Abstract
Prior international studies have shown mixed results regarding the association of hepatitis B and hepatitis C with adverse pregnancy outcomes. We performed an updated evaluation of the prevalence of associated adverse pregnancy outcomes and evaluated trends over time of diagnosis of chronic hepatitis B (HBV) and chronic hepatitis C (HCV) in pregnant women in a national database. All pregnant women with HBV and HCV were identified from the National Inpatient Sample database 2012 to 2018. Multivariate logistic regression analyses were performed to compare pregnancy‐related complications, including rates of preeclampsia/eclampsia, gestational diabetes, intrauterine growth restriction, antepartum/intrapartum hemorrhage, preterm labor, and Cesarean section. We evaluated all‐cause in‐hospital mortality, length of stay, and total cost of hospitalizations. A total of 28.7 million pregnancy‐related hospitalizations that met our eligibility criteria were identified, including 51,200 with HBV and 131,695 with HCV. In comparison with the uninfected controls, the HBV group was significantly more likely to develop gestational diabetes (12.94% vs. 6.94%, p < 0.001). The HCV group was more likely to have preterm labor (9.63% vs. 6.27%, p < 0.001), intrauterine growth restriction (6.04% vs. 2.89%, p < 0.001), longer length of stay (3.4 days vs. 2.7 days, p < 0.001), and higher hospitalization cost (15,052 dollars vs. 14,258 dollars, p < 0.001). These findings should inform counseling of women who are found to have HBV or HCV during pregnancy regarding the risk of adverse pregnancy outcomes and support the need for an interdisciplinary approach to optimize maternal and neonatal outcomes.
Chronic HBV is independently associated with gestational diabetes. Chronic HCV infection increases the risk of intrauterine growth restriction and preterm labor and is associated with a longer length of hospitalization, and an increase in hospitalization costs.

INTRODUCTION
Chronic hepatitis B virus (HBV) and chronic hepatitis C virus (HCV) are both major international public health concerns, as evidenced by the World Health Organization's recently developed plan for viral hepatitis elimination by 2030.[ 1 ] As of 2015, there were globally an estimated 257 million people living with HBV and 71 million people living with HCV.[ 2 ] In the United States, chronic HBV and HCV affect approximately 0.3% (~850,000) and 1% (2.4 million) of the population, respectively.[ 3 , 4 , 5 ] There is an emerging interest in characterizing HBV and HCV in women of childbearing age (WOCA), with regard to changes in prevalence, clinical manifestations, and impact on pregnancy outcomes.
WOCA represents a priority group for surveillance and viral hepatitis elimination efforts, due to the risk of mother‐to‐child transmission as well as the potential for adverse pregnancy outcomes. Multiple studies have reported an increased nationwide prevalence of HCV among WOCA,[ 6 , 7 ] and suggest that although nationally there is a decline in HBV, there may be increases in HBV among WOCA, particularly in specific regions within the United States such as Appalachia.[ 8 ] Both of these epidemiologic trends have likely been driven by an increase in injection drug use, leading to an increased risk for viral hepatitis among young individuals.[ 9 ]
Few studies have examined trends in the prevalence of HBV and HCV among pregnant women, and available literature on the associations between viral hepatitis during pregnancy and specific adverse pregnancy outcomes remains conflicting.[ 10 , 11 ] With a rise in injection drug users among WOCA potentially driving epidemiologic changes in viral hepatitis in WOCA, a clearer understanding of the prevalence of HBV and HCV in pregnancy and their respective effects on pregnancy outcomes in women and neonates is needed. We used data from the National Inpatient Sample to provide an updated and comprehensive evaluation of the impact of HBV and HCV on pregnancy and delivery outcomes.
METHODS
Data source
We conducted a retrospective cohort study using National Inpatient Sample (NIS) data from 2012 to 2018. Sponsored by the Agency for Healthcare Research and Quality, NIS is the largest publicly available all‐payer inpatient care database in the United States. It approximates a 20% stratified sample of all discharges from short‐term, nonfederal hospitals in the United States, excluding rehabilitation and long‐term acute care hospitals.[ 12 ] The NIS contains more than 7 million hospital stays yearly when unweighted, and estimates more than 35 million hospitalizations nationally when weighted.[ 12 , 13 ] Each discharge in the NIS is weighted to ensure the results are nationally representative. This study was considered an institutional review exempt, as it used deidentified and publicly available databases.
