Abstract
Objective:
Cannabis use is increasing among midlife and older adults. We tested the hypotheses that long-term cannabis use is associated with cognitive deficits and smaller hippocampal volume in midlife, which is important because midlife cognitive deficits and smaller hippocampal volume are risk factors for dementia.
Methods:
Participants are members of a representative cohort of 1,037 individuals born in Dunedin, New Zealand in 1972–73 and followed to age 45 years, with 94% retention. Cannabis use and dependence were assessed at ages 18, 21, 26, 32, 38 and 45 years. IQ was assessed at ages 7, 9, 11, and 45 years. Specific neuropsychological functions and hippocampal volume were assessed at age 45 years.
Results:
Long-term cannabis users showed IQ decline from childhood to midlife (mean=−5.5 IQ points), poorer learning and processing speed relative to their childhood IQ, and informant-reported memory and attention problems. These deficits were specific to long-term cannabis users because they were either not present or smaller among long-term tobacco users, long-term alcohol users, midlife recreational cannabis users, and cannabis quitters. Cognitive deficits among long-term cannabis users could not be explained by persistent tobacco, alcohol, or other illicit drug use; childhood SES; low childhood self-control; or family history of substance dependence. Long-term cannabis users showed smaller hippocampal volume, but smaller hippocampal volume did not statistically mediate cannabis-related cognitive deficits.
Conclusions:
Long-term cannabis users showed cognitive deficits and smaller hippocampal volume in midlife. Research is needed to ascertain whether long-term cannabis users show elevated rates of dementia in later life.
In case-control studies, cannabis users exhibit subtle cognitive deficits and structural brain differences (1,2). These findings come largely from studies of adolescents and young adults (3,4). It is unclear if the subtle cognitive and brain differences observed in young cannabis users might be larger in midlife and older adult cannabis users with longer histories of use (5). This issue is timely because cannabis use is increasing among baby boomers (born 1946–64), a group who used cannabis at historically high rates as young adults (6), and who now uses cannabis at historically high rates as midlife and older adults (7). This issue is important because mild cognitive deficits and greater hippocampal atrophy in midlife are risk factors for later dementia (8,9).
We identified four longitudinal studies and seven cross-sectional studies that reported on cannabis users in midlife or older adulthood (Table S1) (3,10–19). Limitations include use of crude or retrospective measures of cannabis exposure and a lack of neuroimaging data. Further, the studies did not address four questions of policy significance. First, are all midlife and older adult cannabis users at risk? Older adults in the United States are increasingly using cannabis (7), but only 10–15% of users are cannabis dependent (20). Distinguishing problem versus non-problem users is important, because non-problem users may not exhibit differences. Second, are cognitive deficits and brain differences among cannabis users minor compared with those observed for alcohol or tobacco users, as some proponents of cannabis legalization claim (21)? Third, do differences among cannabis users persist after cessation? If so, this could increase risk for dementia. Fourth, do brain differences among long-term cannabis users underlie cognitive deficits? In adolescent and young adult cannabis users, brain differences, if observed, are inconsistently related to cognitive deficits. Research is needed in midlife and older adult cannabis users.
We addressed these questions by assessing cannabis use, cognitive function, and hippocampal volume in a population-representative cohort followed prospectively from birth to age-45 years. We compared long-term cannabis users against five groups: (i) lifelong cannabis non-users (to replicate the control group most often reported in the case-control literature); (ii) midlife recreational cannabis users (to ascertain if cognitive deficits and structural brain differences are apparent in non-problem users -- the majority of cannabis users); (iii) long-term tobacco and (iv) long-term alcohol users (to serve as benchmark comparisons for any cannabis findings and to help disentangle potential cannabis effects from tobacco and alcohol effects); and (v) cannabis quitters (to ascertain if differences are apparent after cessation). Importantly, we also conducted tests of dose-response associations using continuously-measured persistence of cannabis use, and rigorously adjusted for numerous confounders derived from multiple longitudinal waves and data sources. Robust dose-response associations would be expected if associations were causal. Finally, we tested if associations between continuously-measured persistence of cannabis use and cognitive deficits were mediated by hippocampal volume differences, a hypothesis that is fairly ubiquitous in the literature (22–24). We focused on the hippocampus because it has a high density of cannabinoid receptors, is instrumental for learning and memory (one of the most consistently impaired cognitive domains in cannabis users), and has been shown though meta-analysis to be the brain region that most consistently emerges as smaller in cannabis users vs. comparison individuals (2).
Methods
Participants
Participants are members of the Dunedin Longitudinal Study, a representative birth cohort (N=1,037; 91% of eligible births; 52% male) born April 1972-March 1973 in Dunedin, New Zealand (NZ), who were eligible based on residence in the province and who participated in the first assessment at age 3 years. The cohort represents the full range of socioeconomic status (SES) in the general population of NZ’s South Island (25). As adults, the cohort matches the NZ National Health and Nutrition Survey on key health indicators (e.g., body mass index, smoking, physical activity, physician visits) (25), and the NZ Census of citizens the same age on educational attainment (26). The cohort is primarily white (93%), which matches South Island demographics. Assessments were carried out at birth and ages 3, 5, 7, 9, 11, 13, 15, 18, 21, 26, 32, 38, and most recently (completed April 2019) 45 years. Participants gave written informed consent. Study protocols were approved by the NZ Health and Disability Ethics Committee.
Measures
Measures are briefly described here. Details are in Table S2.
Long-Term Cannabis Users and Five Comparison Groups.
At ages 18, 21, 26, 32, 38, and 45, study members were interviewed about their substance use using the Diagnostic Interview Schedule (27,28), and past-year substance-use dependencies were assessed following Diagnostic and Statistical Manual of Mental Disorders criteria (29,30). This information was used to identify long-term cannabis users and 5 comparison groups (Figure S1).
Long-term cannabis users (n=86; 64% male) used cannabis weekly or more frequently in the past year at age 45, or were dependent on cannabis at age 45, and also used weekly or more frequently at one or more previous assessment waves. Of these, 31.4% used cannabis before age 18; 89.5% used regularly (4+ days per week) at one or more waves (M=3.4 waves, SD=1.4); and 72% met criteria for cannabis dependence at one or more waves. Age-45 cannabis consumption was a median of 300 days in the past year, with 64% using 4+ days per week.
Lifelong cannabis non-users (n=202; 41% male) never used cannabis, never had a diagnosis of any substance-use disorder, and never used tobacco daily.
Long-term tobacco users (n=75; 40% male) smoked tobacco daily at age 45 and also smoked daily at one or more previous waves; were mostly free from cannabis at age 45 (Table 1); and had no history of weekly cannabis use or dependence.
Table 1.
Sociodemographic characteristics and substance use for the full cohort, long-term cannabis users, and five informative comparison groups.
