ABSTRACT
Streptococcus pneumoniae, an opportunistic human pathogen, is the leading cause of community-acquired pneumonia and an agent of otitis media, septicemia, and meningitis. Although genomic and transcriptomic studies of S. pneumoniae have provided detailed perspectives on gene content and expression programs, they have lacked information pertaining to the translational landscape, particularly at a resolution that identifies commonly overlooked small open reading frames (sORFs), whose importance is increasingly realized in metabolism, regulation, and virulence. To identify protein-coding sORFs in S. pneumoniae, antibiotic-enhanced ribosome profiling was conducted. Using translation inhibitors, 114 novel sORFs were detected, and the expression of a subset of them was experimentally validated. Two loci associated with virulence and quorum sensing were examined in deeper detail. One such sORF, rio3, overlaps with the noncoding RNA srf-02 that was previously implicated in pathogenesis. Targeted mutagenesis parsing rio3 from srf-02 revealed that rio3 is responsible for the fitness defect seen in a murine nasopharyngeal colonization model. Additionally, two novel sORFs located adjacent to the quorum sensing receptor rgg1518 were found to impact regulatory activity. Our findings emphasize the importance of sORFs present in the genomes of pathogenic bacteria and underscore the utility of ribosome profiling for identifying the bacterial translatome.
KEYWORDS: ribosome profiling, Streptococcus pneumoniae D39, small proteins, small open reading frames, virulence, quorum sensing, translation inhibitors, translational control
INTRODUCTION
Streptococcus pneumoniae, a major human pathogen, uses signaling mechanisms and gene regulation to alter global gene expression in response to dynamic environments during infection and colonization. Advanced transcriptomic technologies have permitted the identification of novel short RNA molecules in the highly studied model strain D39 of S. pneumoniae, some of which have been implicated in virulence (1–3). However, there is a distinct lack of information about small proteins encoded in the S. pneumoniae genome.
While conventional computational and experimental approaches have been well optimized for the prediction of protein-coding sequences in bacterial genomes, the identification and characterization of small open reading frames (sORFs) encoding proteins of less than 50 amino acids have been limited due to constraints in methodology and analysis. Computational algorithms used to annotate genomes require distinct size cutoffs to prevent an excess of predicted ORFs, leading to a trade-off between strict criteria that limit discovery and weaker stringencies that produce many false-positive predictions (4–6). Furthermore, the intrinsic properties of small proteins, such as their low molecular weight, insufficient ionic charge, low abundance, or poor stability, complicate their isolation and characterization using standard biochemical methodologies (7, 8).
Despite the difficulty in their identification, some small proteins or “microproteins” have been identified in a wide range of organisms and shown to impact metal homeostasis, virulence, cell development, metabolism, intracellular signaling, and other important physiological properties (9–11). For instance, in Bacillus subtilis, the small protein SpoVM (26 amino acids) is involved in spore coat and cortex development, and the deletion of spoVM results in a significant decrease in the sporulation efficiency (12). In Staphylococcus aureus, the small RNA (sRNA) RNA III encodes the 26-amino-acid cytotoxic peptide delta-hemolysin (hld) whose activity targets host cell membranes for lysis (13). In Escherichia coli, the 42-amino-acid protein MntS regulates intracellular manganese homeostasis, and the 49-amino-acid protein AcrZ enhances resistance to antibiotics through its interaction with the AcrAB-TolC efflux complex (14–16).
Quorum sensing (QS), a mode of bacterial cell-to-cell communication, operates through the production and sensing of low-molecular-weight molecules (pheromones) as intercellular signals for the purpose of coordinating activities among community members (17). Gram-positive bacteria employ peptides as pheromones that are secreted to the extracellular space and subsequently detected by neighboring bacteria. Early studies of natural transformation in S. pneumoniae led to the first discovery of intercellular signaling in bacteria, whereupon the competence-stimulating peptide (CSP) pheromone stimulates a histidine kinase in the development of the competent state for DNA uptake (18, 19). More recently, QS systems of the RRNPP (Rap, Rgg, NprR, PlcR, and PrgX) family, which are widespread among Firmicutes (17), have also been identified in S. pneumoniae as determined by genomic evaluation, with as many as eight paralogous systems being present. The RRNPP receptor proteins reside in the cytoplasm; therefore, precursors of the QS peptides are secreted, and the accumulated extracellular peptide ligands must then be reimported into the cell for direct interaction with cognate receptors to control gene expression (20–23). While phenotypes associated with the inactivation of these systems are starting to emerge, their roles in gene regulation remain largely unknown (20, 23, 24). A considerable barrier to identifying and characterizing these and other quorum sensing networks is the lack of appropriate techniques for detecting sORFs encoding signaling peptides (17). There are approximately 42,000 hypothetical sORFs in the S. pneumoniae D39 genome that encode peptides 8 to 50 amino acids in length. Which, if any, of these putative ORFs encode QS pheromones is unknown (17).
Although extensive analyses of the S. pneumoniae transcriptome and random transposon mutagenesis have resulted in the identification of several novel genes and noncoding RNAs (ncRNAs), a thorough understanding of the S. pneumoniae translatome is still missing (3, 25). To identify short protein-coding sORFs, some of which might encode uncharacterized peptide QS pheromones, virulence-related proteins, or other physiologically important microproteins, we conducted antibiotic-assisted ribosome profiling (Ribo-seq). Conventional Ribo-seq identifies actively translated ORFs by deep sequencing ribosome-protected mRNA fragments, providing a global view of all the genomic sites undergoing active translation (26–28). A specialized version of ribosome profiling exploits the ability of pleuromutilin antibiotics (e.g., retapamulin [Ret]) to specifically arrest ribosomes at initiation codons enhancing the signal-to-noise readout at a gene’s start, thus facilitating the detection of protein-coding genes, including sORFs (11, 29). Using Ribo-seq and retapamulin-enhanced Ribo-seq (Ribo-RET) analyses, we identified 114 novel sORFs in the genome of S. pneumoniae D39 and validated translation for a subset thereof. Among these, we identified two sORFs encoding short peptides involved in QS signaling and sORFs within nine previously annotated sRNAs, at least one of which contributes to the fitness and virulence of S. pneumoniae D39.
RESULTS
Retapamulin and lefamulin stall the ribosome during translation initiation in S. pneumoniae D39.
A recent study used the pleuromutilin antibiotic retapamulin in combination with ribosome profiling (Ribo-RET) to identify the start codons of protein-coding genes in the E. coli genome and discovered 41 novel sORFs in E. coli (11). Retapamulin, a protein synthesis inhibitor, binds to the 50S subunit during ribosome assembly and traps the ribosome during translation initiation, providing an increased signal-to-noise ratio at translation start sites.
We applied Ribo-RET to identify translated sORFs in the S. pneumoniae D39 genome. A D39 capsule mutant was used for all Ribo-seq experiments, as the presence of capsule complicated the rapid isolation of cells and, hence, ribosomes by filtration or centrifugation for downstream processing. Prior to cell lysis, mid-exponential-phase cultures were treated with 62.5 ng mL−1 retapamulin for 2.5 min, which corresponds to 100 times the MIC of the drug (see Fig. S1A in the supplemental material). The metabolic labeling experiments established that a 2.5-min treatment with retapamulin is sufficient to completely stop translation in the cell (Fig. S1B). The polysomes were isolated from the cells using the procedures described in Materials and Methods. However, we found that S. pneumoniae ribosomes tended to dissociate into subunits under conventional conditions of sucrose gradient centrifugation. To preserve ribosome integrity, we increased the concentration of MgCl2 in the lysis buffer from 10 mM to 50 mM. This adjustment significantly stabilized the ribosomes and slightly diminished the activity of micrococcal nuclease (MNase) used for the preparation of the ribosome footprints (Fig. S1C). This resulted in a less-precise trimming of the footprints to the ribosome edge and a broader distribution of the footprint lengths. Notably, even after digestion with MNase, all sucrose gradient centrifugation profiles showed the presence of an additional peak whose sedimentation properties are consistent with either underdigested disomes or, as observed in S. aureus, hibernating ribosome pairs (Fig. S1D, E, G, and H) (30). Despite these potential complications, monomeric 70S:mRNA footprint complexes were isolated, and the Ribo-seq library was prepared for Illumina deep sequencing.
Retapamulin and lefamulin are effective at arresting translation in S. pneumoniae D39. (A) MICs of retapamulin and lefamulin determined in CDM. (B) Assessment of protein synthesis in D39 Δcps treated with 100-fold the MIC of either retapamulin or lefamulin by measuring the incorporation of [35S]methionine at specific time points. (C to H) Monosomes were isolated by sucrose gradient fractionation. Bacterial cultures were either untreated (C and F) or treated with 100-fold the MIC of either retapamulin (D and G) or lefamulin (E and H) for 2.5 min. Lysates were digested (F to H) or not (C to E) with 450 U MNase and loaded onto 10 to 40% sucrose gradients. Download FIG S1, TIF file, 2.9 MB (3MB, tif) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Metagene analysis for all read lengths. (A) Metagene analysis comparing all read lengths positioned relative to the start codon with an offset of 15 nucleotides from untreated and retapamulin- and lefamulin-treated cells. (B) Metagene analysis of all read lengths. Download FIG S2, TIF file, 2.4 MB (2.5MB, tif) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
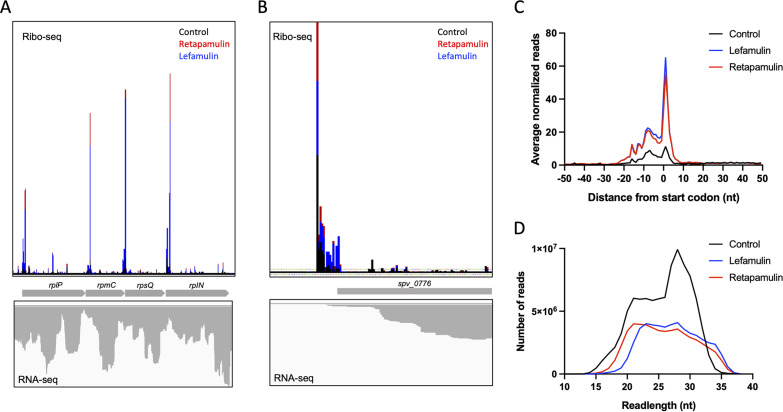
Ribo-seq data sets from untreated cultures revealed the translation of many annotated S. pneumoniae genes as well as the presence of ribosome footprints in some intergenic regions (Fig. 1A). As predicted, treatment of cells with retapamulin led to the accumulation of ribosomes at the start codons of genes (Fig. 1A). Metagene analysis showed that many ribosome footprints mapped to annotated start codons (Fig. S2B); however, a sizeable fraction of reads placed the ribosome as far as 20 nucleotides (nt) upstream from the annotated translation start sites (Fig. 1B and C). We observed in the raw sequencing data that the distribution of ribosome footprint read lengths ranged from 15 to 35 nucleotides, a surprise considering that studies performed on E. coli typically produce lengths of 28 nucleotides (Fig. 1D) (31). To determine whether the footprint size correlated with the ribosome’s location, read lengths were compared to start codon positioning, and it was found that the reads (27 to 35 nt) aligned best with annotated gene start sites (Fig. S2A). When only reads in this size range were used for mapping, two-thirds of the reads were situated at annotated start codons (Fig. 1C). Finally, to test the possibility that ribosome positioning was dependent on the initiation inhibitor used, we repeated the profiling experiment using lefamulin (Ribo-LEF), another pleuromutilin antibiotic reported to bind tightly to the ribosomal peptidyl transferase center (PTC) (32). The sequencing data sets generated from retapamulin- and lefamulin-treated samples produced outcomes that were nearly identical, reinforcing our confidence in the results of our ribosome profiling (Fig. S1E and H and Fig. S7).
FIG 1.
Retapamulin and lefamulin trap the ribosome near the start codon. (A) Ribosome footprint density of S. pneumoniae treated with and without retapamulin and lefamulin. (B) Example of a ribosome footprint stalled prior to the annotated start codon for spv_0776. (C) Metagene analysis of ribosome density reads (27 to 35 nt) distributed relative to the annotated start codon. (D) Distribution of the ribosome footprint length.
The ribosome consistently stalls in the 5′ UTR between different sample preparations and antibiotic treatments. Shown is a snapshot of the ribosome footprint density for the araT gene. Each picture represents a biological replicate, which was either untreated or treated with retapamulin or lefamulin. Download FIG S7, TIF file, 2.1 MB (2.2MB, tif) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Similar to Ribo-RET results obtained with E. coli (29), we also identified peaks of ribosomal footprints at putative internal start sites located within annotated ORFs. Such peaks may indicate instances of alternative initiation of translation or nested ORFs, as observed previously in E. coli (29) (Fig. S3).
Ribosome footprints are present within genes. Examples of Ribo-seq profiles in Streptococcus pneumoniae indicate alternative translation start sites within genes. The gray arrows represent previously annotated genes, and the green arrows indicate alternative open reading frames within annotated genes. Download FIG S3, TIF file, 2.8 MB (2.9MB, tif) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Overall, the ribosome profiling results indicated that 85% of the annotated S. pneumoniae genome is actively translated under laboratory conditions. As such, antibiotic-assisted ribosome profiling provides a powerful tool to identify the S. pneumoniae translatome.
Ribo-RET identifies unannotated sORFs in S. pneumoniae D39.
The S. pneumoniae D39 genome encodes ~2,700 intergenic coding sequences with the potential to encode small proteins 10 to 50 amino acids long. To identify true protein-coding regions, three independent sequencing data sets, two utilizing Ribo-RET and one utilizing Ribo-LEF, were used to map the translation initiation sites. Using a computational approach that mapped and quantified ribosome footprints, normalized to genome-wide sequence reads, we used the following criteria to define putative translation start sites of sORF candidates: at least 1 sequence read per million (rpm) mapped within 10 nucleotides of a theoretical sORF start codon (AUG, GUG, CUG, or UUG), and the respective full-length sORF did not overlap an annotated gene. By these criteria, we identified 117 (RET) and 103 (LEF) sORF candidates. In some instances of neighboring putative start codons, manual assessment of the coding region resulted in a refined list of 114 novel sORF candidates, designated rio (Ribo-seq-identified ORFs) (Tables 1 and 2).
TABLE 1.
