Abstract
HIV/AIDS remains a looming presence in public health across the world, particularly in Sub-Saharan Africa. The HIV Care Cascade hinges on testing and knowledge of HIV status. Though significant advances have been made in diagnosing people living with HIV (PLHIV), limitations in understanding which strategies are best suited to certain regions or populations have contributed to the uneven distribution in the success of various HIV testing strategies. Here, we present a conceptual framework that outlines effective HIV testing strategies for four target groups. This framework is based on a systematic literature review of articles published from January 1st, 2008, to December 31st, 2019. The effectiveness of HIV testing strategies depends on various factors including the setting, type of test and service providers. Multiple strategies are needed to reach the UNAIDS target of 95% of individuals knowing their HIV status. Expansion of community-based approaches, self-testing and HIV testing services in antenatal care will further improve the state of HIV testing in Sub-Saharan Africa.
Keywords: HIV testing, Sub-Saharan Africa, treatment cascade, healthcare delivery
INTRODUCTION:
Globally, 75 million people are estimated to have been infected with human immunodeficiency virus (HIV), and 30 million people have died from this infection (1). In 2020, an estimated 0.8% of adults aged 15–49 years worldwide are living with HIV, and the global HIV burden remains concentrated across Sub-Saharan Africa. About 4% of adults in Sub-Saharan Africa are estimated to be living with HIV, with Eastern and Southern Africa estimated to have more than half of all people living with HIV (PLHIV) (2, 3).
The UNAIDS 95-95-95 targets represent the goals of diagnosing 95% of all PLHIV, provide treatment for 95% of persons diagnosed, and achieving viral suppression in 95% of those treated by 2030 (4). In 2018, 79% of PLHIV knew their status, 62% were being treated and 53% were virally suppressed (5). These corresponding statistics were 76%, 79% and 83% in Eastern and Southern Africa, and 42%, 83% and 73% in Western and Central Africa (6). Many strategies have been developed along the HIV Care Cascade to reach these targets. Identifying PLHIV through testing remains the crucial first step to linking people to services that can reduce HIV burden in networks, across countries, and around the world (7). Innovations in HIV counseling and testing (HCT) include self-testing, rapid testing, community-based approaches, mobile testing, home-based counseling and testing (HBHCT), universal testing, testing targeted to high-risk or missed populations, and contract tracing. These strategies seek to target different recipient groups, improve the types of testing assays, and assess new venues in which testing is offered (8). However, it is unclear which strategies are best suited to increase the portion of individuals who know their HIV status.
More than $85 billion by the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) with a focus on 13 highly-burdened countries (9), $3 billion by the Bill and Melinda Gates Foundation (10), and increasing domestic funding surpassing international donations (1) have led to a 43% reduction in HIV-related mortality since 2003 (2). However, this success is unevenly and inequitably distributed both geographically and among certain demographics (2). In Sub-Saharan Africa, many interventions are less successful in engaging men, adolescents, and key populations in the push to reach the end the AIDS epidemic (6). Given the plethora of approaches to increase testing uptake, it is unclear what strategies work in various settings and who to specifically serve with different intervention strategies. The purpose of this manuscript is to synthesize the many studies focused on improving HIV testing services in low resource settings. Using an implementation lens, we highlight the real-world effectiveness and challenges to inform future HIV testing programming.
METHODS:
We performed a systematic review of studies examining interventions along the HIV care cascade following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11). The included studies were sorted along the HIV Care Cascade: Prevention, Testing, Linkage to Care, and Adherence. This manuscript synthesizes studies on HIV testing implementation strategies.
Eligibility Criteria
Articles evaluating interventions across the HIV care cascade were eligible for inclusion if they: a) were in English; b) took place in Sub-Saharan Africa; c) reported on the outcomes of an intervention; and d) included measures related to the HIV Care Cascade. We excluded studies: a) that did not report results (e.g., study protocols); b) were strictly descriptive or qualitative; c) where HIV prevention, HIV testing, or PLHIV were not the focus of the study; d) where the study did not occur in Sub-Saharan Africa; e) the intervention was not on the care cascade; f) the study was a simulation and was not implemented in the field; or g) were systematic reviews or meta-analyses. We limited our search to articles published in peer-reviewed journals between January 1st, 2008, and December 31st, 2019.
Search Strategy
A query of search terms was completed in PubMed©, EMBASE©, Scopus©, Web of Science©, and the Cochrane Library© on May 19th, 2020. A four-concept search focused on HIV/AIDS, HIV Care Cascade, service/program delivery, and Sub-Saharan Africa was utilized. Search strategy concepts were developed in collaboration with a professional librarian (KL) and the Johns Hopkins University EAWA study team. Only articles published from January 1st, 2008, to December 31st, 2019, were included. An overview of the complete search strategies is available (Electronic Supplemental Material 1).
Study Selection
Duplicate results were removed. Authors then independently screened each title and abstract and removed items that were not relevant to the review. Two authors read the full text of potentially eligible articles and removed those that did not meet the specified eligibility criteria. Eligible articles were then sorted along the HIV Care Cascade. Articles related to HIV testing are included in this review.
Data Extraction
Primary data were used for data extraction. Authors independently extracted the following data: year of publication, country of study, study design, study population(s) (ex: pregnant women or household contacts of PLHIV), sample size, type of intervention implemented, and key findings. Key findings included number of individuals eligible for and offered testing, percent accepting testing, and HIV positivity rates. Areas of potential bias and confounding were also identified.
Quality Assessment of Included Studies
In duplicate, the two principal authors (I.M. and D.A.) evaluated the overall quality of every study in the review using a predetermined scoring criterion. Similar to a previously described method, the criteria evaluated the studies’ design, magnitude, and applicability, and an overall ‘quality’ score (low, moderate, or high) was given based on these factors (12). This scoring system has a foundation in the GRADE framework (13).
Synthesis of Results
From the broad spectrum of populations targeted and interventions evaluated, two authors (I.M. and D.A.) developed a conceptual framework regarding HIV testing strategies, organizing and synthesizing the articles accordingly. This includes the target population: a) general population (ex: adults, adolescents, men); b) pregnant women, partners, and infants; c) high risk populations (ex: female sex workers (FSWs), men who have sex with men (MSM), people who inject drugs (PWID), transgender women, and truck drivers). The framework considers venue of testing including facility-based, community-based, home-based testing, and mobile testing. Lastly, the framework includes adjuvant strategies such as contract tracing, community mobilization, incentives, and stigma reduction.
RESULTS:
We identified 8,025 articles using the specified search criteria. 85 duplicates were removed. 7,940 titles and abstracts were reviewed, and 7,030 not relevant to the HIV Care Cascade were excluded. We performed a full text review on 833 articles. Of these, 378 were excluded due to lack of intervention, lack of results, wrong setting, not acting on the HIV care cascade, reviews or meta-analyses, duplicates, or were simulation studies. 387 included studies were then sorted along the HIV care cascade with a total of n=134 studies were included in this review of HIV testing strategies (Figure 1, Electronic Supplemental Material - Table I). Studies were performed in 20 Sub-Saharan African countries in urban, peri-urban, and rural settings. Specific populations addressed include pregnant women, partners, and infants (n=28, 21%), children and adolescents (n=19, 14%), and sexual partners and household contacts of PLHIV (n=17, 13%). Several studies targeted key populations: FSW (n=11, 8%), MSM (n=7, 5%), and PWID (n=3, 2%). Of the 134 studies, 31received an overall quality rating of High, 90 Moderate, and 13 Low (Electronic Supplemental Material - Table 2).
Figure 1.
