and lifestyle factors, were self‐reported. Diabetes was determined based on a combination of self‐report and/or glycohemoglobin ≥6.5%. Prediabetes was determined based on glycohemoglobin ≥5.7%. The smoking metric was used to characterize smoking status into past smoking history or current smoking. Clinically measured information obtained during mobile examination was used to calculate BMI. Subjects were further characterized into ideal and normal weight (BMI, 18.5–22.9 kg/m2 for Asian Americans; BMI, 18.5–24.9 kg/m2 for non‐Asian Americans), overweight (BMI, 23–27.4 kg/m2 for Asian Americans; BMI, 25–29.9 kg/m2 for non‐Asian Americans), obese (BMI, 27.5–32.4 kg/m2 for Asian Americans; BMI, 30–34.9 kg/m2 for non‐Asian Americans), and morbidly obese (BMI, ≥32.5 kg/m2 for Asian Americans; BMI ≥ 35 kg/m2 for non‐Asian Americans) categories based on World Health Organization (WHO) BMI cut‐off points and proposed BMI cut‐off points for public health action in Asians.[ 15 ]
During the mobile examination, up to four blood pressure measurements were performed. Blood pressure was categorized as ideal (blood pressure < 140/<90 mm Hg) or high (systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg). Blood samples were collected from each subject and measured for total cholesterol, high‐density lipoprotein (HDL), low‐density lipoprotein (LDL), and triglycerides.
The prevalence of demographic, metabolic, and biochemical characteristics, including sex, diabetes, prediabetes, obesity, past smoking history, current smoking, high blood pressure, total cholesterol ≥ 200 mg/dL, HDL < 40 mg/dL, LDL ≥ 160 mg/dL, triglycerides ≥200 mg/dL, and fibrosis stage were analyzed among Asian Americans and non‐Asian Americans.[ 12 , 13 ]
Statistical analysis
The 2‐year NHANES survey weights from 2017 to 2018 were used to compute population estimates. Descriptive statistics were reported as proportion (95% confidence interval [CI]) for categorical variables and mean (±SD) for continuous variables. To compare between two categories, Rao‐Scott chi‐squared test was used for categorical variables and the weighted Student t test was used for continuous variables. Potential risk factors for NAFLD, defined as CAP ≥ 274 dB/m, were estimated using univariable and multivariable logistic regression. Variables on the multivariable regression model were selected based on clinical experience. Two‐sided p ≤ 0.05 was significant. Analyses were carried out using survey procedures and testing in SAS version 9.4 (SAS Institute, Cary, NC) and R. Figures were generated using Microsoft Excel.
RESULTS
We included 8704 respondents from the 2017–2018 NHANES cycle who completed both the interview and mobile examination. After stepwise exclusions for lack of a transient elastography examination (n = 3210), age < 18 years (n = 748), viral hepatitis (n = 813), HIV (n = 7), and excessive alcohol use (n = 287), 3639 subjects were included for final analyses (Figure S1).
Of 3639 participants, 450 (12.37%) were Asian Americans and 3189 (87.63%) were non‐Asian Americans (Table S2). A comparison of demographic and clinical variables between Asian Americans and non‐Asian Americans with NAFLD, defined as CAP ≥ 274 dB/m, is shown in Table 1.
TABLE 1.
