Abstract
Nonselective beta‐blockers are used as prophylaxis for variceal bleeding in patients with advanced chronic liver disease (ACLD). The acute hemodynamic response to intravenous propranolol (i.e., ≥10% reduction in hepatic venous pressure gradient [HVPG]) is linked to a decreased risk of variceal bleeding. In this study, we aimed to investigate the overall prognostic value of an acute response in compensated and decompensated ACLD. We analyzed the long‐term outcome of prospectively recruited patients with ACLD following a baseline HVPG measurement with an intraprocedural assessment of the acute hemodynamic response to propranolol. Overall, we included 98 patients with ACLD (mean ± SD age, 56.4 ± 11.5 years; 72.4% decompensated; 88.8% varices; mean ± SD HVPG, 19.9 ± 4.4 mm Hg) who were followed for a median of 9.6 (interquartile range, 6.5–18.2) months. Fifty‐seven patients (58.2%) demonstrated an acute hemodynamic response to propranolol that was associated with a decreased risk of variceal bleeding (at 12 months, 3.6% vs. 15% in nonresponder; log‐rank, p = 0.038) and hepatic decompensation (at 12 months, 23% vs. 33% in nonresponder; log‐rank, p = 0.096). On multivariate analysis, the acute response was an independent predictor of first/further hepatic decompensation (adjusted hazards ratio, 0.31; 95% confidence interval [CI], 0.13–0.70; p = 0.005). Importantly, there was a tendency toward a prolonged transplant‐free survival in acute responders compared to nonresponders (34.2; 95% CI, 29.2–39.2 vs. 25.2; 95% CI, 19.8–30.6 months; log‐rank, p = 0.191). Conclusions: Patients with ACLD who achieve an acute hemodynamic response to intravenous propranolol experience a lower risk of variceal bleeding and nonbleeding hepatic decompensation events compared to nonresponders. An assessment of the acute hemodynamic response to intravenous propranolol provides important prognostic information in ACLD.
While the acute haemodynamic response to intravenous propranolol has been linked to a reduced risk of variceal bleeding, its value in predicting first/further hepatic decompensation is unclear. In this study we observed that in addition to a decreased risk of variceal bleeding, patients with an acute haemodynamic response experienced a lower incidence of hepatic decompensation, which translated into a better survival. Thus, the acute response is a valuable prognostic tool that can be assessed in a single haemodynamic study, provides early risk stratification, and facilitates personalised therapy.
INTRODUCTION
The natural history of cirrhosis is characterized by the progression from a compensated to a decompensated stage.[ 1 ] The occurrence of hepatic decompensation, such as ascites development, variceal bleeding, or hepatic encephalopathy (HE), marks a watershed moment in the clinical course of advanced chronic liver disease (ACLD) and is associated with a steep increase in mortality.[ 1 ] The main pathophysiological driver of disease progression and subsequent decompensation in patients with ACLD is the development of portal hypertension.[ 2 ] Particularly the development of clinically significant portal hypertension (CSPH), defined by a hepatic venous pressure gradient (HVPG) ≥10 mm Hg, is paralleled by an increased risk of portal hypertension‐related complications.[ 2 , 3 ]
Nonselective beta‐blockers (NSBBs) are a cornerstone of the treatment of patients with portal hypertension.[ 4 ] In terms of monitoring the hemodynamic response to NSBB therapy and assessing the severity of portal hypertension, an invasive HVPG measurement is currently seen as the gold standard.[ 4 , 5 ] A reduction in HVPG values to below 12 mm Hg or a decrease in HVPG of 10% in primary prophylaxis[ 6 , 7 ] and 20% in secondary prophylaxis[ 8 , 9 ] indicate a chronic hemodynamic response to NSBB treatment. Achieving a hemodynamic response to NSBBs has been linked to a decreased incidence of variceal bleeding and other forms of hepatic decompensation as well as increased survival.[ 10 , 11 ] However, the assessment of the HVPG response to NSBB treatment requires a second invasive procedure and in some cases, patients may experience variceal bleeding prior to the second HVPG measurement.[ 12 ] This is of particular importance for patients on secondary prophylaxis for whom the short‐term rebleeding risk is high.[ 13 ]
Alternatively, evaluating the acute hemodynamic response to intravenous propranolol (≥10% decrease of HVPG) may help circumvent these limitations and potentially predict the chronic response to oral NSBB intake, thus allowing for early risk stratification within a single hemodynamic study.[ 7 , 14 ] This could substantially reduce the need for repeated HVPG measurements.[ 7 , 14 ] The prognostic role of an acute hemodynamic response to intravenous propranolol in primary[ 7 , 14 ] and secondary prophylaxis[ 14 ] has been assessed by two previous studies. Both studies yielded promising results and linked the acute response to a reduced incidence of variceal bleeding and rebleeding, as well as prolonged survival.[ 7 , 14 ] Furthermore, the acute hemodynamic response has also been linked to decreased rates of ascites formation, worsening of ascites,[ 6 , 7 ] refractory ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome.[ 6 ] However, the value of the acute response in predicting hepatic decompensation in general, and further decompensation in particular, has not yet been established.
We aimed to investigate the prognostic value of a single hemodynamic assessment of the acute response to intravenous propranolol in order to allow for a simplified assessment of NSBB benefits during a single invasive procedure and advance personalized NSBB therapy in patients with CSPH.
MATERIALS AND METHODS
Patient cohort and study design
In this study, we analyzed prospectively recruited patients with ACLD who underwent a baseline hemodynamic HVPG measurement combined with an assessment of the acute hemodynamic response to intravenous propranolol at the Hepatic Hemodynamic Laboratory of the Medical University of Vienna between January 2017 and March 2020.
ACLD was diagnosed based on HVPG and either unequivocal radiologic/clinical data, histologic findings, or vibration‐controlled transient elastography. All patients signed an informed consent form and were subsequently included in the Vienna Cirrhosis Study (VICIS; NCT03267615).
Inclusion criteria for this study were the presence of CSPH, the initiation of NSBB treatment (carvedilol or propranolol) following the baseline hemodynamic evaluation, and an adequate adherence to therapy, defined as an intake of NSBBs for more than 50% of the duration of the observation period (per‐protocol analysis). Patients with hepatocellular carcinoma, splanchnic venous thrombosis (including portal vein thrombosis), porto‐sinusoidal vascular disease, previous transjugular intrahepatic portosystemic shunt insertion, previous liver transplantation, active NSBB therapy at baseline, or a contraindication to NSBB therapy were excluded.
The study was approved by the local ethics committee at the Medical University of Vienna (EK1262/2017) and was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments as well as the guidelines for good scientific practice of the Medical University of Vienna.
Hemodynamic measurements
The HVPG measurement was performed by following a standardized protocol through the insertion of an angled‐tip balloon catheter[ 15 ] into the right internal jugular vein.[ 5 ] The catheter was advanced through the right atrium into the inferior vena cava under fluoroscopic guidance. Once the catheter had been placed into the lumen of a large hepatic vein, the vessel was occluded by inflating the attached balloon and the wedged hepatic venous pressure (WHVP) was measured. Both the correct catheter location and the wedge position were confirmed by x‐ray following the injection of a contrast medium. After measuring the WHVP, the balloon was deflated and the free hepatic venous pressure (FHVP) was measured. To maximize accuracy, all measurements were performed in triplicates. The HVPG was calculated by subtracting the FHVP from the WHVP. The mean of three calculations rounded to the nearest integer was used for all further analyses. After the measurement of WHVP and FHVP, the pressure within the inferior vena cava (IVCP) and the central venous pressure (CVP; measured from within the right atrium) were assessed. Permanent tracings were recorded for all parameters.