List of sORFs
| Ribo-seq-identified sORF |
Coordinates | Upstream flanking gene |
Downstream flanking gene |
Strand | Expression (highest peak) |
Start codon | Nucleotide sequence | Peptide sequence | Small protein length (amino acids) |
Theoretic mol wt (kDa)a |
Presence of secretion signalb |
Found within/overlapping sRNAc |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rio1 | 24644–24742 | spv_0025 | spv_0027 | + | 48 | CTG | CTGCGTGAAGCGGGTCAGGGGAGGAATCCAGCAGCCCTAAGCGATTTGAATTGTGTGCTCTTTTTTTCGTGCTTTTTCCGAATAAATAAGATAGAATAA | MREAGQGRNPAALSDLNCVLFFSCFFRINKIE | 32 | 3.6 | No | scRNA |
| rio2 | 29719–29778 | spv_0033 | spv_0034 | + | 6.25 | ATG | ATGACTGTACGTCATCAGAAGTTTCAGCGACCATCATTTTTGAACAGTGATAGCACTTGA | MTVRHQKFQRPSFLNSDST | 19 | 2.2 | No | srf-01 |
| rio3 | 39961–39996 | spv_0047 | spv_2085 | + | 53 | ATG | ATGAATCGTAATTTAGAACGGTGTTATCTATTCTGA | MNRNLERCYLF | 11 | 1.4 | No | srf-02 |
| rio4 | 39885–39971 | spv_0047 | spv_2084 | + | 24.4 | ATG | ATGATGAATCTGAATCAAAATTATTTTGCGCATGTAAAGAGGAGTCTTATAGTAACGAGTCAAAAAAGGAGTAACTATGAATCGTAA | MMNLNQNYFAHVKRSLIVTSQKRSNYES | 28 | 3.3 | Yes | |
| rio5 | 78879–78992 | spv_0077 | spv_2092 | + | 2.7 | ATG | ATGAAAATCAAAGATCAAACTAGGAAACTAGCTACGGGCTGCTCAAAACACTGTTTTGAGGTTGCAGATAGAACTGACGAAGTCAGTAACATCTATACGGCAAGGCGACGTTGA | MKIKDQTRKLATGCSKHCFEVADRTDEVSNIYTARRR | 37 | 4.3 | No | spd sr8 |
| rio6 | 86107–86193 | spv_0082 | spv_0083 | + | 10 | ATG | ATGAAGCTCGTCAACAGGTGTCTTATGACAAGTAACCTTGGCTGTTTAGGCGAAGGGCATCTGCACGAATCAGGGCTTTCTAAGTGA | MKLVNRCLMTSNLGCLGEGHLHESGLSK | 28 | 3 | No | |
| rio7 | 113831–113908 | spv_0112 | spv_0113 | + | 13 | TTG | TTGTTAAAGGAGGCTCAGTCCTTGGGCAAAAATCTGCTGTACTACAATGCTTGGAAAGATATCAAGAACAAAGAATGA | MLKEAQSLGKNLLYYNAWKDIKNKE | 25 | 2.9 | No | |
| rio8 | 113958–113987 | spv_0112 | spv_0113 | + | 5.4 | TTG | TTGGGTGACATCAAAGAAAAGTTAGATTAA | MGDIKEKLD | 9 | 1 | No | |
| rio9 | 113770–113814 | spv_0112 | spv_0113 | + | 4.2 | TTG | TTGATTAGTCTGGCTATAATCTTTCCATGGGGCTGGCCGATATAA | MISLAIIFPWGWPI | 14 | 1.6 | No | |
| rio10 | 118625–118672 | spv_0116 | spv_2099 | + | 3.8 | ATG | ATGTGTAGGAATAGCCCCTTTTTTTCACGTATATATAATAGGTTCTGA | MCRNSPFFSRIYNRF | 15 | 1.9 | No | |
| rio11 | 118721–118750 | spv_0116 | spv_2099 | + | 2.5 | TTG | TTGTATTTTAACTATTGCTCGAATTTATAG | MYFNYCSNL | 9 | 1.1 | No | |
| rio12 | 119733–119777 | spv_0116 | spv_2099 | + | 40 | TTG | TTGATTAGTCTAGCTATAATCTTTCCATGGGGCTGGTCGATATAA | MISLAIIFPWGWSI | 14 | 1.6 | No | |
| rio13 | 122716–122766 | spv_0119 | spv_0120 | + | 15 | ATG | ATGGCTGATGAATCTTATTTATACTGTTGCAGTAGGCATTGTCTAGGATAA | MADESYLYCCSRHCLG | 16 | 1.8 | No | |
| rio14 | 122780–122845 | spv_0119 | spv_0120 | + | 8 | ATG | ATGGGGGAAATTGGAATAATTTTAGTAGAAACATTAAAGGATTATGGACAAGTAGCGCAGGATTAG | MGEIGIILVETLKDYGQVAQD | 21 | 2.2 | No | |
| rio15 | 127035–127055 | spv_0124 | spv_2103 | + | 57.5 | ATG | ATGGGGATTGTATTACTCTGA | MGIVLL | 7 | 0.64 | No | |
| rio16 | 132259–132294 | spv_0127 | spv_0128 | + | 25 | ATG | ATGTTGCAGGCTTTTTTGTCCTGCACTTCTTTGTAG | MLQAFLSCTSL | 11 | 1.2 | No | srf-04 |
| rio17 | 149686–149748 | spv_0143 | spv_0144 | − | 2.1 | ATG | ATGAGGTGTGAGCTCAAAATATCCTCCAGTTATGTTTTTCCTAATAGTATACCGGAAGAGTGA | MRCELKISSSYVFPNSIPEE | 20 | 2.3 | No | srf-05 |
| rio18 | 165545–165586 | spv_0160 | spv_0161 | + | 12.5 | TTG | TTGAAGTTGTTCAACTGTTTTTTGAGTATAAACAGTCTTTAA | MKLFNCFLSINSL | 13 | 1.5 | No | |
| rio19 | 174277–174363 | spv_0169 | spv_0170 | − | 2.2 | ATG | ATGAAAGAAGATAAAAGTATATTTGTGCTTTTTGCGTGCTCTGAAATGATTACTTGTCATTTCAGAGCATTTTTGTTAATCGCATAA | MKEDKSIFVLFACSEMITCHFRAFLLIA | 28 | 3.2 | No | |
| rio20 | 186024–186104 | spv_0182 | spv_0183 | + | 105 | CTG | CTGGGAGACTGTATCAGCCCAACTCCCAGAAATGGGGAAGGGTTGGAACGTCCAGTGGATGTTTTTAGCCTAGCTCTTTGA | MGDCISPTPRNGEGLERPVDVFSLAL | 26 | 2.7 | No | |
| rio21 | 186133–186174 | spv_0182 | spv_0183 | + | 33 | ATG | ATGTTTTACTCCACGTTAATATTCATAGTTGCTAGTGATTAG | MFYSTLIFIVASD | 13 | 1.5 | No | |
| rio22 | 185918–185992 | spv_0182 | spv_0183 | + | 12.5 | CTG | CTGGTAAGTCCAGTGGACGTTTTTAGCCTGACGCTAAAAATAAAAACCGTCAGTAAGTTCGGGGGACGTTTTTAG | MVSPVDVFSLTLKIKTVSKFGGRF | 24 | 2.6 | No | |
| rio23 | 188045–188092 | spv_0184 | spv_0185 | + | 5.75 | ATG | ATGCAAGTAGAAAGGGCTGGATTTTTCAGCCTTTTTACTTTTACCTAG | MQVERAGFFSLFTFT | 15 | 1.7 | No | |
| rio24 | 195914–195985 | spv_0191 | spv_0192 | + | 5.5 | TTG | TTGCGACACGCTCGGTTGCATTGCCACGCAACACCGCGTCGGTTTTCTTGTGGAGCTAGCCTATTATCTTAA | MRHARLHCHATPRRFSCGASLLS | 23 | 2.6 | Yes | |
| rio25 | 195901–195972 | spv_0191 | spv_0192 | + | 20 | TTG | TTGATGCAAGAGGTTGCGACACGCTCGGTTGCATTGCCACGCAACACCGCGTCGGTTTTCTTGTGGAGCTAG | MMQEVATRSVALPRNTASVFLWS | 23 | 2.5 | No | |
| rio26 | 202954–203037 | spv_0206 | spv_0207 | + | 40 | TTG | TTGACGAAGAAACTAAAGTTTCTAGGAAAGTTTATCTTTTTCACACAGAGTTTAGCCCGGGTTCAATTGGGCTTGCCAATTTGA | MTKKLKFLGKFIFFTQSLARVQLGLPI | 27 | 3.1 | Yes | |
| rio27 | 203084–203146 | spv_0206 | spv_0207 | + | 8.8 | TTG | TTGCTTCTGCATTCAATTGTCTATTTTTGCTCGTGCTGTTACGCTCTTTGTATCATGTATTAA | MLLHSIVYFCSCCYALCIMY | 20 | 2.3 | No | |
| rio28 | 249098–249157 | spv_0252 | spv_0253 | + | 1.68 | ATG | ATGAATACAAGGAGTTTTATCTTTTTCGCGCAGCATCCCGTTCCAGCTCATATCGGCTAA | MNTRSFIFFAQHPVPAHIG | 19 | 2.1 | No | |
| rio29 | 272815–272874 | spv_0273 | spv_0274 | + | 11.3 | TTG | TTGAAAGGCCCCGGAACCTTCCAAATACTTTTCGATGGGAAGGAACACCCATCACCGTAA | MKGPGTFQILFDGKEHPSP | 19 | 2 | No | |
| rio30 | 306391–306480 | spv_0307 | spv_0308 | + | 18 | ATG | ATGTTTCATCTAGAAATCTTCAGAAGTAAAGATAGTCTACTCCTGCTTGAAAAAGAAAAACCGGAAATAGTACATAGAGTAGCGATTTAG | MFHLEIFRSKDSLLLLEKEKPEIVHRVAI | 29 | 3.4 | No | |
| rio31 | 400274–400312 | spv_0398 | spv_0399 | + | 28 | ATG | ATGGTAAAATCCAATGTAAAAATCATTCTCAGCTATTGA | MVKSNVKIILSY | 13 | 1.3 | No | |
| rio32 | 402069–402098 | spv_0399 | spv_0400 | + | 11.9 | ATG | ATGGTTTTACGAACGAATAGGCGAAAATAA | MVLRTNRRK | 9 | 1.1 | No | |
| rio33 | 401926–401958 | spv_0399 | spv_0400 | + | 11 | TTG | TTGTTGGACGCTAGTCGCTATTTGGCGAACTAG | MLDASRYLAN | 10 | 1.1 | No | |
| rio34 | 408631–408684 | spv_0406 | spv_2159 | + | 18 | ATG | ATGAAAAAGATTATGAGAAAAATTGCATCGTTATTATTGGTTCTAGTTGTATAA | MKKIMRKIASLLLVLVV | 17 | 1.9 | Yes | |
| rio35 | 411179–411250 | spv_0409 | spv_0410 | + | 6.1 | ATG | ATGAATTTGAAGGACATAAGGAATACCTATCTCTCAGATGATTTATTGAGGAAGAAAGATAGGAGTTTTTGA | MNLKDIRNTYLSDDLLRKKDRSF | 23 | 2.8 | No | |
| rio36 | 444912–444977 | spv_0441 | spv_0442 | + | 12.5 | CTG | CTGGTGCAGTCGTCCCAGATTATTCTTATTAGTAGGGTCTTGTTTTCTATATCCCCTCGTAGTTAA | MVQSSQIILISRVLFSISPRS | 21 | 2.3 | No | |
| rio37 | 485231–485263 | spv_0474 | spv_0475 | + | 3.5 | TTG | TTGGAAAGGCTAGAACTAAAGAATGACGTGTAG | MERLELKNDV | 10 | 1.2 | No | |
| rio38 | 497485–497523 | spv_0490 | spv_0491 | + | 10 | ATG | ATGCGGGCTTGGCCCGAAATTGGGTGGTACCGCGGATAA | MRAWPEIGWYRG | 12 | 1.5 | No | |
| rio39 | 508756–508830 | spv_0500 | spv_0501 | + | 30 | ATG | ATGTTATCACTAATACAAGTGAGCAGGAACCTATTTAATCACATCAGAAGAAGTTTCTTGATGTTTTTTAAGTAG | MLSLIQVSRNLFNHIRRSFLMFFK | 24 | 2.9 | No | srf-10 |
| rio40 | 565754–565780 | spv_0553 | spv_2193 | + | 17 | ATG | ATGTATAGTAGACTGAATCTAAAATAG | MYSRLNLK | 8 | 1 | No | |
| rio41 | 569211–569276 | spv_0556 | spv_0557 | + | 24 | ATG | ATGATGTTTGTAATTGAAGAAGTCAAGGATGAAAATCAAAAAAGGCAGTTGTCGCTGAGGTTTTGA | MMFVIEEVKDENQKRQLSLRF | 21 | 2.6 | No | |
| rio42 | 569735–569764 | spv_0557 | spv_2196 | + | 9.4 | TTG | TTGGTAGAGCAAGGCACTCGTAAAGCCTAG | MVEQGTRKA | 9 | 1 | No | |
| rio43 | 616529–616573 | spv_0590 | spv_0510 | + | 4.4 | ATG | ATGAAGCTATTGTTTTATAGTATAATTAATTTGTATAAAATTTAA | MKLLFYSIINLYKI | 14 | 1.7 | No | |
| rio44 | 640777–640818 | spv_0619 | spv_0620 | + | 2.3 | ATG | ATGGGAGTATCGCAAAAAATGACTCATCGTATTCAATTTTGA | MGVSQKMTHRIQF | 13 | 1.5 | No | |
| rio45 | 653885–653935 | spv_0632 | spv_0633 | − | 11.8 | ATG | ATGGAAGTGGAAATGATAATGGGGACTAGCAGTCTTCTATTGCCTTTCTAA | MEVEMIMGTSSLLLPF | 16 | 1.7 | No | |
| rio46 | 657363–657389 | spv_0635 | spv_0636 | + | 275 | TTG | TTGGAGAACGTTTCCAATTCTATGTAA | MENVSNSM | 8 | 0.9 | No | |
| rio47 | 669265–669354 | spv_0649 | spv_0650 | + | 1.3 | ATG | ATGACTTGGAAAAGTATTTCCAGTCACGAAAGGAGGTTGGGTTTTTGTTTCTGTCTAATGAAAGCAGAGCAAAAATTTGACCTTTTTTGA | MTWKSISSHERRLGFCFCLMKAEQKFDLF | 29 | 3.5 | No | |
| rio48 | 676352–676426 | spv_0657 | spv_0658 | + | 182 | ATG | ATGGACATTTCAGTAATTCGTCAGAAAATTGACGCAAATCGTGAAAAATTAGCTTATTTCAGGGGGTCTCTTTGA | MDISVIRQKIDANREKLAYFRGSL | 24 | 2.8 | No | |
| rio49 | 684026–684079 | spv_0663 | spv_0664 | + | 750 | ATG | ATGGTAGGTATTTATTACGAAGAGTTTTCCTATCAGTACTTTGTAACTCTATAA | MVGIYYEEFSYQYFVTL | 17 | 2.1 | No | |
| rio50 | 738388–738480 | spv_0722 | spv_0723 | + | 1.4 | CTG | CTGGAAAGAGATGAACAAATCAAATACATAAAAGATAAACTCATTTTTATTCGTTTGGTAAAAATAAAATGCATATTTTTAAAGAAAGATTGA | MERDEQIKYIKDKLIFIRLVKIKCIFLKKD | 30 | 3.7 | No | |
| rio51 | 764625–764684 | spv_0750 | spv_0751 | + | 2 | ATG | ATGGTATTGATCTTGATAAAATTTTTAAAATACTGTCATTTTGAATATAAAGGAGTTTGA | MVLILIKFLKYCHFEYKGV | 19 | 2.3 | No | |
| rio52 | 767622–767705 | spv_0756 | spv_0757 | + | 20 | TTG | TTGAAAGACGTGAATGATATGAACATGTCCTTGCTGGTGCTTAGGAAAAAAATTATAAGTATGTCAAGTTTAAGAAAAACTTGA | MKDVNDMNMSLLVLRKKIISMSSLRKT | 27 | 3.1 | Yes | |
| rio53 | 773033–773068 | spv_0761 | spv_0762 | + | 57.5 | TTG | TTGCTCTATTTCTGGGGAAATCAGACGTTTTTCTAG | MLYFWGNQTFF | 11 | 1.4 | No | |
| rio54 | 863206–863247 | spv_0846 | spv_0847 | + | 9.6 | CTG | CTGGCCCTACGGATGAAAAGTTTCGAAGAAACGCTATCATAA | MALRMKSFEETLS | 13 | 1.5 | No | |
| rio55 | 865083–865118 | spv_0850 | spv_0851 | + | 14 | TTG | TTGTGTTGTACGATTTTAACTGAGGCCTTGCACTAG | MCCTILTEALH | 11 | 1.2 | No | |