Demographic and clinical characteristics among Asian Americans versus non‐Asian Americans with NAFLD using CAP cutoff ≥ 274 dB/m
| CAP ≥ 274 dB/m | |||
|---|---|---|---|
| Asian American a | Non‐Asian American b | p value | |
| Demographics | |||
| Age (years) | 45.83 (43.47–48.19) | 49.84 (48.72–50.96) | 0.009 |
| Female | 42.38 (34.57–50.19) | 44.94 (41.26–48.63) | 0.532 |
| BMI (kg/m2) | 29.35 (28.83–29.86) | 34.18 (33.45–34.92) | <0.001 |
| Waist circumference (cm) | 98.33 (96.46–100.19) | 111.87 (109.98–113.75) | <0.0001 |
| Any smoking history | 23.46 (16.12–32.05) | 41.81 (37.62–46.09) | <0.001 |
| Current smoking | 9.87 (4.45–18.00) | 13.13 (10.36–16.29) | 0.398 |
| Comorbidities | |||
| Obesity | 58.54 (50.89–66.19) | 70.35 (64.91–75.78) | 0.0168 |
| Prediabetes | 58.84 (48.46–68.72) | 54.97 (50.94–58.97) | 0.466 |
| Diabetes | 25.47 (19.06–32.67) | 22.62 (19.16–26.34) | 0.459 |
| Hypertension | 41.15 (33.51–49.09) | 44.03 (38.13–50.05) | 0.526 |
| WBC (1000 cells/μL) | 7.77 (7.40–8.15) | 7.82 (7.61–8.02) | 0.846 |
| Hemoglobin (g/dL) | 14.24 (13.92–14.56) | 14.43 (14.29–14.58) | 0.300 |
| Platelets (1000 cells/μL) | 252.03 (238.67–265.39) | 251.35(244.38–258.31) | 0.918 |
| Albumin (g/L) | 41.66 (41.11–42.22) | 40.59 (40.19–41.00) | 0.003 |
| ALP (IU/L) | 77.34 (74.91–79.77) | 80.24 (78.12–82.35) | 0.032 |
| ALT (U/L) | 29.56 (26.31–32.81) | 26.48 (25.27–27.68) | 0.050 |
| AST (U/L) | 24.15 (22.05–26.25) | 22.12 (21.3–22.93) | 0.045 |
| Total bilirubin (mg/dL) | 0.45 (0.40–0.51) | 0.45 (0.43–0.48) | 0.995 |
| Total cholesterol ≥ 200 mg/dL | 42.17 (34.11–50.51) | 39.30 (32.91–45.94) | 0.495 |
| HDL < 40 mg/dL | 27.05 (17.96–37.66) | 26.47 (21.39–32.01) | 0.903 |
| LDL ≥ 160 mg/dL | 13.84 (6.87–23.63) | 10.64 (7.47–14.48) | 0.334 |
| Triglycerides ≥ 200 mg/dL | 29.5 (17.16–44.29) | 16.23 (11.75–21.49) | 0.028 |
| HbA1c (%) | 6.02 (5.85–6.20) | 5.97 (5.90–6.05) | 0.659 |
| Median CAP (IQR) | 310.92 (292; 344.02) | 314.70 (292.83; 347.41) | 0.5735 c |
| Median LSM (IQR) | 5.18 (4.30; 6.10) | 5.38 (4.30; 6.81) | 0.1146 c |
| ≥F2 | 10.01 (5.67–15.89) | 15.46 (11.54–20.01) | 0.103 |
| ≥F3 | 7.32 (3.65–12.66) | 10.07 (7.55–13.05) | 0.344 |
| F4 | 2.48 (0.64–6.24) | 5.02 (3.35–7.12) | 0.184 |
| FAST score | 0.18 (0.15–0.21) | 0.17 (0.16–0.18) | 0.390 |
| FAST score ≥ 0.67 | 3.43 (0.03; 6.83) | 2.93 (2.02; 3.83) | 0.7591 |
Note: Values show mean or percentage (95% CI). NAFLD is defined using CAP ≥ 274 dB/m. ≥F2, ≥F3, and F4 were defined by liver stiffness measurements ≥8.2, ≥9.7, and ≥13.6, respectively. Obesity is defined as BMI ≥ 27.5 kg/m2 for Asian Americans or BMI ≥ 30 kg/m2 for non‐Asian Americans.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; CI, confidence interval; FAST, FibroScan aspartate aminotransferase; F2, stage 2 fibrosis; F3, stage 3 fibrosis; F4, stage 4 fibrosis; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; IQR, interquartile range; LDL, low‐density lipoprotein; LSM, liver stiffness measurement; NAFLD, nonalcoholic fatty liver disease; WBC, white blood count.
Unweighted count, n = 183; weighted count, n = 3,426,780.
Unweighted count, n = 1390; weighted count, n = 66,451,377.
This comparison was conducted using Wilcoxon test.