Study population
The study included pregnancy or delivery‐related admissions identified using International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD‐10 CM) diagnostics and procedure codes from NIS 2012 to 2018 (Table S1). Most pregnancy hospitalizations were associated with labor/delivery rather than pregnancy‐related complications alone, as such all hospitalizations resulting from pregnancy‐related complications were analyzed together with labor/delivery hospitalizations. All 7 years were included in the analysis for pregnancy or delivery‐related outcomes, but the trends in the prevalence of diagnoses were only evaluated from 2012 to 2015 and from 2016 to 2018, given differences in diagnosis coding that precluded comparisons between those with ICD‐9 and ICD‐10 coding. Patients with acute liver diseases and coinfection with both HBV and HCV were excluded to evaluate the effect of each virus individually and to focus analysis on the impact of chronic viral hepatitis on pregnancy outcomes. Selected patients were further categorized into three groups: patients with HBV, patients with HCV, and patients with neither HBV nor HCV infection.
Study variables and outcomes
We used NIS variables to identify each patient's age (years), primary payer (Medicare, Medicaid, private insurance, and uninsured), hospital teaching status (rural, urban nonteaching, and urban teaching), and region of the hospital (Northeast, Midwest, South, and West). The patient comorbidities were identified using ICD‐9‐CM or ICD‐10‐CM codes (Table S1).
The primary outcome of interest was the rate of pregnancy and delivery‐related complications, including preeclampsia/eclampsia, gestational diabetes, intrauterine growth restriction, antepartum/intrapartum hemorrhage, preterm labor, and Cesarean section (C‐section).
Secondary outcomes included (1) in‐hospital outcomes, including all‐cause maternal mortality, length of stay, and total charge among three groups, and (2) trend in the prevalence of HBV and HCV from 2012 to 2014 and 2016 to 2018.
Statistical analysis
We analyzed the survey data according to the Agency for Healthcare Research and Quality recommendations.[ 14 ] The entire population of pregnancy‐related admissions in the United States was estimated by implementing a weighting of patient‐level observations. Patient vital status at discharge, which is directly coded in the NIS, was used for the in‐hospital mortality. Length of hospital stay and total charge of the hospitalization were also obtained from the NIS. The Pearson's chi‐square test was used to compare proportions, and the Student t test was used for continuous variables. STATA 15 (StataCorp) was used for analysis. A p‐value of less than 0.05 was considered statistically significant.
Trends in prevalence of HBV and HCV in pregnancy were evaluated using a bivariate logistic regression model, built with HBV and HCV as the dependent variable and year as the independent variable. In addition, a multivariate logistic regression model was used to evaluate the rate of pregnancy and delivery‐related complications among hospitalizations with HBV and HCV, adjusted for potential confounders, including age, race, insurers, hospital teaching status, region, and comorbidities (hypertension, diabetes mellitus, obesity, anemia, alcohol use disorder, smoking, drug abuse, acquired immunodeficiency syndrome/human immunodeficiency virus [AIDS/HIV], and sexually transmitted infection [STI]).
RESULTS
A total of 28,699,580 hospitalizations, which were pregnancy‐related or delivery‐related, were identified from 2012 to 2018, of which a total of 28,681,980 hospitalizations met the criteria (Figure 1). They were further categorized into three groups: HBV (n = 51,200), HCV (n = 131,695), and the cohort without HBV or HCV/the control group (n = 28,499,085).
FIGURE 1.
Flowchart of patient inclusion. Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus; NIS, National Inpatient Sample.
Patient characteristics
Patients with HBV were older compared with the uninfected cohort or HCV (32 years vs. 28 years or 28 years, p < 0.001) (Table 1). Compared with the uninfected cohort, there was a significantly higher rate of Asian or Pacific Islanders in the HBV group (52.05% vs. 5.66%, p < 0.001), and more white individuals in the HCV group (81.99% vs. 52.28%, p < 0.001). Patients with HBV had a significantly higher rate of cirrhosis, anemia, and AIDS/HIV in comparison to the uninfected controls, whereas patients with HCV had significantly higher rates of cirrhosis, hypertension, diabetes mellitus, anemia, alcohol use disorder, smoking, drug abuse, AIDS/HIV, and STI. Compared with HBV, patients with HCV were more likely to have cirrhosis, hypertension, diabetes mellitus, alcohol use disorder, smoking, drug abuse, and STI.
TABLE 1.