| Sociodemographics | Full Cohort (N=938) | Long-Term Cannabis Users (N=86) | Cannabis Non-Users (N=202) | Long-term Tobacco Users (N=75) | Long-term Alcohol Users (N=57) | Midlife Recreational Cannabis Users (N=65) | Cannabis Quitters (N=60 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| %/M | N/SD | %/M | N/SD | %/M | N/SD | %/M | N/SD | %/M | N/SD | %/M | N/SD | %/M | N/SD | |
| 50.5 | 474 | 64.0 | 55 | 40.6 | 82 | 40.0 | 30 | 56.1 | 32 | 58.5 | 38 | 61.7 | 37 | |
| Childhood SES, M (SD) | 3.78 | 1.13 | 3.42 | 1.08 | 3.92 | 1.17 | 3.23 | 0.97 | 3.80 | 1.18 | 3.86 | 1.24 | 3.57 | 1.20 |
| Childhood Low Self-Control, M (SD) | −0.02 | 0.96 | 0.34 | 1.08 | −0.19 | 0.88 | 0.43 | 1.19 | −0.01 | 0.92 | −0.06 | 1.00 | 0.16 | 1.06 |
| Family History of Substance Dependence, M (SD) | 0.15 | 0.17 | 0.21 | 0.21 | 0.10 | 0.13 | 0.20 | 0.18 | 0.14 | 0.15 | 0.13 | 0.14 | 0.19 | 0.18 |
| Age-45 Substance Use | ||||||||||||||
| Cannabis Frequency,a M (SD) | 25.70 | 82.90 | 257.07 | 117.84 | 0.00 | 0.00 | 0.11d | 0.48 | 0.32e | 1.18 | 4.88 | 8.24 | 0.00 | 0.00 |
| Weekly Cannabis Use, % (N) | 9.6 | 89 | 98.8 | 85 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 |
| Regular Cannabis Use,b % (N) | 6.1 | 56 | 64.0 | 55 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 |
| Daily Tobacco Use, % (N) | 21.6 | 199 | 63.5 | 54 | 0.0 | 0 | 100.0 | 75 | 17.5 | 10 | 20.0 | 13 | 33.3 | 20 |
| Weekly Alcohol Use, % (N) | 92.6 | 856 | 88.4 | 76 | 91.1 | 184 | 90.7 | 68 | 100.0 | 57 | 95.4 | 62 | 83.3 | 50 |
| Cannabis Dependence, % (N) | 2.1 | 19 | 22.1 | 19 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 |
| Tobacco Dependence, % (N) | 11.6 | 107 | 44.7 | 38 | 0.0 | 0 | 50.0 | 37 | 10.5 | 6 | 7.7 | 5 | 16.7 | 10 |
| Alcohol Dependence, % (N) | 11.3 | 104 | 19.8 | 17 | 0.0 | 0 | 9.3 | 7 | 52.6 | 30 | 16.9 | 11 | 16.7 | 10 |
| Illicit Drug Dependence, % (N) | 3.4 | 31 | 15.1 | 13 | 0.0 | 0 | 4.0 | 3 | 1.8 | 1 | 3.1 | 2 | 5.0 | 3 |
| Amphetamine Use, %(N)c | 3.1 | 29 | 18.6 | 16 | 0.0 | 0 | 1.3 | 1 | 0.0 | 0 | 3.1 | 2 | 5.0 | 3 |
| Sedatives Use, % (N)c | 1.4 | 13 | 8.1 | 7 | 0.5 | 1 | 1.3 | 1 | 0.0 | 0 | 0.0 | 0 | 1.7 | 1 |
| Cocaine Use, % (N)c | 1.6 | 15 | 3.5 | 3 | 0.0 | 0 | 0.0 | 0 | 3.5 | 2 | 4.6 | 3 | 3.3 | 2 |
| Opioid Use, % (N)c | 1.6 | 15 | 9.3 | 8 | 0.0 | 0 | 2.7 | 2 | 0.0 | 0 | 1.5 | 1 | 0.0 | 0 |
| PCP Use, % (N)c | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 |
| Hallucinogens Use, % (N)c | 2.1 | 19 | 11.6 | 10 | 0.0 | 0 | 0.0 | 0 | 1.8 | 1 | 3.1 | 2 | 1.7 | 1 |
| Inhalants Use, % (N)c | 0.1 | 1 | 1.2 | 1 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 |
| Other Drugs, % (N)c | 0.8 | 7 | 3.5 | 3 | 0.0 | 0 | 0 | 0 | 3.5 | 2 | 1.5 | 1 | 0.0 | 0 |
| Methadone Maintenance, % (N) | 1.1 | 10 | 5.8 | 5 | 0.0 | 0 | 1.3 | 1 | 0.0 | 0 | 0.0 | 0 | 3.3 | 2 |
Note.
Number of days used in past year.
Regular use = 4+ days per week.
Used 6+ times in the past year.
Only 4 long-term tobacco users reported past-year cannabis use, with maximum use of 3 days.
Only 6 long-term alcohol users reported past-year cannabis use, with maximum use of 7 days.
Long-term alcohol users (n=57, 56% male) were weekly drinkers at age 45; had a diagnosis of alcohol dependence at 2+ waves; were mostly free from cannabis at age 45 (Table 1); and had no history of weekly cannabis use or dependence.
Midlife recreational cannabis users (n=65; 59% male) used cannabis between 6–51 days per year (i.e., used more than a few times but less than weekly) in midlife (age 32, 38, or 45), and had no history of weekly cannabis use or dependence.
Cannabis quitters (n=60; 62% male) did not use cannabis at age 45 but previously either diagnosed with cannabis dependence or used regularly (4+ days per week).
Persistence of Cannabis Dependence and Persistence of Regular Cannabis Use.
Persistence of cannabis dependence comprised those who (i) never used cannabis (n=262), (ii) used but never diagnosed (n=498), (iii) diagnosed at one wave (n=85), (iv) two waves (n=39), (v) three waves (n=32), and (vi) 4+ waves (n=16). Persistence of regular cannabis use (i.e., 4+ days per week) comprised those who never used cannabis (n=262), (ii) used but never regularly (n=518), (iii) used regularly at one wave (n=57), (iv) two waves (n=32), (v) three waves (n=33), and (vi) 4+ waves (n=30). Agreement between the two exposures was high but not perfect (weighted κ=0.75), because many regular users did not develop dependence (20). Persistence of tobacco dependence, alcohol dependence, and other illicit drug dependence were similarly defined (Table S2).
Cognitive Tests.
Intelligence was assessed at ages 7, 9, and 11 years, before the onset of cannabis use, and again in adulthood at age 45. We report comparison of the Wechsler Intelligence Scale for Children-Revised (WISC-R) (31), averaged across ages 7–11, and the Wechsler Adult Intelligence Scale-IV at age 45 (WAIS-IV) (32). We also report performance on the WAIS-IV working memory index, perceptual reasoning index, verbal comprehension index, and processing speed index. At age 45, additional neuropsychological tests were administered: the Rey Auditory Verbal Learning Test (33), the Months Backwards Test from the Wechsler Memory Scale-III (WMS-III) (34), Trail Making Test (35), Animal Naming Test (36), and Grooved Pegboard (33). All testing occurred in the morning.
Informant-Reported Memory and Attention Problems.
At age 45, participants nominated people “who knew them well.” Informants completed mailed questionnaire checklists, including whether the participant had problems with memory (e.g., forgets to do errands, return calls, pay bills) and attention (e.g., is easily distracted, gets sidetracked easily) over the past year.
Hippocampal Volume.
Structural MRI was carried out at age 45 for 875 study members (93% of age-45 participants). T1-weighted and fluid-attenuated inversion recovery images were processed with FreeSurfer version 6.0. Mean hippocampal gray-matter volume was extracted using the automatic segmentation (“aseg”) step. Accuracy of subcortical segmentation was confirmed by visual inspection of the “aseg” labels overlaid on the volumes. Mean volumes within 12 hippocampal subfields were estimated with FreeSurfer 6.0’s hippocampal subfields module. We report on bilateral total hippocampal volume and 12 subfield volumes (37) because the hippocampus is composed of anatomically and functionally distinct subfields, and examining them could provide a more nuanced understanding of potential cannabis effects on this structure.
Covariates.
We selected covariates based on theory and documented associations with cannabis use, cognitive functioning, and brain volume: sex, persistent tobacco dependence, persistent alcohol dependence, persistent other illicit drug dependence, childhood SES, low childhood self-control, and family substance dependence history (Table S2).
Statistical Analyses
We used t-tests to compare long-term cannabis users with the five groups. We used ordinary least squares regression to test dose-response associations between persistence of cannabis use (continuously measured) and outcomes, with associations adjusted for sex (Model 1); sex and persistent alcohol, tobacco, and other illicit drug dependence (Model 2); and aforementioned covariates plus childhood SES, low childhood self-control, and family substance dependence history (Model 3). We used path analysis to test mediation (i.e., whether the association between persistence of cannabis use and cognitive deficits arises indirectly through hippocampal volume). Mediation analyses were conducted in MPlus using maximum likelihood estimation and bootstrapped standard errors. Analyses were pre-registered (https://sites.duke.edu/moffittcaspiprojects/files/2021/07/Meier_2020.pdf).
Results
Of 997 cohort members still alive at age 45 years, 938 (94.1%) were assessed at age 45. Age-45 participants did not differ significantly from other participants on childhood SES, childhood self-control, or childhood IQ (Figure S2). Table 1 shows characteristics of the age-45 cohort, long-term cannabis users, and five comparison groups.