Compared with non‐Asian Americans with NAFLD, Asian Americans with NAFLD were younger and had lower BMI, decreased waist circumference, and decreased previous smoking history. Sex, current smoking, and comorbidities, such as obesity, prediabetes, diabetes, and hypertension, were not significantly different between Asian Americans and non‐Asian Americans with NAFLD. Distribution of BMI categories in Asian Americans and non‐Asian Americans with NAFLD are shown in Figure 1. The prevalence of nonobese NAFLD in Asian Americans remained significantly higher than that in non‐Asian Americans (Asian Americans, 14.05%; 95% CI, 11.2%–16.9%; non‐Asian Americans, 7.9%; 95% CI, 5.4%–10.5%; p < 0.001). There was no significant difference in other BMI categories. Compared to non‐Asian Americans with NAFLD, Asian Americans with NAFLD exhibited lower alkaline phosphatase (ALP) and higher albumin, alanine transaminase (ALT), AST, and triglycerides.
FIGURE 1.
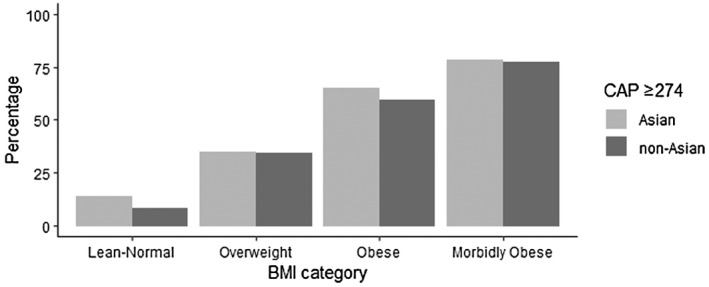
Distribution of prevalence of NAFLD across different BMI categories among Asian Americans versus non‐Asian Americans. The cutoff for NAFLD was defined as CAP ≥ 274 dB/m. Based on WHO BMI cut‐off points and proposed BMI cut‐off points for public health action in Asians, BMI categories were defined as ideal and normal weight (BMI < 23 kg/m2 Asian Americans, BMI < 25 kg/m2 non‐Asian Americans), overweight (BMI 23–27.4 kg/m2 Asian Americans, BMI 25–29.9 kg/m2 non‐Asian Americans), obese (BMI 27.5–32.4 kg/m2 Asian Americans, BMI 30–34.9 kg/m2 non‐Asian Americans), and morbidly obese (BMI ≥ 32.5 kg/m2 Asian Americans, BMI ≥ 35 kg/m2 non‐Asian Americans).[ 15 ] Prevalence was weighted to compute population estimate. BMI, body mass index; CAP, controlled attenuation parameter; NAFLD, nonalcoholic fatty liver disease; WHO, World Health Organization.
The prevalence of NAFLD in Asian Americans was 43.23%, using CAP ≥ 274 dB/m, with 10.01% with F2 or higher, 7.32% with F3 or higher, and 2.48% with F4. In addition, 3.43% of Asian Americans with NAFLD had a FAST score ≥ 0.67, consistent with NASH with significant fibrosis. The prevalence of these clinically significant fibrosis stages and NASH with significant fibrosis was not significantly different between Asian Americans and non‐Asian Americans with NAFLD (Table 1).
Risk factors of NAFLD among Asian Americans
Unadjusted odds ratios (ORs) of characteristics among Asian Americans with NAFLD are illustrated in Table S3. Univariable and multivariable regression, including characteristics among Asian Americans with NAFLD, is demonstrated in Table 2.
TABLE 2.
Independent predictors of NAFLD (CAP ≥ 274) among Asian Americans
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | aOR | 95% CI | p value | |
| Age (every 10 years) | 1.230 | 1.079–1.402 | 0.0042 | 1.144 | 0.910–1.439 | 0.2300 |
| Sex | ||||||
| Female | Reference | |||||
| Male | 1.882 | 1.418–2.498 | 0.0003 | 2.017 | 1.203–3.383 | 0.0111 |
| BMI | ||||||
| <27.5 kg/m2 | Reference | |||||
| ≥27.5 kg/m2 | 6.536 | 4.115–10.417 | <0.0001 | 6.507 | 4.143–10.221 | <0.0001 |
| Diabetes | ||||||
| No | Reference | |||||
| Yes | 4.760 | 0.122–0.363 | <0.0001 | 4.110 | 1.544–10.937 | 0.0077 |
| Hypertension | ||||||
| No | Reference | |||||
| Yes | 0.347 | 0.199–0.606 | 0.0011 | 2.101 | 1.177–3.751 | 0.0155 |
| Platelets (every 1000 cells/μL) | 1.002 | 1.000–1.004 | 0.2188 | 1.002 | 0.999–1.004 | 0.2188 |
| ALT (every 10 U/L) | 1.563 | 1.163–2.102 | 0.0058 | 1.304 | 1.016–1.674 | 0.0388 |
Note: NAFLD defined as CAP ≥ 274 dB/m.