Basic characteristics of pregnancy‐related hospitalizations with chronic HBV or HCV versus uninfected controls
| Control, n = 28,499,085 | HBV, n = 51,200 | HCV, n = 131,695 | Comparison HBV vs. control (p‐value) | Comparison HCV vs. control (p‐value) | Comparison HBV vs. HCV (p‐value) | |
|---|---|---|---|---|---|---|
| Age, median (IQR) | 28 (24, 33) | 32 (28, 35) | 28 (25, 32) | <0.001 | <0.001 | <0.001 |
| Race | <0.001 | <0.001 | <0.001 | |||
| White | 52.28% | 10.97% | 81.99% | |||
| Black | 15.66% | 23.63% | 5.71% | |||
| Hispanic | 20.91% | 5.44% | 7.31% | |||
| Asian or Pacific Islander | 5.66% | 52.05% | 1.28% | |||
| Native American | 0.77% | 0.64% | 1.53% | |||
| Other | 4.72% | 7.27% | 2.18% | |||
| Insurance | <0.001 | <0.001 | <0.001 | |||
| Medicare | 0.82% | 0.63% | 2.59% | |||
| Medicaid | 43.75% | 47.76% | 79.34% | |||
| Private insurance | 49.81% | 44.64% | 13.11% | |||
| Self‐pay | 2.67% | 4.97% | 2.57% | |||
| No charge | 0.09% | 0.09% | 0.09% | |||
| Other | 2.86% | 1.93% | 2.20% | |||
| Hospital teaching status | <0.001 | <0.001 | <0.001 | |||
| Rural | 9.62% | 2.72% | 13.98% | |||
| Urban nonteaching | 27.73% | 19.10% | 18.6% | |||
| Urban teaching | 62.65% | 78.17% | 67.41% | |||
| Region of hospital | <0.001 | <0.001 | <0.001 | |||
| Northeast | 16.03% | 27.46% | 20.20% | |||
| Midwest | 21.01% | 16.28% | 20.56% | |||
| South | 38.93% | 25.93% | 45.47% | |||
| West | 24.04% | 30.33% | 13.77% | |||
| Comorbidities | ||||||
| Cirrhosis | 0.01% | 0.14% | 0.27% | <0.001 | <0.001 | 0.022 |
| Hypertension | 2.89% | 2.85% | 3.99% | 0.822 | <0.001 | <0.001 |
| Diabetes mellitus | 1.30% | 1.20% | 1.78% | 0.374 | <0.001 | <0.001 |
| Obesity | 8.34% | 6.39% | 6.87% | <0.001 | <0.001 | 0.135 |
| Anemia | 1.54% | 1.91% | 2.10% | 0.004 | <0.001 | 0.295 |
| Alcohol use disorder | 0.15% | 0.16% | 2.07% | 0.899 | <0.001 | <0.001 |
| Smoking | 9.54% | 5.25% | 58.58% | <0.001 | <0.001 | <0.001 |
| Drug abuse | 2.32% | 1.88% | 55.95% | 0.006 | <0.001 | <0.001 |
| AIDS/HIV | 0.11% | 0.72% | 0.90% | <0.001 | <0.001 | 0.117 |
| STI | 1.25% | 1.09% | 4.50% | 0.168 | <0.001 | <0.001 |
Abbreviations: AIDS, acquired immunodeficiency syndrome; HIV, human immunodeficiency virus; IQR, interquartile range; STI, sexually transmitted diseases.
Geographically, among all of the patients with HBV, 30.33% were in the West, followed by 27.46% in the Northeast, 25.93% in the South, and 16.28% in the Midwest. In the HCV group, nearly half of the patients (45.47%) were in the South, followed by 20.56% in the Midwest, 20.20% in the Northeast, and 13.77% in the West (Table 1 and Table S2).
Trends over time in the prevalence of HBV and HCV in pregnancy
From 2012 to 2014, the prevalence of HBV remained at 0.15%, and from 2016 to 2018, the prevalence decreased from 0.22% to 0.21% (p = 0.279) (Figure 2). In contrast, there was a significant increase in the prevalence of HCV in pregnant patients from 0.32% in 2012 to 0.43% in 2014 (p < 0.001). Despite being statistically insignificant (p = 0.172), the prevalence of HCV increased from 0.53% to 0.58% from 2016 to 2018. The apparent increase in the prevalence of both HBV and HCV from 2014 to 2016 was likely a function of the change in coding and does not reflect a true change in prevalence.
FIGURE 2.
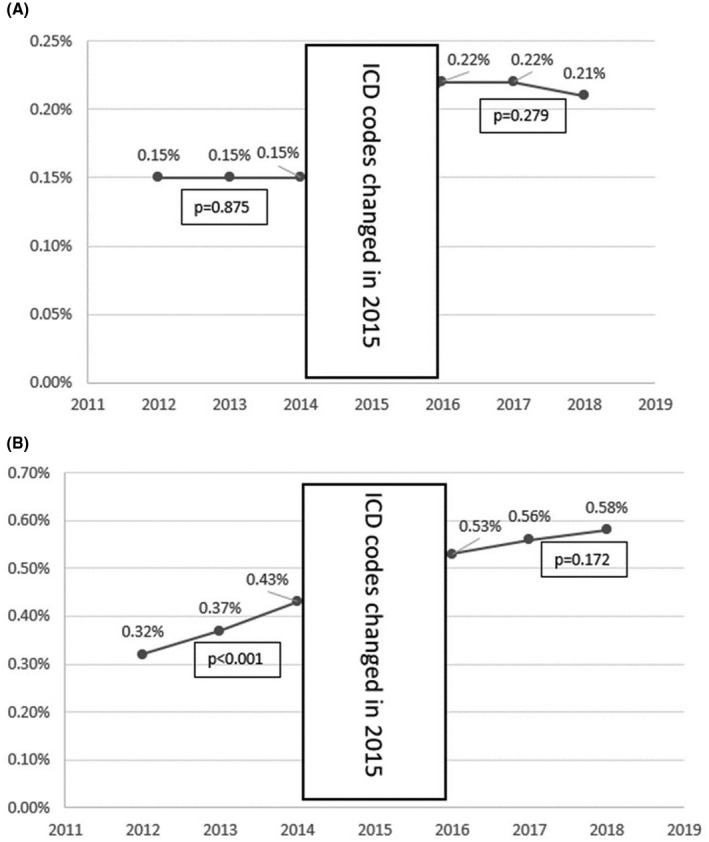
(A,B) Yearly trend of prevalence of HBV. Abbreviation: ICD, International Classification of Diseases.