Following the baseline hemodynamic assessment, propranolol (0.15 mg/kg of body weight; aqueous propranolol‐hydrochloride solution [1 mg/ml] stabilized with citric acid) was administered intravenously by continuous infusion. All hemodynamic measurements were repeated 15 minutes after the administration of propranolol. In accordance with previous studies, we defined the acute hemodynamic response to propranolol as an HVPG decrease of ≥10%.[ 3 , 4 ] The baseline HVPG measurement was performed without active oral NSBB intake at the time of measurement, and any previous ongoing NSBB therapy was paused for at least 5 days before the HVPG evaluation.
A second hemodynamic measurement under oral NSBB therapy (carvedilol or propranolol) for the assessment of the chronic response was conducted in a subgroup of patients. Patients did not receive nitrates in addition to NSBB therapy. A decrease in HVPG by ≥10% or to <12 mm Hg was used to define a chronic treatment response.
Follow‐up
After the baseline HVPG measurement, including the intravenous propranolol response assessment, NSBB treatment with either propranolol or carvedilol was initiated. The choice of NSBB type was made by the treating physician and primarily based on clinical parameters, regardless of the presence of an acute hemodynamic response. In patients who did not demonstrate signs of circulatory dysfunction or refractory ascites, carvedilol was preferred as it has been shown to be more potent in decreasing portal pressure.[ 16 ] Carvedilol was started at a dose of 6.25 mg/day and, if tolerated, subsequently increased to a target dose of 12.5 mg/day.[ 17 ] Higher doses of carvedilol were only used in cases of concomitant arterial hypertension. In propranolol users, the dose was progressively increased until the heart rate decreased to approximately 55 beats per minute.[ 16 , 18 ] In patients with significant ascites, a maximum dose of 80 mg/day of propranolol was administered.[ 18 ]
Follow‐up examinations took place in the outpatient clinic, and all inpatient stays during the observation period were recorded and analyzed. Information regarding the death of patients was either available within the electronic health records or acquired from a nationwide database. Whenever possible, the cause of death was also recorded. Patients were censored at the time of death or liver transplantation.
The primary endpoints of this study were bleeding events from esophageal varices and other events that constitute hepatic decompensation as well as death from any cause. First hepatic decompensation in compensated ACLD (cACLD) or further hepatic decompensation in decompensated ACLD (dACLD) during the observation period were defined as (i) variceal bleeding or rebleeding, (ii) new onset or worsening of ascites, (iii) development of spontaneous bacterial peritonitis, defined by an ascitic polymorphonuclear leucocyte count >250 cells/μl, (iv) new onset or worsening of HE, or (v) liver‐related death. In this study, the definition of a new onset or aggravation of ascites was based on the necessity for paracentesis. Paracentesis is routinely performed in patients with a first onset of ascites as a diagnostic measure, and large‐volume paracentesis denotes a worsening of ascites in patients in whom ascites could previously be controlled with diuretic treatment alone. With regard to HE, the definitive clinical diagnosis of covert HE is difficult; we thus used the occurrence of a West Haven grade III/IV HE event to define a new onset, or in case of previous medication for HE, aggravation of HE. Liver‐related death was included as a decompensating event in order to accurately incorporate data from the nationwide mortality database because disease progression and decompensation before death but not the specific decompensating event are recorded in this database.
Statistical analysis
Statistical analyses were conducted using IBM SPSS 26 (SPSS Inc., Armonk, NY, USA) and R 4.1.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria). Figures were drawn using GraphPad Prism 9 (GraphPad Software, La Jolla, CA, USA). Continuous variables were reported as mean ± SD or as median and interquartile range (IQR) according to parametric distribution tested by the Shapiro‐Wilk test. Categorical variables were reported as the number and proportion of patients with a particular characteristic. Group comparisons for normally distributed data were performed using the Student t test, and not normally distributed data were compared using the Mann‐Whitney U test. Pearson's chi‐square test or Fisher's exact test were conducted to perform group comparisons of categorical data. For the comparison of paired metric variables, a paired t test or a Wilcoxon signed‐rank test was performed. To assess the correlation between metric variables, Spearman correlation coefficients were calculated. Factors associated with an acute hemodynamic response were assessed using a binary logistic regression model. Group comparisons regarding time‐dependent events were performed by the Kaplan‐Meier method and log‐rank test. To identify potential predictors of the occurrence of variceal bleeding, hepatic decompensation, or death during the observation period, univariate and multivariate Cox regressions were performed. All variables were initially assessed in a univariate analysis. Subsequently, variables associated with the event of interest (univariate p < 0.1), the presence of an acute hemodynamic response to intravenous propranolol, and typical parameters of clinical relevance were included in the multivariate model and analyzed using a stepwise backward approach. Additionally, all findings were further analyzed in univariate and multivariate Fine and Gray competing risk regression models. Competing events included (i) liver transplantation (all models), (ii) all‐cause mortality (analysis of variceal bleeding), and (iii) non‐liver‐related mortality (analysis of hepatic decompensation and of liver‐related mortality). Two‐sided p < 0.05 was considered statistically significant.
RESULTS
Acute hemodynamic response
During the study period, a baseline HVPG measurement was performed in 347 patients. Of those, 136 (39.2%) underwent an intraprocedural assessment of the acute hemodynamic response to intravenous propranolol. Nineteen patients presented with ≥1 exclusion criterion at baseline and therefore had to be excluded. During the follow‐up period, 19 patients failed to adhere to the NSBB treatment and subsequently had to be excluded from the study. Thus, a total of 98 patients were included. A detailed flow chart of the patient selection process is provided in Figure S1.
The mean age of the study cohort was 56.4 (SD: ±11.5) years, and 72.4% presented in a decompensated state at baseline. The mean HVPG at baseline was 19.9 (SD: ±4.4) mm Hg. A comprehensive overview of the clinical and hemodynamic characteristics of the study cohort at baseline is presented in Table 1. In order to adjust for differences in baseline characteristics, we additionally performed propensity score matching, the results of which are provided in the Supporting Materials.
TABLE 1.