| rio56 | 1051996–1052016 | spv_0898 | spv_0900 | − | 200 | TTG | TTGAGCATAAGGAGGTCATAA | LSIRRS | 6 | 0.7 | No | srf-17 |
| rio57 | 1036860–1036916 | spv_0915 | spv_2289 | − | 9.9 | CTG | CTGTATCTATTGACAATGATAATTATTATCGATACAATAGACTTGAAATATGTTTAA | MYLLTMIIIIDTIDLKYV | 18 | 2.1 | No | |
| rio58 | 962247–962309 | spv_0992 | spv_0991 | − | 14.35 | TTG | TTGAACCTTTTATCCCGAACCTTGAAATGTAAAGGTGAGGAAGCTAGAAACAGCTTAAAATAA | MNLLSRTLKCKGEEARNSLK | 20 | 2.2 | No | |
| rio59 | 958487–958513 | spv_0997 | spv_0996 | − | 6.7 | TTG | TTGGAAGAAATAGCGTTTTTAACTTGA | MEEIAFLT | 8 | 0.95 | No | |
| rio60 | 921151–921201 | spv_1017 | spv_1018 | − | 13.8 | ATG | ATGAAGAACAAACCAAGATTCAAGCAGGAATTCCTACTGATAATGAAGTAA | MKNKPRFKQEFLLIMK | 16 | 2 | No | |
| rio61 | 917345–917374 | spv_1021 | spv_1023 | − | 25.5 | ATG | ATGAAAAAGAAAACAATAGCAATTATATAG | MKKKTIAII | 9 | 1 | No | |
| rio62 | 1085315–1085347 | spv_1059 | spv_1060 | − | 1.6 | ATG | ATGCCTTTCCTTGTTCACAGGAAATTTATATAA | MPFLVHRKFI | 10 | 1.2 | No | |
| rio63 | 1153159–1153251 | spv_1121 | spv_2314 | − | 5.5 | ATG | ATGATGATACTTTTCGAAAATCTCTTCAAACTACGTCAGCTCAGCTTTGCCTTGCTGTGTTTTGAGCAAGCTACGGTTAGCTTCCGAGTTTGA | MMILFENLFKLRQLSFALLCFEQATVSFRV | 30 | 3.5 | No | |
| rio64 | 1189201–1189269 | spv_1159 | spv_1158 | − | 20 | ATG | ATGGCTATAGTTGAAATTATAAATCTAACAAAAAGCTTTAAAGATATTGAAGTTATTCATAACACTTAA | MAIVEIINLTKSFKDIEVIHNT | 22 | 2.5 | No | |
| rio65 | 1190190–1190255 | spv_1160 | spv_1161 | − | 1.83 | ATG | ATGAAAGATAGTTATTGGTTAAATTATTTTCCGGAGTATAGTTTAGAAACGTTTGAAGTAGAATAG | MKDSYWLNYFPEYSLETFEVE | 21 | 2.6 | No | |
| rio66 | 1242216–1242290 | spv_1212 | spv_1213 | + | 2 | ATG | ATGTGTAAGAATCACAATAAAAAATGCTCTTCCGTCTTGGAGGAGCATTTCTTTTTATCAACGAAAATCAAATAG | MCKNHNKKCSSVLEEHFFLSTKIK | 26 | 2.8 | No | |
| rio67 | 1242341–1242406 | spv_1212 | spv_1213 | + | 0.9 | TTG | TTGTATCAGCAATATGTGTCTGTCAAATTTAGTGACAAAGGTAGTAGAAGAAAGATGAAGAAATAA | MYQQYVSVKFSDKGSRRKMKK | 21 | 2.5 | No | |
| rio68 | 1250407–1250430 | spv_1217 | spv_1218 | − | 1.75 | ATG | ATGGCTTGGATCGACAATACCTAA | MAWIDNT | 7 | 0.8 | No | |
| rio69 | 1250035–1250073 | spv_1217 | spv_1218 | − | 2 | TTG | TTGAATGAAGGTGGTACCGCGGTTTTTCGCCCTTCGTGA | MNEGGTAVFRPS | 12 | 1.2 | No | |
| rio70 | 1264626–1264679 | spv_1232 | spv_2332 | − | 18 | ATG | ATGGGGGAAGGGATAGACAAGAGATTTTATCCACATATGAAAAAAGGAGGTTAG | MGEGIDKRFYPHMKKGG | 17 | 1.9 | No | |
| rio71 | 1274211–1274282 | spv_1245 | spv_1246 | − | 5.4 | ATG | ATGGAAGAAAAAATCAAAATTAAACCGCATTTTTGCTTGACAATTATTCCTTTTACGTGTAGAATGAAATAG | MEEKIKIKPHFCLTIIPFTCRMK | 23 | 2.8 | No | |
| rio72 | 1277771–1277803 | spv_1248 | spv_2334 | − | 1.4 | ATG | ATGATGACGCTGACAATGGGAGTATTATTGTAA | MMTLTMGVLL | 10 | 1.1 | No | |
| rio73 | 1287975–1288013 | spv_1258 | spv_1259 | + | 32 | ATG | ATGACCGAATTAGAAAGAAAAAATCGAAAAATTAGCTAA | MTELERKNRKIS | 12 | 1.5 | No | |
| rio74 | 1297923–1297994 | spv_1267 | spv_2337 | + | 9 | ATG | ATGATATGGGATTTTCATATAATAAATTGTAACCGCCCAATAACGAAGTCTATTGAAAAATCTCCAGATTAG | MIWDFHIINCNRPITKSIEKSPD | 23 | 2.7 | No | |
| rio75 | 1357604–1357621 | spv_1342 | spv_1343 | − | 5 | ATG | ATGTTGTTGATATTTTAA | MLLIF | 5 | 0.6 | No | |
| rio76 | 1357729–1357752 | spv_1342 | spv_1343 | − | 30 | ATG | ATGTATATGAGTAACTATCGATAA | MYMSNYR | 7 | 0.9 | No | |
| rio77 | 1404509–1404604 | spv_1383 | spv_1384 | − | 27 | CTG | CTGGCAGAAACCTGTGATAGTGTCGTCATTCCGAATTTTATGCTGAAAAGTATGCTTTCCGGCCCTATCTTAAACAGCGAGACTTGTTATGATTAA | MAETCDSVVIPNFMLKSMLSGPILNSETCYD | 31 | 3.4 | No | |
| rio78 | 1433395–1433523 | spv_1413 | spv_1414 | − | 5.4 | TTG | TTGCTCTGGTCTTGTGTTATACTAGATAGGTTGCAAAGAAAACAGTACTTTTCTTTTGTGGAAAAGAAGCAACACGATTTTATATCTAGTTATATGAAAATACGTCATAAAAAGAAAAGTATAAACTAA | MLWSCVILDRLQRKQYFSFVEKKQHDFISSYMKIRHKKKSIN | 42 | 5.2 | No | |
| rio79 | 1456875–1456949 | spv_1438 | spv_1439 | − | 117 | ATG | ATGCTTTCCTACGTTCGACATTACCCACTAGCGATAGCTAAATTAATGTGTCTGTGCTCTCCTAAAATCTGCTGA | MLSYVRHYPLAIAKLMCLCSPKIC | 24 | 2.7 | Yes | |
| rio80 | 1457861–1457905 | spv_1439 | spv_1440 | − | 14.9 | TTG | TTGGTTACAGGCATGCCAACCTGTCACTCGGATGAAGCCAAATAA | MVTGMPTCHSDEAK | 14 | 1.5 | No | |
| rio81 | 1480252–1480278 | spv_1464 | spv_1465 | + | 1.6 | TTG | TTGAAACGGAGGATTTTTGAATATTAG | MKRRIFEY | 8 | 1.1 | No | |
| rio82 | 1528556–1528618 | spv_1506 | spv_1507 | − | 120 | ATG | ATGACAGTAACGATTAAAGTAAATTACCAAACCACTTTCCAAAAGAAGGAAGCAAAAAACTAG | MTVTIKVNYQTTFQKKEAKN | 20 | 2.3 | No | srf-21 |
| rio83 | 1539897–1539920 | spv_1517 | spv_1518 | − | 775 | ATG | ATGATATACCATCGTTTAGAATAA | MIYHRLE | 7 | 0.9 | No | |
| rio84 | 1539963–1540064 | spv_1517 | spv_1518 | − | 358 | ATG | ATGGGCTTTAAAAAATATTTGAAGAATTTACCGAAAAACTCTGGATTTTTGATTTGGAGTTGGATTCAACTTATCTGGTTTGAAACATGGTTTTGGGGATAA | MGFKKYLKNLPKNSGFLIWSWIQLIWFETWFWG | 33 | 4.1 | Yes | |
| rio85 | 1579688–1579789 | spv_1558 | spv_1559 | − | 140 | CTG | CTGAATTTGGGCGAGCAAGGCGAGCCCCATAGAGAATACTTTTCGCTGTGGTGTAAGTTGGTACAAGTGATTGTACCAACTGCGGAAAATTTGAGACCTTAG | MNLGEQGEPHREYFSLWCKLVQVIVPTAENLRP | 33 | 3.8 | No | |
| rio86 | 1673721–1673771 | spv_1661 | spv_1662 | − | 16 | TTG | TTGACATTCTATCAAGCTGTCGGTCAGTTCGTTCAGTACAAGGAATCATAA | MTFYQAVGQFVQYKES | 16 | 1.9 | No | |
| rio87 | 1721860–1721904 | spv_1725 | spv_1726 | − | 5.6 | ATG | ATGCTTGCGACAAAAAGAGGCGATGATCTCTCTGCGGATATCTGA | MLATKRGDDLSADI | 14 | 1.5 | No | |
| rio88 | 1731142–1731255 | spv_1737 | spv_1738 | − | 46 | ATG | ATGAAAAGGACATATAGAGACTGTAAAAATATACTTTTGAAAAGCTTTTTAGTCTGGGGTGTTATTGTAGATAGAATGCAGACCTTGTCAGTCCTATTTACAGTGTCAAAATAG | MKRTYRDCKNILLKSFLVWGVIVDRMQTLSVLFTVSK | 37 | 4.3 | Yes | |
| rio89 | 1786300–1786332 | spv_1790 | spv_1791 | − | 5 | TTG | TTGCATTCGAAAAAGCTGGAAACATTTGCCTAG | MHSKKLETFA | 10 | 1.1 | No | |
| rio90 | 1796868–1796975 | spv_1803 | spv_1804 | − | 9.3 | TTG | TTGGTTCTCTCTTTTTTGATTTTCAGTAATTCATTTTGGCAGCGTATACTTTTTGTCTCCAGTCTTTTCAATAAATACCGTAGTTTAGGTATACACATTGAAATTTAA | MVLSFLIFSNSFWQRILFVSSLFNKYRSLGIHIEI | 35 | 4.2 | No | |
| rio91 | 1814148–1814210 | spv_1828 | spv_1829 | − | 2.8 | ATG | ATGAATTTCAATCTCCCAATTTATTTGTTCAAATATTGTTTTAATATATTGAATAAATTCTGA | MNFNLPIYLFKYCFNILNKF | 21 | 2.5 | No | |
| rio92 | 1814385–1814408 | spv_1828 | spv_1829 | − | 1.6 | ATG | ATGTTGAAAAATATCTTCGTATAG | MLKNIFV | 7 | 0.8 | No | |
| rio93 | 1858646–1858711 | spv_1878 | spv_1879 | − | 41.08 | TTG | TTGTTATTCTTCGTTCCTTTTTTATATTATTTTTGGTATAATTATAGTTATTCAAATTTTATTTAG | MLFFVPFLYYFWYNYSYSNFI | 21 | 2.8 | No | |
| rio94 | 1907589–1907618 | spv_1933 | spv_1934 | − | 29.8 | ATG | ATGCTTGAAAAGGAGTATACTTATAAGTAA | MLEKEYTYK | 9 | 1.2 | No | |
| rio95 | 2006759–2006857 | spv_2027 | spv_2028 | − | 37 | CTG | CTGAGAGGAAGTGTTAAACTTCGACCGCACCTGATCTGGGTAATGCCAGCGGAGGGAACGATACTTAGTCTAATTTTGCACCTTTTCCATGTATGGTAA | MRGSVKLRPHLIWVMPAEGTILSLILHLFHVW | 32 | 3.7 | No | |
| rio96 | 2012589–2012618 | spv_2032 | spv_2033 | − | 5 | ATG | ATGTGTAAAGGTAGGTTTACTGAATTGTAA | MCKGRFTEL | 9 | 1 | No | |
| rio97 | 105596–105616 | spv_2096 | spv_0106 | + | 425 | ATG | ATGGACTACGATACTTGTTGA | MDYDTC | 6 | 0.7 | No | |
| rio98 | 120796–120822 | spv_2100 | spv_0118 | + | 15 | ATG | ATGAGGTTGGGAAAAAACTCAATTTGA | MRLGKNSI | 8 | 0.9 | No | |
| rio99 | 120952–120984 | spv_2100 | spv_0118 | + | 5.5 | ATG | ATGACTTTATTTAGGTGTTTGACATTACTATAG | MTLFRCLTLL | 10 | 1.2 | No | |
| rio100 | 121373–121429 | spv_2101 | spv_0119 | + | 8.5 | ATG | ATGATGACTAAAGTTTTTATCAATAATTTGGGCTCCTTGTCAACTGTAGTGGGTTGA | MMTKVFINNLGSLSTVVG | 18 | 1.9 | Yes | |
| rio101 | 141806–141937 | spv_2113 | spv_2115 | + | 38 | ATG | ATGAGTTGGTTAGACGCTTTTCATTATAGGTCATATGGGGCTTTTTTCTACAAGAAACGACCCTATAATTCCTGGGGTGGGATTACCCACTACAGAAATTATAGAGCCAAAGCATTCCAAAGTCTTGTCTGA | MSWLDAFHYRSYGAFFYKKRPYNSWGGITHYRNYRAKAFQSLV | 43 | 5.2 | No | |
| rio102 | 420072–420098 | spv_2164 | spv_0420 | + | 2 | CTG | CTGTTACTAGAAAAAAGAGGACATTAA | MLLEKRGH | 8 | 0.9 | No | |
| rio103 | 742170–742214 | spv_2226 | spv_2227 | + | 55 | ATG | ATGAAAATTGGTCAACGAATTATGCGCTTTGGCATAAAAAATTAA | MKIGQRIMRFGIKN | 14 | 1.6 | No | |
| rio104 | 811459–811533 | spv_2240 | spv_0792 | + | 27 | ATG | ATGAACACATTAAATGAGAAAGTAATCAATATCTGTAAAGCAGTAGTTAAAGAAACTTTAATCCAAGACATTTAG | MNTLNEKVINICKAVVKETLIQDI | 24 | 2.7 | No | |
| rio105 | 849735–849788 | spv_2251 | spv_0833 | + | 35 | CTG | CTGATTGGCTTTTTCAATGTGAATCTTAACTTCATACTCCCAAAGAGGTATTAG | MIGFFNVNLNFILPKRY | 17 | 2 | No | |
| rio106 | 1032903–1032932 | spv_2288 | spv_0918 | − | 12 | ATG | ATGGCAAATACAGCACAGAATTTAAGATAA | MANTAQNLR | 9 | 1 | No | |
| rio107 | 1037861–1037899 | spv_2291 | spv_0914 | − | 9.2 | TTG | TTGTTGGTTTCGTGTCATAACAGTTATAGAGGCAAATAG | MLVSCHNSYRGK | 12 | 1.3 | No | |
| rio108 | 1037928–1037963 | spv_2291 | spv_0914 | − | 7.9 | ATG | ATGATAAAGTTTGTGAATATCTTAGTCCTCATTTGA | MIKFVNILVLI | 11 | 1.3 | No | |
| rio109 | 1038016–1038045 | spv_2291 | spv_0914 | − | 18 | ATG | ATGATTGATAAAGGCAACAAAAAATTTTAG | MIDKGNKKF | 9 | 1 | No | |
| rio110 | 1278108–1278194 | spv_2334 | spv_1249 | − | 9.1 | ATG | ATGAGTGAAAATTATCAAGTTGGAATGTTTGTATCTAAATATATTAGCATGTATTTAGATAAGATGTCTGCAATCCTTTATATATGA | MSENYQVGMFVSKYISMYLDKMSAILYI | 28 | 3.3 | No | |
| rio111 | 1469164–1469196 | spv_2369 | spv_2370 | − | 6.8 | ATG | ATGGAAAGAGGAACGAATGAAGATGAAAGCTAG | MERGTNEDES | 10 | 1.1 | No | |
| rio112 | 1619475–1619567 | spv_2394 | spv_1605 | − | 4.9 | TTG | TTGAGGTGGCACCGCGTTACCAACGCCCTCACACGGAAGTATATTCTGTGTGTGGGCTTTTTTCTATCCGTCGTTTGGTTTATCTTTTATTAG | MRWHRVTNALTRKYILCVGFFLSVVWFIFY | 30 | 3.7 | Yes | |
| rio113 | 1619912–1619941 | spv_2394 | spv_1605 | − | 12.5 | ATG | ATGGAGTGTTCTAAAATAAGTTCTGTTTAG | MECSKISSV | 9 | 0.9 | No | |
| rio114 | 1751386–1751403 | spv_2420 | spv_1757 | + | 1.2 | ATG | ATGTTGTTTCAGTATTGA | MLFQY | 5 | 0.7 | No |
TABLE 2.