Abbreviations: ALT, alanine aminotransferase; aOR, adjusted odds ratio; BMI, body mass index; CAP, controlled attenuation parameter; CI, confidence interval; NAFLD, nonalcoholic fatty liver disease; OR, odds ratio.
NAFLD in Asian Americans was associated with male sex (OR, 2.017; 95% CI, 1.203–3.383), BMI ≥ 27.5 kg/m2 (OR, 6.507; 95% CI, 4.143–10.221), diabetes (OR, 4.11; 95% CI, 1.544–10.937), hypertension (OR, 2.101; 95% CI, 1.177–3.751), and ALT (OR, 1.304; 95% CI, 1.016–1.674).
Risk factors of fibrotic NASH and advanced fibrosis in Asian Americans with NAFLD
Unadjusted ORs of characteristics among Asian Americans with fibrotic NASH and advanced fibrosis, together defined as CAP ≥ 274 dB/m and LSM ≥ 9.7 kPa, are illustrated in Table 3.
TABLE 3.
Unadjusted ORs with 95% CIs for CAP ≥ 274 dB/m and LSM ≥ 9.7 kPa among Asian Americans
| Variable | OR | 95% CI | p value |
|---|---|---|---|
| Age by 10 years | 1.45 | 0.97–2.16 | 0.0659 |
| Sex (male:female) | 2.258 | 0.497–10.261 | 0.2694 |
| BMI ≥ 27.5 kg/m2 | 5.314 | 0.734–38.492 | 0.0923 |
| Diabetes | 27.018 | 7.990–91.354 | <0.0001 |
| Hypertension | 14.153 | 3.440–58.231 | 0.0012 |
| Previous smoking history | 4.033 | 1.066–15.261 | 0.0412 |
| Platelets (cells/μL) | 0.989 | 0.982–0.995 | 0.0011 |
| ALT per 10 U/L | 1.434 | 1.212–1.697 | 0.0004 |
| ALP per 10 IU/L | 1.366 | 1.017–1.834 | 0.0394 |
| AST per 20 U/L | 3.111 | 1.220–7.935 | 0.0208 |
| Triglycerides per 10 mg/dL | 1.055 | 0.984–1.131 | 0.1225 |
Note: Fibrotic NASH and advanced fibrosis is defined as CAP ≥ 274 dB/m and LSM ≥ 9.7 kPa, respectively.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CAP, controlled attenuation parameter; CI, confidence interval; LSM, liver stiffness measurement; NASH, nonalcoholic steatohepatitis; OR, odds ratio.
Fibrotic NASH and advanced fibrosis in Asian Americans with NAFLD were significantly associated with diabetes (OR, 27.018; 95% CI, 7.990–91.354), hypertension (OR, 14.153; 95% CI, 3.440–58.231), previous smoking history (OR, 4.033; 95% CI, 1.066–15.261), decreased platelets (OR, 0.989; 95% CI, 0.982–0.995), and increased levels of ALT by 10 U/L (OR, 1.434; 95% CI, 1.212–1697), ALP by 10 IU/L (OR, 1.366; 95% CI, 1.017–1.834), and AST per 20 U/L (OR, 3.111; 95% CI, 1.220–7.935).
Sensitivity analysis using CAP cutoffs
A comparison of demographic and clinical variables between Asian Americans and non‐Asian Americans with NAFLD, defined as CAP ≥ 248 and ≥302 dB/m, for sensitivity analysis is shown in Table S4.