Cannabis and Cognitive Functioning
Long-term Cannabis Users and Five Comparison Groups.
Relative to normative IQ of 100, long-term cannabis users had average IQ as children (M=99.3) but below-average IQ as adults (M=93.8). Their mean 5.5-point childhood-to-adulthood IQ decline was significantly larger than that observed among lifelong cannabis non-users (M=0.70), long-term tobacco users (M=−1.5), and long-term alcohol users (M=−0.50) (Table 2, Panel A). Long-term cannabis users’ IQ decline was not significantly larger than midlife recreational cannabis users’ (M=−3.5) or cannabis quitters’ (M=−3.3).
Table 2. Panel A.
Child IQ, Adult IQ, and IQ Change: Group comparisons. A comparison of long-term cannabis users and 5 informative subgroups on IQ.
| Statistical Tests of Difference Between Long-term Cannabis Users and Comparison Groups | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IQ | Long-term Cannabis Users (N=84) | Comparison Group 1: Cannabis Non-Users (N=196) | Comparison Group 2: Long-term Tobacco Users (N=75) | Comparison Group 3: Long-term Alcohol Users (N=57) | Comparison Group 4: Midlife Recreational Cannabis Users (N=65) | Comparison Group 5: Cannabis Quitters (N=59) | LT vs 1 | LT vs 2 | LT vs 3 | LT vs 4 | LT vs 5 | ||||||
| M | 95% CI | M | 95% CI | M | 95% CI | M | 95% CI | M | 95% CI | M | 95% CI | p | p | p | p | p | |
| 99.3 | 96.4, 102.2 | 101.4 | 99.4, 103.4 | 93.0 | 89.8, 96.2 | 99.3 | 96.1, 102.5 | 105.1 | 102.0, 108.3 | 97.6 | 93.7, 101.5 | .14 | .01 | .99 | .006 | .48 | |
| Adult IQ | 93.8 | 90.6, 97.0 | 102.1 | 99.9, 104.2 | 91.5 | 88.2, 94.7 | 98.8 | 95.8, 101.8 | 101.6 | 98.1, 105.2 | 94.3 | 90.6, 98.0 | <.001 | .44 | .03 | .001 | .85 |
| IQ Δ | −5.5 | −7.4, −3.6 | 0.70 | −0.67, 2.0 | −1.5 | −3.8, 0.75 | −0.50 | −2.8, 1.8 | −3.5 | −5.8, −1.2 | −3.3 | −6.7, 0.01 | <.001 | .02 | <.001 | .17 | .24 |
| ES IQ Δ | −0.37 | −0.57, −0.18 | 0.25 | 0.12, 0.39 | 0.03 | −0.20, 0.26 | 0.13 | −0.10, 0.37 | −0.17 | −0.40, 0.06 | −0.15 | −0.49, 0.18 | - | - | - | - | - |
Note. Statistical tests of group comparisons are adjusted for sex but means are unadjusted. IQ Δ = IQ change (adult IQ minus child IQ). ES IQ Δ = effect size for IQ change (IQ change scores were standardized on the full sample [M=0, SD=1]). LT=Long-term cannabis users. Bolded p-values indicate a statistically significant difference (p<.05) compared with long-term cannabis users. Dashes indicate the results are the same as results for IQ Δ.
To ascertain whether long-term cannabis users showed deficits in specific neuropsychological functions, we examined age-45 test performance, with estimates adjusted for sex and childhood IQ (Figure 1A, Table S3). Long-term cannabis users performed significantly worse than lifelong cannabis non-users on most tests; worse than long-term tobacco users on tests of learning and memory (Rey Total and Delayed Recall) and processing speed (PSI); worse than long-term alcohol users on tests of learning and memory (Rey Total and Recall), executive function (WMS, Trails B), perceptual reasoning (PRI), verbal comprehension (VCI), and processing speed (PSI); and worse than midlife recreational cannabis users on tests of learning and memory. Long-term cannabis users did not perform significantly worse than cannabis quitters on any test.
Figure 1A.
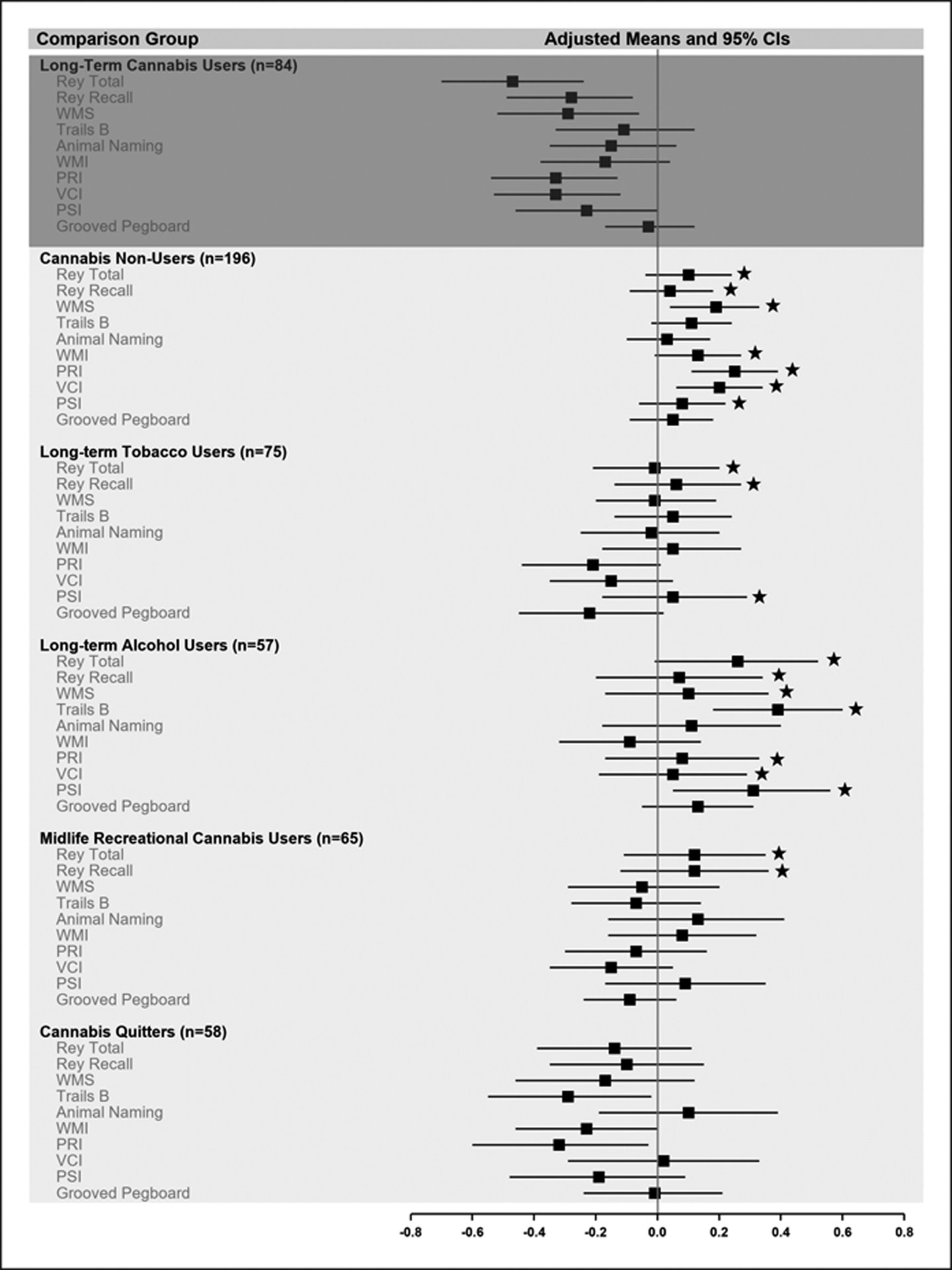
Long-Term Cannabis Use and Neuropsychological Functions. A comparison of long-term cannabis users with 5 informative subgroups on age-45 test performance across specific neuropsychological domains. This figure shows means (and 95% CIs) on age-45 neuropsychological tests, which were adjusted for sex and childhood IQ and standardized on the full cohort (M=0, SD=1). Average normative performance is indicated by the reference line at the representative cohort mean of 0. Estimates below zero indicate poorer than average test performance. Stars indicate mean scores that were statistically significantly better (p<.05) as compared with long-term cannabis users, after adjustment for sex and childhood IQ. Rey Total=Rey Auditory Verbal Learning Test total score (learning). Rey Recall=Rey Auditory Verbal Learning Test delayed recall (memory). WMS=Wechsler Memory Scale Months Backwards test. WMI=Working Memory Index. PRI=Perceptual Reasoning Index. VCI=Verbal Comprehension Index. PSI= Processing Speed Index.