Patient characteristics
| All patients N = 98 | Acute responders n = 57 | Acute nonresponders n = 41 | p value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Sex, male | 68 (69.4%) | 35 (61.4%) | 33 (80.5%) | 0.043* |
| Age, years | 56.39 ± 11.49 | 54.47 ± 11.54 | 59.05 ± 11.01 | 0.051 |
| Etiology | 0.202 | |||
| ALD | 51 (52%) | 25 (43.9%) | 26 (63.4%) | |
| Viral | 12 (12.2%) | 9 (15.8%) | 3 (7.3%) | |
| Mix (ALD/viral) | 12 (12.2%) | 9 (15.8%) | 3 (7.3%) | |
| Other | 23 (23.5%) | 14 (24.6%) | 9 (22.0%) | |
| Weight, kg | 79 (68.75–90)1 | 78 (65–86.75)1 | 84.47 ± 18.36 | 0.054 |
| Height, cm | 172.51 ± 9.391 | 170.89 ± 9.621 | 174.71 ± 8.7 | 0.047* |
| BMI, kg/m2 | 26.6 (22.7–29.8) | 24.7 (22.5–29.3) | 27.5 ± 4.6 | 0.108 |
| Varices | 87 (88.8%) | 51 (89.5%) | 36 (87.8%) | 1.000 |
| Decompensated disease | 71 (72.4%) | 43 (75.4%) | 28 (68.3%) | 0.435 |
| Previous variceal bleeding | 14 (14.3%) | 8 (14%) | 6 (14.6%) | 0.933 |
| Severe/refractory ascites | 18 (18.4%) | 10 (17.5%) | 8 (19.5%) | 0.804 |
| Previous HE episode | 10 (10.2%) | 4 (7%) | 6 (14.6%) | 0.312 |
| CPS | 7 (6–8) | 7 (6–8.5) | 7 (5.5–8) | 0.408 |
| CP stage (A/B/C) | 35.7%/52%/12.2% | 31.6%/52.6%/15.8% | 41.5%/51.2%/7.3% | 0.357 |
| UNOS MELD | 12 (10–16) | 12 (10–16) | 12 (9.5–15) | 0.905 |
| Liver stiffness, kPa | 48 (28.2–72.75)18 | 48 (28.3–72.5)8 | 45.7 (27.7–75)10 | 0.870 |
| Hemodynamic evaluation | ||||
| FHVP, mm Hg | 6.75 (3–10) | 6.5 (3.5–10) | 7 (3–9) | 0.726 |
| Acute change, % | +28.6 ± 134.8% a | +21.0 ± 34.3% a | +38.2 ± 200.5% a | 0.184 |
| WHVP, mm Hg | 26.57 ± 5.05 | 27.23 ± 5.19 | 25.66 ± 4.78 | 0.130 |
| Acute change, % | −2.7 ± 12.3% | −7.2 ± 5.6% | +3.0 ± 15.8% | <0.001* |
| HVPG, mm Hg | 19.90 ± 4.35 | 20.49 ± 4.34 | 19.07 ± 4.28 | 0.112 |
| Acute change, % | −10.1 ± 9.0% | −16.1 ± 5.2% | −1.6 ± 5.5% | <0.001* |
| HVPG ≥20 mm Hg | 53 (54.1%) | 33 (57.9%) | 20 (48.8%) | 0.372 |
| IVCP, mm Hg | 6 (3–9)8 | 6 (3–9)5 | 6 (2–8)3 | 0.422 |
| Acute change, % | +24.3 ± 132.9% a | +14.2 ± 32.3% a | +37.0 ± 197.5% a | 0.112 |
| CVP, mm Hg | 5 (2.75–7)16 | 6 (3–9)12 | 4 (1–5.5)4 | 0.013* |
| Acute change, % | +7.3 ± 22.1% a | +11.5 ± 28.0% a | +2.3 ± 9.7% a | 0.122 |
| Heart rate, bpm | 77 (68–90.5)1 | 79 (67.5–93.5) | 76 (68.5–88.5)1 | 0.758 |
| Acute change, % | −11.4 ± 17.6% | −12.1 ± 19.4% | −10.6 ± 15.3% | 0.538 |
| SAP, mm Hg | 136.18 ± 20.21 | 133.35 ± 20.79 | 140.2 ± 18.861 | 0.100 |
| Acute change, % | −5.7 ± 8.0% | −5.8 ± 7.5% | −5.5 ± 8.6% | 0.859 |
| DAP, mm Hg | 80.03 ± 11.611 | 78.68 ± 12.55 | 81.95 ± 9.961 | 0.174 |
| Acute change, % | −1.7 ± 9.5% | −2.6 ± 9.2% | −0.5 ± 9.9% | 0.325 |
| MAP, mm Hg | 101.14 ± 14.094 | 99.25 ± 14.061 | 103.92 ± 13.853 | 0.123 |
| Acute change, % | −4.4 ± 8.5% | −5.3 ± 7.9% | −3.1 ± 9.3% | 0.272 |
| NSBB prescribed | 0.826 | |||
| Carvedilol | 61 (62.2%) | 36 (63.2%) | 25 (61.0%) | |
| Propranolol | 37 (37.8%) | 21 (36.8%) | 16 (39.0%) | |
| Baseline laboratory parameters | ||||
| Hemoglobin, mg/dl | 11.47 ± 2.03 | 11.23 ± 2.07 | 11.81 ± 1.95 | 0.164 |
| Platelets, G/L | 98.5 (69–127) | 94 (62–123.5) | 111.46 ± 49.07 | 0.230 |
| White blood cells, G/L | 4.5 (3.57–6.02) | 4.25 (3.17–5.39) | 4.83 (3.96–6.31) | 0.076 |
| Sodium, mmol/L | 138 (136–141) | 138 (135.5–140.5) | 138 (136–141) | 0.573 |
| Creatinine, mg/dl | 0.71 (0.58–1) | 0.63 (0.54–0.82) | 0.85 (0.62–1.06) | 0.004* |
| Bilirubin, mg/dl | 1.09 (0.82–2.1) | 1.06 (0.81–2.16) | 1.2 (0.85–2.13) | 0.837 |
| Albumin, g/L | 34.5 ± 5.15 | 34.02 ± 5.38 | 36.2 (32.05–38.7) | 0.183 |
| Prothrombin time, % | 51.88 ± 13.7732 | 51.5 ± 14.9617 | 52.46 ± 11.9815 | 0.784 |
| CRP, mg/dl | 0.37 (0.16–0.83)1 | 0.28 (0.14–0.76)1 | 0.43 (0.19–0.97) | 0.443 |
Notes: Data are listed as number n (%), mean ± SD, or median (IQR). Missing data are noted as a superscript number. Three acute responders were not followed at our center after the baseline hemodynamic assessment.
Abbreviations: ALD, alcohol‐related liver disease; BMI, body mass index; bpm, beats per minute; CPS, Child‐Pugh score; CRP, C‐reactive protein; CVP, central venous pressure; DAP, diastolic arterial pressure; FHVP, free hepatic venous pressure; HE, hepatic encephalopathy; HVPG, hepatic venous pressure gradient; IVCP, inferior vena cava pressure; MAP, mean arterial pressure; MELD, Model for End‐Stage Liver Disease; NSBB, nonselective beta‐blocker; SAP, systolic arterial pressure; UNOS, United Network for Organ Sharing; WHVP; wedged hepatic venous pressure.
Patients with 0 mm Hg at baseline assessment had to be excluded in order to analyze percentage change.
Statistically significant at p < 0.05.
Overall, 57 patients (58.2%) demonstrated an acute response to intravenous propranolol, defined by a decrease in HVPG of ≥10%, and were consequently classified as acute responders (ivP‐Rs). Besides a lower proportion of male patients, a lower height, lower creatinine levels, and a higher CVP among hemodynamic responders, there were no significant differences in baseline characteristics between ivP‐Rs and nonresponders (ivP‐NRs).