In‐hospital outcomes
In comparison to the uninfected cohort, the HBV group did not show a statistical difference in maternal mortality, length of stay, or total hospitalization cost. On the other hand, the HCV group had a longer length of hospitalization (3.39 days vs. 2.66 days; adjusted Coeff 0.46 [95% confidence interval (CI): 0.40, 0.52]), and a higher total cost of hospitalization than uninfected controls ($20,991 vs. $18,289; adjusted Coeff 2,018 [95% CI: 1,613, 2,424]).
Pregnancy and delivery‐related outcomes
Compared with uninfected control, the most significant difference in the HBV group was a higher rate of gestational diabetes (12.94% vs. 6.94%). After adjusting for potential confounders, the adjusted odds ratio (OR) was 1.19 (95% CI: 1.12, 1.27) (Table 2). To further evaluate the association between HBV and gestational diabetes, we also performed propensity score matching for the HBV group and control group by age, race, insurance status, hospital teaching status, and comorbidities, which revealed that HBV was still associated with a significant risk of gestational diabetes with an OR of 1.16 (95% CI: 1.07, 1.27) (Table S3). There was no difference in intrauterine growth restriction, antepartum/intrapartum hemorrhage, and C‐section rate between HBV and uninfected groups, while the HBV group had a small decrease in the rate of preeclampsia/eclampsia (4.48% vs. 5.28%, p = 0.035) and preterm labor (5.52% vs. 6.27%, p = 0.002) than the uninfected group.
TABLE 2.
Outcomes of pregnancy‐related hospitalizations with chronic HBV or HCV versus uninfected controls
| Control, n = 28,499,085 | HBV, n = 51,200 | HCV, n = 131,695 | Comparison HBV vs. control, adjusted OR/coeff. (95% CI) | Comparison HCV vs. control, adjusted OR/coeff. (95% CI) | Comparison HBV vs. HCV, adjusted OR/coeff. (95% CI) | |
|---|---|---|---|---|---|---|
| Preeclampsia/eclampsia | 5.28% | 4.48% | 4.75% | 0.90 (0.81, 0.99) | 0.89 (0.84, 0.96) | 0.92 (0.77, 1.09) |
| Gestational diabetes | 6.94% | 12.94% | 4.57% | 1.19 (1.12, 1.27) | 0.87 (0.81, 0.93) | 1.26 (1.09, 1.46) |
| Intrauterine growth restriction | 2.89% | 3.07% | 6.04% | 0.91 (0.81, 1.02) | 1.27 (1.19, 1.34) | 0.77 (0.63, 0.94) |
| Antepartum/intrapartum hemorrhage | 0.66% | 0.95% | 0.91% | 1.06 (0.85, 1.30) | 0.97 (0.84, 1.12) | 0.77 (0.54, 1.12) |
| Preterm labor | 6.27% | 5.52% | 9.63% | 0.87 (0.80, 0.95) | 1.12 (1.06, 1.17) | 0.71 (0.62, 0.83) |
| C‐section | 29.87% | 31.97% | 29.48% | 1.00 (0.96, 1.04) | 1.09 (1.05, 1.12) | 0.92 (0.84, 1.00) |
| Maternal mortality | 0.01% | 0.03% | 0.05% | 1.94 (0.61, 6.17) | 1.86 (0.99, 3.47) | 2.59 (0.61, 11.04) |
| Length of stay (days), median (IQR) | 2 (2, 3) | 2 (2, 3) | 3 (2, 3) | 0.05 (‐0.00, 0.11) | 0.46 (0.40, 0.52) | −0.20 (−0.32. −0.08) |
| Length of stay (days), mean | 2.66 | 2.81 | 3.39 | |||
| Total charge ($), median (IQR) | 14,258 (9,400, 21,913) | 16,305 (10,342, 25,618) | 15,052 (9,753, 23,646) | 291 (−199, 780) | 2,018 (1,613, 2,424) | −1,389 (−2,252, −526) |
| Total charge ($), mean | 18,289 | 20,925 | 20,991 |
Abbreviations: CI, confidence interval; C‐section, Cesarean‐section; OR, odds ratio.