Dose-response Associations.
Participants who used cannabis more persistently showed greater IQ decline than less persistent users, even after adjustment for persistent use of other substances, childhood SES, low childhood self-control, and family substance dependence history (Table 2, Panel B).
Table 2. Panel B.
IQ Change: Dose-response associations. Dose-response associations between persistence of cannabis use from age 18–45 and IQ change from childhood to adulthood.
| Panel B1. Exposure: Persistence of Cannabis Dependence | Statistical Tests | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | Means for IQ Change from Childhood to Adulthood as a Function of Persistence of Cannabis Dependencea | Model 1: Adjusted for sex | Model 2: +Adjustment for other substance useb | Model 3: + Adjustment for childhood SES, low childhood self-control, and family history of substance-dependencec | |||||||||||
| Persistence of Cannabis Dependence | Never used (n=255) | Used but never diagnosed (N=496) | 1 diagnosis (N=83) | 2 diagnoses (N=39) | 3 diagnoses (N=32) | 4+ diagnoses (N=15) | β | 95% CI | p | β | 95% CI | p | β | 95% CI | p |
| 0.21 | −0.02 | −0.18 | −0.17 | −0.40 | −0.66 | −0.16 | −0.23, −0.10 | <.001 | −0.09 | −0.18, −0.01 | .02 | −0.10 | −0.18, −0.01 | .02 | |
| Panel B2. Exposure: Persistence of Regular Cannabis Use | Statistical Tests | ||||||||||||||
| Exposure | Means for IQ Change from Childhood to Adulthood as a Function of Persistence of Regular Cannabis Usea | Model 1: Adjusted for sex | Model 2: +Adjustment for other substance useb | Model 3: + Adjustment for childhood SES, low childhood self-control, and family history of substance-dependencec | |||||||||||
| Persistence of Regular Cannabis Use | Never used (n=255) | Used but never regularly (n=516) | Regularly used 1x (n=55) | Regularly used 2x (n=32) | Regularly used 3x (n=33) | Regularly used 4+x (n=29) | β | 95% CI | p | β | 95% CI | p | β | 95% CI | p |
| 0.21 | −0.01 | −0.26 | −0.29 | −0.27 | −0.52 | −0.16 | −0.23, −0.10 | <.001 | −0.10 | −0.18, −0.02 | .01 | −0.10 | −0.18, −0.03 | .01 | |
Note.
Means represent unadjusted IQ change scores (adult IQ minus child IQ) that were standardized on the full sample prior to analysis (M=0, SD=1).
Statistical tests were adjusted for sex and persistent use of tobacco, alcohol, and other illicit drugs.
Statistical tests were adjusted for sex; persistent use of tobacco, alcohol, and other illicit drugs; low childhood SES; low childhood self-control; and family history of substance dependence. Beta coefficients represent standardized estimates. Bolded estimates are statistically significant (p<.05).
For specific neuropsychological functions, participants who used cannabis more persistently performed worse on most age-45 tests than less persistent users after adjusting for sex and childhood IQ (Table 3, Model 1). Associations were attenuated after adjustment for persistent use of other substances (Table 3, Model 2) and, to a lesser extent, after additional adjustment for childhood covariates (Table 3, Model 3). However, even after adjustment for all covariates, more persistent cannabis users performed worse than less persistent users on tests of learning (Rey Total), processing speed (PSI), and, to a lesser extent, verbal memory (Rey Recall) and perceptual reasoning (Table 3, Model 3).
Table 3.
Specific neuropsychological functions: Dose-response associations. Dose-response associations between persistence of cannabis use from age 18–45 and age-45 test performance across different neuropsychological domains.
| Panel A. Exposure: Persistence of Cannabis Dependence | Statistical Tests | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Means for Neuropsychological Tests as a Function of Persistence of Cannabis Dependencea | Model 1: Adjusted for sex and childhood IQ | Model 2: +Adjustment for other substance useb | Model 3: + Adjustment for childhood SES, low childhood self-control, and family history of substance dependencec | ||||||||||||
| Exposure: Persistence of Cannabis Dependence | Never used (n=261) | Used but never diagnosed (N=498) | 1 diagnosis (N=85) | 2 diagnoses (N=39) | 3 diagnoses (N=32) | 4+ diagnoses (N=16) | β | 95% CI | p | β | 95% CI | p | β | 95% CI | p |
| Age-45 Tests | |||||||||||||||
| Rey Total | 0.10 | 0.11 | −0.37 | −0.53 | −0.46 | −0.72 | −0.14 | −0.19, −0.08 | <.001 | −0.12 | −0.19, −0.05 | <.001 | −0.11 | −0.18, −0.04 | .002 |
| Rey Recall | 0.09 | 0.08 | −0.36 | −0.55 | −0.26 | −0.32 | −0.08 | −0.13, −0.02 | .01 | −0.05 | −0.12, 0.02 | .18 | −0.05 | −0.12, 0.03 | .23 |
| WMS | 0.15 | 0.05 | −0.48 | −0.06 | −0.41 | −0.71 | −0.13 | −0.19, −0.07 | <.001 | −0.08 | −0.15, −0.01 | .05 | −0.07 | −0.14, 0.01 | .09 |
| Trails B | 0.07 | 0.05 | −0.32 | −0.20 | −0.05 | −0.43 | −0.07 | −0.12, −0.01 | .02 | −0.06 | −0.13, 0.01 | .11 | −0.06 | −0.13, 0.01 | .11 |
| Animal Naming | −0.01 | 0.02 | −0.01 | 0.01 | −0.12 | −0.12 | 0.00 | −0.06, 0.06 | .99 | −0.01 | −0.08, 0.08 | .98 | 0.00 | −0.08, 0.08 | .99 |
| WMI | 0.02 | 0.05 | −0.23 | −0.11 | −0.08 | −0.24 | −0.08 | −0.14, −0.03 | .002 | −0.05 | −0.12, 0.02 | .13 | −0.05 | −0.11, 0.02 | .17 |
| PRI | 0.03 | 0.07 | −0.29 | −0.27 | −0.13 | −0.28 | −0.11 | −0.16, −0.06 | <.001 | −0.05 | −0.11, 0.02 | .16 | −0.04 | −0.11, 0.02 | .19 |
| VCI | −0.02 | 0.06 | −0.21 | 0.17 | −0.43 | −0.04 | −0.07 | −0.12, −0.02 | .007 | 0.00 | −0.06, 0.06 | .97 | 0.00 | −0.06, 0.06 | .93 |
| PSI | 0.04 | 0.11 | −0.35 | −0.39 | −0.38 | −0.60 | −0.11 | −0.17 −0.06 | <.001 | −0.10 | −0.17, −0.03 | .006 | −0.10 | −0.17, −0.03 | .006 |
| Grooved Pegboard | −0.01 | 0.07 | −0.10 | −0.41 | −0.23 | −0.15 | −0.05 | −0.10, 0.01 | .11 | −0.01 | −0.08, 0.06 | .85 | 0.00 | −0.07, 0.07 | .91 |
| Panel B. Exposure: Persistence of Regular Cannabis Use | Statistical Tests | ||||||||||||||
| Means for Neuropsychological Tests as a Function of Persistence of Regular Cannabis Usea | Model 1: Adjusted for sex and childhood IQ | Model 2: +Adjustment for other substance useb | Model 3: + Adjustment for childhood SES, low childhood self-control, and family history of substance dependencec | ||||||||||||
| Exposure: Persistence of Regular Cannabis Use | Never used (n=261) | Used but never regularly (n=518) | Regularly used 1x (n=57) | Regularly used 2x (n=32) | Regularly used 3x (n=33) | Regularly used 4+x (n=30) | B | 95% CI | p | β | 95% CI | p | β | 95% CI | p |
| Age 45-Tests | |||||||||||||||
