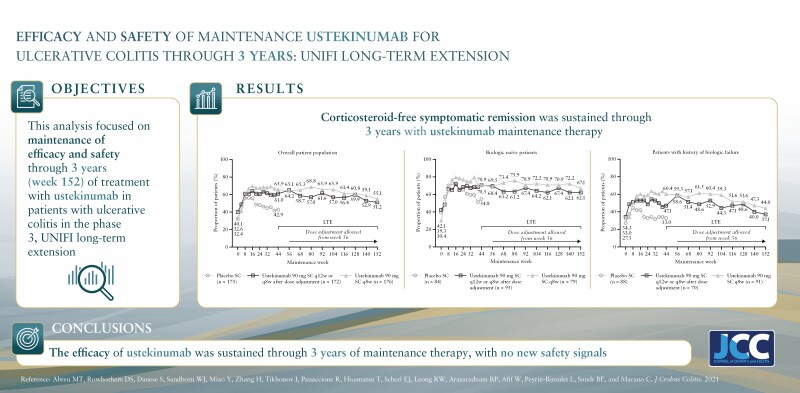
Abstract
Background and Aims
The UNIFI long-term extension [LTE] study reports the efficacy and safety of subcutaneous 90 mg ustekinumab through 3 years of maintenance therapy.
Methods
Patients randomised to ustekinumab every 12 weeks [q12w] or every 8 weeks [q8w] at maintenance baseline [N = 348] and randomised ustekinumab-treated patients in the LTE [N = 284] were evaluated. Symptomatic remission [Mayo stool frequency = 0/1, rectal bleeding = 0] was assessed. Safety included all LTE patients [N = 188 placebo and N = 457 ustekinumab].
Results
Among patients randomised to the ustekinumab q12w and q8w groups at maintenance baseline, 54.1% and 56.3% achieved symptomatic remission at Week 152, respectively. Overall, 20% of patients discontinued ustekinumab, 10% of biologic-naïve and 30% of biologic-exposed patients. Among patients in symptomatic remission at Year 3, 94.6% and 98.0% of patients were also corticosteroid free, respectively. Corticosteroid-free symptomatic remission rates in the ustekinumab q12w and q8w groups were 51.2% and 55.1% at Week 152, respectively. Remission rates were higher for biologic-naïve patients than for those with a history of biologic failure. Biochemical evidence of response was demonstrated by stable, decreased C-reactive protein and faecal calprotectin measurements over 3 years. From Weeks 96 to 156, no deaths, major adverse cardiovascular events, or tuberculosis occurred. Nasopharyngitis, ulcerative colitis, and upper respiratory tract infection were most frequently reported. One ustekinumab-treated patient with a history of basal cell carcinoma [BCC] reported two BCCs. One patient in the q8w ustekinumab group, who was receiving concomitant 6-mercaptopurine, experienced serious adverse events of neutropenic sepsis and oral herpes.
Conclusions
Efficacy of ustekinumab in patients with ulcerative colitis was confirmed through 3 years. No new safety signals were observed.
Keywords: Ustekinumab, ulcerative colitis, symptomatic remission
Graphical Abstract
1. Introduction
Ulcerative colitis [UC] is a chronic inflammatory bowel disease [IBD] that generally requires long-term medical therapy to control symptoms and prevent disease-related complications.1 The introduction of inhibitors of tumour necrosis factor-alpha [TNF-α],2–5 interleukin [IL]-12/23,6 α4β7 integrin,7 and the Janus kinase pathway8 has improved the ability to control UC inflammation and patient symptoms. Given the need for long-term treatment in UC, the durability of response to biologic therapy is a critical clinical question regarding these agents.
Ustekinumab has been shown to be safe and effective for maintaining remission through 2 years of maintenance therapy after an intravenous [IV] induction infusion followed by subcutaneous [SC] dosing.9 An ongoing ustekinumab clinical treatment programme for patients with UC included an initial 8-week induction study followed by a maintenance study through Week 44. Both randomised, double-blind, placebo-controlled studies were conducted under one protocol [UNIFI]. The long-term extension [LTE] of UNIFI evaluates the efficacy and safety of continued ustekinumab SC 90 mg through 3 years of follow-up. The efficacy results for the UNIFI study through Week 152 and safety results through Week 156, presented here, provide additional data on the long-term effects of ustekinumab, highlighting the sustained clinical benefit of ustekinumab through 3 years.