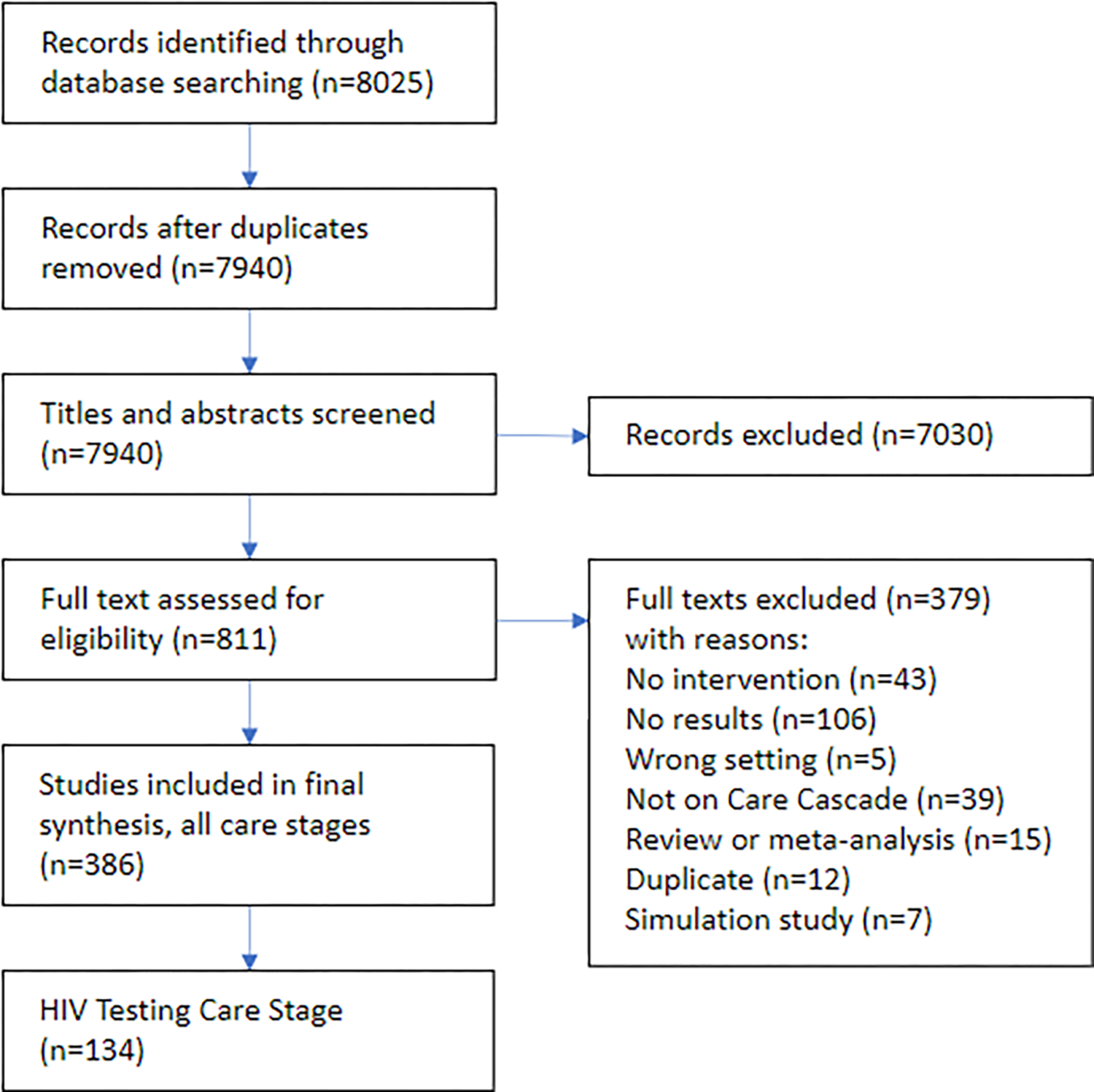
Systematic search procedure and results.
Facility-Based Testing
Nearly half of studies (n=64, 47%) evaluated facility-based testing, spanning a wide geographical range including Southern Africa (n=26, 41%), Western Africa (n=10, 16%), Eastern Africa (n=23, 36%) and Central Africa (n=5, 8%). Two main approaches are provider-initiated testing and counseling (PITC) and voluntary counseling and testing (VCT). PITC can have a blanket approach (bPITC) where providers initiate or recommend testing for every patient, also called universal testing, routine PITC, or opt-out VCT. Providers can also target specific populations (tPITC) at higher risk for HIV (i.e. children of PLHIV). Several studies found that PITC increased test uptake compared to VCT (14–19). Dalal et al showed that in rural and urban health centers in South Africa, PITC increased uptake by 2.85-fold (95% CI: 1.71–4.76) compared to VCT (14). Similarly, opt-out VCT is effective in health facilities. Among children and adolescents, both blanket PITC and targeted PITC resulted in high testing rates. Rates testing uptake in blanket PITC surpassed targeted PITC (90.3 vs 56.7%; p < 0.0001, CI not reported), but targeted PITC’s HIV positivity rate was twice that of blanket PITC. (23, 24). These increased rates of testing can be challenging for healthcare workers with many responsibilities to integrate into their workflow, and task shifting HCT to designated counselors has been a popular approach to mitigate this barrier. Bochner et al showed that deploying a PITC-provider to facilities resulted in 16.7 additional tests per week, while Flick et al attributed 2.6 million additional tests within 2.5 years to the presence of lay workers focused on HCT (21, 22). In rural South Africa, losing a lay counsellor led to 29.7 (95% CI: 21.2 – 38.2, p<0.001)fewer HIV tests per month (25). In addition to task shifting, some health facilities in rural Mozambique provided HIV self-testing kits for in-clinic use, which had high uptake among adolescents (60.3%) (26).
Although PITC often leads to higher testing uptake than VCT, VCT led to higher rates of linkage to care and faster antiretroviral treatment (ART) initiation in Zambia, partially due to higher risks of severe immunosuppression and higher rates of unrecorded mortality in PITC clients. Additionally, PITC clients may be less psychologically prepared for HIV care and treatment compared to VCT clients.(15). Effective approaches to VCT include streamlining clinic flow by offering services to patients waiting to see a provider (27), clinics with adolescent friendly hours (28), or VCT tailored to couples (29). Couples’ HIV testing and counselling is a World Health Organization-recommended strategy to increase testing uptake, especially among males, reduce risky sexual behavior, and improve entry into treatment (30, 31). In Zambia, this strategy was estimated to prevent 17 times the number of infections compared to treatment as prevention for discordant couples at 86% of the cost (32).
Integrating HCT with other healthcare services also impacted testing uptake. Embedding routine PITC in emergency department services in South Africa resulted in 72.8% testing uptake with the largest limiting component being sufficient counselors to offer testing to all patients (33). Integration of HIV testing and tuberculosis (TB) services in Ghana led to high levels of screening on-site and at referral facilities (98.6% vs 72.5%) (27) and, in Tanzania, improved rates of couples’ testing and counselling (34). However, the impact of integrating HIV testing with family planning varies, with some interventions resulting in increased testing (35) and others finding no improvement in testing uptake (30, 31).
In South Africa, media and SMS communication about HIV testing resulted in exposure-dependent effects (n=2), with higher exposure increasing likelihood of testing. de Tolly et al. found that 10 motivational messages sent 3 days apart had 1.7-fold increased odds of testing while 3 strictly informational messages regarding HIV had no significant effect (38). Additionally, exposure to TV programs aimed to promote HIV prevention behavior was linked to increased likelihood of HIV testing due to the increased perception that one’s friends were tested and increased discussions about HTS (39).
Mobile, Home, and Community Testing
Moving HIV testing out of healthcare facilities and to people is the core of mobile, home-based, and community testing. Mobile clinics have been studied in South Africa, Mawali, Lesotho, Swaziland, Tanzania, and Zimbabwe in both rural (n=8, 5.8%) and urban settings (n=3, 2.2%). In both rural and urban South Africa, participants felt comfortable, had high levels of satisfaction, would refer others to mobile testing (40). A South African intervention successfully tested 72,220 participants in 18 months. Forty percent of testers were male, and individuals aged 20 to 29 made up the largest group of testers (41). Similarly, when reviewing South African program data, Mabuto et al found urban mobile testing reached the greatest proportion of men (52%) compared to clinics and stand-alone testing sites (42). In rural regions of Kenya and Uganda, 81% of children were tested through mobile testing (43). Similar to integrated facility-based testing, many mobile clinics offered additional services including malaria screening, deworming, prenatal care, and TB testing (43, 44). Of note, few studies reported linkage to care rates after mobile testing.