| Rey Total | 0.10 | 0.11 | −0.30 | −0.72 | −0.79 | −0.55 | −0.15 | −0.21, −0.10 | <.001 | −0.14 | −0.21, −0.08 | <.001 | −0.13 | −0.20, −0.06 | <.001 |
| Rey Recall | 0.09 | 0.09 | −0.25 | −0.67 | −0.64 | −0.39 | −0.10 | −0.16, −0.04 | <.001 | −0.09 | −0.16, −0.02 | .01 | −0.09 | −0.16, −0.01 | .02 |
| WMS | 0.15 | 0.04 | −0.26 | −0.55 | −0.45 | −0.45 | −0.11 | −0.18, −0.05 | <.001 | −0.06 | −0.13, 0.01 | .11 | −0.05 | −0.12, 0.02 | .18 |
| Trails B | 0.07 | 0.03 | −0.10 | −0.14 | −0.32 | −0.39 | −0.05 | −0.11, 0.01 | .07 | −0.04 | −0.11, 0.03 | .29 | −0.03 | −0.10, 0.03 | .33 |
| Animal Naming | −0.01 | 0.05 | 0.02 | −0.41 | −0.18 | −0.25 | −0.03 | −0.10, 0.03 | .30 | −0.05 | −0.13, 0.02 | .17 | −0.05 | −0.13, 0.02 | .18 |
| WMI | 0.02 | 0.04 | −0.04 | −0.27 | −0.18 | −0.22 | −0.06 | −0.11, −0.01 | .03 | −0.02 | −0.09, 0.04 | .50 | −0.02 | −0.08, 0.05 | .64 |
| PRI | 0.03 | 0.07 | −0.03 | −0.39 | −0.42 | −0.55 | −0.13 | −0.18, −0.08 | <.001 | −0.07 | −0.13, −0.02 | .009 | −0.07 | −0.12, −0.01 | .01 |
| VCI | −0.02 | 0.07 | 0.16 | −0.39 | −0.59 | −0.31 | −0.09 | −0.14, −0.05 | <.001 | −0.05 | −0.11, 0.01 | .10 | −0.04 | −0.10, 0.01 | .13 |
| PSI | 0.04 | 0.09 | −0.16 | −0.64 | −0.40 | −0.57 | −0.11 | −0.16, −0.05 | <.001 | −0.09 | −0.15, −0.02 | .01 | −0.09 | −0.15, −0.02 | .01 |
| Grooved Pegboard | −0.01 | 0.07 | −0.21 | −0.23 | −0.21 | −0.32 | −0.05 | −0.10, 0.01 | .12 | −0.01 | −0.08, 0.06 | .81 | 0.00 | −0.07, 0.06 | .91 |
Note.
Means represent unadjusted test scores that were standardized (M=0, SD=1) on the full sample prior to analyses. Lower scores indicate poorer test performance.
Statistical tests were adjusted for sex, childhood IQ, and persistent use of tobacco, alcohol, and other illicit drugs.
Statistical tests were adjusted for sex; childhood IQ; persistent use of tobacco, alcohol, and other illicit drugs; low childhood SES; low childhood self-control; and family history of substance dependence. Beta coefficients represent standardized estimates. Bolded estimates are statistically significant (p<.05). Rey Total=Rey Auditory Verbal Learning Test total score (learning). Rey Recall = Rey Auditory Verbal Learning Test delayed recall (memory). WMS=Wechsler Memory Scale Months Backwards test. WMI=Working Memory Index. PRI=Perceptual Reasoning Index. VCI=Verbal Comprehension Index. PSI = Processing Speed Index.
Associations between persistent cannabis use and cognitive functioning could not be explained by recent cannabis use (Table S4).
Cannabis and Informant-Reported Cognitive Problems
Long-term Cannabis Users and Five Comparison Groups.
Long-term cannabis users showed significantly more informant-reported memory and attention problems at age-45 years than all groups except long-term tobacco users and cannabis quitters (Table 4, Panel A).
Table 4. Panel A.
Informant-reported memory and attention problems: Group comparisons. A comparison of long-term cannabis users and 5 informative subgroups on informant-reported memory and attention problems at age 45 years.
| Statistical Tests of Difference Between Long-term Cannabis Users and Comparison Groups | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Informant Report | Long-term Cannabis Users (N=74) | Comparison Group 1: Cannabis Non-Users (N=199) | Comparison Group 2: Long-term Tobacco Users (N=69) | Comparison Group 3: Long-term Alcohol Users (N=56) | Comparison Group 4: Midlife Recreational Cannabis Users (N=74) | Comparison Group 5: Cannabis Quitters (N=54) | LT vs 1 | LT vs 2 | LT vs 3 | LT vs 4 | LT vs 5 | ||||||
| M | 95% CI | M | 95% CI | M | 95% CI | M | 95% CI | M | 95% CI | M | 95% CI | p | p | p | p | p | |
| 0.53 | 0.20, 0.86 | −0.19 | −0.31, −0.08 | 0.19 | −0.13, 0.51 | 0.05 | −0.16, 0.26 | 0.03 | −0.20, 0.26 | 0.18 | −0.11, 0.47 | <.001 | 0.21 | .03 | .02 | .15 | |
Note. Means represent unadjusted informant-reported memory and attention scores that were standardized on the full sample (M=0, SD=1) prior to analyses. Higher scores indicate worse memory and attention problems. Statistical tests of group comparisons are adjusted for sex. Bolded p-values indicate a statistically significant difference (p<.05) compared with long-term cannabis users. LT=Long-term cannabis users.
Dose-response Associations.
Participants who used cannabis more persistently had more memory and attention problems than less persistent users, according to informants, even after covariate adjustment (Table 4, Panel B).
Table 4. Panel B.
Informant-reported memory and attention problems: Dose-response associations. Dose-response associations between persistence of cannabis use from age 18–45 and age-45 informant-reported memory and attention problems.
| Panel B1. Exposure: Persistence of Cannabis Dependence | Statistical Tests | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Means for Informant-Reported Memory and Attention Problems as a Function of Persistence of Cannabis Dependencea | Model 1: Adjusted for sex | Model 2: +Adjustment for other substance useb | Model 3: + Adjustment for childhood SES, low childhood self-control, and family history of substance dependencec | ||||||||||||
| Exposure: Persistence of Cannabis Dependence | Never used (n=249) | Used but never diagnosed (N=487) | 1 diagnosis (N=73) | 2 diagnoses (N=31) | 3 diagnoses (N=28) | 4+ diagnoses (N=14) | B | 95% CI | p | β | 95% CI | p | β | 95% CI | p |
| Informant Report | |||||||||||||||
| Attention | −0.13 | −0.09 | 0.36 | 0.85 | 0.69 | 0.21 | 0.20 | 0.13, 0.27 | <.001 | 0.16 | 0.07, 0.24 | <.001 | 0.15 | 0.07, 0.23 | <.001 |
| Panel B2. Exposure: Persistence of Regular Cannabis Use | Statistical Tests | ||||||||||||||
| Means for Informant-Reported Memory and Attention Problems as a Function of Persistence of Regular Cannabis Usea | Model 1: Adjusted for sex | Model 2: +Adjustment for other substance useb | Model 3: + Adjustment for childhood SES, low childhood self-control, and family history of substance dependencec | ||||||||||||
| Exposure: Persistence of Regular Cannabis Use | Never used (n=249) | Used but never regularly (n=503) | Regularly used 1x (n=48) | Regularly used 2x (n=30) | Regularly used 3x (n=29) | Regularly used 4+x (n=23) | B | 95% CI | p | β | 95% CI | p | β | 95% CI | p |
| Informant Report | |||||||||||||||
| Attention | −0.13 | −0.06 | 0.30 | 0.67 | 0.48 | 0.60 | 0.19 | 0.12, 0.26 | <.001 | 0.13 | 0.05, 0.21 | .002 | 0.11 | 0.03, 0.19 | .006 |
Note.
