Abstract
In Salmonella enterica serovar Typhimurium, PurF-independent thiamine synthesis (or alternative pyrimidine biosynthesis) allows strains, under some growth conditions, to synthesize thiamine in the absence of the first step in the purine biosynthetic pathway. Mutations have been isolated in a number of loci that prevent this synthesis and thus result in an Apb− phenotype. Here we identify a new class of mutations that prevent PurF-independent thiamine synthesis and show that they are defective in the nuo genes, which encode the major, energy-generating NADH dehydrogenase of the cell. Data presented here indicated that a nuo mutant has reduced flux through the oxidative pentose phosphate pathway that may contribute to, but is not sufficient to cause, the observed thiamine requirement. We suggest that reduction of the oxidative pentose phosphate pathway capacity in a nuo mutant is an attempt to restore the ratio between reduced and oxidized pyridine nucleotide pools.
As our knowledge of discrete biochemical pathways increases, it has become important to understand how these pathways are integrated to result in an effective physiology. We have examined thiamine synthesis in Salmonella enterica serovar Typhimurium as a model system for detecting metabolic pathway integration (8, 13, 15, 23). Thiamine monophosphate is synthesized by the condensation of 4-amino-5-hydroxymethyl-2-methyl pyrimidine (HMP) pyrophosphate and 4-methyl-5-(β-hydroxyethyl)-thiazole phosphate. Thiamine monophosphate phosphorylation produces thiamine pyrophosphate, the coenzymic form of the vitamin (5, 6). As depicted in Fig. 1A, HMP pyrophosphate and 4-methyl-5-(β-hydroxyethyl)-thiazole phosphate are synthesized by independent pathways. Despite rigorous in vivo labeling experiments, the biochemical reactions that result in the synthesis of these moieties are not defined.
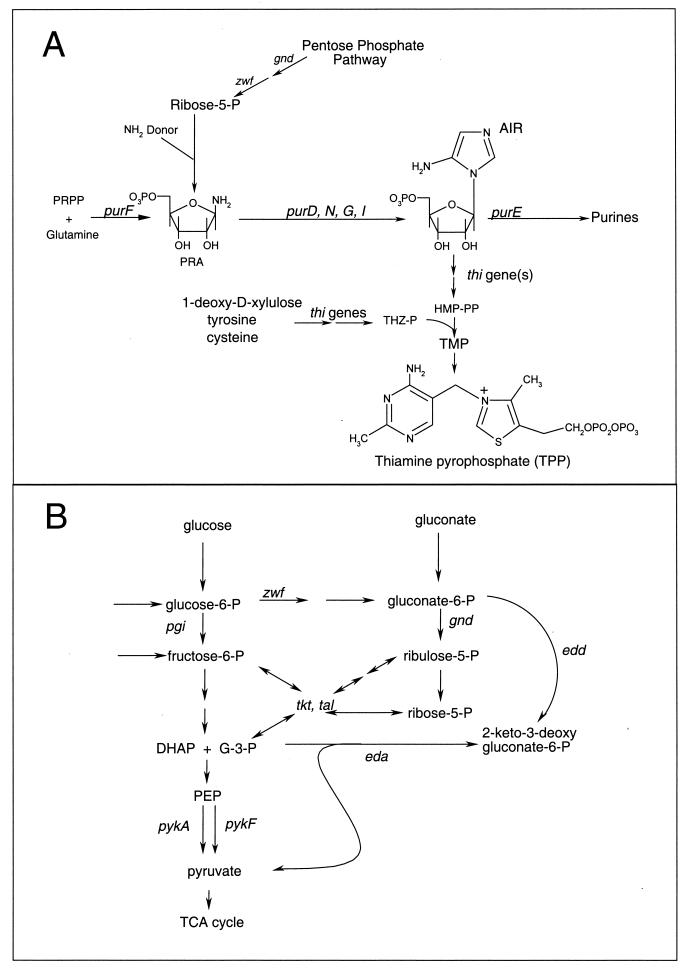
FIG. 1.