sRNAs involved in virulence
| sRNAa in Tigr4 | Tigr4 flanking gene | Tigr4 flanking gene | sRNA D39 homolog | Host | Fitness (<1, fitness defect) | sORF | Expression (rpm) |
|---|---|---|---|---|---|---|---|
| F38 | sp_1012 | sp_1013 | srf-17 | Nasopharynx | 0 | rio56 | 115 |
| SN39 | sp_0761 | sp_0762 | Nasopharynx | 0 | rio49 | 550 | |
| F52 | sp_0041 | sp_0042 | srf-02 | Nasopharynx | 0 | rio3 | 208 |
| trn0760 | sp_1625 | sp_1626 | Nasopharynx | 0 | rio79 | 73 |
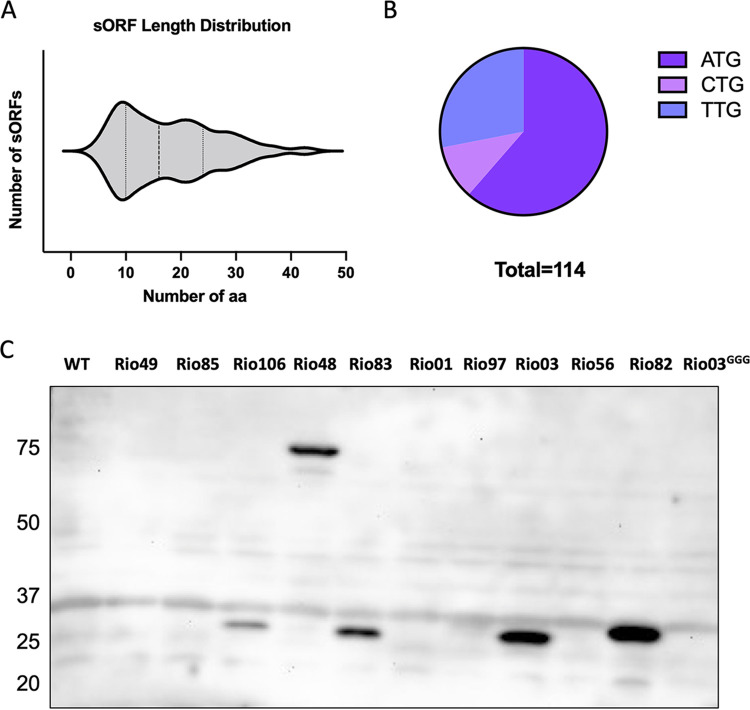
The identified sORFs range in length from 5 to 43 amino acids, with the majority having an AUG start codon (Fig. 2A and B). Using tBLASTn analysis (33), we investigated the conservation of the sORFs among six clinically relevant S. pneumoniae serotypes (1, 4, 14, 19A, 23F, and 19F). We found that 49 sORFs were conserved in all six serotypes, with the remaining sORFs being conserved in at least one of the serotypes except for rio34, rio72, and rio89 (see Table 3). We also identified 10 sORFs encoding proteins containing putative signal peptides as determined by SignalP analysis (34) suggested their insertion into or translocation across the cytoplasmic membrane (Table 1). One of these, rio84, was found adjacent to an Rgg family member gene, and we hypothesized that it encodes the signaling peptide for a pheromone receptor QS system (see below). Nine sORFs are located within or overlap the previously annotated noncoding RNAs (ncRNAs), two of which were previously associated with fitness defects determined by transposon-insertion sequencing (TIS) (Tables 1 and 2) (1, 3).
FIG 2.
Identification and validation of unannotated sORFs. (A) Violin plot showing the distribution of protein lengths (amino acids [aa]) encoded by the 114 sORFs identified. (B) Start codon identity distribution of the sORFs. (C) Western blotting of C-terminally sfGFP-tagged sORFs expressed from their native locus.
TABLE 3.
sORFs identified by Ribo-seq are conserved among other Streptococcus pneumoniae serotypesa
| sORF length (nt) | sORF | Presence of sORF in strain |
|||||
|---|---|---|---|---|---|---|---|
| P1031 (serotype 1; GenBank accession no. CP000920) | TIGR4 (serotype 4; GenBank accession no. AE005672.3) | JJA (serotype 14; GenBank accession no. CP000919.1) | Hungary 19A-6 (serotype 19A; GenBank accession no. CP000936.1) | ATCC 700669 (serotype 23F; GenBank accession no. FM211187.1) | Taiwan 19F-14 (serotype 19F; GenBank accession no. CP000921.1) | ||
| 32 | rio1 | X | X | X | |||
| 19 | rio2 | X | X | X | X | X | |
| 11 | rio3 | X | X | X | X | X | X |
| 28 | rio4 | X | X | X | X | X | X |
| 37 | rio5 | X | X | X | X | X | X |
| 28 | rio6 | X | X | X | X | X | X |
| 25 | rio7 | X | X | ||||
| 9 | rio8 | X | |||||
| 14 | rio9 | X | X | ||||
| 15 | rio10 | X | X | ||||
| 9 | rio11 | X | X | ||||
| 14 | rio12 | X | X | X | X | ||
| 16 | rio13 | X | X | ||||
| 21 | rio14 | X | X | X | |||
| 7 | rio15 | X | X | X | |||
| 11 | rio16 | X | X | ||||
| 20 | rio17 | X | X | X | X | X | X |
| 13 | rio18 | X | X | X | X | X | X |
| 28 | rio19 | X | X | X | X | X | X |
| 26 | rio20 | X | X | ||||
| 13 | rio21 | X | X | X | |||
| 24 | rio22 | X | |||||
| 15 | rio23 | X | X | X | X | X | X |
| 23 | rio24 | X | X | X | X | X | X |
| 23 | rio25 | X | X | X | X | X | X |
| 27 | rio26 | X | X | X | X | X | X |
| 20 | rio27 | X | X | X | X | X | X |
| 19 | rio28 | X | X | X | X | X | X |
| 19 | rio29 | X | X | X | X | X | X |
| 29 | rio30 | X | X | X | |||
| 13 | rio31 | X | X | X | X | X | X |
| 9 | rio32 | X | X | X | X | ||
| 10 | rio33 | X | X | X | X | X | |
| 17 | rio34 b | ||||||
| 23 | rio35 | X | X | X | X | X | X |
| 21 | rio36 | X | X | X | X | X | X |
| 10 | rio37 | X | X | X | X | X | |
| 12 | rio38 | X | X | ||||
| 24 | rio39 | X | |||||
| 8 | rio40 | X | X | X | X | ||
| 21 | rio41 | X | |||||
| 9 | rio42 | X | X | ||||
| 14 | rio43 | X | X | X | X | X | X |
| 13 | rio44 | X | X | X | X | X | X |
| 16 | rio45 | X | X | X | X | X | X |
| 8 | rio46 | X | X | X | |||
| 29 | rio47 | X | X | X | X | X | X |
| 24 | rio48 | X | X | X | X | X | X |
| 17 | rio49 | X | X | X | X | ||
| 30 | rio50 | X | X | X | |||
| 19 | rio51 | X | X | X | X | X | X |
| 27 | rio52 | X | X | X | X | X | |
| 11 | rio53 | X | X | ||||
| 13 | rio54 | X | X | X | X | X | X |
| 11 | rio55 | X | X | ||||
| 6 | rio56 | X | X | X | X | ||
| 18 | rio57 | X | X | X | X | ||
| 20 | rio58 | X | X | X | X | X | X |
| 8 | rio59 | X | |||||
| 16 | rio60 | X | X | X | |||
| 9 | rio61 | X | X | X | |||
| 10 | rio62 | X | X | X | X | ||
| 30 | rio63 | X | X | X | X | X | X |
| 22 | rio64 | X | X | X | X | X | |
| 21 | rio65 | X | |||||
| 26 | rio66 | X | X | X | X | X | X |
| 21 | rio67 | X | X | X | X | X | X |
| 7 | rio68 | X | X | X | |||
| 12 | rio69 | X | X | X | X | X | X |
| 17 | rio70 | X | X | X | X | X | X |
| 23 | rio71 | X | X | X | X | X | X |
| 10 | rio72 b | ||||||
| 12 | rio73 | X | X | X | X | X | X |
| 23 | rio74 | X | X | X | X | X | |
| 5 | rio75 | X | X | X | X | ||
| 7 | rio76 | X | X | X | X | ||
| 31 | rio77 | X | X | X | X | X | X |
| 42 | rio78 | X | X | X | X | X | X |
| 24 | rio79 | X | X | X | X | X | X |
| 14 | rio80 | X | X | X | X | X | X |
| 8 | rio81 | X | X | ||||
| 20 | rio82 | X | X | X | X | X | X |
| 7 | rio83 | X | X | X | |||
| 33 | rio84 | X | X | X | |||
| 33 | rio85 | X | |||||
| 16 | rio86 | X | X | X | X | X | X |
| 14 | rio87 | X | X | X | X | X | X |
| 37 | rio88 | X | X | X | X | X | X |
| 10 | rio89 b | ||||||
| 35 | rio90 | X | X | X | X | X | X |
| 21 | rio91 | X | X | X | X | ||
| 7 | rio92 | X | X | X | X | ||
| 21 | rio93 | X | X | X | X | X | X |
| 9 | rio94 | X | X | X | |||
| 32 | rio95 | X | X | X | X | X | X |
| 9 | rio96 | X | X | ||||
| 6 | rio97 | X | X | X | X | ||
| 8 | rio98 | X | |||||
| 10 | rio99 | X | |||||
| 18 | rio100 | X | X | X | |||
| 43 | rio101 | X | X | X | X | X | X |
| 8 | rio102 | X | X | X | |||
| 14 | rio103 | X | X | X | X | X | X |
| 24 | rio104 | X | X | X | X | X | X |
| 17 | rio105 | X | X | X | X | ||
| 9 | rio106 | X | X | X | X | X | |
| 12 | rio107 | X | X | X | X | X | X |
| 11 | rio108 | X | X | X | X | ||
| 9 | rio109 | X | X | X | X | ||
| 28 | rio110 | X | X | X | X | X | X |
| 10 | rio111 | X | |||||
| 30 | rio112 | X | X | X | X | X | X |
| 9 | rio113 | X | X | X | X | X | |
| 5 | rio114 | X | X | X | X | ||
sORFs highlighted in boldface type were too short for tBLASTn analysis, so we assessed their conservation by looking for conserved nucleotide sequences.
Nucleotide sequence not conserved in the 6 serotypes but found in other strains.
To validate the Ribo-RET results and demonstrate sORF translation, 6 sORFs displaying the highest number of read counts at start codons (rio48, rio49, rio83, rio85, rio97, and rio106) and 4 sORFs located within documented ncRNAs (rio01, rio3, rio82, and rio56) were selected to be tagged with translation reporters (Table 1). A sequence encoding superfolder green fluorescent protein (sfGFP) (lacking its own start codon) was placed at the 3′ end of each selected sORF at its native chromosomal locus to generate in-frame translational fusions. If translated, the addition of sfGFP should increase the molecular weight of each sORF peptide by ~27 kDa. Cells containing the tagged constructs were cultured to mid-log phase in chemically defined medium (CDM) to mimic the conditions used in ribosome profiling experiments, and the expressed proteins were evaluated by Western blotting using an anti-GFP antibody. Of the 10 sfGFP-tagged constructs, 5 produced a strong band with the expected mobility on an SDS gel, verifying their translation (Fig. 2C). To demonstrate that sfGFP was not independently translated when placed in frame with sORFs, the start codon of the sORF rio3 fused to sfGFP was mutated from ATG to GGG. The production of the fusion protein was completely abolished, demonstrating not only the translation of the identified sORFs but also the accuracy of mapping its start codon by Ribo-RET/Ribo-LEF.
Unexpectedly, rio48::sfGFP, located immediately upstream of the gene encoding peptide release factor 2 (RF2), prfB, produced a strong band of ~70 kDa. In E. coli, the expression of RF2 is autoregulated by programmed frameshifting; RF2 deficiency stimulates a +1 frameshift resulting in the readthrough of the in-frame UGA stop codon and the translation of the full-size functional RF2 protein (35). In E. coli, previous studies have demonstrated that the frameshift mechanism exploits several key features of the prfB mRNA: a Shine-Dalgarno (SD) sequence 3 nucleotides upstream of the frameshift site (AGG GGG), the frameshift site (CUU UGA), and the context of the UGA stop codon flanked with a 3′ C (Fig. S4) (36–38). The short distance between the SD sequence and the frameshifting site creates tension destabilizing the interactions between the P-site and the anticodon of the ribosome, resulting in a +1 frameshift. Furthermore, the genetic context of the UGA stop codon in proximity to a C nucleotide has been demonstrated to be the least efficient termination signal (37–39). These key mRNA features are also conserved in rio48, suggesting that prfB in S. pneumoniae is regulated in a similar manner. Likewise, a +1 frameshift at the UGA stop codon of rio48 is in frame with downstream prfB, and therefore, programmed frameshifting during Rio48 translation could stimulate the expression of RF2. The rio48::sfGFP construct retains the UGA stop codon of rio48 after sfGFP and likely results in readthrough and the generation of the larger gene product corresponding to ~70 kDa seen on the immunoblot. Thus, in this instance, Ribo-RET likely identified the correct translation start site for prfB.
Sequence alignment of prfB in E. coli and S. pneumoniae D39. Shown is a schematic representation of prfB in E. coli and S. pneumoniae. The stop sign represents the internal UGA stop codon where a +1 frameshift occurs. The mRNA sequence is of the 5′ end of prfB, and the underlined sequence depicts the conserved sequence in both bacteria. SD highlights the Shine-Dalgarno sequence, FS highlights the frameshift site, and the asterisk identifies the UGA stop codon. Download FIG S4, TIF file, 1.8 MB (1.8MB, tif) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Peptides associated with an Rgg-type quorum sensing system.