Home-based HIV counseling and testing (HBHCT) has been tested in rural (n=14, 10.2%) and urban (n=10, 7.3%) settings. Uptake of testing was generally high and ranged from 26% (45) to 91% (46). Compared to standard clinic based VCT in rural South Africa and Zambia, participants were more likely to be tested when offered home-based strategies (RR 1.5, 95% CI: 1.32 to 1.81 and RR 1.6, 95% CI: 1.4 to 1.8 respectively) (47, 48). HBHCT improved testing in children (OR = 1.97, 95% CI = 1.34–2.92) (49) and first-time testers (testing accepted by 68% vs 29% in standard testing services, p<0.0001, χ2 = 82.0) (50). Linkage to care after HBHCT frequently was not reported, and reported rates ranged from 25.6% (51) to 56.3% (52). Of note, van Rooyen et al obtained a linkage to care rate of 99% at 1 month and 100% at 3 months after providing point-of-care CD4 testing and facilitated referrals to HIV clinics in rural South Africa (46). Interestingly, in follow-up surveys, HBHCT participants reported less sexual risk behaviors including a reduction in the proportion of people with multiple partners (0.45, 95% CI 0.22 to 0.62)(48), reduction in the percent of people who exchanged money for sex (12.0% CI: 10.2 to 13.8 to 4.1% CI: 3.0 to 5.2, p<0.0001), and increase in the proportion who used a condom when money was exchanged during a sexual act (39.3% CI: 31.6 to 37.4 to 79.6% CI: 67.1 to 88.2, p<0.001) (53).
Testing strategies in the community (urban: n=3, 2.2%, rural, n=11, 8.0%) varied greatly. Comprehensive community mobilization strategies increased testing in control and intervention groups without significant increase between groups (54, 55). Large one-time events tested high proportions of community members (93% in rural South Africa) (56). Lugada et al engaged 96% of people with preventative care packages with 99.7% of these individuals receiving HIV testing in rural Uganda (57). A stand-alone permanent testing center near a transportation hub in Johannesburg, South Africa reached nearly 29,000 individuals in 3.5 years (42). Parishes were also often involved in testing and successfully tested52% and 76% of men (58, 59). In Tanzania, venue-based testing tested more men (69%) and young adults aged 15–24 years (42%) compared to health facility-based testing and home-based testing (60). Streamlining HIV testing with existing community-based services led to high rates of HIV testing (61). After training CHWs in rural South Africa on HTC, testing uptake increased from 55% to 78% (p<0.001, CIs not reported) (62).
Self-Testing
In recent years, there have been an increasing number of studies evaluating HIV self-testing (HIVST) (n= 15, 10.9%) (63). HIVST with pre-test counseling and demonstration of how to use the test in urban Malawi led to 76.5% of participants reporting they had completed self-testing within the past year (52). In Zambia, providing both self-testing and HBHCT compared to just HBHCT increased the percentage of people who knew their HIV status (adjusted odds ratio 1.30, 95% CI 1.03–1.65; p=0.03) (64). In Ugandan fishing communities, a pilot trial of peer distribution of self-tests showed 81.9% of men offered the tests returned the used kit (65). In Senegal, 94.3% of individuals reported using an HIVST kit after distribution (66).
HIV Testing for Pregnant Women, Partners, and Infants
Interventions to increase HIV testing uptake among pregnant women, partners of pregnant women and infants are predominantly carried out in antenatal clinics or health facilities with antenatal services to prevent mother-to-child transmission. Provision of VCT in labor wards increased HIV testing. In labor wards of two hospitals in South Africa, offering rapid HIV testing intrapartum or postpartum led to increased testing – 66.8% (95% CI: 60.5–72.7) and 60.5% (95% CI: 59.1–67.4) respectively (67). In Togo, 92% of pregnant women accepted rapid HIV testing in labor wards (68). The integration of testing services for malaria, sickle cell genotype, syphilis, and hepatitis B with HIV services increased HIV testing uptake among pregnant women 11-fold (AOR= 11.2; 95% CI: 8.77–14.25) at church-held baby showers in the community, compared to referral to a health facility (69). Of note, routine opt-out VCT in Uganda was reported to have higher uptake compared to opt-in VCT, increasing testing among pregnant women from 22% to 87.6% (p = 0.002, CI not reported) (70).
In addition to increasing testing of pregnant women, many studies (n=13, 10%) evaluated interventions to increase partner testing, which generally have low testing rates (71). Invitations to male partners to attend clinic sessions with their pregnant partners increased attendance and testing uptake, even when invites included information on VCT (72–75). Marwa et al. found that provision of self-testing kits for male partners of pregnant women increased male partner testing 12-fold (76–78). Orne-Gliemann et al showed couple-oriented post-test HIV counselling led to higher partner testing uptake compared to standard post-test counselling (OR=1.97; CI: 1.24–3.13) (79). Couples’ VCT reduced loss to follow-up of pregnant women (80) and increase likelihood of using protective measures against transmission compared to individual VCT (90% vs 60%; p = 0.14, CI not reported) (81).
Strategies to test infants were mostly clinic-based and targeted HIV-exposed infants (HEI) (n=11, 8.0%). Early infant diagnosis (EID) programs successfully increased testing rates (82, 83). Point-of-care EID also provided shorter intervals between testing and receiving results, faster time to ART initiation, and increased number of infants started on ART (84, 85). Technological outreach approaches in urban settings also improved testing of infants. Maternal health SMSs sent to HIV-infected pregnant women increased the likelihood of infant testing within 6 weeks after birth compared to control in South Africa (81.3% vs 75.4%; p = 0.064, CI not reported) and within 8 weeks after birth in Kenya (92.0%; 95% CI 87.5–95.3) (86, 87). Additionally, web-based tracing of HEI led to increased testing and ART initiation in Kenya (88). The evidence on routine HIV testing for infants is mixed. In Côte d’Ivoire, routine HIV screening offered to all infants and mothers was inefficient; though 61% of mothers received HIV tests, only 15% of infants were tested (89). In contrast, integrating routine HIV testing led to 90.4% uptake among infants at an immunization clinic in South Africa (90) and a 55% increase in testing among pediatric population aged 0–5 years across 33 health facilities in Zimbabwe (91). Lastly, active case finding and door-to-door HIV testing by community health workers, led to a 23-fold increase in the number of HIC identified and receiving care in Malawi (92).
Contact Tracing
Contact tracing of newly diagnosed individuals can be an effective method of identifying new cases (n=16, 11.7%). Cameroon, Nigeria, and Zimbabwe initiated regional or country-wide index testing programs and partner notification services (93–95). In Cameroon, health advisors successfully contacted 83.8% of sexual partners and 66.7% of partners notified were tested (93). Nigeria’s program found a disclosure rate of 68.3%, successfully traced 97.7% of sexual contacts, and tested 85.2% of these contacts. The HIV positivity rate was 51% among sexual contacts (94). Additional outreach methods at the facility-level include passive notification in which the index case notifies partner(s), contract notification in which a healthcare provider notifies the partner if the partner does not present for testing within a specified timeframe, or provider notification (96–99). Contract and provider referral are more resource-intensive but had higher rates of partner testing (51%; 95% CI: 41% to 62% and 51%; 95% CI: 40% to 62% respectively) compared to passive referral (24%; 95% CI: 15% to 34%) (P < 0.001) (97). A Ugandan study found that household members of PLHIV were more likely to receive testing when offered HBVT than facility-based testing (55.8% vs 10.9%, odds ratio: 10.41 CI: 7.89 to 13.73, P<0.001) (57). Although index testing tested fewer individuals compared to mobile, home, and work-place testing in several South African districts, index testing identified higher HIV positivity rates(10.3%, 95% CI 10.0–10.6 vs. 7.3% 95% CI 7.25–7.36) (100).