The prevalence of NAFLD in Asian Americans was 57.38%, using CAP ≥ 248 dB/m, with 8.20% with F2 or higher, 5.98% with F3 or higher, and 2.40% with F4, whereas the prevalence of NAFLD in Asian Americans was 28.03%, using CAP ≥ 302 dB/m, with 14.14% with F2 or higher, 9.74% with F3 or higher, and 3.38% with F4. The prevalence of NAFLD with FAST score ≥ 0.67 (NASH with significant fibrosis) was 2.50% and 5.78% in Asian Americans for CAP ≥ 248 and ≥302 dB/m, respectively. The prevalence of these clinically significant fibrosis stages and NASH with significant fibrosis was not significantly different between Asian Americans and non‐Asian Americans with NAFLD for either CAP ≥ 248 or ≥302 dB/m.
DISCUSSION
Among individuals with NAFLD, Asian Americans were younger, had a less prevalent smoking history, and had lower BMI and waist circumference but had a significantly higher prevalence of prediabetes and numerically higher prevalence of type 2 diabetes compared to non‐Asian Americans. As Asian Americans with NAFLD are younger, have a lower BMI and waist circumference, and have a decreased previous smoking history compared to non‐Asian Americans with NAFLD, this suggests that Asian Americans may be vulnerable to developing NAFLD; this would likely be secondary to a combination of genetics, cultural influence, and dietary choices. The vulnerability of Asian Americans to NAFLD is alarming as studies have demonstrated that less than 5% of non‐Hispanic Asians with NAFLD are aware of having liver disease.[ 16 ] Educational programs to enhance awareness and development of guidelines for NAFLD screening among susceptible individuals are essential for early detection and management.
In this large nationally representative study with VCTE‐proven NAFLD, Asian Americans had a NAFLD prevalence that was overall comparable to that of non‐Asian Americans; however, Asian Americans had a higher prevalence for NAFLD than non‐Asian Americans in the lean/normal BMI and morbidly obese categories, even after using the lower WHO BMI thresholds for Asian Americans.[ 15 ] Earlier NHANES studies that used the US‐fatty liver index (US‐FLI) showed that Asian Americans had the lowest prevalence of NAFLD (below 20%) among all ethnicities and races from 2011 to 2016.[ 9 ] In our current study with VCTE measurement and a CAP ≥ 274 dB/m cutoff, the prevalence of Asian Americans with NAFLD was about 40%, which is similar to estimates of NAFLD prevalence based on a recent prospective study that used magnetic resonance imaging proton density fat fraction in the general population in Texas.[ 17 ] There was no significant difference in NAFLD prevalence between Asian Americans and non‐Asian Americans. The differences between our study and Golabi et al.[ 9 ] could be explained by the increased prevalence of NAFLD among Asian Americans and the varied performance of diagnostic methods. Of note, the diagnostic accuracy of the US‐FLI for Asian Americans remains unknown as Asian Americans were not included when developing the US‐FLI.[ 18 ] Further studies validating the US‐FLI among Asian Americans are warranted.[ 16 , 18 ]
Metabolic abnormalities and diabetes are known risk factors associated with NAFLD. In this study, the prevalence of type 2 diabetes and prediabetes was higher overall in Asian Americans. Additionally, factors significantly associated with a higher risk of NAFLD in Asian Americans were male sex, diabetes, hypertension, and increased ALT, whereas risk factors of fibrotic NASH in Asian Americans with NAFLD included diabetes, hypertension, previous smoking history, decreased platelets, and increased levels of ALT, AST, and ALP. This suggests that Asian Americans may be predisposed to increased liver injury from metabolic abnormalities compared to non‐Asian Americans and that considerable effort should be made to control metabolic abnormalities or diseases in Asian Americans. The reasons why these factors increase risk are not completely understood. However, genetic or, more importantly, dietary factors, such as a carbohydrate‐enriched diet (e.g., rice), can contribute to this risk.[ 19 ] Indeed, because studies have shown that Asian populations experience increased risk of obesity‐related comorbidities, including diabetes, hypertension, and cardiovascular disease, at lower BMI cutoffs, WHO has proposed lower BMI cut‐off thresholds for public health action in Asians.[ 15 , 20 , 21 , 22 ] These lower BMI cutoffs redefine the ideal/normal weight, overweight, obese, and morbidly obese categories for Asians in order to identify those who are at increased risk for cardiometabolic diseases at an early stage. We have previously shown that Japanese Americans are at an increased risk of NAFLD and NAFLD cirrhosis.[ 2 ] Further studies investigating the genetic and dietary factors of other Asian American populations are warranted.