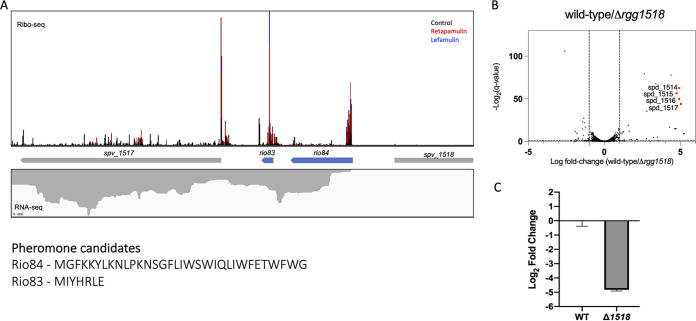
Previous studies identified and characterized RRNPP transcriptional regulators in streptococci and demonstrated their importance in regulating genes associated with virulence, immunosuppression, lysozyme resistance, and competence (40–43). Ribo-RET detected the presence of two sORFs encoding polypeptides of 33 amino acids (rio84) and 7 amino acids (rio83) in length that are adjacent to an Rgg-like transcriptional regulator (spv_1518, referred to as rgg1518 here) (Fig. 3A). The peptide encoded by rio84 has characteristics resembling those of other streptococcal pheromones, such as a positively charged N terminus and a Trp-X-Trp (WXW) motif at the C terminus (44), leading us to hypothesize that rio84 may encode the pheromone for Rgg1518 (Fig. 3A). To verify that Rgg1518 functions as a transcriptional regulator and to identify the genes under its regulation, transcriptome sequencing (RNA-seq) analysis was conducted to compare the gene expression of wild-type D39 to that of an isogenic deletion mutant, Δrgg1518 (Fig. 3B). The expression of the spv_1513-1517 operon located adjacent to rgg1518 and immediately downstream from rio83 was substantially decreased in the deletion mutant, a trend that we verified by quantitative real-time PCR (qRT-PCR) (Fig. 3C). The operon of genes spv_1513 to spv_1517 (hereafter spv_1513-1517) encodes proteins predicted to comprise an ABC transporter of an unknown substrate(s), suggesting that Rgg1518 could be a regulator of nutrient acquisition. A previous report found that the spv_1513-1517 operon was significantly upregulated when wild-type D39 bacteria were applied to A549 lung epithelial cells, suggesting a role during interactions with the host (25). We tested the impact that deleting the operon would have on adherence to or invasion of A549 cells but found no difference in attachment, internalization, or viability from the wild type, at least over short infection times (up to 4 h) (Fig. S5A and B). An independent recent report demonstrated that the presence of intact Rgg1518 is important for colonization of the murine nasopharynx by S. pneumoniae (23). To assess whether the spv_1513-1517 operon is responsible for this phenotype, we coinfected CD1 mice with 105 CFU of wild-type D39 and 105 CFU of the Δspv_1513-1517 mutant in the nasopharynx and determined the bacterial burden in the nasal passage over a span of 7 days. The Δspv_1513-1517 mutant decreased over time in comparison with wild-type S. pneumoniae; however, the difference was not statistically significant, suggesting that the conditions in our experiment were not conducive to show whether this operon plays a critical role in colonizing the murine nasal passage (Fig. S5C).
FIG 3.
Identification of two novel sORFs found near the uncharacterized transcriptional regulator Rgg1518. (A) Ribosome footprint density profiles of rio83 and rio84 found near spv_1518 (Rgg1518). Blue arrows represent sORFs identified by Ribo-RET, and gray arrows represent previously annotated ORFs. (B) Volcano plot of wild-type D39 versus Δrgg1518 transcript fold changes. Genes of interest with the highest fold change differences are indicated on the graph. (C) qRT-PCR validation of spv_1517 expression in wild-type (WT) D39 versus the Δrgg1518 mutant.
The Rgg1518-regulated operon spv_1513-1517 is not involved in adhesion, intracellular survival, or colonization of the murine nasopharynx. (A and B) A549 alveolar epithelial cells were infected with the Δcps (positive control) and Δcps Δspv_1513-1517 strains at an MOI of 1:100 to assess their role in adhesion (A) and intracellular survival (B). (C) CD1 mice were coinfected with 2 × 105 CFU/20 μL of the wild-type D39 and Δspv_1513-1517 strains, and the bacterial burden in the nasopharynx was determined over a span of 7 days. Download FIG S5, TIF file, 1.7 MB (1.8MB, tif) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
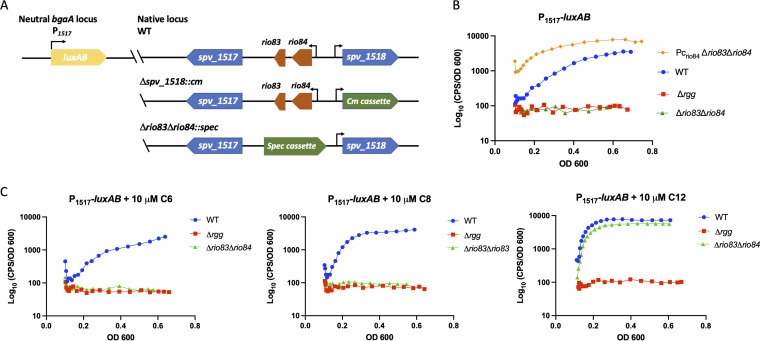
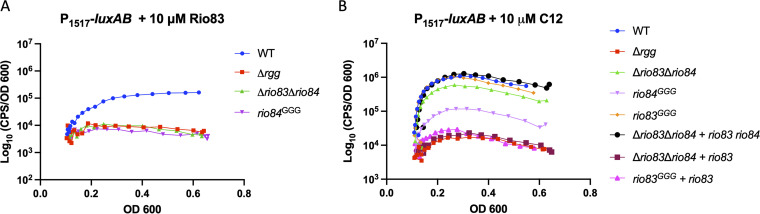
To assist in evaluating the potential contributions of Rgg1518, Rio83, and Rio84 to mediating cell-to-cell signaling, we constructed a luciferase-based transcription reporter using the promoter (P1517) identified by 5′ rapid amplification of cDNA ends (RACE) upstream of rio84 (Fig. 4A). The promoter-reporter construct was placed into an unlinked, neutral location in the chromosome of isogenic strains with deletions of rgg1518 or a combined deletion of its affiliated sORFs rio83 and rio84 (45). During growth in CDM, the wild-type reporter strain produced strong luminescence as the culture density increased, whereas the luminescence of the isogenic Δrgg1518 and Δrio83 Δrio84 mutants remained at low levels throughout the cultures’ growth (Fig. 4B; Fig. S6A). The expression of rio84 from a constitutive promoter (Pc-rio84) in the Δrio83 Δrio84 mutant background led to enhanced luciferase activity (Fig. 4B, yellow curve; Fig. S6A), indicating that the expression of rio84 in trans was sufficient to complement the Δrio83 Δrio84 mutant. These results support a model in which rio84 encodes a functional pheromone for Rgg1518, consistent with the results of a recent independent study (23). To identify the mature form of the pheromone, synthetic peptides of various lengths encompassing the C-terminal region of rio84 (C6, C8, and C12) were added to cultures. While the 6- and 8-amino-acid-long peptides were unable to stimulate transcription from the P1517 promoter, the C12 variant (IQLIWFETWFWG) efficiently induced the expression of P1517 in the wild-type or Δrio83 Δrio84 strain but not in the Δrgg1518 strain (Fig. 4C; Fig. S6B). Thus, the active form of the rio84 pheromone is likely confined within or is equivalent to this sequence.
FIG 4.
rio84 encodes the signaling peptide for the Rgg1518 quorum sensing system. (A) Schematic of the luciferase reporter integrated into the bgaA locus of S. pneumoniae D39. The black arrows indicate the promoter. (B) P1517 is induced when grown in CDM and upon the constitutive expression of rio84 in the background of the Δrio83 Δrio84 strain. (C) Induction of P1517 upon the addition of 10 μM synthetic C6, C8, and C12 Rio84 peptides. The data shown are representative of results from three independent experiments.
Biological replicates of luciferase assays. (A) Biological replicates of the luciferase assays depicted in Fig. 4B. (B) Biological replicates of the luciferase assays depicted in Fig. 4C. (C) Biological replicates of the luciferase assays in Fig. 5A. (D) Biological replicates of the luciferase assays in Fig. 5B. Download FIG S6, TIF file, 2.9 MB (2.9MB, tif) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
RNA-seq results indicated that rio83 is downregulated in the absence of Rgg1518, suggesting that rio83 might be involved in the regulation of Rgg1518-based QS regulation. The translation of the rio83 sORF was validated by fusing it to sfGFP and was detected by Western blotting when cultures were treated with exogenous pheromone (Fig. 2C). The addition of the full-length synthetic rio83 peptide to cultures did not alter luciferase activity (Fig. 5A; Fig. S6C). Intriguingly, the reporter activity in a Δrio84 mutant, grown in the presence of C12, did not reach the level of luciferase activity seen in the wild-type or Δrio83 Δrio84 strain (Fig. 5B; Fig. S6D). Furthermore, complementing the Δrio83 Δrio84 strain with rio83 resulted in a complete loss of luminescence activity. These results suggest that rio83 serves as a negative regulator. However, the extent of its impact on the control of the putative ABC transporter (spv_1513-1517) remains unclear.
FIG 5.
Expression of rio83 in the absence of rio84 represses luciferase activity. (A) P1517 induction in the presence of 10 μM full-length synthetic Rio83. (B) P1517 induction in different knockout strains in the presence of 10 μM synthetic C12. The data shown are representative of results from three independent experiments.
The Ribo-RET data set also identified the known signaling peptide (rio9) for the Rgg0112 transcriptional regulator (44) as well as additional sORFs found downstream of Rgg0112 (rio7, rio8, and rio10-15) (Table 1), which appear to be part of the Rgg0112 regulon based on our RNA-seq data comparing wild-type D39 to an rgg0112 mutant. Manual assessment of the Ribo-RET data set near other known Rgg-like transcriptional regulators identified sORFs that did not meet our initial search criteria (Table S3). rio119, found within the current annotation of srf-06 and partially overlapping Rgg144, encodes the previously characterized pheromone for Rgg0144 (21, 22). Additional sORFs (rio120, rio121, and rio122) were identified within the same locus, downstream of the Rgg0144 pheromone and overlapping the transcriptional regulator on the opposite strand. To date, the roles that these additional sORFs may play in the QS systems are unknown.
sORFs overlapping the gene on the opposite strand. Download Table S3, DOCX file, 0.1 MB (97.3KB, docx) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
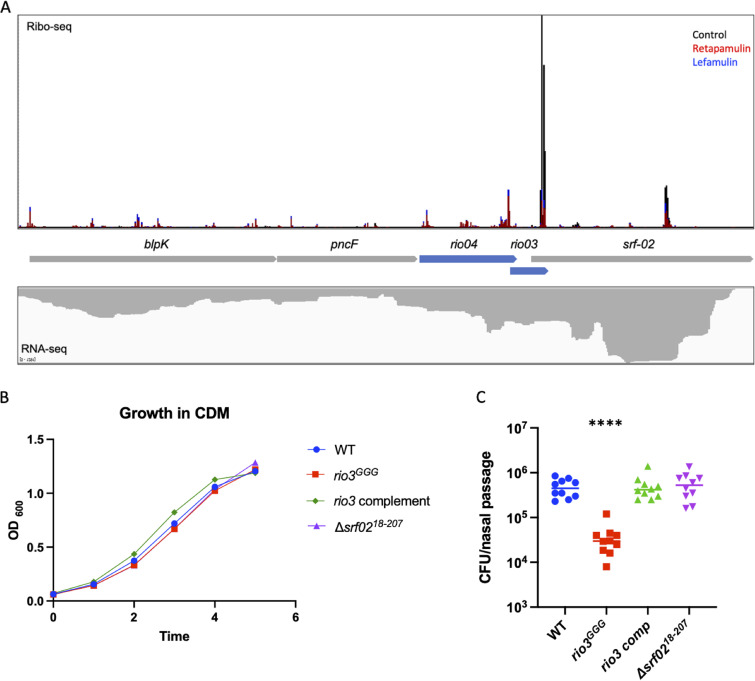
The sORF rio3, contained within the ncRNA srf-02, contributes to nasopharyngeal colonization.
A previous TIS study identified a noncoding RNA, F52, in S. pneumoniae TIGR4 whose disruption negatively impacted the fitness of the pathogen in a mouse model of pneumonia (1). The S. pneumoniae reference strain D39 contains an ortholog of this ncRNA, which is referred to as srf-02 (3). One of the sORFs identified and confirmed in our experiments (Fig. 2C), rio3, overlaps the annotated ncRNA srf-02 (Fig. 6A). Given this overlap, we wondered if the fitness defect described in the TIS study might be attributable to a disruption of rio3 rather than the ncRNA. To test this hypothesis, the start codon of rio3 was mutated (ATG→GGG) to prevent the translation of the sORF. Separately, a deletion was generated (Δsrf-0218–207) that extended through the srf-02 gene, which removed 188 3′-terminal nucleotides of the ncRNA while ensuring that the rio3 sORF remained intact. Neither mutant strain displayed a growth defect compared to the wild type in culture (Fig. 6B). In order to assess whether rio3 has an impact on nasopharyngeal colonization, 6-week-old BALB/c mice were infected intranasally with the wild type, the rio03GGG mutant, the Δsrf-0218–207 mutant, or the rio03GGG complemented strain. The bacterial burdens in the nasal passage were enumerated at 24 h postinfection. Minimal differences were seen between the abilities of the wild-type, the Δsrf-0218–207 mutant, and the rio03GGG complemented strains to colonize the nasopharynx; however, the rio03GGG mutant displayed a significant defect in colonizing the murine nasopharynx (Fig. 6C). These data indicate that the fitness defects attributed to srf-02 on the basis of the TIS experiments are instead related to disruption of the sORF rio3 identified in the S. pneumoniae genome by our Ribo-RET/Ribo-LEF approach.
FIG 6.
rio3 is important for nasopharyngeal colonization in a pneumonia mouse model. (A) Ribosome footprint of the blpK operon. Arrows in blue represent sORFs identified by Ribo-RET, and arrows in gray represent ORFs annotated previously. (B) Growth curve of wild-type and mutant strains in CDM over a span of 6 h. (C) Six-week-old BALB/c mice were inoculated with 1 × 107 CFU/25 μL of either the wild type, the rio03GGG mutant, the rio03GGG complemented strain, or the Δsrf-0218–207 mutant. The nasal passages were collected at 24 h postinfection, homogenized, and plated to determine the bacterial burden. Statistical significance was determined using Kruskal-Wallis analysis. **** denotes a P value of <0.0001.
DISCUSSION
Ribosome profiling has been conducted and optimized extensively in E. coli; however, its application to other bacteria, including Gram-positive pathogens like S. pneumoniae, has seen limited reports (46–48). Here, we set out to identify actively translated unannotated sORFs using antibiotic-assisted ribosome profiling in S. pneumoniae D39, an approach that was successfully used to identify translation start sites in the E. coli genome (11, 29). We conducted profiling on samples without and with two translation inhibitors, retapamulin and lefamulin; identified 114 novel sORFs in the D39 genome; and confirmed that translation occurs for a subset of them. Although this is a considerable addition to the number of genes deserving future study in the S. pneumoniae genome, ribosome profiling provides only limited information regarding gene function. We drew upon genome context and published genomic studies to initiate a functional characterization of four sORFs: two associated with the Rgg1518 quorum sensing system, one attributed to colonization, and one serving as a leader peptide that governs that translation of peptide release factor. A total of 89% of the remaining sORFs were conserved in at least 2 genomes, and 42% were conserved in all 6 additional S. pneumoniae strains that we searched, representing diverse serotypes. Given the dynamic plasticity of the S. pneumoniae metagenome, the retention of sORFs among multiple genomes implies that they contribute to fitness, at least in some niches (Table 3). Identifying appropriate conditions under which an sORF contributes to fitness is not trivial, but having their identity known or proposed will stimulate hypothesis-driven mechanistic studies of bacterial processes in which sORFs are suspected to play a role.