Using a binary logistic regression model, a univariate analysis was performed to detect variables linked to an acute response. Sex (male vs. female; odds ratio [OR], 0.39; 95% confidence interval [CI], 0.15–0.99; p = 0.047), weight (per kg; OR, 0.98; 95% CI, 0.95–1.00; p = 0.039), CVP (per mm Hg; OR, 1.18; 95% CI, 1.03–1.35; p = 0.015), and baseline creatinine levels (per mg/dl; OR, 0.27; 95% CI, 0.08–0.91; p = 0.035) were significantly associated with an increased probability of achieving a hemodynamic response. In the multivariate analysis, only CVP (per mm Hg; adjusted OR [aOR], 1.24; 95% CI, 1.07–1.45; p = 0.006) and creatinine levels (per mg/dl; aOR, 0.25; 95% CI, 0.07–0.97; p = 0.046) remained significant predictors of an acute response (Table S1).
Following the acute administration of intravenous propranolol, FHVP increased while heart rate and systolic arterial pressure (SAP) decreased significantly in both ivP‐Rs and ivP‐NRs. However, only ivP‐Rs demonstrated a significant reduction in WHVP, diastolic arterial pressure (DAP), and mean arterial pressure (MAP), combined with a significant increase in CVP and IVCP compared to the baseline measurement (Table S2). A decrease in HVPG did not correlate with a decrease in heart rate (Spearman's ρ = 0.154; p = 0.174) or with a decrease in MAP (Spearman's ρ = 0.156; p = 0.179).
Chronic hemodynamic response and concordance with acute hemodynamic response
After the initial hemodynamic assessment, carvedilol was administered in 63.2% of ivP‐Rs and 61% of ivP‐NRs. In order to assess the chronic hemodynamic response to NSBB therapy, a second hemodynamic study was performed in 54 patients (58.2%; n = 31 ivP‐Rs [54.4%] and n = 23 ivP‐NRs [56.1%]). The second measurement was performed after a median time of 9.4 (IQR: 5.0–18.1) weeks. There were no significant differences in the baseline characteristics of the patients who underwent a second hemodynamic assessment and those who did not. Overall, 30 patients (55.6% of those with a second measurement) demonstrated a decrease in HVPG by ≥10% or to <12 mm Hg and were thus classified as chronic responders. Of note, a decrease in HVPG to <12 mm Hg was achieved in two patients, both of whom also showed a decrease of ≥10%. While DAP and heart rate decreased significantly under NSBB therapy in chronic responders and nonresponders, a significant reduction in WHVP, SAP, and MAP only occurred in chronic responders (Table S3). At baseline, there were no significant differences between chronic responders and nonresponders, except for lower levels of DAP (mean ± SD: 78.1 ± 8.3 vs. 83.6 ± 9.9 mm Hg; p = 0.031) and sodium (136.33 ± 5.92 vs. 139.08 ± 3.26 mmol/L; p = 0.035) in chronic responders (Table S4). Endoscopic band ligation for high‐risk varices was performed in 45.8% of chronic nonresponders.
Of all ivP‐Rs that underwent a second hemodynamic assessment, 20 patients (64.5%) also demonstrated a chronic response to NSBB treatment. However, an acute response did not predict a chronic response in univariate analysis using binary logistic regression (OR, 2.36; 95% CI, 0.78–7.14; p = 0.127). While acute responders also tended to demonstrate a more pronounced HVPG decrease at the chronic response measurement, this finding did not reach statistical significance. Importantly, ivP‐NR treated with carvedilol achieved a chronic response in 50% of cases, while a chronic response only occurred in 28.6% of acute nonresponders who were subsequently treated with propranolol. Additional data pertaining to the chronic hemodynamic response assessment is provided in the Supporting Materials.
Variceal bleeding according to acute hemodynamic response
Following the baseline evaluation, patients were followed up for a median of 9.6 (IQR: 6.5–18.2) months. Eighty‐seven patients (88.8%) presented with esophageal varices at baseline and were included in the analysis of bleeding events. Six patients (6.9%) suffered a variceal bleeding event during the follow‐up period, all in the setting of primary prophylaxis. Of these events, five occurred in acute nonresponders and one in an acute responder. Accordingly, ivP‐Rs demonstrated a significantly lower cumulative probability of variceal bleeding, as evidenced by a 24‐month bleeding risk of 3.6% in ivP‐Rs compared to 14.9% in ivP‐NRs (log‐rank p = 0.038; Figure 1). Additional risk factors associated with an increased risk of variceal bleeding were assessed in a univariate Cox regression analysis. This model revealed a significant link between increased risk of variceal bleeding and high baseline levels of HVPG (hazard ratio [HR], 1.207; 95% CI, 1.019–1.430; p = 0.029) and C‐reactive protein (CRP; HR, 1.588; 95% CI, 1.028–2.454; p = 0.037). Both variables were subsequently included in a multivariate model, together with the acute hemodynamic response and additional hemodynamic and clinical parameters of prognostic importance. In this multivariate analysis, only the baseline HVPG (adjusted HR [aHR], 1.385; 95% CI, 1.108–1.731; p = 0.004) and the presence of an acute response (aHR, 0.042; 95% CI, 0.004–0.487; p = 0.011) were independently predictive of a variceal bleeding event (Table 2). Importantly, we observed a similar trend in our univariate (subdistribution HR [SHR], 0.141; 95% CI, 0.018–1.130; p = 0.065) and multivariate (adjusted SHR [aSHR], 0.047; 95% CI, 0.002–1.350; p = 0.074; adjusted for baseline HVPG) competing risk regression models.
FIGURE 1.
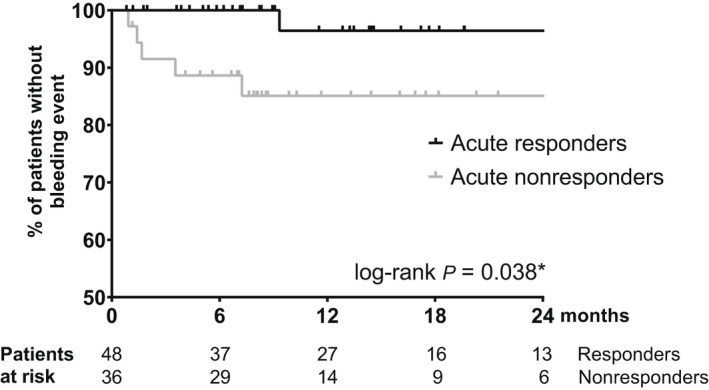
Variceal (re‐)bleeding stratified by acute hemodynamic response. *, significant.
TABLE 2.
Predictors of variceal bleeding
| Univariable analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | aHR | 95% CI | p value | |
| Age (per year) | 0.960 | 0.893–1.032 | 0.266 | |||
| Large varices (vs. small) | 1.591 | 0.291–8.710 | 0.592 | |||
| CPS (per point) | 1.216 | 0.818–1.810 | 0.333 | |||
| Baseline HVPG (per mm Hg) | 1.207 | 1.019–1.430 | 0.029* | 1.385 | 1.108–1.731 | 0.004* |
| Baseline MAP (per mm Hg) | 0.989 | 0.931–1.052 | 0.730 | |||
| Acute hemodynamic response | 0.141 | 0.017–1.213 | 0.074 | 0.042 | 0.004–0.487 | 0.011* |
| Sodium (per mmol/L) | 0.892 | 0.770–1.032 | 0.125 | |||
| Creatinine (per mg/dl) | 1.190 | 0.161–8.795 | 0.865 | |||
| CRP (per mg/dl) | 1.588 | 1.028–2.454 | 0.037* | |||
Note: The final step of the multivariate logistic regression analysis is shown.