Despite lower BMI thresholds and a decreased prevalence of comorbid BMI ≥ 30 kg/m2, Asian Americans with NAFLD paradoxically exhibit significantly increased hepatocellular injury and triglyceridemia, as demonstrated by higher ALT/AST and higher triglycerides, respectively, compared to non‐Asian Americans with NAFLD. Notably, fibrosis stages, FAST scores, and the prevalence of FAST scores ≥ 0.67 were not significantly different between Asian Americans and non‐Asian Americans with NAFLD. This is not surprising as liver enzymes are not the best indicator of the degree of severity or damage (fibrosis) seen in patients with NAFLD.[ 23 ] One study that used the NAFLD fibrosis score indicated a lower risk of fibrosis in Asian Americans with NAFLD.[ 16 ] Another study, TARGET‐NASH, to analyze overall lean NAFLD found that the odds of cirrhosis in Asian Americans with lean NAFLD was almost half the odds of cirrhosis in non‐Asian Americans with lean NAFLD (OR, 0.47; 95% CI, 0.29–0.77) after adjusting for sex, age, and center type and site.[ 5 ] However, staging by liver biopsy was not available for all patients.[ 5 ] In contrast, a study that included biopsy‐proven NAFLD showed that Asian Americans had a trend toward more severe steatosis and inflammation but not fibrosis, although this study was limited by a small sample size.[ 24 ] Overall, our findings indicate that neither NASH with F2 or higher (as reflected by a FAST score and prevalence of a FAST score ≥ 0.67) nor clinically significant stages of fibrosis (F2 or higher, F3 or higher, or F4 as reflected by LSM) are less prevalent in Asian Americans compared to non‐Asian Americans. This is plausible given that the prevalence of prediabetes and type 2 diabetes was not less in the Asian American population.
This study has limitations. First, the NHANES measures of smoking were based on self‐reported data, which may be subject to recall bias. Nevertheless, self‐assessments of smoking status have been shown to be accurate and valid.[ 25 ] Second, the NHANES surveys lack genetic information that would allow improved differentiation among Asian Americans. Given the small sample sizes of Asian Americans, the assessment of NAFLD, its comorbidities, and its disease severity among individual Asian subgroups (such as the Chinese, Japanese, and Vietnamese populations), was not possible. However, to our knowledge, this cross‐sectional study encompasses the largest nationally representative population of Asian Americans to date with access to VCTE assessments that allow for accurate measurements of steatosis by CAP and fibrosis by LSM. As this study cohort is nationally representative, our study holds increased generalizability and applicability in the clinical setting.
In conclusion, the prevalence of NAFLD may vary based on the CAP score cutoff used in the Asian American population. Despite younger age, lower BMI cutoffs, and decreased prevalence of obesity, Asian Americans with NAFLD demonstrate similar disease prevalence with comparable severity of disease as evidenced by the FAST score, prevalence of FAST score ≥ 0.67, and LSM on VCTE compared to non‐Asian Americans with NAFLD. Given the diversity of cultures in the United States, culturally responsive health care specific to individual populations is necessary for diagnosis, evaluation, and treatment of NAFLD.
CONFLICTS OF INTEREST
Mazen Noureddin has been on the advisory board for 89BIO, Gilead, Intercept, Pfizer, Novartis, Novo Nordisk, Allergan, Blade, EchoSens, Fractyl, Terns, OWL, Siemens, Roche Diagnostic, and Abbott; he has received research support from Allergan, BMS, Gilead, Galmed, Galectin, Genfit, Conatus, Enanta, Madrigal, Novartis, Shire, Viking, and Zydus; he is a minor shareholder or has stock in Anaetos and Viking. Naim Alkhouri has been on the advisory board for Gilead and Allergan; he is on the speaker's bureau for Intercept and Gilead. Vinay Sundaram is on the speaker's bureau for Gilead, AbbVie, and Intercept and is a consultant for Saol Therapeutics. Vincent Wong has served as an advisory board member or consultant for 3 V‐BIO, AbbVie, Allergan, Boehringer Ingelheim, Echosens, Gilead Sciences, Inventiva, Merck, Novartis, Novo Nordisk, Pfizer, ProSciento, Sagimet Biosciences, TARGET PharmaSolutions, and Terns and is on the speaker's bureau for Abbott, AbbVie, Echosens, Gilead Sciences, and Novo Nordisk; he has received a grant from Gilead Sciences for fatty liver research and is a cofounder of Illuminatio Medical Technology Limited. The other authors have nothing to report.