For instance, substantial effort has gone into identifying sORF-encoded pheromones of peptide-mediated QS systems (11, 22, 23, 29, 49–52). The number of putative pheromone receptors identified in genomes greatly outnumbers recognizable pheromone genes. Cognate pheromones for a majority of RRNPP proteins remain elusive since most receptor genes do not have an obvious pheromone-encoding sORF in their proximity; intergenic regions are typically replete with several theoretical sORFs, making it difficult to identify actual pheromone genes. In addition to the two sORFs associated with the Rgg1518 QS system, the Ribo-RET/LEF data set identified sORFs near previously characterized Rgg-mediated QS systems (Table 1), providing an empirical basis to test their role in QS systems. Unfortunately, translation profiling was still not powerful enough to predict pheromone sORFs for all RRNPP systems in S. pneumoniae, as the genes rgg0999, rgg1786, and rgg1916 remain orphan receptors following our study. Transcription profiling (RNA-seq) indicated that the loci encoding these systems were silent under the conditions that we used to collect RNA and ribosomes. Thus, having conditions under which communication networks are universally active remains elusive and is a primary weakness of genome-wide expression studies.
Previous genomic studies conducted in S. pneumoniae D39, like those using transcriptional profiling tools and algorithms to annotate novel sRNAs (1, 2) and transposon insertion sequencing that correlates insertion mutants with fitness, were the primary sources of information for us to prioritize a deeper study of sORF function. Traditionally, sRNAs provide mechanisms of posttranscriptional regulation governing a variety of processes such as metabolism, the stress response, and virulence (53, 54). sRNAs are thought to be noncoding and function through base pair interactions with target mRNA molecules, either preventing or enhancing translation or influencing mRNA stability. The Ribo-RET and Ribo-LEF data sets identified sORFs within nine previously annotated sRNA loci, indicating that they either are protein-coding mRNAs or have a dual function as messengers and regulators. Our results argue that rio3 is a protein-coding gene whose expression accounts for the in vivo fitness attribute first identified by TIS (1). It is possible that the srf-02 RNA also plays a regulatory role in some fashion; however, we did not observe a phenotype supporting this possibility. Another ncRNA, srf-21, was found to contain the protein-coding gene rio82. Previous studies have shown that srf-21 is regulated by the CiaRH two-component system known to regulate genes involved in competence, biofilm formation, antibiotic resistance, and stress tolerance (55, 56), suggesting a possible function of rio82 in these processes.
An unexpected observation from the Ribo-RET/LEF data sets was the finding of a substantial number of genes for which ribosomes mapped to regions as far as 20 nt upstream of start codons (Fig. 1C); this was consistently observed among all 5 biological replicates (see Fig. S7 in the supplemental material). We have yet to determine whether these patterns are due to an unforeseen artifact of the modified techniques that we employed (i.e., elevated concentration of MgCl2 in the cell lysis buffer) or if they are attributable to a biological phenomenon. Since S. pneumoniae is an AT-rich organism, and the nuclease used to isolate the ribosome footprint (MNase) cleaves at A and U more efficiently than at G and C, we suspect that some mRNAs undergo aberrant digestion, leading to the incorrect mapping of the ribosome footprint. Our attempt to filter data based on footprint length improved the percentage of genes with aligned start sites, but a pattern of footprints in the 5′ untranslated region (UTR) remained albeit to a lesser extent. The use of a different nuclease, e.g., RNase I, or a combination of different nucleases could be a potential solution to mitigate the nuclease bias of AT-rich genomes in future ribosome profiling studies. However, we also cannot exclude that the presence of upstream ribosome footprints reflects an alternative mode of translation initiation in S. pneumoniae. The initiation of translation involves the recruitment of the ribosome to the ribosome binding site (RBS) in mRNA, aided sometimes by the recognition of a purine-rich SD sequence preceding the start codon (57–59). However, not every RBS contains conventional SD sequences, and a recent genome-wide study demonstrated that recognition of the SD motif is not crucial for translation initiation in E. coli (60). Additional factors might govern ribosome recruitment to the start codons of the ORFs. It is possible that the initiation of the translation of some genes in S. pneumoniae requires the loading of the ribosome upstream from the ORF, with the subsequent migration of the 70S initiation complex to the start codon.
Taken together, Ribo-RET is a powerful technique utilizing the initiation inhibitor retapamulin or lefamulin to reveal a genome-wide view of the translational landscape of S. pneumoniae D39. These data sets identify small proteins or microproteins whose contributions span a spectrum of activities that include cell-to-cell communication, host-microbe interactions, and physiological homeostasis.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
All strains and plasmids used in this study are listed in Table S1 in the supplemental material. S. pneumoniae D39 was routinely grown on tryptic soy agar (TSA) supplemented with 5% sheep blood or cultured in Todd-Hewitt broth with 0.2% yeast (THY) and 0.5% Oxyrase (catalog number OB-0100; Oxyrase) or in a chemically defined medium (CDM) (50) supplemented with 1% glucose, 10% choline, and 0.5% Oxyrase at 37°C in an atmosphere of 5% CO2. When appropriate, chloramphenicol (4 μg/mL), spectinomycin (150 μg/mL), kanamycin (200 μg/mL), erythromycin (0.3 μg/mL), or neomycin (20 μg/mL) was added to S. pneumoniae D39 cultures.
Strains and plasmids used in this study. Download Table S1, DOCX file, 0.02 MB (26.4KB, docx) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Transformation.
To generate competent S. pneumoniae D39 cells, wild-type D39 cells were grown in 7.5 mL THY supplemented with 0.013 N HCl and 0.05% glycine at 37°C in an atmosphere of 5% CO2 to an optical density at 600 nm (OD600) of 0.05 to 0.1. Cells were diluted into 1 mL THY to an OD600 of 0.03; supplemented with a solution containing 10 mM NaOH, 0.2% bovine serum albumin (BSA), 1 mM CaCl2, and 0.2 μg/mL competence-stimulating peptide 1 (CSP-1); and placed in a 37°C water bath for 14 min. Following incubation, ~850 ng of donor DNA was added, and cells were allowed to recover at 37°C with 5% CO2 for 1 h, followed by plating onto TSA plates supplemented with 5% sheep blood and the appropriate antibiotic.
Construction of mutant strains.
All S. pneumoniae D39 deletion mutants, listed in Table S1, were generated by transforming competent S. pneumoniae D39 cells with linear DNA containing upstream and downstream sequences that facilitate homologous recombination and were generated by Gibson assembly of PCR amplicons using the primers listed in Table S2. All strains were confirmed by sequencing the locations of the chromosome containing the relevant alterations. Specific constructs are described further here. To delete rgg1518 (strain IL20), a PCR-generated upstream flanking region (UFR) amplicon and a downstream flanking region (DFR) amplicon were joined with a chloramphenicol resistance cassette by Gibson assembly using NEBuilder HiFi DNA assembly master mix (New England BioLabs [NEB]). Strain IL40 (Δrio83 Δrio84::spec) was constructed by Gibson assembly using a spectinomycin resistance cassette. Strain IL108 contains a deletion of the noncoding RNA srf-02 (Δsrf-0218–207::erm) without disrupting the overlapping sORF rio3; the UFR encompasses the first 17 nucleotides of srf-02. To generate the missense point mutations in strains IL91 (rio03ATG-GGG-spec) and IL101 (rio83ATG-GGG-spec), special oligonucleotides were designed to replace the start codon ATG with the glycine codon GGG. To generate strain IL91, two DNA fragments were generated using primer pairs ILp355/ILp356 and ILp354/KTp043, and overlapping PCR was performed to generate a PCR amplicon with the start codon mutation in rio3, which was subsequently used as the template to amplify the UFR for the construct. To generate strain IL101, overlapping PCR was performed as described above, using primer pairs ILp170/ILp161 and ILp169/ILp166.
Primers used for the construction of the strains used in this study. Download Table S2, DOCX file, 0.03 MB (34KB, docx) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Construction of chromosomal luxAB reporters.
To assess the expression levels of spv_1517, the intergenic region between spv_1517 and spv_1518 was amplified using a DNA template containing start codon mutations (GGG in place of ATG) in both rio83 and rio84. To attain the DNA amplicon containing the missense mutations, overlapping PCR was performed using primer pairs ILp166/ILp167 and ILp168/ILp161 for rio84 and primer pairs ILp170/ILp161 and ILp169/ILp166 for rio83. Overlapping PCR combined the two mutations on one DNA amplicon. The resulting linear piece of DNA was then used as a template to amplify the promoter region for spv_1517 using primer pair ILp161/ILp166. Using Gibson assembly, the upstream region of the bga locus was fused to the promoter fragment and linked to luxAB of pJC156, followed by Pc and the kanamycin resistance cassette from CP1296 and flanked downstream by 2,000 bp of the bgaA gene. The resulting reporter construct was transformed into wild-type D39, IL20 (Δrgg1518), IL40 (Δrio83 Δrio84::spec), and IL101 (rio83ATG-GGG-spec).
To generate strain IL93 (Δrio83 Δrio84::spec bgaA::P1517rio83-GGG,rio84-GGG-luxAB-Pc-kan-Pc-rio84), the luciferase reporter constitutively expressing rio84 driven by the Pc promoter and genomic DNA from strain IL81 (bgaA::P1517rio83-GGG,rio84-GGG-luxAB-Pc-kan) were used as the template to amplify the reporter construct using primer pair ILp387/ILp264, which was then linked to the constitutive promoter Pc and fused to rio84 using Gibson assembly. This construct was transformed into wild-type D39 and IL40 (Δrio83 Δrio84::spec).
Restoration of mutations in rio83 and rio84 containing luxAB reporters.
Start codon mutations in rio83 and rio84 were restored by transforming strains IL40 (Δrio83 Δrio84::spec) and IL101 (rio83ATG-GGG-spec) with DNA fragments containing wild-type sequences, generating strains IL52 (Δrio83 Δrio84::spec bgaA::P1517rio83-ATG,rio84-ATG-luxAB-Pc-kan), IL106 (Δrio83 Δrio84::spec bgaA::P1517rio83-GGG,rio84-ATG-luxAB-Pc-kan), and IL113 (rio83ATG-GGG-spec bgaA::P1517rio83-GGG,rio84-ATG-luxAB-Pc-kan).
Generation of chromosomal sfGFP-tagged constructs.
To generate the chromosomal superfolder GFP (sfGFP)-tagged constructs, we performed transformations using linear DNA amplicons as described above. Each construct fused sfGFP in frame in front of the stop codon, followed by a spectinomycin resistance cassette, and was flanked by UFR and DFR homologous sequences. Strain IL75 (D39 rio03ATG-GGG-sfGFP) was constructed using strain IL91 (rio03ATG-GGG-spec) as a template to amplify the missense mutation with primer pair ILp323/ILp324.
SDS-PAGE and Western blotting for sfGFP-tagged sORFs.
The sfGFP-tagged strains were grown in 10 mL CDM to an OD600 of 0.4, and cells were collected at 4,000 × g for 10 min. Cell pellets were resuspended in 250 μL loading buffer (0.0625 M Tris [pH 8], 2% SDS, 10% glycerol, 5% 2-mercaptoethanol, 50 mg bromophenol blue) and lysed using a BioSpec bead beater for 10 min at maximum speed. Gel loading volumes of each sample were normalized by culture OD readings and resolved on a 12% SDS-PAGE gel at 150 V for 1.5 h. Gels were blotted onto 0.2-μm polyvinylidene difluoride (PVDF) membranes at 350 mA for 1.5 h, and the membranes were blocked overnight at 4°C with rocking in Tris-buffered saline plus 0.1% Tween (TBST) containing 5% BSA. Membranes were subsequently incubated for 1 h at room temperature, with rocking, with anti-sfGFP antibody (catalog number AE011; ABclonal) at a dilution of 1:3,000 in TBST plus 5% BSA. The membranes were then washed three times in TBST, followed by the addition of goat anti-rabbit IgG(H+L) (Thermo Fisher) at a dilution of 1:80,000 in TBST plus 5% BSA for 1 h with rocking at room temperature. The membranes were then washed three times, and sfGFP-tagged proteins were detected using the SuperSignal West Femto maximum-sensitivity substrate (catalog number 34094; Thermo Fisher). To prepare the working solution, equal volumes of the stable peroxide solution and the luminol-enhancer solution were mixed and incubated with the blot for 5 min, followed by exposure on a ProteinSimple FluorChem imaging system.
Synthesis of pheromone peptides.
Synthetic peptides were purchased from ABclonal. All peptides were reconstituted in dimethyl sulfoxide (DMSO) at a final concentration of 10 mM and stored at −80°C. Peptide purity ranged from 50 to 80%.
MIC assay.
Dilutions of the antibiotics retapamulin and lefamulin were prepared in CDM and loaded into a 96-well microtiter plate. D39 Δcps was grown in CDM to an OD600 of 0.5 and diluted 10-fold to an OD600 of 0.05 into the antibiotic-containing medium. Plates were incubated at 37°C in a microplate reader (Synergy 2; BioTek), and the OD was measured every 15 min over a span of 10 h.
Metabolic labeling.
Inhibition of translation by retapamulin and lefamulin was determined using metabolic labeling. All manipulations were performed at 37°C. D39 Δcps was inoculated from a starter culture (OD600 of 1) into 6 mL and grown in CDM lacking methionine and containing 0.5% Oxyrase to an OD600 of 0.5 at 37°C with 5% CO2. Cells were diluted 10-fold into CDM without methionine and containing 0.5% Oxyrase and grown until the culture density reached an OD600 of ~0.2, and three 350-μL aliquots were transferred to microcentrifuge tubes (two drug conditions and one control group). Retapamulin and lefamulin were individually added to Eppendorf tubes at a final concentration of 100× MIC. Prior to and immediately following the addition of antibiotics (0, 1, 2.5, 5, and 15 min), 28 μL of the culture was added to microcentrifuge tubes containing 0.3 μCi [35S]l-methionine (specific activity of 1,175 Ci/mmol; MP Biomedicals) in 2 μL of CDM. After a 1-min incubation, 25 μL of the mixture was spotted onto Whatman 3MM paper discs prewetted with 7% trichloroacetic acid (TCA). The discs were boiled twice in 7% TCA for 5 min, soaked in 100% acetone for 2 min, and then air dried prior to being placed into a 5-mL scintillation cocktail and being read using a scintillation counter.
Ribosome profiling.
Ribosome profiling was conducted as previously described, with the following modifications (29, 61). D39 Δcps cells were grown to an OD600 of 0.4 in 100 mL CDM supplemented with 0.5% Oxyrase at 37°C in an atmosphere of 5% CO2. Retapamulin or lefamulin was added to individual 100-mL cultures at final concentrations of 100× MIC for 2.5 min. No antibiotic was added to the untreated control group. After 2.5 min, bacteria were harvested by centrifugation at 6,300 × g at 37°C for 4 min and flash-frozen in liquid nitrogen. Cells were cryo-lysed in 650 μL lysis buffer (20 mM Tris-HCl [pH 8.0], 50 mM MgCl2, 100 mM NH4Cl,5 mM CaCl2) supplemented with 65 U RNase-free DNase I (catalog number 04716728001; Roche), 208 U SUPERase In RNase inhibitor (catalog number AM2694; Invitrogen), and 3 mM Guanosine 5′ –[b,g-imido] triphosphate trisodium salt hydrate (GMPPNP; catalog number G0635; Sigma-Aldrich). Pulverized cells were thawed at 30°C and spun at 20,000 × g for 10 min at 4°C to pellet insoluble debris. Clarified lysates were subjected to treatment with 450 U MNase (catalog number 10107921001; Roche), 120 U SUPERase In RNase inhibitor was added to the clarified lysates, and the reaction mixtures were incubated for 1 h at 25°C with shaking. The reaction mixtures were quenched with 5 mM EGTA, and the 70S monosome peak was isolated by sucrose gradient centrifugation (10 to 40% sucrose gradient) for 2 h 45 min at 39,000 × g. RNA was isolated by acid-phenol extraction and run on a 15% Tris-borate-EDTA (TBE)-urea polyacrylamide gel. RNA fragments ranging from 20 nucleotides to 38 nucleotides were excised, eluted, and used for library preparation as previously described, which included the addition of barcodes for multiplexing (31).