Key Populations
Populations at higher risk for HIV, such as MSM (n=7, 5.1%), PWID (n=3, 1.5%), FSW (n=11, 8.0%), or mobile populations such as truck drivers (n=4, 2.9%) may benefit from targeted outreach methods. In Malindi, Kenya, 97.3% of self-identified MSM and 98.1% of self-identified PWIDs agreed to venue-based HIV testing in venues identified by community informants as places where MSM and PWID socialize and meet new partners (101). Herce et al, developed venue-based testing in Angola and Malawi using the PLACE method, a 5-step methodology to identify where to reach people at highest risk for HIV. They found that FSWs, MSM, and transgender women that encountered outreach worker-delivered HIV/AIDS education were 3.15 (95% CI: 1.99 to 5.01), 3.12 (95% CI: 2.17 to 4.48), and 1.80 (95% CI: 0.67 to 4.87), more likely to have undergone HTS in the past 6 months, respectively (102). Using social networking strategies to refer individuals and peer educators providing HTS were also successful among MSM in Nigeria and Ghana (103, 104). Other interventions included self-testing (97.7% acceptance in 3 months among 319 MSM in Nigeria, increased likelihood of HIV testing in past month among FSWs in Uganda, risk ratio: 1.33, 95% CI 1.17–1.51, p < 0.001)(105, 106) and SMS messaging (truck drivers, FSWs, and community members near a transportation hub more likely to report HIV testing in last 6 months after receiving 35 messages on HIV reduction and testing: 86.1% vs. 77.7%; AOR 1.71, 95% CI 1.11–2.66) (107). More comprehensive interventions such as city-based diagonal interventions with sensitization training for providers increased HIV testing access in cities that had less FSW-targeted services (FSWs reporting HIV testing in past 6 months increased from 40.9% to 83.2% in Durban, South Africa, adjusted odds ratio: 7.51, 95% CI 3.84–14.7) (108). In Zimbabwe, after public health workers received “sex worker friendly” training, testing increased in all site locations, most notably from 13.4% (95% CI 8.7% to 19.9%) to 80.8% (95% CI 74.0 to 87.7) in Victoria Falls from the start of the program to analysis four years later (109).
DISCUSSION:
A wide range of HIV testing strategies have been evaluated in countries across Sub-Saharan Africa. Many interventions increased testing, though few achieved greater than 95% uptake. A combination of interventions is needed to optimize uptake and ultimately coverage (Figure 2).
Figure 2.
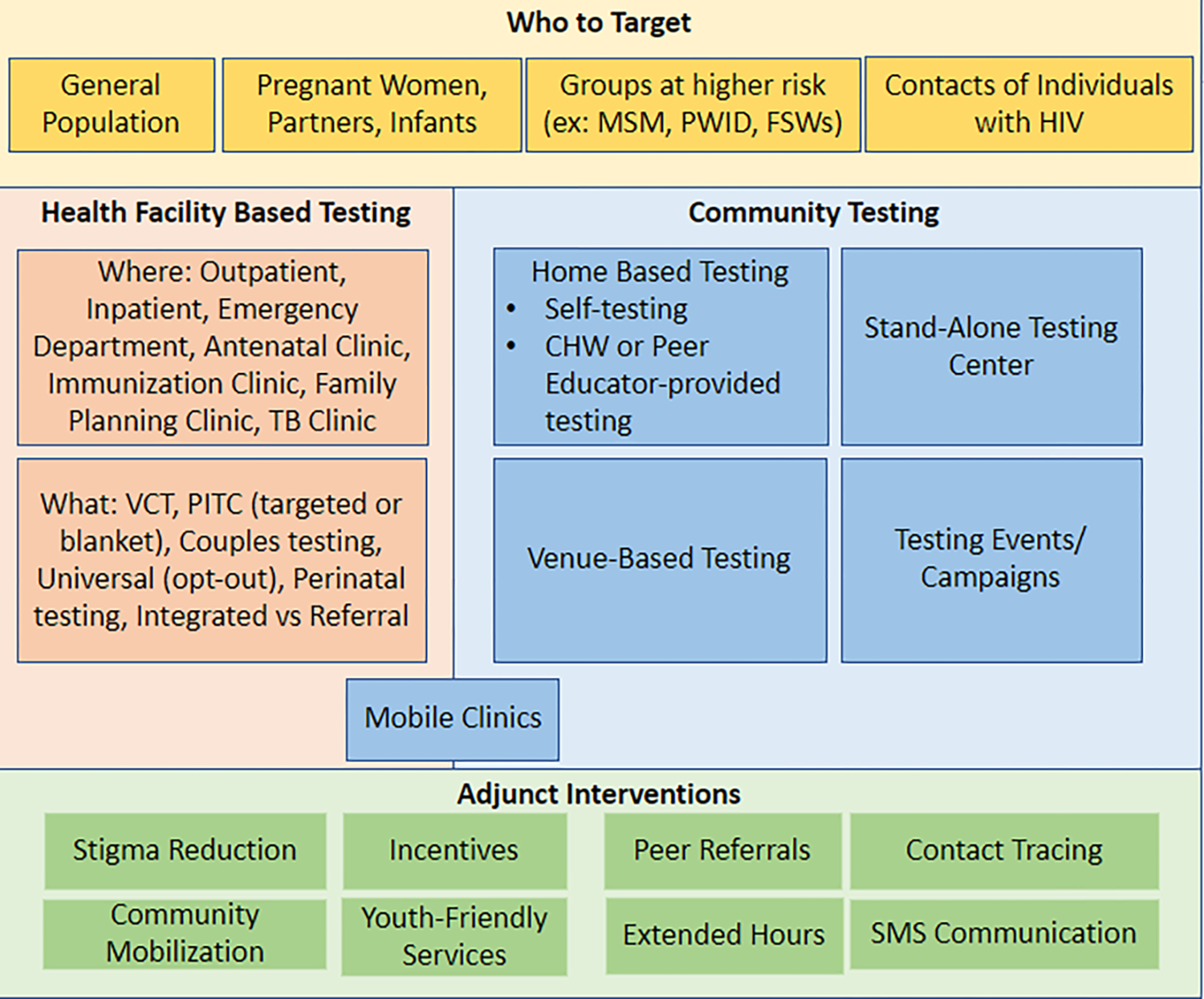
Conceptual framework of HIV testing strategies.
In health facilities, routine provision of HTS had higher uptake than VCT. Opt-out approaches could be perceived as standard of care in clinical practice and reduce the fear of stigma associated with VCT (16). Routine PITC also helps in early diagnosis of PLHIV and allows providers to initiate HCT without fearing consequences of causing offense (36). Since these approaches can be resource intensive (22), they should be gradually ushered in to avoid disrupting facility operations (37). Economical approaches include replacing traditional counseling with abbreviated counseling (110). Additionally, HTS could be offered by trained CHW while patients wait to see their provider (37). Overall, home-based interventions, mobile testing, and community-based interventions generally were able to test a greater proportion of individuals, as well as more first-time testers, compared to facility-based interventions (57). These methods may also reach potentially more marginalized groups including men and young adults better than facility-based testing (41, 42, 58). However, there is large variability in outcomes of these studies. For examples, two HBHTC studies in rural South African regions tested 26% and 91% of the eligible individuals, respectively (45, 46). The two studies varied in greatly in the number of eligible participants (38,827 compared to 739), rate of HIV prevalence in the communities (e.g.- 30% compared to 15.3% among 15–24-year-olds and 23.5% among ≥25-year-olds), other services provided, or tasks performed (e.g. - in conjunction with a triannual household-based survey and an annual anonymous HIV prevalence survey compared to just HBHCT and point-of-care CD4 count testing). It is difficult to assess further differences in methods due to differences in reporting. In recent years, self-testing appears to be a beneficial method or supplement to testing, though further research on linkage-to-care may need to be done (52, 64).
In addition to privacy concerns, low risk perception, needing partner permission and time constraints, declining a test was associated with fear of stigma and intimate partner violence (16, 18, 82, 96, 111, 112). This indicates the need for HIV awareness campaigns to reduce stigma and intimate partner violence associated with HIV-positive status and also promote autonomy among women(18, 56, 113). Couples oriented counseling should be provided in antenatal clinics and incorporated in family planning services to empower women to share test results with partners in a safe environment to mitigate intimate partner violence (79). Contact tracing can be done at a regional or national level or by individual facilities and can represent an efficient method for reaching individuals at higher risk for HIV (93–99). Provider-initiated referrals are more effective than passive referrals but are more time-consuming (96–98). Key populations at higher risk for HIV, including MSM, PWID, FSW and transgender individuals, may not be effectively served by non-adaptive intervention strategies. Venue-based testing, self-testing, and peer educators can all increase testing coverage (66, 101, 102, 111, 114, 115). Additionally, stigma-mitigation trainings for healthcare workers can have a positive impact on HIV testing (108).