Relevant metabolic pathways. (A) Illustrated are the biosynthetic pathways involved in purine and thiamine synthesis. Names of genes whose products are known are indicated. The reaction(s) involved in the formation of PRA independent of PurF has not been defined biochemically or genetically. (B) Glycolysis, the pentose phosphate pathway, and the Entner-Doudoroff pathway are schematically represented. Names of genes whose products are required are indicated. Abbreviations: P, phosphate; PRPP, 5-phosphoribosyl-1-pyrophosphate AIR, aminoimidazole ribotide; PP, pyrophosphate; THZ-P, 4-methyl-5-(β-hydroxyethyl)-thiazole phosphate; TMP, thiamine monophosphate; G-3-P, glyceraldehyde-3-phosphate; DHAP, dihydroxyacetone phosphate; PEP, phosphoenolpyruvate; TCA, tricarboxylic acid.
The HMP moiety of thiamine is synthesized from the de novo purine pathway intermediate aminoimidazole ribotide, the last intermediate common to the two pathways (16, 20, 21). Work in our laboratory demonstrated that thiamine synthesis could occur independently of the purF gene product, and this synthesis was attributed to the alternative pyrimidine biosynthetic (APB) pathway (10, 12). Recent work has demonstrated that the four purine biosynthetic reactions after that catalyzed by PurF are needed for HMP synthesis via the APB pathway, making it synonymous with PurF-independent thiamine synthesis (13). However, the proposed step(s) forming phosphoribosylamine (PRA) in the absence of PurF has not been defined genetically or biochemically. To date, mutations preventing PurF-independent thiamine synthesis (Apb−) have been involved in the pantothenate biosynthetic pathway (e.g., panE) (11, 17), in loci thought to affect the conversion of aminoimidazole ribotide to HMP (i.e., apbC and apbE) (4, 22), or in genes encoding enzymes of the oxidative pentose phosphate (OPP) pathway (e.g., gnd and zwf) (15, 23). Based on analyses of the last class of mutants, it was proposed that the OPP pathway contributed ribose-5-phosphate for the formation of PRA via the APB pathway (14, 24). Work presented here was initiated to identify genes encoding functions needed to synthesize PRA in the absence of PurF.
Herein we describe a new class of mutations that block PurF-independent thiamine synthesis. These lesions were located in the nuo operon, which encodes NADH dehydrogenase I (NADH dHI). NADH dHI transfers electrons to ubiquinone in the electron transport chain and, unlike NADH dHII (encoded by ndh), produces a proton motive force (18). Two general classes of metabolic phenotypes have been reported for nuo mutants: those due to lack of energy generation (2, 27) and those due to the resulting imbalance in the ratio of reduced to oxidized pyridine nucleotide pools (25). We report here that, in addition to having a defect in thiamine synthesis, nuo mutants have a reduced capacity for flux through the OPP pathway and suggest that this is a consequence of the imbalance in pyridine nucleotide pools in these mutants.
Rationale for mutant isolation.
In the majority of mutants isolated for their defect in PurF-independent thiamine synthesis, a purE block was able to restore thiamine-independent growth to the levels of wild-type strains (4, 22, 24). To eliminate this predominant class of mutants, we screened MudJ(Km) insertion mutations for those that prevented strain DM2353 (purF purE) (Table 1) from growing on gluconate-adenine medium lacking thiamine. Of the approximately 35,000 Kmr transductants screened, 11 mutants carried insertions that blocked thiamine synthesis in a purF purE mutant but that had no effect on the ability of a wild-type strain to grow on minimal medium. Three of these mutants had lesions in gnd (encoding gluconate-6-phosphate dehydrogenase), and one had a lesion in panE (which encodes ketopantoate reductase), both of which were expected based on previously described phenotypes (11, 15, 17). The remaining seven mutants had insertions that were unlinked to loci previously shown to prevent PurF-independent thiamine synthesis and thus represented a new class of mutants.
TABLE 1.