Means represent unadjusted informant-reported memory and attention scores that were standardized (M=0, SD=1) on the full sample prior to analyses. Higher scores indicate worse memory and attention problems.
Statistical tests were adjusted for sex and persistent use of tobacco, alcohol, and other illicit drugs.
Statistical tests were adjusted for sex; persistent use of tobacco, alcohol, and other illicit drugs; low childhood SES; low childhood self-control; and family history of substance dependence. Beta coefficients represent standardized estimates. Bolded estimates are statistically significant (p<.05).
Cannabis and Hippocampal Volume
Long-term Cannabis Users and Five Comparison Groups.
Long-term cannabis users showed significantly smaller volume than cannabis non-users in bilateral total hippocampus and 5 of 12 subfields (tail, HATA, CA1, molecular layer, dentate gyrus), and significantly smaller volume than midlife recreational cannabis users in bilateral hippocampus and 3 of 12 subfields (tail, CA1, and molecular layer) (Figure 1B, Table S5). Long-term cannabis users generally did not show significantly smaller volume in bilateral total hippocampus or hippocampal subfields than long-term tobacco users, long-term alcohol users, or cannabis quitters.
Figure 1B.
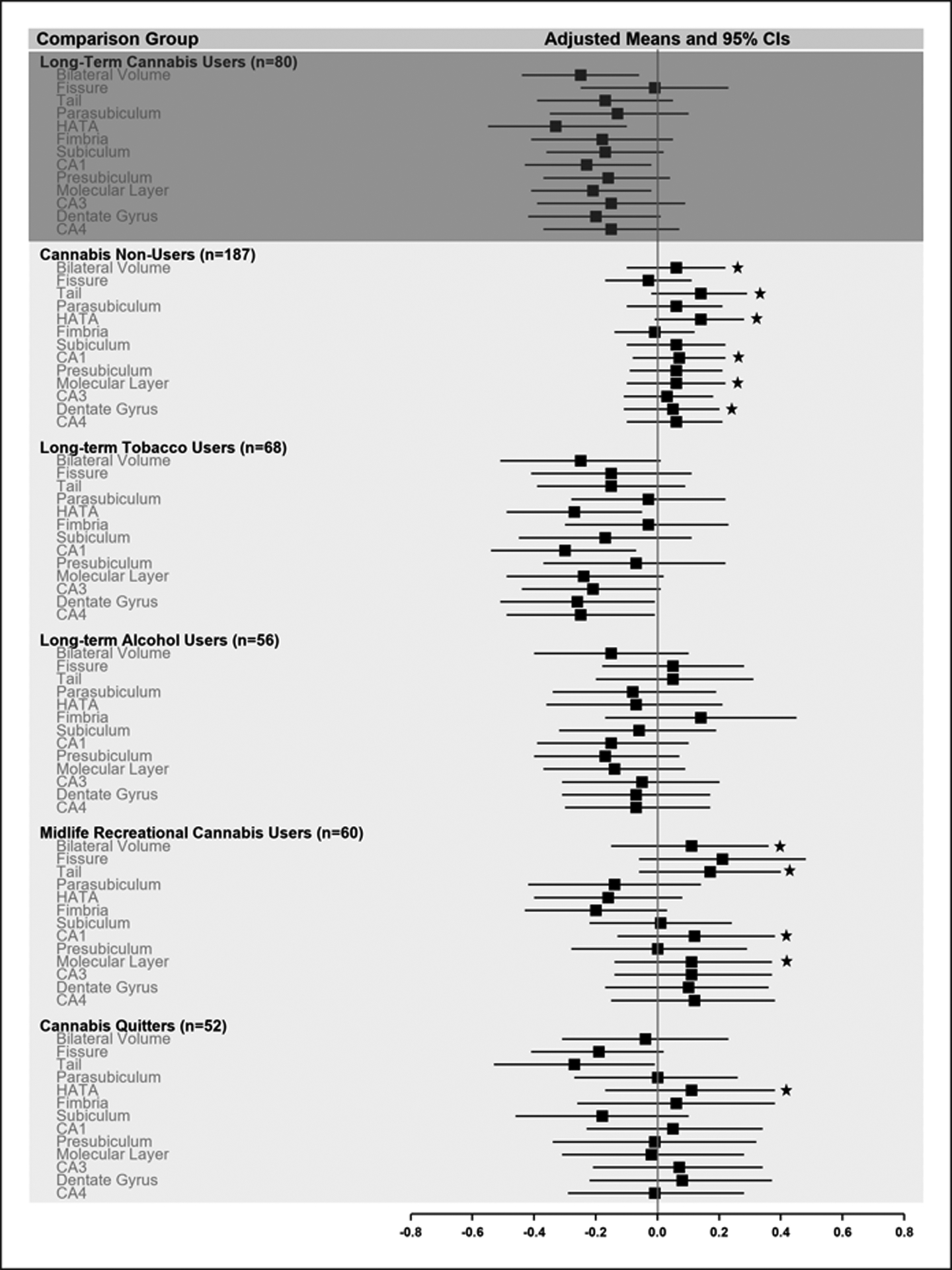
Long-Term Cannabis Use and Hippocampal Volume. A comparison of long-term cannabis users with 5 informative subgroups on age-45 hippocampal volume. This figure shows means (and 95% CIs) on age-45 hippocampal volume, which were adjusted for sex and standardized on the full cohort (M=0, SD=1). Average normative volume is indicated by the reference line at the representative cohort mean of 0. Estimates below zero indicate smaller than average volume. Stars indicate mean volumes that were statistically significantly larger (p<.05) as compared with long-term cannabis users, after adjustment for sex. CA1-CA4=cornu ammonis 1–4. HATA=hippocampal amygdala transition area.
Dose-response Associations.
Participants who used cannabis more persistently had smaller volume than less persistent users in bilateral hippocampus and numerous hippocampal subfields, after adjusting for sex. Most associations were non-significant after additional covariate adjustment (Table S6).
Does Hippocampal Volume Mediate Associations Between Persistence of Cannabis Use and Cognitive Deficits?
Persistence of cannabis use was associated with cognitive deficits and, to a lesser extent, smaller hippocampal volume. Larger hippocampal volume was related to better cognitive test performance (Table S7). However, smaller hippocampal volume did not statistically mediate associations between persistence of cannabis use and cognitive deficits (Table S8).
Robustness to Unmeasured Confounding
To ascertain the robustness of associations to unmeasured confounding, we computed E-values for dose-response associations that were statistically significant after covariate adjustment (Table S9) (38). E-values estimate how large a relative risk ratio would need to be between an unmeasured confounder and both persistence of cannabis use and outcomes to fully account for observed associations. E-values ranged from 1.33–1.56, which represent the risk ratios needed for unmeasured confounders after adjustment for measured confounders.
Discussion
This prospective study followed a population-representative birth cohort for five decades, generating a unique evidence base for evaluating whether long-term cannabis users show cognitive deficits and smaller hippocampal volume in midlife. The longitudinal design enabled a comparison of a person’s midlife cognitive abilities to their childhood cognitive abilities before cannabis initiation. The study also enabled a test of the role of hippocampal gray matter volume in mediating associations between long-term cannabis use and cognitive deficits. Six findings stand out.
First, long-term cannabis users exhibited IQ decline and poorer learning and processing speed in midlife relative to their childhood IQ. People who knew them well described them as having memory and attention problems. These associations were not explained by prospectively-assessed persistent tobacco, alcohol, and other illicit drug dependence or by childhood SES, low childhood self-control, and family substance dependence history. Associations were also not explained by recent cannabis use. Findings were consistent across two cannabis exposures (persistence of cannabis dependence, persistence of regular use), and in tests comparing long-term cannabis users to five groups (cannabis non-users, tobacco users, alcohol users, recreational cannabis users, and cannabis quitters). (Table S10 summarizes findings across tests of dose-response associations and group comparisons.) This suggests that cannabis-related IQ decline, poorer learning and processing speed, and informant-reported memory and attention problems are not artifacts of analytic approach, or of measured confounders, but rather are more likely to be consequences of long-term use. Cognitive child-to-adult changes such as we observed have been shown to predict steeper cognitive decline from age 70–82, and do so better than adult cognitive level (39).