This review of prospective studies, retrospective studies, randomized and non-randomized controlled trials assessed the effectiveness of various HIV testing interventions in Sub-Saharan Africa. Findings from individual studies may not be generalizable to other settings or populations in Sub-Saharan Africa or elsewhere. Transportability frameworks are being developed to characterize specific contextual factors’ effects on program outcomes and what the outcomes may be in an alternative specific context (116). The approach used to promote HIV testing uptake should take into consideration the values and culture of the program benefactors. This review was also limited to sources available in the peer-reviewed literature in English, and do not include any grey literature publications on interventions by healthcare programs. Due to the broad range of study designs included, many standard quality assessment tools were unable to assess all studies. The quality assessment method used evaluated study design, magnitude, and applicability and did not evaluate other indicators of risk of bias.
The HIV care cascade hinges on testing, and increasing testing is a key component to reducing the HIV burden in people living in countries across Sub-Saharan Africa. While PITC is necessary, it should be supplemented alongside other community-based approaches, including providing self-testing kits. Incorporating HIV testing into antenatal care can be effective in reaching pregnant women. Of note, general strategies may miss key populations at higher risk for acquiring HIV and strategies to specifically target these groups should be considered. Overall, the effectiveness of HTS strategies is context-dependent, and multiple strategies are likely needed to reach the target of 95% in any given region.
Supplementary Material
REFERENCES:
- 1.Joint United Nations Programme on HIV/AIDS. Fact Sheet—Global HIV Statistics; UNAIDS Joint United Nations Programme on HIV. AIDS: Geneva, Switzerland. 2020. [Google Scholar]
- 2.World Health Organization. Global health sector strategy on HIV 2016–2021. Towards ending AIDS. World Health Organization; 2016. [Google Scholar]
- 3.UNAIDS D, Update AE. Geneva: Joint United Nations Programme on HIV. AIDS. 2019. [Google Scholar]
- 4.UNAIDS. Fast Track: Ending the AIDS Epidemic by 2030. 2014.
- 5.Joint United Nations Programme on HIV/AIDS. Fact Sheet—Global HIV Statistics; UNAIDS Joint United Nations Programme on HIV. AIDS: Geneva, Switzerland. 2017. [Google Scholar]
- 6.UNAIDS. Ending AIDS: Progress towards the 90–90-90 targets. 2017.
- 7.UNAIDS. 90–90-90 An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland; 2014. [Google Scholar]
- 8.World Health Organization. Consolidated Guidelines on HIV Testing Services: 5Cs: Consent, Confidentiality, Counselling, Correct Results and Connection 2015. 2015. [PubMed]
- 9.PEPFAR. PEPFAR & Global AIDS. HIV.gov; 2020.
- 10.Bill & Melinda Gates Foundation. HIV Strategy Overview. HIV.gov.
- 11.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. [DOI] [PubMed] [Google Scholar]
- 12.Jenson A, Hansoti B, Rothman R, de Ramirez SS, Lobner K, Wallis L. Reliability and validity of emergency department triage tools in low- and middle-income countries: a systematic review. Eur J Emerg Med. 2018;25(3):154–60. [DOI] [PubMed] [Google Scholar]
- 13.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. Bmj. 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalal S, Lee CW, Farirai T, Schilsky A, Goldman T, Moore J, et al. Provider-initiated HIV testing and counseling: increased uptake in two public community health centers in South Africa and implications for scale-up. PLoS One. 2011;6(11):e27293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topp SM, Li MS, Chipukuma JM, Chiko MM, Matongo E, Bolton-Moore C, et al. Does provider-initiated counselling and testing (PITC) strengthen early diagnosis and treatment initiation? Results from an analysis of an urban cohort of HIV-positive patients in Lusaka, Zambia. J Int AIDS Soc. 2012;15(2):17352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogbo FA, Mogaji A, Ogeleka P, Agho KE, Idoko J, Tule TZ, et al. Assessment of provider-initiated HIV screening in Nigeria with sub-Saharan African comparison. BMC Health Serv Res. 2017;17(1):188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ijadunola K, Abiona T, Balogun J, Aderounmu A. Provider-initiated (Opt-out) HIV testing and counselling in a group of university students in Ile-Ife, Nigeria. Eur J Contracept Reprod Health Care. 2011;16(5):387–96. [DOI] [PubMed] [Google Scholar]
- 18.Abdurahman S, Seyoum B, Oljira L, Weldegebreal F. Factors affecting acceptance of provider-initiated HIV testing and counseling services among outpatient clients in selected health facilities in Harar Town, Eastern Ethiopia. HIV AIDS (Auckl). 2015;7:157–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nsirim R, Ugochukwu G, Onuoha M, Okoroezi I, Ani C, Peters E. Effectiveness of provider-initiated testing and counseling in increasing HIV testing and counselling utilization and HIV detection rates in Ebonyi State, South-Eastern Nigeria. International Journal of STD and AIDS. 2018;29(14):1362–7. [DOI] [PubMed] [Google Scholar]
- 20.Baisley K, Doyle AM, Changalucha J, Maganja K, Watson-Jones D, Hayes R, et al. Uptake of voluntary counselling and testing among young people participating in an HIV prevention trial: comparison of opt-out and opt-in strategies. PLoS One. 2012;7(7):e42108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bochner AF, Tippett Barr BA, Makunike B, Gonese G, Wazara B, Mashapa R, et al. Strengthening provider-initiated testing and counselling in Zimbabwe by deploying supplemental providers: a time series analysis. BMC Health Serv Res. 2019;19(1):351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flick RJ, Simon KR, Nyirenda R, Namachapa K, Hosseinipour MC, Schooley A, et al. The HIV diagnostic assistant: early findings from a novel HIV testing cadre in Malawi. AIDS. 2019;33(7):1215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yumo HA, Kuaban C, Ajeh RA, Nji AM, Nash D, Kathryn A, et al. Active case finding: comparison of the acceptability, feasibility and effectiveness of targeted versus blanket provider-initiated-testing and counseling of HIV among children and adolescents in Cameroon. BMC Pediatr. 2018;18(1):309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yumo HA, Ajeh RA, Beissner M, Ndenkeh JN Jr., Sieleunou I, Jordan MR, et al. Effectiveness of symptom-based diagnostic HIV testing versus targeted and blanket provider-initiated testing and counseling among children and adolescents in Cameroon. PLoS ONE. 2019;14(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu J, Geldsetzer P, Steele SJ, Matthews P, Ortblad K, Solomon T, et al. The impact of lay counselors on HIV testing rates: quasi-experimental evidence from lay counselor redeployment in KwaZulu-Natal, South Africa. AIDS (London, England). 2018;32(14):2067–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hector J, Davies MA, Dekker-Boersema J, Aly MM, Abdalad CCA, Langa EBR, et al. Acceptability and performance of a directly assisted oral HIV self-testing intervention in adolescents in rural Mozambique. PLoS ONE. 2018;13(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ansa GA, Walley JD, Siddiqi K, Wei X. Delivering TB/HIV services in Ghana: a comparative study of service delivery models. Trans R Soc Trop Med Hyg. 2014;108(9):560–7. [DOI] [PubMed] [Google Scholar]