2. Methods
2.1.Study design
The ustekinumab programme included two randomised, double-blind, placebo-controlled studies: an 8-week induction study and a maintenance study through week 44 [UNIFI]. Detailed study design and efficacy results from the induction and maintenance studies6 and LTE through Week 969 have been published previously.
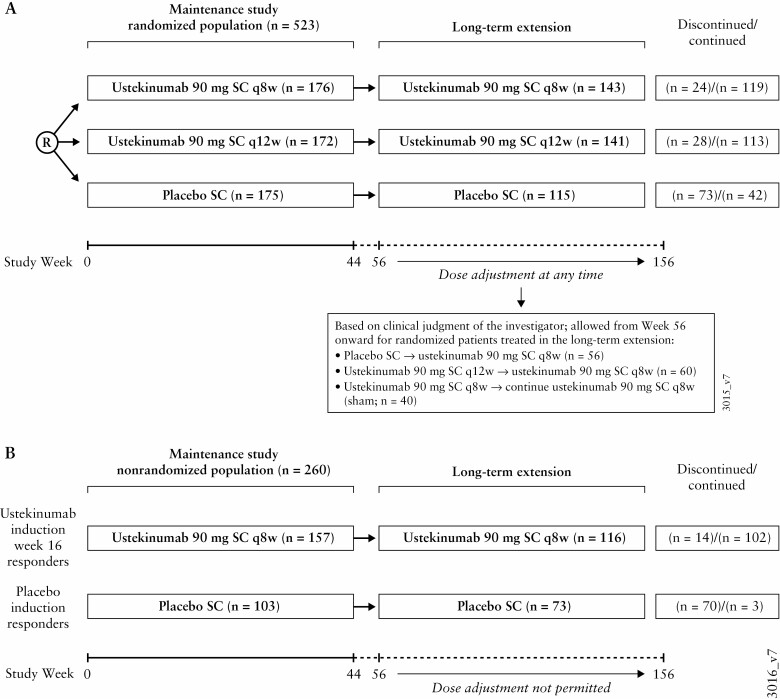
All patients completing treatment through Week 44 of UNIFI could enter the LTE and receive the same treatment they were receiving at Week 44 (SC placebo, ustekinumab 90 mg every 8 weeks [q8w], or every 12 weeks [q12w]) [Figure 1A]. Study unblinding occurred after the Week 44 analyses were completed. After unblinding, ustekinumab-treated patients continued in the LTE, whereas patients remaining on placebo were discontinued. Patients whose UC disease activity worsened [in the clinical opinion of the investigator] were eligible for a single dose adjustment after Week 56 as follows: placebo SC to ustekinumab 90 mg SC q8w [prior to unblinding]; ustekinumab 90 mg SC q12w to ustekinumab 90 mg SC q8w; ustekinumab 90 mg SC q8w continued on ustekinumab 90 mg SC q8w [sham dose adjustment]. Efficacy assessments were conducted every 12 weeks until unblinding and then q8w or q12w at dosing visits.
Figure 1.
Study flow. a. Patients who were assigned to placebo SC during the maintenance study were discontinued after study unblinding, which occurred after analysis of the maintenance study. R, patients who responded 8 weeks after ustekinumab intravenous induction were re-randomised in the maintenance study; SC, subcutaneous; q8w, every 8 weeks; q12w, every 12 weeks.
2.2.Efficacy endpoints
From Weeks 44 through 152, partial Mayo scores were collected every 12 weeks and at each dosing visit after unblinding. Symptomatic remission, defined as a stool frequency subscore of 0 or 1 and a rectal bleeding subscore of 0, was evaluated every 12 weeks. Corticosteroid use was assessed throughout the study, including the LTE. Patients who were in symptomatic remission and not receiving corticosteroids [including beclomethasone dipropionate and budesonide] over time were analysed; corticosteroid dose [excluding beclomethasone dipropionate and budesonide] over time is also reported. Inflammatory biomarker samples (serum C-reactive protein [CRP], faecal calprotectin) were collected every 3 months.
Disease-specific health-related quality of life was evaluated using the Inflammatory Bowel Disease Questionnaire [IBDQ]10 and completed every 6 months. Patients with an IBDQ score ≥170 points were considered to be in IBDQ remission.11
2.3.Safety
Safety (adverse events [AEs], serious AEs [SAEs], and laboratory assessments) was evaluated through Week 156.