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; CPS, Child‐Pugh score; CRP, C‐reactive protein; HR, hazard ratio; HVPG, hepatic venous pressure gradient; MAP, mean arterial pressure.
Statistically significant at p < 0.05.
During the follow‐up period, variceal ligation was performed in 12 patients without an acute hemodynamic response to propranolol and with varices at baseline (33.3%; nine under primary prophylaxis and three under secondary prophylaxis). Of note, of the five variceal bleeding events that occurred in nonresponders during the study period, four occurred in patients without variceal ligation during follow‐up and one in a patient with variceal ligation. In a univariate analysis within this subgroup, variceal ligation did not reach statistical significance with regard to a potential decrease in the risk of variceal bleeding (HR, 0.430; 95% CI, 0.048–3.864; p = 0.451).
Hepatic decompensation according to acute hemodynamic response
At baseline, 72.4% of all patients presented with dACLD, with ascites being the most common cause of previous decompensation. During follow‐up, 14 ivP‐Rs (24.6%) and 16 ivP‐NRs (39%) suffered an episode of first or further hepatic decompensation. In the overall study population, the leading cause of hepatic decompensation was the development or worsening of hepatic encephalopathy (30%). Patients who experienced decompensation during the observation period showed a significantly higher Child‐Pugh score (CPS), HVPG, CRP, creatinine, and interleukin‐6 (IL‐6) and a lower hemoglobin level at baseline (Table S5). Compared to ivP‐NR, the likelihood of developing any form of hepatic decompensation tended to be lower in ivP‐R, with a 22.8% versus 33.1% cumulative decompensation rate at 1 year and 22.8% versus 48.8% at 2 years, respectively (log‐rank p = 0.096; Figure 2A). In a multivariate analysis, in addition to the baseline HVPG, the presence of an acute hemodynamic response was independently predictive of hepatic decompensation (aHR, 0.306; 95% CI, 0.133–0.701; p = 0.005; Table 3). This finding also remained true in the multivariate competing risk regression analysis (aSHR, 0.340; 95% CI, 0.151–0.765; p = 0.009; Table S6).
FIGURE 2.
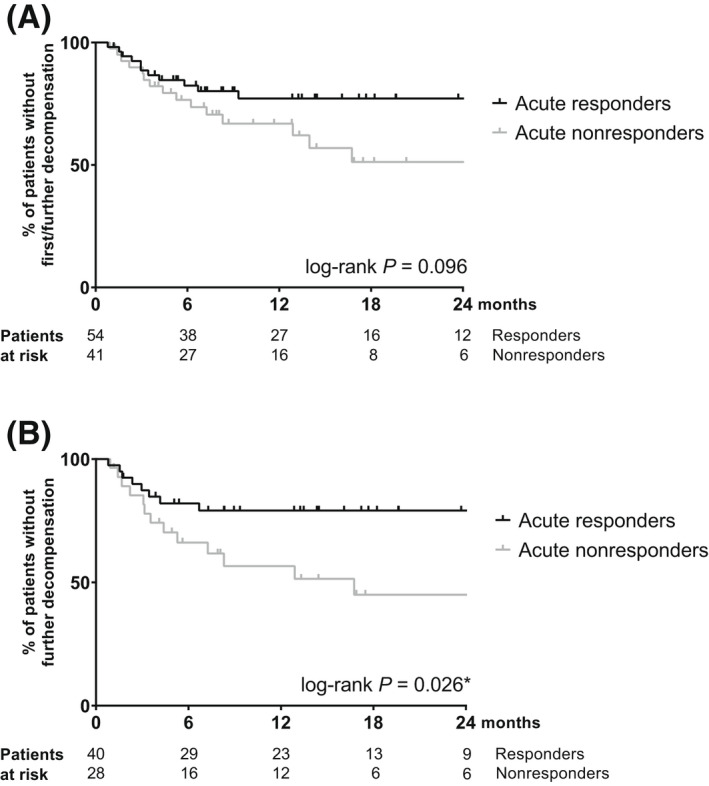
Hepatic decompensation stratified by acute hemodynamic response. (A) Overall cohort. (B) Patients with previous decompensation. *, significant.
TABLE 3.
Predictors of hepatic decompensation
| Univariable analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | aHR | 95% CI | p value | |
| Age (per year) | 1.007 | 0.975–1.040 | 0.689 | |||
| CPS (per point) | 1.251 | 1.044–1.498 | 0.015* | 1.244 | 0.996–1.553 | 0.054 |
| Baseline HVPG (per mm Hg) | 1.103 | 1.016–1.197 | 0.019* | 1.102 | 1.005–1.209 | 0.040* |
| Baseline MAP (per mm Hg) | 0.969 | 0.939–0.999 | 0.043* | 0.967 | 0.932–1.002 | 0.065 |
| Acute hemodynamic response | 0.541 | 0.260–1.127 | 0.101 | 0.306 | 0.133–0.701 | 0.005* |
| Sodium (per mmol/L) | 0.963 | 0.895–1.036 | 0.314 | |||
| Creatinine (per mg/dl) | 1.577 | 0.671–3.706 | 0.294 | |||
| CRP (per mg/dl) | 1.258 | 0.967–1.638 | 0.088 | |||
Note: The final step of the multivariate logistic regression analysis is shown.
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; CPS, Child‐Pugh score; CRP, C‐reactive protein; HR, hazard ratio; HVPG, hepatic venous pressure gradient; MAP, mean arterial pressure.
Statistically significant at p < 0.05.
When considering patients with dACLD separately, the prognostic value of an acute response became even more apparent. The probability of further decompensation during follow‐up was significantly lower in ivP‐Rs compared to ivP‐NRs, with 20.9% versus 43.4% decompensating further at 1 year and 20.9% versus 55% at 2 years, respectively (log‐rank p = 0.026; Figure 2B). Furthermore, the acute response remained an independent predictor of further decompensation in the multivariate model (aHR, 0.229; 95% CI, 0.088–0.594; p = 0.002; Table S7).
Transplant‐free survival according to acute hemodynamic response
During the observation period, 20 patients (20.5%) died; nine were ivP‐Rs (15.8%) and 11 ivP‐NRs (26.8%). The cause of death was liver‐related in 70% of all cases (77.8% in ivP‐Rs; 63.6% in ivP‐NRs). The majority of non‐liver‐related deaths were related to cardiovascular events, namely cardiac arrest in two patients, stroke in one patient, and postsurgical complications in two patients. In one patient, the cause of death was unknown.
All‐cause mortality tended to be reduced in ivP‐Rs, with a mean survival time of 34.2 (95% CI, 29.2–39.2) months compared to 25.2 (95% CI, 19.8–30.6) months for ivP‐NRs (log‐rank p = 0.191) (Figure 3A). The survival benefit associated with an acute response seemed more pronounced in patients with dACLD at baseline, as shown by an 83.9% survival rate in ivP‐Rs versus 54.5% in ivP‐NR after 2 years (log‐rank p = 0.061) (Figure 3B). In the overall cohort, a higher CPS (aHR, 1.520; 95% CI, 1.157–1.998; p = 0.003), a lower baseline MAP (aHR, 0.943; 95% CI, 0.900–0.989; p = 0.016), and the presence of an acute hemodynamic response (aHR, 0.297; 95% CI, 0.109–0.811; p = 0.018) were independent predictors of transplant‐free survival (Table S8). Importantly, an acute response remained a significant predictor of all‐cause mortality in a multivariate competing risk model adjusted for CPS and MAP (aSHR, 0.294; 95% CI, 0.097–0.885; p = 0.029).