- 28.Kose J, Tiam A, Ochuka B, Okoth E, Sunguti J, Waweru M, et al. Impact of a Comprehensive Adolescent-Focused Case Finding Intervention on Uptake of HIV Testing and Linkage to Care Among Adolescents in Western Kenya. J Acquir Immune Defic Syndr. 2018;79(3):367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Darbes LA, McGrath NM, Hosegood V, Johnson MO, Fritz K, Ngubane T, et al. Results of a Couples-Based Randomized Controlled Trial Aimed to Increase Testing for HIV. J Acquir Immune Defic Syndr. 2019;80(4):404–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hewett PC, Nalubamba M, Bozzani F, Digitale J, Vu L, Yam E, et al. Randomized evaluation and cost-effectiveness of HIV and sexual and reproductive health service referral and linkage models in Zambia. BMC Public Health. 2016;16:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Church K, Warren CE, Birdthistle I, Ploubidis GB, Tomlin K, Zhou W, et al. Impact of Integrated Services on HIV Testing: A Nonrandomized Trial among Kenyan Family Planning Clients. Stud Fam Plann. 2017;48(2):201–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wall KM, Inambao M, Kilembe W, Karita E, Vwalika B, Mulenga J, et al. HIV testing and counselling couples together for affordable HIV prevention in Africa. International Journal of Epidemiology. 2019;48(1):217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansoti B, Stead D, Parrish A, Reynolds SJ, Redd AD, Whalen MM, et al. HIV testing in a South African Emergency Department: A missed opportunity. PLoS One. 2018;13(3):e0193858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Courtenay-Quirk C, Pals S, Howard AA, Ujamaa D, Henjewele C, Munuo G, et al. Increasing partner HIV testing and linkage to care in TB settings: findings from an implementation study in Pwani, Tanzania. AIDS Care. 2018;30(12):1600–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liambila W, Askew I, Mwangi J, Ayisi R, Kibaru J, Mullick S. Feasibility and effectiveness of integrating provider-initiated testing and counselling within family planning services in Kenya. Aids. 2009;23 Suppl 1:S115–21. [DOI] [PubMed] [Google Scholar]
- 36.Ferrand RA, Meghji J, Kidia K, Dauya E, Bandason T, Mujuru H, et al. Implementation and Operational Research: The Effectiveness of Routine Opt-Out HIV Testing for Children in Harare, Zimbabwe. J Acquir Immune Defic Syndr. 2016;71(1):e24–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McNaghten AD, Schilsky Mneimneh A, Farirai T, Wamai N, Ntiro M, Sabatier J, et al. Implementation and Operational Research: Strengthening HIV Test Access and Treatment Uptake Study (Project STATUS): A Randomized Trial of HIV Testing and Counseling Interventions. J Acquir Immune Defic Syndr. 2015;70(4):e140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Tolly K, Skinner D, Nembaware V, Benjamin P. Investigation into the use of short message services to expand uptake of human immunodeficiency virus testing, and whether content and dosage have impact. Telemed J E Health. 2012;18(1):18–23. [DOI] [PubMed] [Google Scholar]
- 39.Do M, Kincaid DL, Figueroa ME. Impacts of four communication programs on HIV testing behavior in South Africa. AIDS Care. 2014;26(9):1109–17. [DOI] [PubMed] [Google Scholar]
- 40.van Rooyen H, McGrath N, Chirowodza A, Joseph P, Fiamma A, Gray G, et al. Mobile VCT: reaching men and young people in urban and rural South African pilot studies (NIMH Project Accept, HPTN 043). AIDS Behav. 2013;17(9):2946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Daniels J, Komarek A, Forgreive B, Pahl K, Stafford S, Bruns LC, et al. Shout-It-Now: A Mobile HCT Model Employing Technology and Edutainment in South Africa. J Int Assoc Provid AIDS Care. 2017;16(5):506–11. [DOI] [PubMed] [Google Scholar]
- 42.Mabuto T, Latka MH, Kuwane B, Churchyard GJ, Charalambous S, Hoffmann CJ. Four models of HIV counseling and testing: utilization and test results in South Africa. PLoS One. 2014;9(7):e102267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ayieko J, Chamie G, Balzer L, Kwarisiima D, Kabami J, Sang N, et al. Mobile, Population-wide, Hybrid HIV Testing Strategy Increases Number of Children Tested in Rural Kenya and Uganda. Pediatric Infectious Disease Journal. 2018;37(12):1279–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindgren TG, Deutsch K, Schell E, Bvumbwe A, Hart KB, Laviwa J, et al. Using mobile clinics to deliver HIV testing and other basic health services in rural Malawi. Rural Remote Health. 2011;11(2):1682. [PubMed] [Google Scholar]
- 45.Baisley KJ, Seeley J, Siedner MJ, Koole K, Matthews P, Tanser F, et al. Findings from home-based HIV testing and facilitated linkage after scale-up of test and treat in rural South Africa: young people still missing. HIV Med. 2019;20(10):704–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Rooyen H, Barnabas RV, Baeten JM, Phakathi Z, Joseph P, Krows M, et al. High HIV testing uptake and linkage to care in a novel program of home-based HIV counseling and testing with facilitated referral in KwaZulu-Natal, South Africa. J Acquir Immune Defic Syndr. 2013;64(1):e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fylkesnes K, Sandoy IF, Jurgensen M, Chipimo PJ, Mwangala S, Michelo C. Strong effects of home-based voluntary HIV counselling and testing on acceptance and equity: a cluster randomised trial in Zambia. Soc Sci Med. 2013;86:9–16. [DOI] [PubMed] [Google Scholar]
- 48.Doherty T, Tabana H, Jackson D, Naik R, Zembe W, Lombard C, et al. Effect of home based HIV counselling and testing intervention in rural South Africa: cluster randomised trial. BMJ. 2013;346:f3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thurman TR, Luckett B, Taylor T, Carnay M. Promoting uptake of child HIV testing: an evaluation of the role of a home visiting program for orphans and vulnerable children in South Africa. AIDS Care. 2016;28 Suppl 2:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jurgensen M, Sandoy IF, Michelo C, Fylkesnes K, Mwangala S, Blystad A. The seven Cs of the high acceptability of home-based VCT: results from a mixed methods approach in Zambia. Soc Sci Med. 2013;97:210–9. [DOI] [PubMed] [Google Scholar]
- 51.Labhardt ND, Motlomelo M, Cerutti B, Pfeiffer K, Kamele M, Hobbins MA, et al. Home-based versus mobile clinic HIV testing and counseling in rural Lesotho: a cluster-randomized trial. PLoS Med. 2014;11(12):e1001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choko AT, MacPherson P, Webb EL, Willey BA, Feasy H, Sambakunsi R, et al. Uptake, Accuracy, Safety, and Linkage into Care over Two Years of Promoting Annual Self-Testing for HIV in Blantyre, Malawi: A Community-Based Prospective Study. PLoS Med. 2015;12(9):e1001873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nuwaha F, Kasasa S, Wana G, Muganzi E, Tumwesigye E. Effect of home-based HIV counselling and testing on stigma and risky sexual behaviours: serial cross-sectional studies in Uganda. J Int AIDS Soc. 2012;15(2):17423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khumalo-Sakutukwa G, Morin SF, Fritz K, Charlebois ED, van Rooyen H, Chingono A, et al. Project Accept (HPTN 043): a community-based intervention to reduce HIV incidence in populations at risk for HIV in sub-Saharan Africa and Thailand. J Acquir Immune Defic Syndr. 2008;49(4):422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lippman SA, Neilands TB, MacPhail C, Peacock D, Maman S, Rebombo D, et al. Community Mobilization for HIV Testing Uptake: Results From a Community Randomized Trial of a Theory-Based Intervention in Rural South Africa. J Acquir Immune Defic Syndr. 2017;74 Suppl 1:S44–S51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.SEARCH Collaboration. Evaluating the feasibility and uptake of a community-led HIV testing and multi-disease health campaign in rural Uganda. J Int AIDS Soc. 2017;20(1):21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lugada E, Levin J, Abang B, Mermin J, Mugalanzi E, Namara G, et al. Comparison of home and clinic-based HIV testing among household members of persons taking antiretroviral therapy in Uganda: results from a randomized trial. J Acquir Immune Defic Syndr. 2010;55(2):245–52. [DOI] [PubMed] [Google Scholar]
- 58.Jobson G, Khoza S, Mbeng R, Befula N, Struthers HE, Kerongo G, et al. Bridging the Gap: Reaching Men for HIV Testing Through Religious Congregations in South Africa. Journal of Acquired Immune Deficiency Syndromes. 2019;81(5):E160–E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chamie G, Schaffer EM, Ndyabakira A, Emperador DM, Kwarisiima D, Camlin CS, et al. Comparative effectiveness of novel nonmonetary incentives to promote HIV testing. AIDS. 2018;32(11):1443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cham HJ, MacKellar D, Maruyama H, Rwabiyago OE, Msumi O, Steiner C, et al. Methods, outcomes, and costs of a 2.5 year comprehensive facility-and community-based HIV testing intervention in Bukoba Municipal Council, Tanzania, 2014–2017. PLoS ONE. 2019;14(5):e0215654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brunie A, Wamala-Mucheri P, Akol A, Mercer S, Chen M. Expanding HIV testing and counselling into communities: Feasibility, acceptability, and effects of an integrated family planning/HTC service delivery model by Village Health Teams in Uganda. Health Policy Plan. 2016;31(8):1050–7. [DOI] [PubMed] [Google Scholar]