Intrauterine growth restriction was higher in the HCV group (6.04% vs. 2.89%) with a crude OR of 2.15 (95% CI: 2.04, 2.28) and adjusted OR of 1.27 (95% CI: 1.19, 1.34). The HCV group also had significantly higher rates of preterm labor (9.63% vs. 6.27%) with a crude OR of 1.59 (95% CI: 1.52, 1.66), and after adjusting for confounders, an OR of 1.12 (95% CI: 1.06, 1.17). Bivariate analyses evaluating the impact of each confounding factor is given in Tables S4 and S5. The rate of preeclampsia/eclampsia (4.75% vs. 5.28%, p = 0.001) and gestational diabetes (4.57% vs. 6.94%, p < 0.001) was slightly lower in the HCV group than in the uninfected cohort. There was no significant difference in antepartum/intrapartum hemorrhage between HCV and the uninfected cohort.
Between HBV and HCV, HCV had a higher rate of intrauterine growth restriction (p = 0.009) and preterm labor (p < 0.001) than the HBV group, whereas HBV was significantly more likely to be associated with gestational diabetes (p = 0.002).
DISCUSSION
Using the largest publicly available inpatient database in the United States, we found that in comparison to uninfected controls, patients with HBV were significantly more likely to develop gestational diabetes, and patients with HCV were more likely to have intrauterine growth restriction and preterm labor after adjusting for known confounders, including reported drug use. The all‐cause in‐hospital mortality, length of stay, and total hospitalization costs were significantly higher in the HCV group, demonstrating that patients with HCV during pregnancy are particularly at risk for significant morbidity and adverse pregnancy outcomes. In addition, we identified a trend toward an increase in the prevalence of HCV in pregnant patients, particularly from 2012 to 2014, which was not observed in the HBV group.
An understanding of HCV prevalence and disease burden in pregnant women is crucial to improving guidelines on disease surveillance and treatment in pregnancy. In our study, we provided an updated assessment of epidemiological trends, showing not only that the prevalence of HCV in pregnant women has significantly increased from 2012 to 2014, but also that rates are higher in the Southern and Northeastern regions of the United States (see Table S2 for a description of regions). This is consistent with a growing body of literature demonstrating an increase in the detection of HCV in both WOCA and pregnant women.[ 6 , 7 , 15 , 16 ] For example, population‐based retrospective study using National Center for Health Statistics birth records also observed a greater than 100% increase in maternal HCV infection rates in primarily Southern and Midwestern states.[ 16 ] These shifts in the epidemiologic trends of HCV in pregnant women, likely driven by an increase in injection drug use,[ 9 , 17 ] further underscores the importance of implementing universal HCV screening recommendations for pregnant women.
In our analysis of NIS data, we found that HCV was associated with higher rates of preterm labor and intrauterine growth restriction after adjusting for confounders. Interestingly, Reddick et al.[ 10 ] also found such an association with preterm labor; however, it was not significant after their own adjustments.[ 10 ] Other studies, including a nationwide study of pregnant Swedish women by Stokkeland et al.,[ 11 ] performed a meta‐analysis of international observational studies and also described an increased risk of preterm labor[ 11 , 18 , 19 ] as well as intrauterine growth restriction[ 11 ] with maternal HCV infection. The link between maternal HCV infection and adverse pregnancy outcomes such as intrauterine growth restriction and preterm labor may be explained by an excessive inflammatory state, with a higher ratio of pro‐inflammatory to anti‐inflammatory cytokines,[ 20 , 21 , 22 ] which in turn has been shown to impair uteroplacental dynamics, an important mediator of perinatal outcomes.[ 23 , 24 ] However, further mechanisms and direct viral impact on pregnancy outcomes need to be further evaluated. Altogether, when also considering the increased cost of hospitalization and increased risk of maternal mortality in pregnant women with HCV, it is clear that HCV is associated with adverse pregnancy outcomes, more so than HBV. Thus, women with HCV present a high‐risk pregnant population and should be closely monitored and counseled on their increased pregnancy risks.