AUTHOR CONTRIBUTIONS
Emily Truong and Yee Hui Yeo participated in the design of the study, interpreted the data, and drafted the manuscript. Galen Cook‐Wiens performed the statistical analysis. Mark Muthiah, Ju Dong Yang, Vinay Sundaram, Devon Chang, Tsuyoshi Todo, Irene K. Kim, Shelly C. Lu, Veronica Wendy Setiawan, Vincent W. S. Wong, Stephen A Harrison, and Naim Alkhouri interpreted the data and critically revised the manuscript for important intellectual content. Mazen Noureddin participated in the design of the study, supervised the study, interpreted the data, and drafted the manuscript. All authors read and approved the final manuscript.
Supporting information
Supplementary Figure 1 Flowsheet for Study Exclusion
Supplemental Table 1 Demographic and clinical characteristics among participants and non‐participants of VCTE examinations
Supplementary Table 2: Demographic and Clinical Characteristics among Asian Americans vs non‐Asian Americans
Supplementary Table 3: Unadjusted Odds Ratios of Characteristics among Asian Americans with NAFLD
Supplementary Table 4: Demographic and clinical characteristics among Asian Americans vs non‐Asian Americans with NAFLD using CAP cutoffs ≥ 248 dB/m and ≥302 dB/m
ACKNOWLEDGMENT
None.
Truong E, Yeo YH, Cook‐Wiens G, Muthiah M, Yang JD, Sundaram V, et al. Nonalcoholic fatty liver disease prevalence and severity in Asian Americans from the national health and nutrition examination surveys 2017–2018. Hepatol Commun. 2022;6:2253–2261. 10.1002/hep4.1981
Emily Truong and Yee Hui Yeo contributed equally to this work.
REFERENCES
- 1. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 2. Setiawan VW, Stram DO, Porcel J, Lu SC, Le Marchand L , Noureddin M. Prevalence of chronic liver disease and cirrhosis by underlying cause in understudied ethnic groups: the multiethnic cohort. Hepatology. 2016;64:1969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dietrich P, Hellerbrand C. Non‐alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28:637–53. [DOI] [PubMed] [Google Scholar]
- 4. Younes R, Bugianesi E. NASH in lean individuals. Semin Liver Dis. 2019;39:86–95. [DOI] [PubMed] [Google Scholar]
- 5. Weinberg EM, Trinh HN, Firpi RJ, Bhamidimarri KR, Klein S, Durlam J, et al. Lean Americans with nonalcoholic fatty liver disease have lower rates of cirrhosis and comorbid diseases. Clin Gastroenterol Hepatol. 2021;19:996–1008.e6. [DOI] [PubMed] [Google Scholar]
- 6. Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non‐alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2019;4:389–98. [DOI] [PubMed] [Google Scholar]
- 7. Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non‐obese or lean non‐alcoholic fatty liver disease: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2020;5:739–52. [DOI] [PubMed] [Google Scholar]
- 8. Budiman A, Ruiz N. Key facts about Asian Americans, a diverse and growing population. Washington, DC: Pew Research Center; 2021. [cited 2021 December]. https://www.pewresearch.org/fact‐tank/2021/04/29/key‐facts‐about‐asian‐americans/. [Google Scholar]
- 9. Golabi P, Paik J, Hwang JP, Wang S, Lee HM, Younossi ZM. Prevalence and outcomes of non‐alcoholic fatty liver disease (NAFLD) among Asian American adults in the United States. Liver Int. 2019;39:748–57. [DOI] [PubMed] [Google Scholar]
- 10. Zhang X, Heredia NI, Balakrishnan M, Thrift AP. Prevalence and factors associated with NAFLD detected by vibration controlled transient elastography among US adults: Results from NHANES 2017–2018. PLoS One. 2021;16:e0252164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Paulose‐Ram R, Burt V, Broitman L, Ahluwalia N. Overview of Asian American data collection, release, and analysis: National Health and Nutrition Examination Survey 2011–2018. Am J Public Health. 2017;107:916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1717–30. [DOI] [PubMed] [Google Scholar]