FIGURE 3.
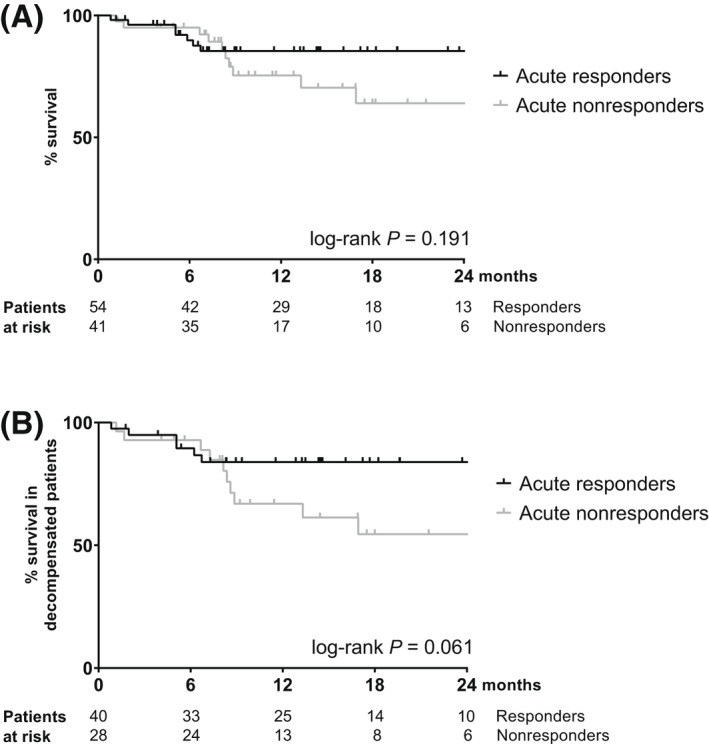
Survival stratified by acute hemodynamic response. (A) Overall cohort. (B) Patients with previous decompensation.
With regard to the analysis of liver‐related mortality, we observed a significantly reduced risk in ivP‐Rs in a multivariate Cox regression model adjusted for CPS and MAP (aHR, 0.291; 95% CI, 0.088–0.995; p = 0.042). Similarly, in a multivariate competing risk model adjusted for CPS and MAP, an acute hemodynamic response was associated with a reduced risk of liver‐related mortality (aSHR, 0.336; 95% CI, 0.095–1.189; p = 0.091).
DISCUSSION
Our study demonstrates that the acute hemodynamic response to intravenous propranolol in patients with ACLD and CSPH is associated with a significantly reduced risk of variceal bleeding and hepatic decompensation, which also translates into a trend toward an increased probability of survival.
The value of an acute hemodynamic response for the prediction of variceal bleeding in the setting of primary[ 7 , 14 ] and secondary prophylaxis[ 14 ] has been examined by two previous studies. These demonstrated that achieving an acute response is linked to a decreased risk of variceal bleeding. In the study performed by La Mura et al.,[ 14 ] which, similarly to our study, included patients on primary and secondary prophylaxis, the incidence of variceal bleeding or rebleeding at 1 year was 7% in acute responders and 21% in nonresponders. Accordingly, we also found a decreased risk of variceal bleeding in ivP‐Rs, specifically a 1‐year cumulative bleeding probability of 4% in ivP‐Rs compared to 15% in ivP‐NRs. Importantly, achieving an acute hemodynamic response remained independently predictive of variceal bleeding on multivariate analysis. In accordance with previous studies, our results indicate that an acute hemodynamic response is protective with regard to variceal bleeding and thus provides prognostic information which might allow physicians to detect patients at an increased risk of treatment failure.
Another significant finding of our study is that the risk of hepatic decompensation was reduced by more than 50% in ivP‐Rs. The decreased rate of (further) decompensation was most apparent in the cohort of patients with previously decompensated disease. In light of the considerable 5‐year mortality following further decompensation, estimated at up to 88%,[ 19 ] this finding is of particular importance. Interestingly, the leading cause of hepatic decompensation in our overall collective was a new onset or worsening of HE. A possible explanation for this observation is that a majority of patients included in our study presented with ascites at inclusion and the need for diuretic treatment may have promoted the development of HE.[ 20 ] Furthermore, because only the first decompensating event was recorded, it is possible that a subsequent development or worsening of ascites may have occurred.
Our results are in line with previous research, as Villanueva et al.[ 7 ] observed a lower probability of developing ascites (i.e., a form of hepatic decompensation) in ivP‐Rs as well. However, the impact on other forms of hepatic decompensation was not discussed in this study, and the authors did not specify the diagnostic criteria for ascites. Thus it is unclear whether a worsening of preexisting ascites was included in their definition. A more comprehensive assessment of the risk of developing ascites in patients with compensated cirrhosis was provided by Hernández‐Gea and colleagues.[ 6 ] They not only observed a significantly reduced risk of de novo ascites formation in ivP‐Rs but also demonstrated a decrease in the incidence of ascites‐related complications, including refractory ascites and hepatorenal syndrome.[ 6 ] In addition to the aforementioned studies, the value of an HVPG‐guided therapy with regard to the risk of further hepatic decompensation was evaluated in another study that used the acute hemodynamic response to guide the choice of treatment in patients after a variceal bleeding event.[ 21 ] There, Villanueva et al.[ 21 ] demonstrated a significant reduction in the risk of hepatic decompensation in acute hemodynamic responders, which was also reflected by a higher probability of survival. However, the treatment regimen varied greatly between the groups. While acute responders were initially treated with nadolol alone, nonresponders and the control group received a combination therapy consisting of nadolol, nitrates, and variceal ligation.[ 21 ] In light of the abovementioned limitations of these previous publications, our study provides new insights into the prognostic role of an acute hemodynamic response with regard to all causes of first/further hepatic decompensation and particularly highlights its relevance in patients with decompensated disease.