Second, long-term cannabis users showed significantly larger IQ decline, poorer learning and memory, and poorer processing speed than long-term tobacco or alcohol users. Thus, some cognitive deficits were more pronounced for long-term cannabis users than for long-term tobacco or alcohol users, contrary to some claims (21,40).
Third, cognitive functioning among midlife recreational cannabis users was similar to representative cohort norms. This suggests that infrequent, non-problem recreational cannabis use in midlife is unlikely to compromise cognitive functioning. Results highlight the importance of not conflating long-term and recreational cannabis users in future studies.
Fourth, cannabis quitters showed subtle cognitive deficits that may explain inconsistent findings on the benefits of cessation (11,14,41–45).
Fifth, long-term cannabis users showed smaller volume in bilateral total hippocampus and 5 of 12 structurally and functionally distinct subregions compared with non-users, consistent with case-control studies (2).
Sixth, although persistence of cannabis use showed dose-response associations with cognitive deficits and smaller hippocampal volume in the representative sample, smaller hippocampal volume did not statistically mediate associations between persistence of cannabis use and cognitive deficits. Smaller hippocampal volume has been suggested as a possible mediator of cannabis-related cognitive deficits (24), because the hippocampus is rich in type-1 cannabinoid (CB1) receptors and is involved in learning and memory. However, smaller hippocampal volume may be a reductionistic explanation for cannabis-related cognitive deficits. For example, in addition to the hippocampus, other CB1-rich brain regions, including those involved in reward and motivation, may play a role (2). Further, neurobiological mechanisms likely extend beyond gray matter volume differences to include differences in structural and functional connectivity (46). Finally, social mechanisms could also play a role.
Our findings conflict with some studies (including one by us) that compared the cognitive functioning of twins who were discordant for cannabis use and found little evidence of cannabis-related cognitive deficits (47–50). Discordant twin comparisons represent a compelling approach to controlling for shared genetics and family background. However, a limitation is that the size of the differences between twins in cannabis use and in cognitive functioning is much smaller than between unrelated individuals. As such, it is unclear whether associations that are attenuated in twin-difference comparisons, relative to comparisons between unrelated individuals, are an indication of true confounding or are an artifact of reduced statistical power.
In the present study, we tackled confounding by incorporating the most notable confounding variables identified in the literature, including childhood SES, low self-control, low childhood IQ, family substance dependence history, and persistent dependence on other substances, using unusually strong measures derived from multiple waves and data sources. These obvious confounders, considered together, could not account for many of the observed associations. We also reported E-values, with larger E-values indicating that considerable unmeasured confounding would be needed to explain associations. E-values ranged from 1.33–1.56. These E-values represent the risk ratios needed after adjustment for measured confounders, raising the bar for unmeasured confounding to play a role.
This study has limitations. First, cannabis use was self-reported. Under-reporting for fear of admitting to illegal drug use is unlikely because participants were interviewed repeatedly over a lifetime and learned to trust the confidentiality guarantee. Second, some group sizes were small, raising concerns about low statistical power. These concerns were minimized through powerful tests of dose-response associations and through transparent reporting of effect sizes in a representative cohort. Third, long-term cannabis users also use tobacco, alcohol, and other illicit drugs. Disentangling cannabis effects from other substances is challenging. We did not limit analyses to cannabis-only users because they are unrepresentative of cannabis users (51). Instead, we used two complementary approaches: (i) we reported no midlife cognitive deficits for long-term tobacco and alcohol users, groups who showed polysubstance use, like long-term cannabis users, but were free from cannabis, and (ii) we controlled for persistent dependence on tobacco, alcohol, and other illicit drugs in analyses of dose-response associations and found that a number of associations were robust to covariate control. Collectively, findings suggest that use of other substances cannot fully account for cognitive deficits observed in long-term cannabis users.
Fourth, we focused on hippocampal volume as a key MRI outcome based on theory and prior research (2). Elsewhere, we report results of exploratory analyses of associations between long-term cannabis use and comprehensive MRI measures of global and regional gray and white matter (52). Fifth, results are based on a single birth cohort who began using cannabis in the 1980s-1990s. The concentration of THC, the psychoactive constituent of cannabis, has risen in recent years (53). Therefore, if THC exposure underlies associations, we may have underestimated effect sizes in contemporary users. Finally, observational studies cannot conclusively demonstrate causality.
This study has implications. First, long-term cannabis use is robustly associated with cognitive deficits in midlife. These may be consequential given that mild cognitive deficits in midlife are a risk factor for dementia (8). The deficits we observed are comparable to midlife cognitive deficits of individuals who developed dementia in the Atherosclerosis Risk in Communities Study (ARIC) (8). Older adults who developed dementia showed midlife cognitive deficits that ranged from 0.32 to 0.53 standard deviations below the cohort mean on tests of memory, processing speed, and word fluency (8). Second, research is needed to ascertain whether long-term cannabis users show elevated rates of dementia in later life. This is important given the huge burden of dementia, and is timely given the confluence of two trends: the growth of the aging population, and the record high rates of cannabis use among today’s older adults.
Supplementary Material
Acknowledgments:
This work was supported by NIA grants R01AG069939, R01AG049789 and R01AG032282, and U.K. Medical Research Council grant MR/P005918. The Dunedin Multidisciplinary Health and Development Research Unit is supported by the New Zealand Health Research Council and the New Zealand Ministry of Business, Innovation and Employment (MBIE). We thank members of the Advisory Board for the Dunedin Neuroimaging Study, Dunedin Study members, unit research staff, and Dunedin Study founder Phil Silva. The authors have no conflicts of interest to report.