Strain list
| Strain | Genotype |
|---|---|
| LT2 | Wild type |
| DM574 | purF2085 gnd175::Tn10d(Tc) |
| DM837 | nuo-351::Tn10d(Tc) |
| DM1936 | purF2085 |
| DM2353 | purF2085 purE3041 |
| DM3702 | zec-8611::MudJ edd |
| DM4134 | purF2085 purE3041 nuo-353::MudJ |
| DM4484 | purF2085 purE3041 nuoN355::MudJ |
| DM4485 | nuoN355::MudJ |
| DM4489 | nuoA357::MudJ |
| DM4615 | purF2085 nuoN355::MudJ |
| DM5247 | nuo-351::Tn10d(Tc) zec-8611::MudJ edd |
| DM5248 | purF2085 ndh::Kmr |
| DM5399 | edd zec-8611::MudJ nuo-351::Tn10d(Tc) |
| DM5400 | zec-8611::MudJ nuo-351::Tn10d(Tc) |
| DM5411a | edd zec-8611::MudJ nuo-351::Tn10d(Tc)/pMN6 (Gnd) |
| DM5479 | purF2085 nuoN355::MudJ/pMN6 (Gnd) |
| MWC233b | cyd ndh::Kmr (E. coli) |
pMN6 was obtained from R. E. Wolf, Jr. (19).
E. coli MWC233 was obtained from Robert Gennis.
Mutations in the nuo operon prevent PurF-independent thiamine synthesis.
A Tn10d(Tc) insertion [zeg-8661::Tn10d(Tc)] was isolated 50% linked to MudJ(Km) in strain DM4134 (purF2085 purE3041 nuo-354::MudJ), and found to be linked to the remaining six MudJ insertion mutations, suggesting that the seven mutants were defective in the same locus.
To identify the physical locations of the insertions, sequence adjacent to two representative insertions was determined by sequencing a PCR product produced with degenerate primers and primers derived from the insertion sequence (7). Based on homology alignment with BLASTX (1), the MudJ(Km) insertions in DM4489 and DM4485 were positioned in nuoA and nuoN, respectively. These genes are the first and last, respectively, in an operon (nuoA-nuoN) located in the minute 51 region of the Salmonella serovar Typhimurium chromosome. The 14-gene nuo operon encodes the subunits of the major energy-generating NADH dehydrogenase complex (NADH dHI) (3). Since the insertions chosen for sequencing disrupted a gene at the beginning and end of the nuo operon and resulted in the same phenotype, it was unlikely that polarity effects were responsible for the observed phenotypes.
The mutations described here were isolated for their ability to prevent growth of a purF purE double mutant on solid medium containing adenine and lacking thiamine with gluconate as the sole carbon and energy source. The insertions were transduced into additional genetic backgrounds, and the resulting phenotypes are illustrated by the growth rates (μ) shown in Table 2. These data reflect two significant points: (i) that mutations in nuo eliminated thiamine synthesis in a purF mutant when gluconate (Table 2) or other sugars such as fructose, glycerol, and mannitol were used as sole carbon sources (data not shown) and (ii) that although growth of a nuo purF purE triple mutant was scored as negative on solid adenine gluconate medium, thiamine-independent growth was detectable in liquid (μ = 0.15), which reflects the sensitivity for quantification afforded by liquid medium, rather than a medium-specific growth.
TABLE 2.
A nuo mutation prevents PurF-independent thiamine synthesis
| Strain | Relevant genotype | Specific μ witha:
|
|
|---|---|---|---|
| Adenine | Adenine + thiamine | ||
| DM1936 | purF | 0.20 | 0.34 |
| DM6415 | purF nuo | 0.03 | 0.26 |
| DM2353 | purF purE | 0.28 | 0.27 |
| DM4484 | purF purE nuo | 0.15 | 0.27 |
Gluconate was used as the sole carbon source, and growth curves were determined as described previously (8). Data shown here are from a representative experiment.
Thiamine-independent growth in previously described mutant classes was either completely restored (i.e., apbE and apbC) or unaffected (i.e., gnd and zwf) by mutations in purE when mutants were tested under the above-described conditions. From these results we considered that mutants defective in nuo represented a new, phenotypically distinct class of mutants defective in PurF-independent thiamine synthesis. Alternatively, nuo mutants may have a unique phenotype because thiamine synthesis was being affected in more than one way by a lack of the major NADH dehydrogenase.
Mutations in nuo reduce flux through the OPP pathway in vivo.