Importantly, the Prevent Decompensation of Cirrhosis in Patients With Clinically Significant Portal Hypertension (PREDESCI) study also used an acute hemodynamic response‐guided approach and compared NSBB treatment—specifically propranolol for ivP‐Rs and carvedilol for ivP‐NRs—to placebo. Interestingly, they not only found a reduced incidence of decompensation or death in the active treatment arm but also showed a more pronounced decrease in HVPG and a slightly more beneficial outcome in patients treated with carvedilol (i.e., acute ivP‐NRs).[ 22 ] In contrast, we primarily administered carvedilol following the baseline hemodynamic measurement regardless of whether a patient demonstrated an acute response to intravenous propranolol or not as long as the patient did not show clinical signs of circulatory dysfunction or refractory ascites. This may have decreased the bias of the outcome comparison between responders and nonresponders because the choice of NSBB treatment regimen during follow‐up remained largely unaffected by the acute response. When compared to traditional NSBBs, such as nadolol and propranolol, which have been used in the majority of previous studies evaluating the value of an acute response,[ 6 , 7 , 14 , 21 ] carvedilol has been shown to be more efficient at decreasing portal pressure, due to its additional anti‐α1‐adrenergic effect.[ 16 ]
In addition to a significantly decreased risk of variceal bleeding and hepatic decompensation, we observed a trend toward a prolonged transplant‐free survival in ivP‐Rs. These results are in accordance with previous studies, which suggest a survival benefit for acute responders.[ 7 , 14 , 21 ]
Establishing the acute response assessment as a tool for early risk stratification and estimation of treatment efficacy during a single hemodynamic study is more cost‐ and time‐effective, as well as less invasive than two consecutive HVPG measurements with and without NSBB therapy. Furthermore, chronic response evaluations are susceptible to the dynamics of the underlying liver disease (e.g., alcohol abstinence[ 23 ] or sustained virological response[ 24 , 25 , 26 ]). Although the evolution of the underlying liver disease may also confer prognostic information and may theoretically strengthen the prognostic value of the chronic hemodynamic response status,[ 27 ] it hinders the assessment of a potential benefit of NSBB therapy in the individual patient. This is particularly relevant in the setting of hemodynamic response‐guided treatment approaches, as applied in the PREDESCI study.[ 22 ]
In order to evaluate the chronic response to NSBB treatment, a second hemodynamic measurement was performed in a subset of patients. Even though acute responders demonstrated a tendency toward a more pronounced decrease in HVPG at the second measurement, this finding did not reach statistical significance. Even in previous studies, which used conventional NSBBs for both assessments, the correlation between the acute and chronic response was not always consistent. While Villanueva et al. observed a significant correlation and demonstrated that 85% of acute responders were also chronic responders,[ 7 ] La Mura et al.[ 14 ] found that the acute response persisted in only 35% of all acute responders. Thus, a possible correlation between an acute and chronic response, as well as potential influences on the change of response status, warrant further research. Importantly, even though we did not detect a significant correlation between the acute and chronic decrease in HVPG, we observed that ivP‐NRs treated with carvedilol achieved a chronic hemodynamic response in 50% of all cases. This finding is in line with our previous study[ 28 ] demonstrating that carvedilol can be used to achieve a hemodynamic response in 56% of oral propranolol nonresponders. Interestingly, NSBB therapy may also ameliorate intestinal permeability[ 29 ] and systemic inflammation,[ 30 ] at least in part independent from their hemodynamic effects.
The main limitations of our study are linked to the heterogeneous study cohort because we included patients with compensated and decompensated disease and patients with and without a history of variceal bleeding. Therefore, our findings might not apply to all patients with ACLD, and further studies in specific subgroups are required. Importantly, all patients were included in the prospective VICIS study[ 31 ] and thus followed up closely at our center. Nevertheless, as the VICIS study is an observational study, there was no fixed study protocol defining when to perform an acute hemodynamic response measurement, or what subsequent NSBB treatment regimen to use. Finally, the duration of follow‐up was short, and the limited number of events may have precluded statistical significance in some analyses that only found nonsignificant trends.
In conclusion, our study demonstrates the important prognostic value of an acute hemodynamic response to intravenous propranolol, which can be evaluated during a single hemodynamic study. Acute responders experienced a considerably lower incidence of variceal bleeding, further hepatic decompensation, and even showed a tendency toward a prolonged transplant‐free survival. Thus, our results suggest that the assessment of the acute hemodynamic response allows for early risk stratification and may facilitate personalized therapy.
AUTHOR CONTRIBUTIONS
Benedikt S. Hofer, Mattias Mandorfer, and Thomas Reiberger contributed to study concept and design, analysis, and manuscript draft. All authors contributed to data acquisition and interpretation, critically revised the draft, and read and approved the final manuscript.
FUNDING INFORMATION
The study was co‐supported by the Austrian Federal Ministry for Digital and Economic Affairs, the National Foundation for Research, Technology and Development, the Christian Doppler Research Association, and Boehringer Ingelheim.
CONFLICT OF INTEREST
Benedikt Simbrunner received travel support from AbbVie and Gilead. Bernhard Scheiner received travel support from Gilead, AbbVie, and Ipsen. Albert F. Staettermayer served as a speaker and/or consultant and/or advisory board member for Boehringer Ingelheim, Gilead, and MSD. Michael Trauner received grant support from Albireo, Alnylam, Cymabay, Falk, Gilead, Intercept, MSD, Takeda, and UltraGenyx; he received honoraria for consulting from Albireo, Boehringer Ingelheim, BiomX, Falk, Genfit, Gilead, Intercept, MSD, Novartis, Phenex, Pliant, Regulus, and Shire; he received speaker fees from Bristol‐Myers Squibb, Falk, Gilead, Intercept, and MSD as well as travel support from AbbVie, Falk, Gilead, and Intercept; he is coinventor of patents on the medical use of 24‐noursodeoxycholic acid. Mattias Mandorfer served as a speaker and/or consultant and/or advisory board member for AbbVie, Collective Acumen, Gilead, and W. L. Gore & Associates and received travel support from AbbVie and Gilead. Thomas Reiberger received grant support from AbbVie, Boehringer‐Ingelheim, Gilead, Philips Healthcare, Siemens, Pliant, and Gore; he received speaking honoraria from AbbVie, Gilead, Gore, Intercept, Roche, and MSD; he received consulting/advisory board fees from AbbVie, Boehringer‐Ingelheim, Gilead, and Siemens and travel support from Gilead and AbbVie. The other authors declare no conflict of interest.
CLINICAL TRIAL NUMBER
Supporting information
Appendix S1.
ACKNOWLEDGMENT
The financial co‐support by the Austrian Federal Ministry for Digital and Economic Affairs, the National Foundation for Research, Technology and Development, the Christian Doppler Research Association, and Boehringer Ingelheim is gratefully acknowledged. Furthermore, we would like to thank Kerstin Zinober and Martha Seif for their excellent support for the Vienna Cirrhosis Study (VICIS). We also thank the nurses of the Vienna Hepatic Hemodynamic Laboratory for their professional assistance and care for the patients during the HVPG measurements.