- 62.Uwimana J, Zarowsky C, Hausler H, Swanevelder S, Tabana H, Jackson D. Community-based intervention to enhance provision of integrated TB-HIV and PMTCT services in South Africa. Int J Tuberc Lung Dis. 2013;17(10 Suppl 1):48–55. [DOI] [PubMed] [Google Scholar]
- 63.Asiimwe S, Oloya J, Song X, Whalen CC. Accuracy of un-supervised versus provider-supervised self-administered HIV testing in Uganda: A randomized implementation trial. AIDS Behav. 2014;18(12):2477–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mulubwa C, Hensen B, Phiri MM, Shanaube K, Schaap AJ, Floyd S, et al. Community based distribution of oral HIV self-testing kits in Zambia: a cluster-randomised trial nested in four HPTN 071 (PopART) intervention communities. Lancet HIV. 2018;6(2):e81–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choko AT, Nanfuka M, Birungi J, Taasi G, Kisembo P, Helleringer S. A pilot trial of the peer-based distribution of HIV self-test kits among fishermen in Bulisa, Uganda. PLoS ONE. 2018;13(11):e0208191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lyons CE, Coly K, Bowring AL, Liestman B, Diouf D, Wong VJ, et al. Use and Acceptability of HIV Self-Testing Among First-Time Testers at Risk for HIV in Senegal. AIDS and Behavior. 2019;23:130–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Theron GB, Shapiro DE, Van Dyke R, Cababasay MP, Louw J, Watts DH, et al. Rapid intrapartum or postpartum HIV testing at a midwife obstetric unit and a district hospital in South Africa. Int J Gynaecol Obstet. 2011;113(1):44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ekouevi DK, Kariyiare BG, Coffie PA, Jutand MA, Akpadza K, Lawson-Evi A, et al. Feasibility and acceptability of rapid HIV screening in a labour ward in Togo. J Int AIDS Soc. 2012;15(2):17380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ezeanolue EE, Obiefune MC, Ezeanolue CO, Ehiri JE, Osuji A, Ogidi AG, et al. Effect of a congregation-based intervention on uptake of HIV testing and linkage to care in pregnant women in Nigeria (Baby Shower): a cluster randomised trial. Lancet Glob Health. 2015;3(11):e692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Byamugisha. Dramatic and sustained increase in HIV-testing rates among antenatal attendees in Eastern Uganda after a policy change from voluntary counselling and testing to routine counselling and testing for HIV: a retrospective analysis of hospital records, 2002–2009. BMC health services research. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choko AT, Fielding K, Stallard N, Maheswaran H, Lepine A, Desmond N, et al. Investigating interventions to increase uptake of HIV testing and linkage into care or prevention for male partners of pregnant women in antenatal clinics in Blantyre, Malawi: study protocol for a cluster randomised trial. Trials. 2017;18(1):349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Theuring S, Jefferys LF, Nchimbi P, Mbezi P, Sewangi J. Increasing Partner Attendance in Antenatal Care and HIV Testing Services: Comparable Outcomes Using Written versus Verbal Invitations in an Urban Facility-Based Controlled Intervention Trial in Mbeya, Tanzania. PLoS One. 2016;11(4):e0152734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mohlala BK, Boily MC, Gregson S. The forgotten half of the equation: randomized controlled trial of a male invitation to attend couple voluntary counselling and testing. AIDS. 2011;25(12):1535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Byamugisha R, Astrom AN, Ndeezi G, Karamagi CA, Tylleskar T, Tumwine JK. Male partner antenatal attendance and HIV testing in eastern Uganda: a randomized facility-based intervention trial. J Int AIDS Soc. 2011;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jefferys LF, Nchimbi P, Mbezi P, Sewangi J, Theuring S. Official invitation letters to promote male partner attendance and couple voluntary HIV counselling and testing in antenatal care: an implementation study in Mbeya Region, Tanzania. Reprod Health. 2015;12:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Choko AT, Corbett EL, Stallard N, Maheswaran H, Lepine A, Johnson CC, et al. HIV self-testing alone or with additional interventions, including financial incentives, and linkage to care or prevention among male partners of antenatal care clinic attendees in Malawi: An adaptive multi-arm, multi-stage cluster randomised trial. PLoS Med. 2019;16(1):e1002719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marwa T, Karanja S, Osero J, Orago A. The effects of HIV self-testing kits in increasing uptake of male partner testing among pregnant women attending antenatal clinics in Kenya: a randomized controlled trial. Pan Afr Med J. 2019;33:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gichangi A, Wambua J, Mutwiwa S, Njogu R, Bazant E, Wamicwe J, et al. Impact of HIV self-test distribution to male partners of ANC clients: results of a randomized controlled trial in Kenya. JAIDS, Journal of Acquired Immune Deficiency Syndromes. 2018;79(4):467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Orne-Gliemann J, Balestre E, Tchendjou P, Miric M, Darak S, Butsashvili M, et al. Increasing HIV testing among male partners. Aids. 2013;27(7):1167–77. [DOI] [PubMed] [Google Scholar]
- 80.Conkling M, Shutes EL, Karita E, Chomba E, Tichacek A, Sinkala M, et al. Couples’ voluntary counselling and testing and nevirapine use in antenatal clinics in two African capitals: a prospective cohort study. J Int AIDS Soc. 2010;13:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Becker S, Mlay R, Schwandt HM, Lyamuya E. Comparing couples’ and individual voluntary counseling and testing for HIV at antenatal clinics in Tanzania: a randomized trial. AIDS Behav. 2010;14(3):558–66. [DOI] [PubMed] [Google Scholar]
- 82.Dube Q, Dow A, Chirambo C, Lebov J, Tenthani L, Moore M, et al. Implementing early infant diagnosis of HIV infection at the primary care level: experiences and challenges in Malawi. Bull World Health Organ. 2012;90(9):699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nuwagaba-Biribonwoha H, Werq-Semo B, Abdallah A, Cunningham A, Gamaliel JG, Mtunga S, et al. Introducing a multi-site program for early diagnosis of HIV infection among HIV-exposed infants in Tanzania. BMC Pediatr. 2010;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mwenda R, Fong Y, Magombo T, Saka E, Midian D, Mwase C, et al. Significant Patient Impact Observed Upon Implementation of Point-Of-Care Early Infant Diagnosis Technologies in an Observational Study in Malawi. Clin Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bianchi F, Cohn J, Sacks E, Bailey R, Lemaire JF, Machekano R. Evaluation of a routine point-of-care intervention for early infant diagnosis of HIV: an observational study in eight African countries. Lancet HIV. 2019;6(6):e373–e81. [DOI] [PubMed] [Google Scholar]
- 86.Coleman J, Bohlin KC, Thorson A, Black V, Mechael P, Mangxaba J, et al. Effectiveness of an SMS-based maternal mHealth intervention to improve clinical outcomes of HIV-positive pregnant women. AIDS Care. 2017;29(7):890–7. [DOI] [PubMed] [Google Scholar]
- 87.Odeny TA, Bukusi EA, Geng EH, Hughes JP, Holmes KK, Scott McClelland R. Participation in a clinical trial of a text messaging intervention is associated with increased infant HIV testing: A parallel-cohort randomized controlled trial. PLoS ONE. 2018;13(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Finocchario-Kessler S, Odera I, Okoth V, Bawcom C, Gautney B, Khamadi S, et al. Lessons learned from implementing the HIV infant tracking system (HITSystem): A web-based intervention to improve early infant diagnosis in Kenya. Healthc (Amst). 2015;3(4):190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ndondoki C, Brou H, Timite-Konan M, Oga M, Amani-Bosse C, Menan H, et al. Universal HIV screening at postnatal points of care: which public health approach for early infant diagnosis in Côte d’Ivoire? PLoS One. 2013;8(8):e67996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rollins N, Mzolo S, Moodley T, Esterhuizen T, van Rooyen H. Universal HIV testing of infants at immunization clinics: an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. Aids. 2009;23(14):1851–7. [DOI] [PubMed] [Google Scholar]