2.4.Immunogenicity
Serum blood samples for immunogenicity assessments were collected every 6 months. A validated drug-tolerant electrochemiluminescent immunoassay on the MesoScale Discovery platform was used to evaluate antibodies to ustekinumab. This assay can detect anti-drug antibodies [ADAs] in the presence of up to 100 mg/mL of ustekinumab in the sample. Patients were classified as positive if antibodies were detected in their serum sample at any time.
2.5.Statistical analysis
The LTE efficacy analysis populations are: [1] intent-to-treat population of all patients randomised to ustekinumab q12w [N = 172] or q8w [N = 176] at maintenance Week 0 (placebo [N = 175] is shown for reference); and [2] randomised patients treated with ustekinumab in the LTE: q12w [N = 141] or q8w [N = 143]. Three distinct methods were used for analyses of symptomatic remission: [1] an analysis applying nonresponder imputation for patients who met treatment failure criteria or had missing data; [2] an observed case analysis of patients with available data applying nonresponder imputation for patients who met treatment failure criteria; and [3] an observed case analysis of patients with available data without applying nonresponder imputation for treatment failure. Supplementary Table S1 provides a detailed description of analysis populations and methods, including treatment failure criteria and handling of missing data. An additional analysis of randomised patients who were treated in the LTE and dose adjusted before unblinding was also conducted.
For continuous endpoints of CRP concentrations, faecal calprotectin concentrations, and prednisone-equivalent corticosteroid dose, last observation carried forward was used for missing data, and induction baseline observation was carried forward from the time of first treatment failure onward.
Because patients receiving placebo discontinued the study after unblinding of investigative sites [after the last patient completed Week 44 and database lock and subsequent analyses were completed], direct comparisons and/or statistical comparisons of efficacy results between placebo and ustekinumab treatment groups were not done.
Demographic and baseline disease characteristics, efficacy, and safety analyses included all patients treated with at least one administration of study agent during the LTE. Descriptive statistics [e.g. mean, median, standard deviation, interquartile range, minimum, and maximum] were used to summarise continuous variables. Counts and percentages were used to summarise categorical variables. Immunogenicity was summarised for all patients who were treated in the LTE, received at least one dose of ustekinumab [either in the induction or maintenance study], and had appropriate samples for detection of antibodies to ustekinumab [i.e., patients with at least one sample obtained after their first dose of study drug]. Patients were considered positive if antibodies were detected at any time point. Safety was evaluated by calculating the number of AEs, SAEs, infections, serious infections, AEs leading to discontinuation of study agent, and malignancies, per 100 patient-years [PY] of follow-up among all patients who were treated in the LTE. Event rates per 100 PY for the events in ustekinumab-treated patients were summarised for the maintenance study [first year of the study; Weeks 0 through 44], the second year [LTE Weeks 44 through 96], and the third year [LTE Weeks 96 through 156].
3. Results
3.1.Patient demographics and baseline characteristics
Among 399 patients randomised at maintenance baseline and treated in the LTE, clinical disease characteristics at LTE baseline [maintenance Week 44] for patients in the ustekinumab q12w and q8w groups, respectively were: patients in clinical remission [i.e., Mayo score ≤2 points, with no individual subscore >1], 46.1% and 52.4%; patients with endoscopic healing [Mayo endoscopy subscore 0/1], 56.7% and 61.5%; median Mayo score: 2.0 for both groups; median IBDQ score, 193.0 and 194.0; median CRP concentration, 1.47 mg/L and 1.41 mg/L; and median faecal calprotectin concentration, 118.00 mg/kg and 158.00 mg/kg.9
Overall, 44.1% [176/399] of patients had a history of biologic failure: 32.1% [128/399] to only anti-TNF-α treatment, 12.0% [48/399] in combination with vedolizumab [regardless of anti-TNF-α], and 11.8% [47/399] to a TNF-α antagonist and vedolizumab. Overall, 53.1% [212/399] were biologic-naïve; 2.8% [11/399] biologic-experienced patients without a documented history of biologic failure were excluded from efficacy assessments because of the limited number of these patients. During LTE, concomitant medications were administered at the discretion of the investigator, as was the decision to taper corticosteroids.9
Approximately 80% of ustekinumab-treated and 35% of placebo-treated patients continued treatment through Week 156. In the placebo group, 55/115 [47.8%] patients were discontinued after study unblinding. Discontinuation rates were 21.3% [30/141] and 18.9% [27/143] in the q12w and q8w groups, respectively. The discontinuation rate for patients with a history of biologic failure [31.5%; 39/124] was numerically greater than for biologic-naïve patients [10.7%; 16/149] with reasons primarily related to lack of efficacy or UC worsening [Table 1].