References
- 1.Kroon E, Kuhns L, Hoch E, Cousijn J. Heavy cannabis use, dependence and the brain: a clinical perspective. Addiction 2020; 115(3):559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lorenzetti V, Chye Y, Silva P, Solowij N, Roberts CA. Does regular cannabis use affect neuroanatomy? An updated systematic review and meta-analysis of structural neuroimaging studies. Eur Arch Psy Clin N 2019; 269(1):59–71. [DOI] [PubMed] [Google Scholar]
- 3.McKetin R, Parasu P, Cherbuin N, Eramudugolla R, Anstey KJ. A longitudinal examination of the relationship between cannabis use and cognitive function in mid-life adults. Drug Alcohol Depen 2016; 169:134–40. [DOI] [PubMed] [Google Scholar]
- 4.Scott EP, Brennan E, Benitez A. A systematic review of the neurocognitive effects of cannabis use in older adults. Current Addiction Reports 2019;6(4):443–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morin J-FG, Afzali MH, Bourque J, Stewart SH, Séguin JR, O’Leary-Barrett M, et al. A population-based analysis of the relationship between substance use and adolescent cognitive development. Am J Psychiat 2019; 176(2):98–106. [DOI] [PubMed] [Google Scholar]
- 6.Han BH, Palamar JJ. Marijuana use by middle-aged and older adults in the United States, 2015–2016. Drug Alcohol Depem 2018; 191:374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han BH, Palamar JJ. Trends in cannabis use among older adults in the United States, 2015–2018. JAMA Intern Med 2020; 180(4):609–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knopman DS, Gottesman RF, Sharrett AR, Tapia AL, DavisThomas S, Windham BG, et al. Midlife vascular risk factors and midlife cognitive status in relation to prevalence of mild cognitive impairment and dementia in later life: The Atherosclerosis Risk in Communities Study. Alzheimer’s & Dementia 2018; 14(11):1406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Research Reviews 2004;3(4):369–82. [DOI] [PubMed] [Google Scholar]
- 10.Auer R, Vittinghoff E, Yaffe K, Künzi A, Kertesz SG, Levine DA, et al. Association between lifetime marijuana use and cognitive function in middle age: the Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA Intern Med 2016;176(3):352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA 2012;109(40):E2657–E64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dregan A, Gulliford MC. Is illicit drug use harmful to cognitive functioning in the midadult years? A cohort-based investigation. Am J Epidemiol 2012;175(3):218–27. [DOI] [PubMed] [Google Scholar]
- 13.Thayer RE, YorkWilliams SL, Hutchison KE, Bryan AD. Preliminary results from a pilot study examining brain structure in older adult cannabis users and nonusers. Psychiatry Research: Neuroimaging 2019;285:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burggren AC, Siddarth P, Mahmood Z, London ED, Harrison TM, Merrill DA, et al. Subregional hippocampal thickness abnormalities in older adults with a history of heavy cannabis use. Cannabis and Cannabinoid Research 2018;3(1):242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyons MJ, Bar J, Panizzon M, Toomey R, Eisen S, Xian H, et al. Neuropsychological consequences of regular marijuana use: a twin study. Psychol Med 2004;34(7):1239–50. [DOI] [PubMed] [Google Scholar]
- 16.Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depen 2003;69(3):303–10. [DOI] [PubMed] [Google Scholar]
- 17.Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, et al. Cognitive functioning of long-term heavy cannabis users seeking treatment. J Am Med Assoc 2002;287(9):1123–31. [DOI] [PubMed] [Google Scholar]
- 18.Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry 2001;58(10):909–15. [DOI] [PubMed] [Google Scholar]
- 19.Fletcher JM, Page JB, Francis DJ, Copeland K, Naus MJ, Davis CM, et al. Cognitive correlates of long-term cannabis use in Costa Rican men. Arch Gen Psychiatry 1996;53(11):1051–7. [DOI] [PubMed] [Google Scholar]
- 20.Leung J, Chan GC, Hides L, Hall WD. What is the prevalence and risk of cannabis use disorders among people who use cannabis? A systematic review and meta-analysis. Addict Behav 2020:106479. [DOI] [PubMed] [Google Scholar]
- 21.Hall W, Lynskey M. Assessing the public health impacts of legalizing recreational cannabis use: the US experience. World Psychiatry 2020;19(2):179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ashtari M, Avants B, Cyckowski L, Cervellione KL, Roofeh D, Cook P, et al. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res 2011;45(8):1055–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubino T, Realini N, Braida D, Guidi S, Capurro V, Viganò D, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus 2009;19(8):763–72. [DOI] [PubMed] [Google Scholar]
- 24.Paul S, Bhattacharyya S. Cannabis use-related working memory deficit mediated by lower left hippocampal volume. Addict Biol 2020:e12984. [DOI] [PubMed] [Google Scholar]
- 25.Poulton R, Moffitt TE, Silva PA. The Dunedin Multidisciplinary Health and Development Study: Overview of the first 40 years, with an eye to the future. Soc Psychiatry Psychiatr Epidemiol 2015;50(5):679–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Richmond-Rakerd LS, D’Souza S, Andersen SH, Hogan S, Houts RM, Poulton R, et al. Clustering of health, crime and social-welfare inequality in 4 million citizens from two nations. Nature Human Behaviour 2020;4(3):255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule: Its history, characteristics, and validity. Arch Gen Psychiatry 1981;38(4):381–9. [DOI] [PubMed] [Google Scholar]
- 28.Robins LN, Cottler L, Bucholz KK, Compton W. Diagnostic Interview Schedule for DSM-IV. St Louis, MO: Washington University School of Medicine; 1995. [Google Scholar]
- 29.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. Third Edition, Revised. Washington, DC: American Psychiatric Association; 1987. [Google Scholar]
- 30.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 31.Wechsler D Manual for the Wechsler Intelligence Scale for Children—Revised. New York: Psychological Corporation; 1974. [Google Scholar]
- 32.Wechsler D Wechsler Adult Intelligence Scale -- Fourth Edition. San Antonio, TX: Pearson Assessment; 2008. [Google Scholar]
- 33.Lezak MD. Neuropsychological Assessment - Fourth Edition. New York, New York: Oxford University Press; 2004. [Google Scholar]
- 34.Wechsler D Wechsler Memory Scale -- Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 35.Army Individual Test Battery. Manual & directions for scoring. Washington, DC: War Department, Adjutant General’s Office; 1944 [Google Scholar]
- 36.Sager MA, Hermann BP, La Rue A, Woodard JL. Screening for dementia in community-based memory clinics. WMJ: official publication of the State Medical Society of Wisconsin 2006;105(7):25–9. [PubMed] [Google Scholar]
- 37.Van der Meer D, Rokicki J, Kaufmann T, Córdova-Palomera A, Moberget T, Alnæs D, et al. Brain scans from 21,297 individuals reveal the genetic architecture of hippocampal subfield volumes. Mol Psychiatr 2020;25(11):3053–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-value to assess the potential effect of unmeasured confounding in observational studies. J Am Med Assoc 2019;321(6):602–3. [DOI] [PubMed] [Google Scholar]
- 39.Conte F, Okely J, Hamilton O, Corley J, Page D, Redmond P, et al. Cognitive change before old age (11 to 70) predicts cognitive change after old age (70 to 82). PsyArXiv. April 8. doi: 10.31234/osf.io/h8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bourque J, Potvin S. Cannabis and cognitive functioning: From acute to residual effects, from randomized controlled trials to prospective designs. Front Psychiatry 2021;12:919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schreiner AM, Dunn ME. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-snalysis. Exp Clin Psychopharm 2012;20(5):420–9. [DOI] [PubMed] [Google Scholar]
- 42.Scott JC, Slomiak ST, Jones JD, Rosen AF, Moore TM, Gur RC. Association of Cannabis With Cognitive Functioning in Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Psychiat 2018;75(6):585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lovell ME, Akhurst J, Padgett C, Garry MI, Matthews A. Cognitive outcomes associated with long-term, regular, recreational cannabis use in adults: A meta-analysis. Exp Clin Psychopharm 2020;28(4):471. [DOI] [PubMed] [Google Scholar]
- 44.Wallace AL, Wade NE, Lisdahl KM. Impact of two-weeks of monitored abstinence on cognition in adolescent and young adult cannabis users. J Int Neuropsychol Soc 2020;26(8):776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roten A, Baker NL, Gray KM. Cognitive performance in a placebo-controlled pharmacotherapy trial for youth with marijuana dependence. Addict Behav 2015;45:119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bloomfield MA, Hindocha C, Green SF, Wall MB, Lees R, Petrilli K, et al. The neuropsychopharmacology of cannabis: A review of human imaging studies. Pharmacol Therapeut 2019;195:132–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jackson NJ, Isen JD, Khoddam R, Irons D, Tuvblad C, Iacono WG, et al. Impact of adolescent marijuana use on intelligence: results from two longitudinal twin studies. Proc Natl Acad Sci USA 2016;113(5):E500–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meier MH, Caspi A, Danese A, Fisher HL, Houts R, Arseneault L, et al. Associations between adolescent cannabis use and neuropsychological decline: a longitudinal co-twin control study. Addiction 2018;113(2):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ross JM, Ellingson JM, Rhee SH, Hewitt JK, Corley RP, Lessem JM, et al. Investigating the causal effect of cannabis use on cognitive function with a quasi-experimental co-twin design. Drug Alcohol Depen 2020;206:107712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaefer JD, Hamdi NR, Malone SM, Vrieze S, Wilson S, McGue M, et al. Associations between adolescent cannabis use and young-adult functioning in three longitudinal twin studies. Proc Natl Acad Sci USA 2021;118(14): E2013180118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosen AS, Sodos LM, Hirst RB, Vaughn D, Lorkiewicz SA. Cream of the crop: Clinical representativeness of eligible and ineligible cannabis users in research. Subst Use Misuse 2018;53(12):1937–50. [DOI] [PubMed] [Google Scholar]
- 52.Knodt A, Hariri A, Moffitt TE, Meier MH, Harrington HL, Hogan S, Houts R, Caspi A. A portrait of the brains of long-term cannabis users in midlife. Under review. [Google Scholar]
- 53.ElSohly MA, Chandra S, Radwan M, Gon C, Church JC. A comprehensive review of Cannabis potency in the USA in the last decade. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2021;6(6):603–606. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