- 13. Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, et al. Individual patient data meta‐analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–30. [DOI] [PubMed] [Google Scholar]
- 14. Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, et al. FibroScan‐AST (FAST) score for the non‐invasive identification of patients with non‐alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362–73. Erratum in: Lancet Gastroenterol Hepatol. 2020;5:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. WHO Expert Consultation . Appropriate body‐mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–63. Erratum in: Lancet. 2004;363:902. [DOI] [PubMed] [Google Scholar]
- 16. Le MH, Yeo YH, Cheung R, Wong VWS, Nguyen MH. Ethnic influence on nonalcoholic fatty liver disease prevalence and lack of disease awareness in the United States, 2011–2016. J Intern Med. 2020;287:711–22. [DOI] [PubMed] [Google Scholar]
- 17. Harrison SA, Gawrieh S, Roberts K, Lisanti CJ, Schwope RB, Cebe KM, et al. Prospective evaluation of the prevalence of non‐alcoholic fatty liver disease and steatohepatitis in a large middle‐aged US cohort. J Hepatol. 2021;75:284–91. [DOI] [PubMed] [Google Scholar]
- 18. Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther. 2015;41:65–76. [DOI] [PubMed] [Google Scholar]
- 19. Tajima R, Kimura T, Enomoto A, Yanoshita K, Saito A, Kobayahi S, et al. Association between rice, bread, and noodle intake and the prevalence of non‐alcoholic fatty liver disease in Japanese middle‐aged men and women. Clin Nutr. 2017;36:1601–8. [DOI] [PubMed] [Google Scholar]
- 20. Pan WH, Flegal KM, Chang HY, Yeh WT, Yeh CJ, Lee WC. Body mass index and obesity‐related metabolic disorders in Taiwanese and US whites and blacks: implications for definitions of overweight and obesity for Asians. Am J Clin Nutr. Vol 79; Oxford: Oxford University Press; 2004. p. 31–9. [DOI] [PubMed] [Google Scholar]
- 21. Shai I, Jiang R, Manson JAE, Stampfer MJ, Willett WC, Colditz GA, et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20‐year follow‐up study. Diabetes Care. 2006;29:1585–90. [DOI] [PubMed] [Google Scholar]
- 22. Wen CP, Cheng TYD, Tsai SP, Chan HT, Hsu HL, Hsu CC, et al. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr. 2009;12:497–506. [DOI] [PubMed] [Google Scholar]
- 23. Gawrieh S, Wilson LA, Cummings OW, Clark JM, Loomba R, Hameed B, et al. Histologic findings of advanced fibrosis and cirrhosis in patients with nonalcoholic fatty liver disease who have normal aminotransferase levels. Am J Gastroenterol. 2019;114:1626–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tabibian JH, Lazo M, Durazo FA, Yeh HC, Tong MJ, Clark JM. Nonalcoholic fatty liver disease across ethno‐racial groups: do Asian‐American adults represent a new at‐risk population? J Gastroenterol Hepatol. 2011;26:501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self‐reported smoking: a review and meta‐analysis. Am J Public Health. 1994;84:1086–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Flowsheet for Study Exclusion
Supplemental Table 1 Demographic and clinical characteristics among participants and non‐participants of VCTE examinations
Supplementary Table 2: Demographic and Clinical Characteristics among Asian Americans vs non‐Asian Americans
Supplementary Table 3: Unadjusted Odds Ratios of Characteristics among Asian Americans with NAFLD
Supplementary Table 4: Demographic and clinical characteristics among Asian Americans vs non‐Asian Americans with NAFLD using CAP cutoffs ≥ 248 dB/m and ≥302 dB/m