Table 1.
Study agent discontinuation before Week 156; patients randomised at maintenance baseline who were treated in the LTE.
| Ustekinumabb | ||||||
|---|---|---|---|---|---|---|
| Placebo SCa [N = 115] |
90 mg SC q12w [N = 141] |
90 mg SC q8w [N = 143] |
Combined ustekinumab [N = 284] |
Biologic failure [N = 124] |
Biologic-naïve [N = 149] |
|
| Patients who discontinued study agent, N [%] | 75 [65.2] | 30 [21.3] | 27 [18.9] | 57 [20.1] | 39 [31.5] | 16 [10.7] |
| Reason for discontinuation | ||||||
| Adverse event | 7 [6.1] | 16 [11.3] | 7 [4.9] | 23 [8.1] | 17 [13.7] | 5 [3.4] |
| UC worsening | 5 [4.3] | 12 [8.5] | 5 [3.5] | 17 [6.0] | 13 [10.5] | 3 [2.0] |
| Other than UC worsening | 2 [1.7] | 4 [2.8] | 2 [1.4] | 6 [2.1] | 4 [3.2] | 2 [1.3] |
| Lack of efficacy | 8 [7.0] | 5 [3.5] | 7 [4.9] | 12 [4.2] | 10 [8.1] | 2 [1.3] |
| Did not show improvement in UC activity 16 weeks after dose adjustment | 1 [0.9] | 1 [0.7] | 2 [1.4] | 3 [1.1] | 1 [0.8] | 2 [1.3] |
| Lost to follow-up | 0 | 0 | 0 | 0 | 0 | 0 |
| Placebo discontinued after study unblinding | 55 [47.8] | 0 | 0 | 0 | 0 | 0 |
| Death | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 4 [3.5] | 8 [5.7] | 11 [7.7] | 19 [6.7] | 11 [8.9] | 7 [4.7] |
IV, intravenous; LTE, long-term extension; PBO, placebo, q8w, every 8 weeks; q12w, every 12 weeks; SC, subcutaneous; UC, ulcerative colitis; UST, ustekinumab.
aPatients who were in clinical response to UST IV induction dosing and were randomised to PBO SC on entry into this maintenance study.
bEleven patients were exposed but did not have documentation of biologic failure history. These patients are not summarised here.
Of 399 randomised patients who were treated in the LTE, 50.9% of patients were unblinded by Week 92; all patients were unblinded by Week 156 [Supplementary Table S2].
3.2.Efficacy
3.2.1. Randomised patients in the maintenance study [intent-to-treat population]
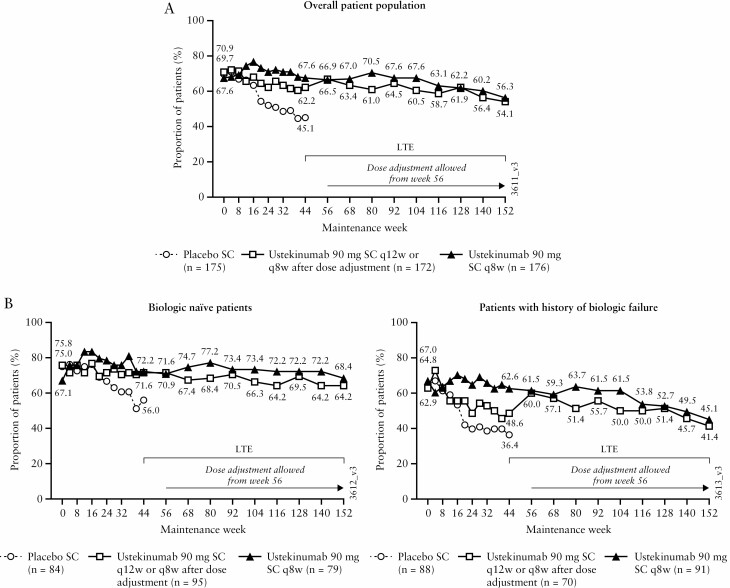
At Week 44, 62.2% of patients randomised to ustekinumab q12w and 67.6% of patients randomised to ustekinumab q8w were in symptomatic remission.9 Symptomatic remission rates ranged from 64.5% and 67.6% at Week 92 to 54.1% and 56.3% at Week 152 for patients in the ustekinumab q12w and q8w groups, respectively [Figure 2A]. Through Week 152, biologic-naïve patients had numerically higher symptomatic remission rates than patients with a history of biologic failure [Figure 2B]. Among patients in symptomatic remission at maintenance baseline, 53.3% [65/122] and 53.8% [64/119] of patients were in symptomatic remission through Week 152 [defined as patients who had achieved symptomatic remission at ≥80% of all visits from Week 4 to Week 140 and at Week 152] in the ustekinumab q12w and q8w groups, respectively.