Computational analysis of ribosome profiling data.
The ribosome footprint reads were analyzed as described previously (61). In brief, samples were demultiplexed, linker barcodes were removed, and 5 nucleotides from the 3′ end and 2 nucleotides from the 5′ end were removed as they were included in the library design (29, 31). The reads were aligned to the S. pneumoniae D39V (GenBank accession number CP027540.1) reference genome by Bowtie2 (v2.2.9) after discarding reads mapping to known tRNAs and rRNAs. Read lengths ranging from 28 to 34 nucleotides were included for the analysis; the first nucleotide of the P-site codon was assigned 15 nucleotides from the 3′ end of the read, as previously suggested (11).
Novel sORFs found within intergenic regions were identified based on the following criteria: a Ribo-RET peak of at least 1 sequence read per million (rpm) that mapped within 10 nucleotides of a theoretical sORF starting with AUG, GUG, CUG, or UUG and whose respective full-length sORF did not overlap an annotated gene. In some instances, multiple start codons were identified in the 10-nucleotide window; therefore, a manual approach was used to inspect each candidate relative to the Ribo-RET peak. The list of sORFs identified can be found in Table 1. The code used to analyze the data set can be found at https://github.com/ilaczk2/D39_ribosome_profile_MS.
Metagene analysis.
Metagene analyses, to evaluate the positions of ribosomes at annotated genes with respect to the 5′ (start) and 3′ (stop) ends of genes, were performed according to a previously described protocol (62). Genes included in the analysis satisfied two criteria: a length of at least 200 nt and a read density of at least 0.005 rpm per nucleotide in all 5 samples (2 retapamulin, 1 lefamulin, and 2 controls). Coverage at each nucleotide position within a gene was normalized to the coverage density of the entire gene plus 50 nt of the flanking up- and downstream regions. The mean of these values was calculated and plotted for the windows around the start and stop codons.
Luciferase assay.
Strains of interest were inoculated from flash-frozen starter cultures in CDM plus 0.5% Oxyrase at 37°C in an atmosphere of 5% CO2 and reached exponential growth to an OD of ~0.5. Strains were then diluted 10-fold in CDM in a total volume of 150 μL in a 96-well white/clear-bottom plate (Sigma). When relevant, synthetic peptides were added to the wells at a final concentration of 10 μM. Dosing assays determined 10 μM to be the optimal concentration to induce the system. Decyl aldehyde (Sigma) was added to the spaces between the wells at a final concentration of 1% in mineral oil. The plate was covered and placed into the microplate reader (Synergy 2; BioTek) at 37°C with intermittent shaking. The luminescence (counts per second [CPS]) and optical density (OD600) were measured every 15 min over a span of 10 h. Relative light units (RLU) were calculated by normalizing the CPS to the OD600. Each assay was conducted in technical triplicates, and each figure shows results representative of data from at least three independent experiments (Fig. S6).
Cell adhesion assay.
A549 lung epithelial cells (ATCC) were routinely cultured in Dulbecco’s modified Eagle’s medium (DMEM) plus 10% fetal bovine serum (FBS) without antibiotics at 37°C in an atmosphere of 5% CO2. For the adhesion assay, A549 cells were seeded into a 24-well plate at 2 × 105 cells/well. Following incubation overnight, each well was washed once with 1× phosphate-buffered saline (PBS). D39 Δcps and D39 Δcps Δspv_1513-1517::spec were grown in CDM plus 10% choline and 0.5% Oxyrase to an OD600 of 0.5, washed in DMEM, and added to the cells at a multiplicity of infection (MOI) of 100:1 for 1 h at 37°C with 5% CO2. Following incubation, cells were gently washed three times with 1× PBS to remove unbound bacteria, treated with 0.025% trypsin for 6 min at 37°C with 5% CO2 to detach the cells, lysed with 0.1% saponin, and plated onto blood agar plates to determine bacterial CFU.
Intracellular survival assay.
The initial steps for the intracellular survival assay were the same as the ones described above for the adhesion assay. To differentiate between internalized and external bacteria, epithelial cells were treated with 100 μg/mL gentamicin for 1, 3, and 4 h to kill the external bacteria. Following antibiotic treatment, the supernatant was aspirated, and cells were gently washed three times with 1× PBS. Cells were removed from the wells by the addition of 0.025% trypsin for 6 min and lysed with 0.1% saponin. The suspension was serially diluted and plated onto sheep blood agar plates to determine the bacterial burden.
Mouse experiment.
Mice were housed at a biosafety level 2 facility and anesthetized with inhaled isoflurane (3%) when necessary. As shown in Fig. 6C, 6-week-old BALB/c mice were intranasally inoculated with wild-type D39, IL91 (rio03ATG-GGG-spec), IL127 (rio03ATG-GGG;rio03GGG-ATG-kan), and IL108 (Δsrf-0218–207::erm) at a dose of 1 × 107 CFU/25 μL. A minimum of 10 mice were used for each bacterial inoculation. Mice were sacrificed 24 h after inoculation using carbon dioxide inhalation followed by cervical dislocation. The nasal passage of each mouse was isolated, homogenized in 500 μL 1× PBS, and plated onto blood agar plates to determine the bacterial CFU burden. As shown in Fig. S6C, 30 6-week-old CD1 mice were intranasally inoculated with bacterial suspensions containing 1:1 mixtures of wild-type D39 and IL97 (Δspv_1513-1517::spec) at a dose of 2 × 105 CFU/20 μL. Ten mice were sacrificed either 24 h, 72 h, or 168 h after inoculation, followed by nasal passage isolation and plating onto blood agar plates to determine bacterial CFU.
RNA isolation and RNA sequencing.
The wild-type D39 and IL20 (Δrgg1518) strains were cultured in CDM supplemented with 10% choline plus 0.5% Oxyrase and grown to an OD600 of 0.4 at 37°C with 5% CO2. Three independent cultures of each strain were prepared. Cultures were harvested by centrifugation, supernatants were discarded, and cell pellets were suspended in 1 mL RNAlater (Ambion) and incubated at room temperature for 10 min. Following incubation, samples were centrifuged at 14,000 × g for 1 min, supernatants were discarded, and cell pellets were stored at −80°C. Total RNAs from wild-type D39, IL20 (Δrgg1518), and the Ribo-seq samples (retapamulin treated, lefamulin treated, and untreated) were extracted using the Ambion RiboPure RNA purification bacterial kit according to the manufacturer’s instructions and as previously described (63). Following the successful extraction of RNA, the Genome Research Core at the University of Illinois at Chicago (UIC) assessed RNA quality and quantity using the Tapestation 2200 system (Agilent), prepared the cDNA libraries, and processed samples on an Illumina HiSeq 4000 platform with 100-bp single reads. The raw sequencing data were analyzed by the Research Informatics Core at UIC.
Preparation of cDNA for qRT-PCR experiments.
cDNA was prepared from RNA using the Superscript III first-strand synthesis system (Thermo Fisher) according to the manufacturer’s instructions and as previously described (63). Total cDNA was diluted 1:10, and reaction mixtures were prepared using 1× Fast SYBR green master mix with the gene-specific primers listed in Table S2. qRT-PCR was performed using the CFX Connect real-time PCR detection system (Bio-Rad). All samples were run in biological and technical triplicates, and relative gene expression was determined using the 2−ΔΔCT method.
5′ RACE.
5′ RACE was conducted as previously described (63). Total RNA was isolated as detailed in the section on RNA isolation and RNA sequencing above. cDNA synthesis of the spv_1517 transcript and template switching were performed using the NEB template-switching RT enzyme mix with primers specific for the spv_1517 operon (ILp151) and the template-switching oligonucleotide (TSO) (BRp311). The 5′ end of the spv_1517 transcript was amplified using IL151 primers and the TSO-specific primer BRp312 using the Q5 high-fidelity enzyme. The resulting PCR product was sequenced by Sanger sequencing.
sORF conservation analysis.
sORF conservation was assessed as previously described (64). tBLASTn analysis was used to assess sORF conservation in six clinically relevant S. pneumoniae serotypes (1, 4, 14, 19A, 23F, and 19F). The amino acid sequence of each sORF was submitted to tBLASTn analysis. The following parameters were modified: the maximum number of target sequences was 250, the expected threshold was set to 100, and no low-complexity filter was used. The search was refined by selecting only sORFs that had 100% query coverage and ≥70% sequence identity. The sORFs highlighted in boldface type in Table 3 were too short for tBLASTn analysis; therefore, BLASTn was used to assess their conservation using the same parameters as the ones described above.
Data availability.
All raw ribosome profiling reads, RNA sequencing reads, and annotation files are available in the NCBI GEO database (https://www.ncbi.nlm.nih.gov/bioproject/857299; Accession #PRJNA857299). Analysis scripts are available on GitHub (https://github.com/ilaczk2/D39_ribosome_profile_MS).
ACKNOWLEDGMENTS
We are grateful for the technical assistance provided by the UIC and Northwestern core facilities, especially Xinkun “Sequen” Wang (NU) and Mark Maienschein-Cline (UIC) for sequencing and data analysis. We thank Dorota Klepacki for technical assistance, Jennifer Chang for critical review of the manuscript, and members of the Chicago Positive Thinking group for their interest and feedback.
Conceived and designed the analysis, I.L., Y.G., A.J.H., A.M., N.V.-L., and M.J.F. Collected the data, I.L., K.M., and X.S. Contributed data or analysis tools, I.L., K.M., Y.G., A.M., and A.J.H. Performed the analysis, I.L. Wrote the paper: I.L. and M.J.F.
We declare that there are no competing interests.
This study was supported by NIH/NIAID grant AI144500.
Contributor Information
Michael J. Federle, Email: mfederle@uic.edu.
N. Luisa Hiller, Carnegie Mellon University.
REFERENCES
- 1.Mann B, van Opijnen T, Wang J, Obert C, Wang Y-D, Carter R, McGoldrick DJ, Ridout G, Camilli A, Tuomanen EI, Rosch JW. 2012. Control of virulence by small RNAs in Streptococcus pneumoniae. PLoS Pathog 8:e1002788. doi: 10.1371/journal.ppat.1002788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinha D, Zimmer K, Cameron TA, Rusch DB, Winkler ME, De Lay NR. 2019. Redefining the small regulatory RNA transcriptome in Streptococcus pneumoniae serotype 2 strain D39. J Bacteriol 201:e00764-18. doi: 10.1128/JB.00764-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slager J, Aprianto R, Veening J. 2018. Deep genome annotation of the opportunistic human pathogen Streptococcus pneumoniae D39. Nucleic Acids Res 46:9971–9989. doi: 10.1093/nar/gky725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sberro H, Fremin BJ, Zlitni S, Edfors F, Greenfield N, Snyder MP, Pavlopoulos GA, Kyrpides NC, Bhatt AS. 2019. Large-scale analyses of human microbiomes reveal thousands of small, novel genes. Cell 178:1245–1259.e14. doi: 10.1016/j.cell.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison PM, Kumar A, Lang N, Snyder M, Gerstein M. 2002. A question of size: the eukaryotic proteome and the problems in defining it. Nucleic Acids Res 30:1083–1090. doi: 10.1093/nar/30.5.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basrai MA, Hieter P, Boeke JD. 1997. Small open reading frames: beautiful needles in the haystack. Genome Res 7:768–771. doi: 10.1101/gr.7.8.768. [DOI] [PubMed] [Google Scholar]
- 7.Congdon RW, Muth GW, Splittgerber AG. 1993. The binding interaction of Coomassie blue with proteins. Anal Biochem 213:407–413. doi: 10.1006/abio.1993.1439. [DOI] [PubMed] [Google Scholar]
- 8.Stellwagen E. 1994. Protein purification: principles and practice, 3rd edition. FEBS Lett 352:400–401. doi: 10.1016/0014-5793(94)80041-3. [DOI] [Google Scholar]
- 9.Su M, Ling Y, Yu J, Wu J, Xiao J. 2013. Small proteins: untapped area of potential biological importance. Front Genet 4:286. doi: 10.3389/fgene.2013.00286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemm MR, Weaver J, Storz G. 2020. Escherichia coli small proteome. EcoSal Plus 9(1):ESP-0031-2019. doi: 10.1128/ecosalplus.ESP-0031-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver J, Mohammad F, Buskirk AR, Storz G. 2019. Identifying small proteins by ribosome profiling with stalled initiation complexes. mBio 10(2):e02819-18. doi: 10.1128/mBio.02819-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin PA, Fan N, Ricca E, Driks A, Losick R, Cutting S. 1993. An unusually small gene required for sporulation by Bacillus subtilis. Mol Microbiol 9:761–771. doi: 10.1111/j.1365-2958.1993.tb01736.x. [DOI] [PubMed] [Google Scholar]
- 13.Verdon J, Girardin N, Lacombe C, Berjeaud J, Héchard Y. 2009. Delta-hemolysin, an update on a membrane-interacting peptide. Peptides 30:817–823. doi: 10.1016/j.peptides.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Du D, Neuberger A, Orr MW, Newman CE, Hsu P-C, Samsudin F, Szewczak-Harris A, Ramos LM, Debela M, Khalid S, Storz G, Luisi BF. 2020. Interactions of a bacterial RND transporter with a transmembrane small protein in a lipid environment. Structure 28:625–634.e6. doi: 10.1016/j.str.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin JE, Waters LS, Storz G, Imlay JA. 2015. The Escherichia coli small protein MntS and exporter MntP optimize the intracellular concentration of manganese. PLoS Genet 11:e1004977. doi: 10.1371/journal.pgen.1004977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hobbs EC, Yin X, Paul BJ, Astarita JL, Storz G. 2012. Conserved small protein associates with the multidrug efflux pump AcrB and differentially affects antibiotic resistance. Proc Natl Acad Sci USA 109:16696–16701. doi: 10.1073/pnas.1210093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neiditch MB, Capodagli GC, Prehna G, Federle MJ. 2017. Genetic and structural analyses of RRNPP intercellular peptide signaling of Gram-positive bacteria. Annu Rev Genet 51:311–333. doi: 10.1146/annurev-genet-120116-023507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomasz A. 1965. Control of the competent state in pneumococcus by a hormone-like cell product: an example for a new type of regulatory mechanism in bacteria. Nature 208:155–159. doi: 10.1038/208155a0. [DOI] [PubMed] [Google Scholar]
- 19.Håvarstein LS, Coomaraswamy G, Morrison DA. 1995. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci USA 92:11140–11144. doi: 10.1073/pnas.92.24.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aggarwal SD, Yesilkaya H, Dawid S, Hiller NL. 2020. The pneumococcal social network. PLoS Pathog 16:e1008931. doi: 10.1371/journal.ppat.1008931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cuevas RA, Eutsey R, Kadam A, West-Roberts JA, Woolford CA, Mitchell AP, Mason KM, Hiller NL. 2017. A novel streptococcal cell-cell communication peptide promotes pneumococcal virulence and biofilm formation. Mol Microbiol 105:554–571. doi: 10.1111/mmi.13721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhi X, Abdullah IT, Gazioglu O, Manzoor I, Shafeeq S, Kuipers OP, Hiller NL, Andrew PW, Yesilkaya H. 2018. Rgg-shp regulators are important for pneumococcal colonization and invasion through their effect on mannose utilization and capsule synthesis. Sci Rep 8:6369. doi: 10.1038/s41598-018-24910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shlla B, Gazioglu O, Shafeeq S, Manzoor I, Kuipers OP, Ulijasz A, Hiller NL, Andrew PW, Yesilkaya H. 2021. The Rgg1518 transcriptional regulator is a necessary facet of sugar metabolism and virulence in Streptococcus pneumoniae. Mol Microbiol 116:996–1008. doi: 10.1111/mmi.14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fleuchot B, Gitton C, Guillot A, Vidic J, Nicolas P, Besset C, Fontaine L, Hols P, Leblond-Bourget N, Monnet V, Gardan R. 2011. Rgg proteins associated with internalized small hydrophobic peptides: a new quorum-sensing mechanism in streptococci. Mol Microbiol 80:1102–1119. doi: 10.1111/j.1365-2958.2011.07633.x. [DOI] [PubMed] [Google Scholar]