- 91.Musarandega R, Mutede B, Mahomva A, Nyamayaro W, Mushavi A, Lindan C, et al. Scaling up Pediatric HIV Testing by Incorporating Provider-Initiated HIV Testing Into all Child Health Services in Hurungwe District, Zimbabwe. Journal of acquired immune deficiency syndromes (1999). 2018;77(1):78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ahmed S, Kim MH, Dave AC, Sabelli R, Kanjelo K, Preidis GA, et al. Improved identification and enrolment into care of HIV-exposed and -infected infants and children following a community health worker intervention in Lilongwe, Malawi. J Int AIDS Soc. 2015;18(1):19305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Henley C, Forgwei G, Welty T, Golden M, Adimora A, Shields R, et al. Scale-up and case-finding effectiveness of an HIV partner services program in Cameroon: an innovative HIV prevention intervention for developing countries. Sex Transm Dis. 2013;40(12):909–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katbi M, Adegboye A, Adedoyin A, Yunusa F, Kayode G, Bello M, et al. Effect of clients Strategic Index Case Testing on community-based detection of HIV infections (STRICT study). International Journal of Infectious Diseases. 2018;74:54–60. [DOI] [PubMed] [Google Scholar]
- 95.Mahachi N, Muchedzi A, Tafuma TA, Mawora P, Kariuki L, Semo BW, et al. Sustained high HIV case-finding through index testing and partner notification services: experiences from three provinces in Zimbabwe. J Int AIDS Soc. 2019;22 Suppl 3(Suppl Suppl 3):e25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kahabuka C, Plotkin M, Christensen A, Brown C, Njozi M, Kisendi R, et al. Addressing the First 90: A Highly Effective Partner Notification Approach Reaches Previously Undiagnosed Sexual Partners in Tanzania. AIDS Behav. 2017;21(8):2551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brown LB, Miller WC, Kamanga G, Nyirenda N, Mmodzi P, Pettifor A, et al. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr. 2011;56(5):437–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Madsen T, Jespersen S, Medina C, Té DDS, Wejse C, Laursen AL, et al. Acceptance and Feasibility of Partner Notification to HIV Infected Individuals in Guinea-Bissau. AIDS Behav. 2019;24(5):1476–85. [DOI] [PubMed] [Google Scholar]
- 99.Masyuko SJ, Cherutich PK, Contesse MG, Maingi PM, Wamuti BM, Macharia PM, et al. Index participant characteristics and HIV assisted partner services efficacy in Kenya: results of a cluster randomized trial. J Int AIDS Soc. 2019;22 Suppl 3(Suppl Suppl 3):e25305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shamu S, Farirai T, Kuwanda L, Slabbert J, Guloba G, Khupakonke S, et al. Comparison of community-based HIV counselling and testing (CBCT) through index client tracing and other modalities: Outcomes in 13 South African high HIV prevalence districts by gender and age. PLoS ONE. 2019;14(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singh K, Brodish P, Mbai F, Kingola N, Rinyuri A, Njeru C, et al. A venue-based approach to reaching MSM, IDUs and the general population with VCT: a three study site in Kenya. AIDS Behav. 2012;16(4):818–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Herce ME, Miller WM, Bula A, Edwards JK, Sapalalo P, Lancaster KE, et al. Achieving the first 90 for key populations in sub-Saharan Africa through venue-based outreach: challenges and opportunities for HIV prevention based on PLACE study findings from Malawi and Angola. Journal of the International AIDS Society. 2018;21:e25132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adebajo S, Eluwa G, Njab J, Oginni A, Ukwuije F, Ahonsi B, et al. Evaluating the effect of HIV prevention strategies on uptake of HIV counselling and testing among male most-at-risk-populations in Nigeria; a cross-sectional analysis. Sex Transm Infect. 2015;91(8):555–60. [DOI] [PubMed] [Google Scholar]
- 104.Girault P, Green K, Clement NF, Rahman YA, Adams B, Wambugu S. Piloting a Social Networks Strategy to Increase HIV Testing and Counseling Among Men Who Have Sex with Men in Greater Accra and Ashanti Region, Ghana. AIDS Behav. 2015;19(11):1990–2000. [DOI] [PubMed] [Google Scholar]
- 105.Tun W, Vu L, Dirisu O, Sekoni A, Shoyemi E, Njab J, et al. Uptake of HIV self-testing and linkage to treatment among men who have sex with men (MSM) in Nigeria: A pilot programme using key opinion leaders to reach MSM. J Int AIDS Soc. 2018;21 Suppl 5(Suppl Suppl 5):e25124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ortblad K, Kibuuka Musoke D, Ngabirano T, Nakitende A, Magoola J, Kayiira P, et al. Direct provision versus facility collection of HIV self-tests among female sex workers in Uganda: A cluster-randomized controlled health systems trial. PLoS Med. 2017;14(11):e1002458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Govender K, Beckett S, Masebo W, Braga C, Zambezi P, Manhique M, et al. Effects of a Short Message Service (SMS) Intervention on Reduction of HIV Risk Behaviours and Improving HIV Testing Rates Among Populations located near Roadside Wellness Clinics: A Cluster Randomised Controlled Trial in South Africa, Zimbabwe and Mozambique. AIDS and Behavior. 2019;23(11):3119–28. [DOI] [PubMed] [Google Scholar]
- 108.Lafort Y, Greener L, Lessitala F, Chabeda S, Greener R, Beksinska M, et al. Effect of a ‘diagonal’ intervention on uptake of HIV and reproductive health services by female sex workers in three sub-Saharan African cities. Trop Med Int Health. 2018;23(7):774–84. [DOI] [PubMed] [Google Scholar]
- 109.Ndori-Mharadze T, Fearon E, Busza J, Dirawo J, Musemburi S, Davey C, et al. Changes in engagement in HIV prevention and care services among female sex workers during intensified community mobilization in 3 sites in Zimbabwe, 2011 to 2015. Journal of the International AIDS Society. 2018;21:e25138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wanyenze RK, Kamya MR, Fatch R, Mayanja-Kizza H, Baveewo S, Szekeres G, et al. Abbreviated HIV counselling and testing and enhanced referral to care in Uganda: a factorial randomised controlled trial. Lancet Glob Health. 2013;1(3):e137–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oldenburg CE, Chanda MM, Ortblad KF, Mwale M, Chongo S, Kamungoma N, et al. Effect of HIV self-testing on the number of sexual partners among female sex workers in Zambia. Aids. 2018;32(5):645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dalal W, Feikin DR, Amolloh M, Ransom R, Burke H, Lugalia F, et al. Home-based HIV testing and counseling in rural and urban Kenyan communities. J Acquir Immune Defic Syndr. 2013;62(2):e47–54. [DOI] [PubMed] [Google Scholar]
- 113.Rosenberg NE, Stanley CC, Rutstein SE, Bonongwe N, Kamanga G, Pettifor A, et al. Recruiting the social contacts of patients with STI for HIV screening in Lilongwe, Malawi: process evaluation and assessment of acceptability. Sex Transm Infect. 2016;92(8):587–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bhattacharjee P, Musyoki H, Prakash R, Malaba S, Dallabetta G, Wheeler T, et al. Micro-planning at scale with key populations in Kenya: Optimising peer educator ratios for programme outreach and HIV/STI service utilisation. PLoS ONE. 2018;13(11):e0205056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kelvin EA, George G, Mwai E, Nyaga E, Mantell JE, Romo ML, et al. Offering self-administered oral HIV testing to truck drivers in Kenya to increase testing: a randomized controlled trial. AIDS Care - Psychological and Socio-Medical Aspects of AIDS/HIV. 2018;30(1):47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mehrotra ML, Petersen ML, Geng EH. Understanding HIV Program Effects: A Structural Approach to Context Using the Transportability Framework. J Acquir Immune Defic Syndr. 2019;82 Suppl 3:S199–S205. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