Data on the epidemiological trends of HBV in pregnant women in the United States is not as robust as for HCV. We demonstrated that while the prevalence of HBV in pregnant women has remained stable or decreased over time, certain geographic regions such as the Northeast and West have a higher prevalence of HBV in pregnant women. One retrospective study using the Quest Diagnostics database noted significant increases in acute and chronic HBV infection in WOCA, particularly in certain Appalachian states (Mississippi, Kentucky, and West Virginia).[ 8 ] Furthermore, in evaluating the impact of HBV on pregnancy outcomes, we found an association between maternal HBV infection and gestational diabetes mellitus (GDM), supporting prior studies in which a higher than 47% risk for GDM in hepatitis B surface antigen (HBsAg)–positive pregnant women was found.[ 25 , 26 ] Although the exact physiological mechanism behind this association remains unclear, certain theories have been proposed. First, high levels of tumor necrosis factor‐alpha (TNF‐α) have been shown to be a predictor of maternal insulin resistance,[ 27 , 28 ] and studies have found that patients with chronic HBV have increased levels of TNF‐α and its receptor.[ 29 , 30 ] Second, a study of Korean adults demonstrated that chronic HBV alone could increase the risk of insulin resistance and the subsequent development of diabetes mellitus,[ 31 ] potentially due to HBV's effects on hepatic steatosis and systemic inflammation, which impair the insulin signaling pathway.[ 32 , 33 ]
Our study demonstrated slightly lower preeclampsia/eclampsia rates in both HBV and HCV groups compared with controls, which has also been seen in prior studies.[ 11 , 19 ] Although the mechanism for these findings is not well understood, immunologic changes on pregnancy course due to HBV, as well as changes in pro‐angiogenic factors in HCV, have been implicated as potential causes.[ 34 , 35 ] Furthermore, our study population had a lower prevalence of obesity among patients with HBV/HCV as compared with controls—providing another explanation for lower rates of preeclampsia/eclampsia, as obesity is a well‐established risk factor for preeclampsia/eclampsia.
One of the main strengths of our study is our updated evaluation of the NIS, providing data on current rates and trends of HBV/HCV, as well as defining associated pregnancy risks that can be used to counsel women (in time for recommendation for universal HCV screening in pregnancy and increased efforts to address elimination among individuals during pregnancy). The NIS provides a large, nationally representative, and diverse study population in which we were able to study both viruses, which are not endemic in the United States. This is a contemporary study using the latest data from the last decade to observe not only the clinical outcomes of HBV‐infected and HCV‐infected pregnant women, but also the economic impact (via cost of hospitalization and length of hospital stay). This study is also unique in that we compared the similarities and differences in pregnancy outcomes of HBV and HCV, allowing for a more complete understanding of the overall impact of these viral diseases on mothers and their infants.
There are some limitations to our study. First, the analysis is limited by its retrospective nature; therefore, we cannot establish the causality between the viral hepatitis and adverse maternal or fetal outcomes. Second, the NIS database relies on correct coding by providers. Potential misclassification might exist; however, results would be biased toward the null, assuming the misclassification is random. Third, the NIS is an inpatient database, therefore, the results cannot generalize to patients who underwent out‐of‐hospital delivery and had not been hospitalized, although out‐of‐hospital deliveries only accounted for about 1% of US deliveries.[ 36 ] Fourth, this database does not provide information about medications, laboratory results, and imaging. Therefore, there are limited data on virological control before or during pregnancy, although studies suggest that viral control may not necessarily improve pregnancy outcomes.[ 37 , 38 ] Fifth, the outcomes were restricted to that particular admission, and long‐term data or outpatient data, such as frequency of outpatient perinatal appointments, death, and other complications that occurred after discharge, were unavailable in the NIS. Finally, universal screening for HCV during pregnancy did not occur before 2018, potentially decreasing the proportion of patients with HCV in our study, as some patients may have been misclassified as being HCV‐negative while others who were screened may have been screened due to underlying bias (e.g., history of drug use).
In summary, we found that HBV was associated with an increased rate of GDM, and HCV was associated with a higher rate of preterm labor, intrauterine growth restriction, length of stay, and total charge in pregnancy or delivery‐related admissions. Given the current recommendations for universal screening for both HBV and HCV in pregnancy, these findings should inform counseling of women who are found to have HBV or HCV during pregnancy regarding the risk of adverse pregnancy outcomes and support the need for comprehensive management by a multidisciplinary team to optimize maternal and neonatal outcomes.
CONFLICT OF INTEREST
T.K. advises for Gilead and Abbvie.