Hofer BS, Simbrunner B, Bauer DJM, Paternostro R, Schwabl P, Scheiner B, et al. Acute hemodynamic response to propranolol predicts bleeding and nonbleeding decompensation in patients with cirrhosis. Hepatol Commun. 2022;6:2569–2580. 10.1002/hep4.2021
REFERENCES
- 1. D'Amico G, Morabito A, D'Amico M, Pasta L, Malizia G, Rebora P, et al. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68(3):563–76. [DOI] [PubMed] [Google Scholar]
- 2. Ripoll C, Groszmann R, Garcia‐Tsao G, Grace N, Burroughs A, Planas R, et al. Hepatic venous pressure gradient predicts clinical decompensation in patients with compensated cirrhosis. Gastroenterology. 2007;133(2):481–8. [DOI] [PubMed] [Google Scholar]
- 3. Simbrunner B, Beer A, Wöran K, Schmitz F, Primas C, Wewalka M, et al. Portal hypertensive gastropathy is associated with iron deficiency anemia. Wien Klin Wochenschr. 2020;132(1–2):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Franchis R, Baveno VI Faculty . Expanding consensus in portal hypertension: report of the Baveno VI Consensus Workshop: stratifying risk and individualizing care for portal hypertension. J Hepatol. 2015;63(3):743–52. [DOI] [PubMed] [Google Scholar]
- 5. Reiberger T, Schwabl P, Trauner M, Peck‐Radosavljevic M, Mandorfer M. Measurement of the hepatic venous pressure gradient and transjugular liver biopsy. J Vis Exp. 2020. 10.3791/58819 [DOI] [PubMed] [Google Scholar]
- 6. Hernández‐Gea V, Aracil C, Colomo A, Garupera I, Poca M, Torras X, et al. Development of ascites in compensated cirrhosis with severe portal hypertension treated with β‐blockers. Am J Gastroenterol. 2012;107(3):418–27. [DOI] [PubMed] [Google Scholar]
- 7. Villanueva C, Aracil C, Colomo A, Hernández‐Gea V, JM L–B, Alvarez–Urturi C, et al. Acute hemodynamic response to beta‐blockers and prediction of long‐term outcome in primary prophylaxis of variceal bleeding. Gastroenterology. 2009;137(1):119–28. [DOI] [PubMed] [Google Scholar]
- 8. Abraldes JG, Tarantino I, Turnes J, Garcia‐Pagan JC, Rodés J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long‐term prognosis of cirrhosis. Hepatology. 2003;37(4):902–8. [DOI] [PubMed] [Google Scholar]
- 9. Feu F, García‐Pagán JC, Bosch J, Luca A, Escorsell A, Rodés J, et al. Relation between portal pressure response to pharmacotherapy and risk of recurrent variceal haemorrhage in patients with cirrhosis. Lancet. 1995;346(8982):1056–9. [DOI] [PubMed] [Google Scholar]
- 10. Mandorfer M, Hernández‐Gea V, Reiberger T, García‐Pagán JC. Hepatic venous pressure gradient response in non‐selective beta‐blocker treatment—is it worth measuring? Curr Hepatol Rep. 2019;18:174–86. [Google Scholar]
- 11. Turco L, Villanueva C, La Mura V, García‐Pagán JC, Reiberger T, Genescà J, et al. Lowering portal pressure improves outcomes of patients with cirrhosis, with or without ascites: a meta‐analysis. Clin Gastroenterol Hepatol. 2020;18(2):313–27.e6. [DOI] [PubMed] [Google Scholar]
- 12. D'Amico G, Garcia‐Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131(5):1611–24. [DOI] [PubMed] [Google Scholar]
- 13. D'Amico G, De Franchis R, Cooperative Study Group . Upper digestive bleeding in cirrhosis. Post‐therapeutic outcome and prognostic indicators. Hepatology. 2003;38(3):599–612. [DOI] [PubMed] [Google Scholar]
- 14. La Mura V, Abraldes JG, Raffa S, Retto O, Berzigotti A, García‐Pagán JC, et al. Prognostic value of acute hemodynamic response to i.v. propranolol in patients with cirrhosis and portal hypertension. J Hepatol. 2009;51(2):279–87. [DOI] [PubMed] [Google Scholar]
- 15. Ferlitsch A, Bota S, Paternostro R, Reiberger T, Mandorfer M, Heinisch B, et al. Evaluation of a new balloon occlusion catheter specifically designed for measurement of hepatic venous pressure gradient. Liver Int. 2015;35(9):2115–20. [DOI] [PubMed] [Google Scholar]
- 16. Mandorfer M, Reiberger T. Beta blockers and cirrhosis, 2016. Dig Liver Dis. 2017;49(1):3–10. [DOI] [PubMed] [Google Scholar]
- 17. Schwarzer R, Kivaranovic D, Paternostro R, Mandorfer M, Reiberger T, Trauner M, et al. Carvedilol for reducing portal pressure in primary prophylaxis of variceal bleeding: a dose‐response study. Aliment Pharmacol Ther. 2018;47(8):1162–9. [DOI] [PubMed] [Google Scholar]
- 18. Reiberger T, Mandorfer M. Beta adrenergic blockade and decompensated cirrhosis. J Hepatol. 2017;66(4):849–59. [DOI] [PubMed] [Google Scholar]
- 19. D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, et al. Competing risks and prognostic stages of cirrhosis: a 25‐year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39(10):1180–93. [DOI] [PubMed] [Google Scholar]
- 20. Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, et al. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–35. [DOI] [PubMed] [Google Scholar]
- 21. Villanueva C, Graupera I, Aracil C, Alvarado E, Miñana J, Puente Á, et al. A randomized trial to assess whether portal pressure guided therapy to prevent variceal rebleeding improves survival in cirrhosis. Hepatology. 2017;65(5):1693–707. [DOI] [PubMed] [Google Scholar]
- 22. Villanueva C, Albillos A, Genescà J, Garcia‐Pagan JC, Calleja JL, Aracil C, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet. 2019;393(10181):1597–608. Erratum in: Lancet. 2019;393(10190):2492. [DOI] [PubMed] [Google Scholar]
- 23. Villanueva C, López‐Balaguer JM, Aracil C, Kolle L, González B, Miñana J, et al. Maintenance of hemodynamic response to treatment for portal hypertension and influence on complications of cirrhosis. J Hepatol. 2004;40(5):757–65. [DOI] [PubMed] [Google Scholar]
- 24. Mandorfer M, Kozbial K, Schwabl P, Freissmuth C, Schwarzer R, Stern R, et al. Sustained virologic response to interferon‐free therapies ameliorates HCV‐induced portal hypertension. J Hepatol. 2016;65(4):692–9. [DOI] [PubMed] [Google Scholar]
- 25. Schwabl P, Mandorfer M, Steiner S, Scheiner B, Chromy D, Herac M, et al. Interferon‐free regimens improve portal hypertension and histological necroinflammation in HIV/HCV patients with advanced liver disease. Aliment Pharmacol Ther. 2017;45(1):139–49. [DOI] [PubMed] [Google Scholar]
- 26. Lens S, Alvarado‐Tapias E, Mariño Z, Londoño MC, Llop E, Martinez J, et al. Effects of all‐oral anti‐viral therapy on HVPG and systemic hemodynamics in patients with hepatitis C virus‐associated cirrhosis. Gastroenterology. 2017;153(5):1273–83.e1. [DOI] [PubMed] [Google Scholar]
- 27. Mandorfer M, Kozbial K, Schwabl P, Chromy D, Semmler G, Stättermayer AF, et al. Changes in hepatic venous pressure gradient predict hepatic decompensation in patients who achieved sustained virologic response to interferon‐free therapy. Hepatology. 2020;71(3):1023–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reiberger T, Ulbrich G, Ferlitsch A, Payer BA, Schwabl P, Pinter M, et al. Carvedilol for primary prophylaxis of variceal bleeding in cirrhotic patients with haemodynamic non‐response to propranolol. Gut. 2013;62(11):1634–41. [DOI] [PubMed] [Google Scholar]
- 29. Reiberger T, Ferlitsch A, Payer BA, Mandorfer M, Heinisch BB, Hayden H, et al. Non‐selective betablocker therapy decreases intestinal permeability and serum levels of LBP and IL‐6 in patients with cirrhosis. J Hepatol. 2013;58(5):911–21. [DOI] [PubMed] [Google Scholar]
- 30. Jachs M, Hartl L, Schaufler D, Desbalmes C, Simbrunner B, Eigenbauer E, et al. Amelioration of systemic inflammation in advanced chronic liver disease upon beta‐blocker therapy translates into improved clinical outcomes. Gut. 2021;70(9):1758–67. [DOI] [PubMed] [Google Scholar]
- 31. Costa D, Simbrunner B, Jachs M, Hartl L, Bauer D, Paternostro R, et al. Systemic inflammation increases across distinct stages of advanced chronic liver disease and correlates with decompensation and mortality. J Hepatol. 2021;74(4):819–28. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1.