Previously we showed that flux through the OPP pathway was required for PurF-independent thiamine synthesis (14). Therefore, we tested whether nuo mutations resulted in reduced flux through the OPP pathway and, if so, whether the reduction was sufficient to cause the observed thiamine requirement. We reasoned that eliminating the major NADH dehydrogenase activity would result in a higher cellular NADH/NAD pool ratio, an idea previously proposed by others (25). If this assumption was correct, the cell might compensate by decreasing NADPH pools in an attempt to restore internal redox balance. Since the OPP pathway provides a source of NADPH, this pathway may be a site for such regulation. Results from the following experiments indicated that the flux capacity of the OPP pathway was reduced in a nuo mutant but that this reduction was not solely responsible for the resulting thiamine requirement.
Gluconate is a non-PTS hexose that is taken up by the cell through a specific gluconate transporter and phosphorylated by an ATP-dependent kinase (26). Gluconate-6-phosphate can then enter central metabolism by one of two routes: (i) it can enter the OPP pathway, where gluconate-6-phosphate dehydrogenase (encoded by gnd) converts it to ribulose-5-phosphate, after which it can enter central metabolism by the actions of transketolase and transaldolase, or (ii) it can be utilized via the gluconate-inducible Entner-Doudoroff pathway, producing pyruvate and glyceraldehyde-3-phosphate (Fig. 1B) (9).
In the presence of an edd block, gluconate will be catabolized exclusively via the OPP pathway and require Gnd (gluconate-6-phosphate dehydrogenase) activity. Thus, a gnd edd double mutant is unable to utilize gluconate as a sole carbon source (μ < 0.03 [data not shown]) (9). To test whether nuo mutants had reduced the flux of the OPP pathway in vivo, we assessed the ability of a nuo edd strain to utilize gluconate as a sole carbon source. The data in Table 3 clearly show that the nuo edd double mutant had a >2-fold reduction in growth rate compared to that of either single parental mutant when gluconate was used as the sole carbon source. Importantly, the growth rate of the double mutant was indistinguishable from that of either of the single mutants when glucose was provided as the sole carbon source (Table 3). The latter result demonstrated that nuo mutations were not simply causing an overall decrease in growth rate independently of the carbon source. Since the structural genes for enzymes of the OPP were intact in the edd nuo double mutant, these results suggested that a gene(s) and/or enzyme(s) of the OPP pathway was down regulated in the nuo mutant.
TABLE 3.
A nuo edd double mutant is unable to efficiently catabolize gluconate
| Strain | Relevant genotype | Specific μ ona:
|
|
|---|---|---|---|
| Gluconate | Glucose | ||
| DM5400 | nuo | 0.23 | 0.18 |
| DM3702 | edd | 0.16 | 0.18 |
| DM5399 | edd nuo | 0.07 | 0.17 |
| DM5411 | edd nuo/pGnd+ | 0.16 | 0.17 |
Growth curves were determined as described previously (8), and data are from a representative experiment.
Gnd is the limiting step of the OPP pathway in nuo mutants.
Based on the described routes for gluconate catabolism, the above results indicated that a nuo mutation resulted in reduced flux through the OPP pathway. The rationale for the model presented above suggested that Gnd, the enzyme directly responsible for production of NADPH, may be the limiting step in gluconate utilization in the nuo edd mutant. To address this possibility, pMN6 (Gnd+) (19) was introduced into DM5399 (nuo edd) and growth of the resulting strain (DM5400) was assessed with gluconate as the sole carbon source. Representative data from these experiments, shown in Table 3, demonstrated that pMN6 restored a wild-type growth rate to the double mutant. Assays in cell-free extracts determined that strains carrying the pMN6 plasmid had ∼10-fold more gluconate-6-phosphate dehydrogenase activity than those with only chromosomal gnd (data not shown). These results were consistent with a reduction of Gnd activity preventing a nuo edd double mutant from utilizing gluconate.
Reduced flux through the OPP pathway does not cause the Thi− phenotype of nuo mutants.