Supporting information
Data S1. xxxx
Chen B, Wang Y, Lange M, Kushner T. Hepatitis C is associated with more adverse pregnancy outcomes than hepatitis B: A 7‐year national inpatient sample study. Hepatol Commun. 2022;6:2465–2473. 10.1002/hep4.2002
REFERENCES
- 1. World Health Organization. Global Hepatitis Programme . Global Health Sector Strategy on Viral Hepatitis, 2016‐2021: Towards Ending Viral Hepatitis. Geneva, Switzerland: WHO; 2016. [Google Scholar]
- 2. World Health Organization. Global Hepatitis Programme . Global Hepatitis Report, 2017. Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 3. Roberts H, Kruszon‐Moran D, Ly KN, Hughes E, Iqbal K, Jiles RB, et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. households: National Health and Nutrition Examination Survey (NHANES), 1988–2012. Hepatology. 2016;63:388–97. [DOI] [PubMed] [Google Scholar]
- 4. Kim HS, Rotundo L, Yang JD, Kim D, Kothari N, Feurdean M, et al. Racial/ethnic disparities in the prevalence and awareness of Hepatitis B virus infection and immunity in the United States. J Viral Hepat. 2017;24:1052–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rosenberg ES, Rosenthal EM, Hall EW, Barker L, Hofmeister MG, Sullivan PS, et al. Prevalence of hepatitis C virus infection in US States and the District of Columbia, 2013 to 2016. JAMA Netw Open. 2018;1:e186371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ly KN, Jiles RB, Teshale EH, Foster MA, Pesano RL, Holmberg SD. Hepatitis C virus infection among reproductive‐aged women and children in the United States, 2006 to 2014. Ann Intern Med. 2017;166:775–82. [DOI] [PubMed] [Google Scholar]
- 7. Koneru A, Nelson N, Hariri S, Canary L, Sanders KJ, Maxwell JF, et al. Increased hepatitis C virus (HCV) detection in women of childbearing age and potential risk for vertical transmission—United States and Kentucky, 2011–2014. MMWR Morb Mortal Wkly Rep. 2016;65:705–10. [DOI] [PubMed] [Google Scholar]
- 8. Kushner T, Chen Z, Tressler S, Kaufman H, Feinberg J, Terrault NA. Trends in hepatitis B infection and immunity among women of childbearing age in the United States. Clin Infect Dis. 2020;71:586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kushner T, Reau N. Changing epidemiology, implications, and recommendations for hepatitis C in women of childbearing age and during pregnancy. J Hepatol. 2021;74:734–41. [DOI] [PubMed] [Google Scholar]
- 10. Reddick KLB, Jhaveri R, Gandhi M, James AH, Swamy GK. Pregnancy outcomes associated with viral hepatitis. J Viral Hepat. 2011;18:e394‐8. [DOI] [PubMed] [Google Scholar]
- 11. Stokkeland K, Ludvigsson JF, Hultcrantz R, Ekbom A, Höijer J, Bottai M, et al. Pregnancy outcome in more than 5000 births to women with viral hepatitis: a population‐based cohort study in Sweden. Eur J Epidemiol. 2017;32:617–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agency for Healthcare Research and Quality . NIS Database Documentation. 2021. https://www.hcup‐us.ahrq.gov/db/nation/nis/nisdbdocumentation.jsp. Accessed 02 Sep 2021.
- 13. Agency for Healthcare Research and Quality . Overview of the National (Nationwide) Inpatient Sample (NIS). 2021. https://www.hcup‐us.ahrq.gov/nisoverview.jsp. Accessed 02 Sep 2021.
- 14. Agency for Healthcare Research and Quality . Calculating National Inpatient Sample (NIS) Variance for Data Years 2012 and Later. 2016. https://www.hcup‐us.ahrq.gov/reports/methods/2015_09.jsp. Accessed 02 Sep 2021.
- 15. Schillie SF, Canary L, Koneru A, Nelson NP, Tanico W, Kaufman HW, et al. Hepatitis C virus in women of childbearing age, pregnant women, and children. Am J Prev Med. 2018;55:633–41. [DOI] [PubMed] [Google Scholar]
- 16. Rossi RM, Wolfe C, Brokamp R, McAllister JM, Wexelblatt S, Warshak CR, et al. Reported prevalence of maternal hepatitis C virus infection in the United States. Obstet Gynecol. 2020;135:387–95. [DOI] [PubMed] [Google Scholar]
- 17. Ko JY, Haight SC, Schillie SF, Bohm MK, Dietz PM. National Trends in hepatitis C infection by opioid use disorder status among pregnant women at delivery hospitalization—United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2019;68:833–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Connell LE, Salihu HM, Salemi JL, August EM, Weldeselasse H, Mbah AK. Maternal hepatitis B and hepatitis C carrier status and perinatal outcomes. Liver Int. 2011;31:1163–70. [DOI] [PubMed] [Google Scholar]