- 25.Aprianto R, Slager J, Holsappel S, Veening J. 2018. High-resolution analysis of the pneumococcal transcriptome under a wide range of infection-relevant conditions. Nucleic Acids Res 46:9990–10006. doi: 10.1093/nar/gky750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brar GA, Weissman JS. 2015. Ribosome profiling reveals the what, when, where and how of protein synthesis. Nat Rev Mol Cell Biol 16:651–664. doi: 10.1038/nrm4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingolia NT, Brar GA, Stern-Ginossar N, Harris MS, Talhouarne GJS, Jackson SE, Wills MR, Weissman JS. 2014. Ribosome profiling reveals pervasive translation outside of annotated protein-coding genes. Cell Rep 8:1365–1379. doi: 10.1016/j.celrep.2014.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ingolia NT, Ghaemmaghami S, Newman JRS, Weissman JS. 2009. Genome-wide analysis in vivo of translation with nucleotide resolution using ribosome profiling. Science 324:218–223. doi: 10.1126/science.1168978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meydan S, Marks J, Klepacki D, Sharma V, Baranov PV, Firth AE, Margus T, Kefi A, Vázquez-Laslop N, Mankin AS. 2019. Retapamulin-assisted ribosome profiling reveals the alternative bacterial proteome. Mol Cell 74:481–493.e6. doi: 10.1016/j.molcel.2019.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueta M, Wada C, Wada A. 2010. Formation of 100S ribosomes in Staphylococcus aureus by the hibernation promoting factor homolog SaHPF. Genes Cells 15:43–58. doi: 10.1111/j.1365-2443.2009.01364.x. [DOI] [PubMed] [Google Scholar]
- 31.McGlincy NJ, Ingolia NT. 2017. Transcriptome-wide measurement of translation by ribosome profiling. Methods 126:112–129. doi: 10.1016/j.ymeth.2017.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eyal Z, Matzov D, Krupkin M, Paukner S, Riedl R, Rozenberg H, Zimmerman E, Bashan A, Yonath A. 2016. A novel pleuromutilin antibacterial compound, its binding mode and selectivity mechanism. Sci Rep 6:39004. doi: 10.1038/srep39004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gertz EM, Yu Y, Agarwala R, Schäffer AA, Altschul SF. 2006. Composition-based statistics and translated nucleotide searches: improving the TBLASTN module of BLAST. BMC Biol 4:41. doi: 10.1186/1741-7007-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nielsen H. 2017. Predicting secretory proteins with SignalP. Methods Mol Biol 1611:59–73. doi: 10.1007/978-1-4939-7015-5_6. [DOI] [PubMed] [Google Scholar]
- 35.Craigen WJ, Caskey CT. 1986. Expression of peptide chain release factor 2 requires high-efficiency frameshift. Nature 322:273–275. doi: 10.1038/322273a0. [DOI] [PubMed] [Google Scholar]
- 36.Baranov PV, Gesteland RF, Atkins JF. 2002. Release factor 2 frameshifting sites in different bacteria. EMBO Rep 3:373–377. doi: 10.1093/embo-reports/kvf065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Poole ES, Major LL, Mannering SA, Tate WP. 1998. Translational termination in Escherichia coli: three bases following the stop codon crosslink to release factor 2 and affect the decoding efficiency of UGA-containing signals. Nucleic Acids Res 26:954–960. doi: 10.1093/nar/26.4.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Major LL, Poole ES, Dalphin ME, Mannering SA, Tate WP. 1996. Is the in-frame termination signal of the Escherichia coli release factor-2 frameshift site weakened by a particularly poor context? Nucleic Acids Res 24:2673–2678. doi: 10.1093/nar/24.14.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pavlov MY, Freistroffer DV, Dincbas V, MacDougall J, Buckingham RH, Ehrenberg M. 1998. A direct estimation of the context effect on the efficiency of termination. J Mol Biol 284:579–590. doi: 10.1006/jmbi.1998.2220. [DOI] [PubMed] [Google Scholar]
- 40.Rahbari KM, Chang JC, Federle MJ. 2021. A Streptococcus quorum sensing system enables suppression of innate immunity. mBio 12(3):e03400-20. doi: 10.1128/mBio.03400-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gogos A, Jimenez JC, Chang JC, Wilkening RV, Federle MJ. 2018. A quorum sensing-regulated protein binds cell wall components and enhances lysozyme resistance in Streptococcus pyogenes. J Bacteriol 200:e00701-17. doi: 10.1128/JB.00701-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chaussee MS, Ajdic D, Ferretti JJ. 1999. The rgg gene of Streptococcus pyogenes NZ131 positively influences extracellular SPE B production. Infect Immun 67:1715–1722. doi: 10.1128/IAI.67.4.1715-1722.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mashburn-Warren L, Morrison DA, Federle MJ. 2012. The cryptic competence pathway in Streptococcus pyogenes is controlled by a peptide pheromone. J Bacteriol 194:4589–4600. doi: 10.1128/JB.00830-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang CY, Medlin JS, Nguyen DR, Disbennett WM, Dawid S. 2020. Molecular determinants of substrate selectivity of a pneumococcal rgg-regulated peptidase-containing ABC transporter. mBio 11(1):e02502-19. doi: 10.1128/mBio.02502-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zähner D, Hakenbeck R. 2000. The Streptococcus pneumoniae beta-galactosidase is a surface protein. J Bacteriol 182:5919–5921. doi: 10.1128/JB.182.20.5919-5921.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barth VC, Zeng J, Vvedenskaya IO, Ouyang M, Husson RN, Woychik NA. 2019. Toxin-mediated ribosome stalling reprograms the Mycobacterium tuberculosis proteome. Nat Commun 10:3035. doi: 10.1038/s41467-019-10869-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miranda-CasoLuengo AA, Staunton PM, Dinan AM, Lohan AJ, Loftus BJ. 2016. Functional characterization of the Mycobacterium abscessus genome coupled with condition specific transcriptomics reveals conserved molecular strategies for host adaptation and persistence. BMC Genomics 17:553. doi: 10.1186/s12864-016-2868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Basu A, Yap MF. 2016. Ribosome hibernation factor promotes staphylococcal survival and differentially represses translation. Nucleic Acids Res 44:4881–4893. doi: 10.1093/nar/gkw180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pérez-Pascual D, Gaudu P, Fleuchot B, Besset C, Rosinski-Chupin I, Guillot A, Monnet V, Gardan R. 2015. RovS and its associated signaling peptide form a cell-to-cell communication system required for Streptococcus agalactiae pathogenesis. mBio 6(1):e02306-14. doi: 10.1128/mBio.02306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chang JC, LaSarre B, Jimenez JC, Aggarwal C, Federle MJ. 2011. Two group A streptococcal peptide pheromones act through opposing rgg regulators to control biofilm development. PLoS Pathog 7:e1002190. doi: 10.1371/journal.ppat.1002190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mashburn-Warren L, Morrison DA, Federle MJ. 2010. A novel double-tryptophan peptide pheromone controls competence in Streptococcus spp. via an rgg regulator. Mol Microbiol 78:589–606. doi: 10.1111/j.1365-2958.2010.07361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Junges R, Salvadori G, Shekhar S, Åmdal HA, Periselneris JN, Chen T, Brown JS, Petersen FC. 2017. A quorum-sensing system that regulates Streptococcus pneumoniae biofilm formation and surface polysaccharide production. mSphere 2(5):e00324-17. doi: 10.1128/mSphere.00324-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Danger JL, Cao TN, Cao TH, Sarkar P, Treviño J, Pflughoeft KJ, Sumby P. 2015. The small regulatory RNA FasX enhances group A Streptococcus virulence and inhibits pilus expression via serotype-specific targets. Mol Microbiol 96:249–262. doi: 10.1111/mmi.12935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Storz G, Vogel J, Wassarman KM. 2011. Regulation by small RNAs in bacteria: expanding frontiers. Mol Cell 43:880–891. doi: 10.1016/j.molcel.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Slager J, Aprianto R, Veening J. 2019. Refining the pneumococcal competence regulon by RNA sequencing. J Bacteriol 201:e00780-18. doi: 10.1128/JB.00780-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He L-Y, Le Y-J, Guo Z, Li S, Yang X-Y. 2021. The role and regulatory network of the CiaRH two-component system in streptococcal species. Front Microbiol 12:693858. doi: 10.3389/fmicb.2021.693858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shine J, Dalgarno L. 1974. The 3′-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci USA 71:1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steitz JA, Jakes K. 1975. How ribosomes select initiator regions in mRNA: base pair formation between the 3′ terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci USA 72:4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vellanoweth RL, Rabinowitz JC. 1992. The influence of ribosome-binding-site elements on translational efficiency in Bacillus subtilis and Escherichia coli in vivo. Mol Microbiol 6:1105–1114. doi: 10.1111/j.1365-2958.1992.tb01548.x. [DOI] [PubMed] [Google Scholar]
- 60.Saito K, Green R, Buskirk AR. 2020. Translational initiation in E. coli occurs at the correct sites genome-wide in the absence of mRNA-rRNA base-pairing. Elife 9:e55002. doi: 10.7554/eLife.55002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mangano K, Florin T, Shao X, Klepacki D, Chelysheva I, Ignatova Z, Gao Y, Mankin AS, Vázquez-Laslop N. 2020. Genome-wide effects of the antimicrobial peptide apidaecin on translation termination in bacteria. Elife 9:e62655. doi: 10.7554/eLife.62655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aleksashin NA, Leppik M, Hockenberry AJ, Klepacki D, Vázquez-Laslop N, Jewett MC, Remme J, Mankin AS. 2019. Assembly and functionality of the ribosome with tethered subunits. Nat Commun 10:930. doi: 10.1038/s41467-019-08892-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rued BE, Covington BC, Bushin LB, Szewczyk G, Laczkovich I, Seyedsayamdost MR, Federle MJ. 2021. Quorum sensing in Streptococcus mutans regulates production of tryglysin, a novel RaS-RiPP antimicrobial compound. mBio 12(2):e02688-20. doi: 10.1128/mBio.02688-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stringer A, Smith C, Mangano K, Wade JT. 2022. Identification of novel translated small open reading frames in Escherichia coli using complementary ribosome profiling approaches. J Bacteriol 204:e00352-21. doi: 10.1128/JB.00352-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A. 2005. Protein identification and analysis tools on the ExPASy server, p 571–607. In Walker JM (ed), The proteomics protocols handbook. Humana Press, Totowa, NJ. [Google Scholar]
- 66.Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods 8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- 67.Kumar R, Shah P, Swiatlo E, Burgess SC, Lawrence ML, Nanduri B. 2010. Identification of novel noncoding small RNAs from Streptococcus pneumoniae TIGR4 using high-resolution genome tiling arrays. BMC Genomics 11:350. doi: 10.1186/1471-2164-11-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Retapamulin and lefamulin are effective at arresting translation in S. pneumoniae D39. (A) MICs of retapamulin and lefamulin determined in CDM. (B) Assessment of protein synthesis in D39 Δcps treated with 100-fold the MIC of either retapamulin or lefamulin by measuring the incorporation of [35S]methionine at specific time points. (C to H) Monosomes were isolated by sucrose gradient fractionation. Bacterial cultures were either untreated (C and F) or treated with 100-fold the MIC of either retapamulin (D and G) or lefamulin (E and H) for 2.5 min. Lysates were digested (F to H) or not (C to E) with 450 U MNase and loaded onto 10 to 40% sucrose gradients. Download FIG S1, TIF file, 2.9 MB (3MB, tif) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Metagene analysis for all read lengths. (A) Metagene analysis comparing all read lengths positioned relative to the start codon with an offset of 15 nucleotides from untreated and retapamulin- and lefamulin-treated cells. (B) Metagene analysis of all read lengths. Download FIG S2, TIF file, 2.4 MB (2.5MB, tif) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The ribosome consistently stalls in the 5′ UTR between different sample preparations and antibiotic treatments. Shown is a snapshot of the ribosome footprint density for the araT gene. Each picture represents a biological replicate, which was either untreated or treated with retapamulin or lefamulin. Download FIG S7, TIF file, 2.1 MB (2.2MB, tif) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Ribosome footprints are present within genes. Examples of Ribo-seq profiles in Streptococcus pneumoniae indicate alternative translation start sites within genes. The gray arrows represent previously annotated genes, and the green arrows indicate alternative open reading frames within annotated genes. Download FIG S3, TIF file, 2.8 MB (2.9MB, tif) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Sequence alignment of prfB in E. coli and S. pneumoniae D39. Shown is a schematic representation of prfB in E. coli and S. pneumoniae. The stop sign represents the internal UGA stop codon where a +1 frameshift occurs. The mRNA sequence is of the 5′ end of prfB, and the underlined sequence depicts the conserved sequence in both bacteria. SD highlights the Shine-Dalgarno sequence, FS highlights the frameshift site, and the asterisk identifies the UGA stop codon. Download FIG S4, TIF file, 1.8 MB (1.8MB, tif) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
The Rgg1518-regulated operon spv_1513-1517 is not involved in adhesion, intracellular survival, or colonization of the murine nasopharynx. (A and B) A549 alveolar epithelial cells were infected with the Δcps (positive control) and Δcps Δspv_1513-1517 strains at an MOI of 1:100 to assess their role in adhesion (A) and intracellular survival (B). (C) CD1 mice were coinfected with 2 × 105 CFU/20 μL of the wild-type D39 and Δspv_1513-1517 strains, and the bacterial burden in the nasopharynx was determined over a span of 7 days. Download FIG S5, TIF file, 1.7 MB (1.8MB, tif) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Biological replicates of luciferase assays. (A) Biological replicates of the luciferase assays depicted in Fig. 4B. (B) Biological replicates of the luciferase assays depicted in Fig. 4C. (C) Biological replicates of the luciferase assays in Fig. 5A. (D) Biological replicates of the luciferase assays in Fig. 5B. Download FIG S6, TIF file, 2.9 MB (2.9MB, tif) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
sORFs overlapping the gene on the opposite strand. Download Table S3, DOCX file, 0.1 MB (97.3KB, docx) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Strains and plasmids used in this study. Download Table S1, DOCX file, 0.02 MB (26.4KB, docx) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Primers used for the construction of the strains used in this study. Download Table S2, DOCX file, 0.03 MB (34KB, docx) .
Copyright © 2022 Laczkovich et al.
This content is distributed under the terms of the Creative Commons Attribution 4.0 International license.
Data Availability Statement
All raw ribosome profiling reads, RNA sequencing reads, and annotation files are available in the NCBI GEO database (https://www.ncbi.nlm.nih.gov/bioproject/857299; Accession #PRJNA857299). Analysis scripts are available on GitHub (https://github.com/ilaczk2/D39_ribosome_profile_MS).