Having determined that nuo mutants had reduced flux through the OPP pathway, we asked whether this reduction was responsible for the thiamine requirement observed in a purF nuo mutant. Two results indicated that the defect in the OPP pathway was not sufficient to generate the thiamine requirement of a purF nuo mutant. First, the presence of pMN6(Gnd+) in a purF nuo double mutant failed to restore thiamine-independent growth. The specific growth rates of the strain with and without this plasmid were not significantly different in medium with gluconate as the sole carbon and energy source and containing adenine (μ = 0.134 and 0.198, respectively). That the pMN6 plasmid restored flux through the OPP pathway suggested that an additional effect of the nuo mutation was involved in causing the thiamine requirement. Second, when exogenous ribose is spotted on a top agar lawn of purF gnd cells on gluconate adenine medium, thiamine-independent growth is restored (14). Exogenous ribose was unable to restore thiamine synthesis in either DM4615 (purF nuo) or DM4484 (purF nuo purE) when the strains were tested in the same way (data not shown). This result also supported the conclusion that a nuo mutation did not cause a thiamine requirement solely by reducing flux through the OPP pathway.
Summary.
The studies described here made two contributions to our understanding of metabolism in Salmonella serovar Typhimurium. First, we have shown that mutations in the nuo operon are unable to perform PurF-independent thiamine synthesis. Second, we showed that strains defective in the major energy-generating NADH dehydrogenase complex are impaired in the OPP pathway. Our data are consistent with the model that lesions in this complex result in an increased NADH/NAD ratio that inhibits the activity of Gnd. Such an inhibition may be designed to reduce the NADPH/NADP ratio and thus restore the balance of reduced to oxidized pyridine nucleotide pools in the cell.
We further showed that the reduced flux through the OPP pathway caused by a nuo mutation was not sufficient to cause the observed thiamine requirement in this strain. This finding predicts that an additional metabolic perturbation(s) caused by a nuo mutation inhibits PurF-independent thiamine synthesis. It is tempting to speculate that the role of the nuo locus in energy generation is involved in causing this phenotype. For instance, in a purF mutant, thiamine synthesis may have an energy requirement that cannot be met in the absence of a functional NADH DHI. Since nuo mutants are proficient at thiamine synthesis in a PurF+ background, such an energy requirement would be specific for conditions of low flux through the purine (and thus the HMP) pathway.
Acknowledgments
The initial sequencing of the nuo mutations was performed by David Gonzales and Aileen Rubio. We thank Jeff Gralnick for helpful discussion.
This work was supported by NIH grant GM47296 and the Shaw Scientist Program of the Milwaukee Foundation.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Archer D, Wang X, Elliott T. Mutants defective in the energy conserving NADH dehydrogenase of Salmonella typhimurium identified by a decrease in energy dependent proteolysis after carbon starvation. Proc Natl Acad Sci USA. 1993;90:9877–9881. doi: 10.1073/pnas.90.21.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archer D C, Elliott T. Transcriptional control of the nuo operon which encodes the energy-conserving NADH dehydrogenase of Salmonella typhimurium. J Bacteriol. 1995;177:2335–2342. doi: 10.1128/jb.177.9.2335-2342.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck B J, Downs D M. The apbE gene encodes a lipoprotein involved in thiamine synthesis in Salmonella typhimurium. J Bacteriol. 1998;180:885–891. doi: 10.1128/jb.180.4.885-891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begley T P, Downs D M, Ealick S, McLafferty F, van Loon D, Taylor S, Chiu H, Kinsland C, Reddick J, Xi J, Campobasso N. Thiamin synthesis in prokaryotes. Arch Microbiol. 1999;171:293–300. doi: 10.1007/s002030050713. [DOI] [PubMed] [Google Scholar]
- 6.Brown G M, Williamson J M. Biosynthesis of folic acid, riboflavin, thiamine, and pantothenic acid. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 521–538. [Google Scholar]
- 7.Caetano-Annoles G. Amplifying DNA with arbitrary oligonucleotide primers. PCR Methods Appl. 1993;3:85–92. doi: 10.1101/gr.3.2.85. [DOI] [PubMed] [Google Scholar]
- 8.Christian T, Downs D M. Defects in pyruvate kinase cause a conditional increase of thiamine synthesis in Salmonella typhimurium. Can J Microbiol. 1999;45:565–572. [PubMed] [Google Scholar]