- 19. Huang Q‐T, Chen JH, Zhong M, Hang LL, Wei SS, Yu YH. Chronic hepatitis B infection is associated with decreased risk of preeclampsia: a meta‐analysis of observational studies. Cell Physiol Biochem. 2016;38:1860–8. [DOI] [PubMed] [Google Scholar]
- 20. Serfaty L, Capeau J. Hepatitis C, insulin resistance and diabetes: clinical and pathogenic data. Liver Int. 2009;29((Suppl 2)):13–25. [DOI] [PubMed] [Google Scholar]
- 21. Butt AA, Xiaoqiang W, Budoff M, Leaf D, Kuller LH, Justice AC. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;49:225–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adinolfi LE, Restivo L, Guerrera B, Sellitto A, Ciervo A, Iuliano N, et al. Chronic HCV infection is a risk factor of ischemic stroke. Atherosclerosis. 2013;231:22–6. [DOI] [PubMed] [Google Scholar]
- 23. Kovo M, Schreiber L, Bar J. Placental vascular pathology as a mechanism of disease in pregnancy complications. Thromb Res. 2013;131((Suppl 1)):S18–21. [DOI] [PubMed] [Google Scholar]
- 24. Kovo M, Schreiber L, Ben‐Haroush A, Wand S, Golan A, Bar J. Placental vascular lesion differences in pregnancy‐induced hypertension and normotensive fetal growth restriction. Am J Obstet Gynecol. 2010;202:561.e1–5.e5. [DOI] [PubMed] [Google Scholar]
- 25. Lao TT, Chan BCP, Leung W‐C, Ho L‐F, Tse K‐Y. Maternal hepatitis B infection and gestational diabetes mellitus. J Hepatol. 2007;47:46–50. [DOI] [PubMed] [Google Scholar]
- 26. Tan J, Mao X, Zhang G, Wang W, Pan T, Liu X, et al. Hepatitis B surface antigen positivity during pregnancy and risk of gestational diabetes mellitus: a systematic review and meta‐analysis. J Viral Hepat. 2018;25:1372–83. [DOI] [PubMed] [Google Scholar]
- 27. Winkler G, Cseh K, Baranyi É, Melczer Z, Speer G, Hajós P, et al. Tumor necrosis factor system in insulin resistance in gestational diabetes. Diabetes Res Clin Pract. 2002;56:93–9. [DOI] [PubMed] [Google Scholar]
- 28. Kirwan JP, Hauguel‐de Mouzon S, Lepercq J, Challier JC, Huston‐Presley L, Friedman JE, et al. TNF‐ is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51:2207–13. [DOI] [PubMed] [Google Scholar]
- 29. Marinos G, Naoumov NV, Rossol S, Torre F, Wong PYN, Gallati H, et al. Tumor necrosis factor receptors in patients with chronic hepatitis B virus infection. Gastroenterology. 1995;108:1453–63. [DOI] [PubMed] [Google Scholar]
- 30. Sheron N, Lau J, Daniels H, Goka J, Eddleston A, Alexander GJM, et al. Increased production of tumour necrosis factor alpha in chronic hepatitis B virus infection. J Hepatol. 1991;12:241–5. [DOI] [PubMed] [Google Scholar]
- 31. Lee JG, Lee S, Kim YJ, Cho BM, Park JS, Kim HH, et al. Association of chronic viral hepatitis B with insulin resistance. World J Gastroenterol. 2012;18:6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murakami S. Hepatitis B virus X protein: structure, function and biology. Intervirology. 1999;42:81–99. [DOI] [PubMed] [Google Scholar]
- 33. Kim KH, Shin H–J, Kim K, Choi HM, Rhee SH, Moon H–B, et al. Hepatitis B virus X protein induces hepatic steatosis via transcriptional activation of SREBP1 and PPARgamma. Gastroenterology. 2007;132:1955–67. [DOI] [PubMed] [Google Scholar]
- 34. Ram M, Anaya JM, Barzilai O, Izhaky D, Porat Katz BS, Blank M, et al. The putative protective role of hepatitis B virus (HBV) infection from autoimmune disorders. Autoimmun Rev. 2008;7:621–5. [DOI] [PubMed] [Google Scholar]
- 35. Talaat RM. Soluble angiogenesis factors in sera of Egyptian patients with hepatitis C virus infection: correlation with disease severity. Viral Immunol. 2010;23:151–7. [DOI] [PubMed] [Google Scholar]
- 36. MacDorman MF, Mathews TJ & Declercq AE Trends in Out‐of‐Hospital Births in the United States, 1990–2012. 2014. https://www.cdc.gov/nchs/products/databriefs/db144.htm. Accessed 17 Mar 2022. [PubMed]
- 37. Money D, Boucoiran I, Wagner E, Dobson S, Kennedy A, Lohn Z, et al. Obstetrical and neonatal outcomes among women infected with hepatitis C and their infants. J Obstet Gynaecol Can. 2014;36:785–94. [DOI] [PubMed] [Google Scholar]
- 38. Brown RS, McMahon BJ, Lok ASF, Wong JB, Ahmed AT, Mouchli MA, et al. Antiviral therapy in chronic hepatitis B viral infection during pregnancy: a systematic review and meta‐analysis. Hepatology. 2016;63:319–33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. xxxx