- 9.Conway T. The Entner-Doudoroff pathway: history, physiology and molecular biology. FEMS Microbiol Rev. 1992;103:1–28. doi: 10.1111/j.1574-6968.1992.tb05822.x. [DOI] [PubMed] [Google Scholar]
- 10.Downs D M. Evidence for a new, oxygen-regulated biosynthetic pathway for the pyrimidine moiety of thiamine in Salmonella typhimurium. J Bacteriol. 1992;174:1515–1521. doi: 10.1128/jb.174.5.1515-1521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downs D M, Petersen L. apbA, a new genetic locus involved in thiamine biosynthesis in Salmonella typhimurium. J Bacteriol. 1994;176:4858–4864. doi: 10.1128/jb.176.16.4858-4864.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downs D M, Roth J R. Synthesis of thiamine in Salmonella typhimurium independent of the purF function. J Bacteriol. 1991;173:6597–6604. doi: 10.1128/jb.173.20.6597-6604.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enos-Berlage J, Downs D M. Biosynthesis of the pyrimidine moiety of thiamine independent of the PurF enzyme (phophoribosylpyrophosphate amidotransferase) in Salmonella typhimurium: incorporation of stable isotope-labeled glycine and formate. J Bacteriol. 1999;181:841–848. doi: 10.1128/jb.181.3.841-848.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enos-Berlage J L, Downs D M. Involvement of the oxidative pentose phosphate pathway in thiamine biosynthesis in Salmonella typhimurium. J Bacteriol. 1996;178:1476–1479. doi: 10.1128/jb.178.5.1476-1479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Enos-Berlage J L, Downs D M. Mutations in sdh (succinate dehydrogenase genes) alter the thiamine requirement of Salmonella typhimurium. J Bacteriol. 1997;179:3989–3996. doi: 10.1128/jb.179.12.3989-3996.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estramareix B, Therisod M. Biosynthesis of thiamine: 5-aminoimidazole ribotide as the precursor of all the carbon atoms of the pyrimidine moiety. J Am Chem Soc. 1984;106:3857–3860. [Google Scholar]
- 17.Frodyma M, Downs D M. The panE gene, encoding ketopantoate reductase, maps at 10 minutes and is allelic to apbA in Salmonella typhimurium. J Bacteriol. 1998;180:4757–4759. doi: 10.1128/jb.180.17.4757-4759.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gennis R B, Stewart V. Respiration. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 217–261. [Google Scholar]
- 19.Nasoff M S, Wolf R E., Jr Molecular cloning, correlation of genetic and restriction maps, and determination of the direction of transcription of gnd of Escherichia coli. J Bacteriol. 1980;143:731–741. doi: 10.1128/jb.143.2.731-741.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Newell P C, Tucker R G. Biosynthesis of the pyrimidine moiety of thiamine. Biochem J. 1968;106:279–287. doi: 10.1042/bj1060279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Newell P C, Tucker R G. Precursors of the pyrimidine moiety of thiamine. Biochem J. 1968;106:271–277. doi: 10.1042/bj1060271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petersen L, Downs D M. Mutations in apbC (mrp) prevent function of the alternative pyrimidine biosynthetic pathway in Salmonella typhimurium. J Bacteriol. 1996;178:5676–5682. doi: 10.1128/jb.178.19.5676-5682.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Petersen L A, Downs D M. Identification and characterization of an operon in Salmonella typhimurium involved in thiamine biosynthesis. J Bacteriol. 1997;179:4894–4900. doi: 10.1128/jb.179.15.4894-4900.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen L A, Enos-Berlage J E, Downs D M. Genetic analysis of metabolic crosstalk and its impact on thiamine synthesis in Salmonella typhimurium. Genetics. 1996;143:37–44. doi: 10.1093/genetics/143.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prub B M, Nelms J M, Park C, Wolfe A J. Mutations in NADH:ubiquinone oxidoreductase of Escherichia coli affect growth on mixed amino acids. J Bacteriol. 1994;176:2143–2150. doi: 10.1128/jb.176.8.2143-2150.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tong S, Porco A, Isturiz T, Conway T. Cloning and molecular genetic characterization of the Escherichia coli gntR, gntK, and gntU genes of gntI, the main system for gluconate metabolism. J Bacteriol. 1996;178:3260–3269. doi: 10.1128/jb.178.11.3260-3269.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zambrano M M, Kolter R. Escherichia coli mutants lacking NADH dehydrogenase I have a competitive disadvantage in stationary phase. J Bacteriol. 1993;175:5642–5647. doi: 10.1128/jb.175.17.5642-5647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]