Figure 2.
Symptomatic remission through Week 152 for all ustekinumab IV induction responders who were randomised in the maintenance study [intent-to-treat population with missing data and treatment failure rules applied]: overall population [A], and biologic treatment history subgroups [B]; data are displayed according to the patients’ randomised treatment group. AE, adverse event; IV, intravenous; LTE, long-term extension; q8w, every 8 weeks; q12w, every 12 weeks; SC, subcutaneous; UC, ulcerative colitis.a. Data are displayed by randomised group at maintenance Week 0 regardless of whether patients had a dose adjustment during the LTE. Between Weeks 56 and 152, 60 patients in the q12w group received dose adjustment to q8w.
b. Symptomatic remission is defined as a stool frequency subscore of 0 or 1 and a rectal bleeding subscore of 0.
c. Patients who had both stool frequency and rectal bleeding subscores missing at a visit were considered not to be in symptomatic remission for that visit.
d. Patients who had a prohibited change in UC medication, an ostomy or colectomy, or used a rescue medication after clinical flare, or discontinued study agent due to lack of therapeutic effect or due to an AE of worsening of UC prior to the Week 44 visit were considered not to be in symptomatic remission.
e. Patients who had an ostomy or colectomy, or discontinued study agent due to lack of therapeutic effect or due to an AE of worsening of UC after Week 44 and prior to the designated visit, were considered not to be in symptomatic remission.
f. Patients who maintained symptomatic remission are defined as those who had achieved symptomatic remission at least 80% of all visits from Week 4 to Week 140 [at least 16 out of 19 visits] and were in symptomatic remission at Week 152.
3.2.1.1.Corticosteroid-free symptomatic remission
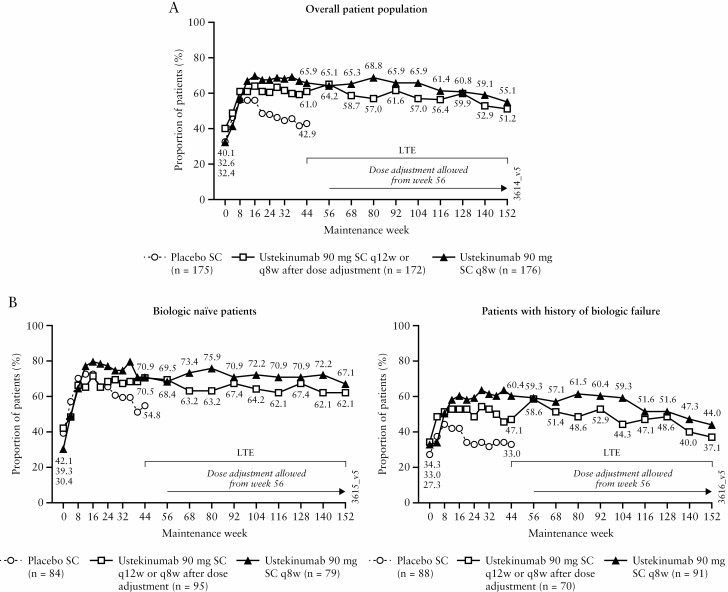
At maintenance baseline, 48.3% [83/172] and 54.0% [95/176] of patients in the ustekinumab q12w and q8w groups, respectively, were receiving corticosteroids [including budesonide and beclomethasone dipropionate].6 Rates of corticosteroid-free symptomatic remission ranged from 61.6% and 65.9% at Week 92 to 51.2% and 55.1% at Week 152 of the LTE for patients in the ustekinumab q12w and q8w groups, respectively [Figure 3A]. Corticosteroid-free symptomatic remission rates were numerically greater for the biologic-naïve patients than for patients with a history of biologic failure [Figure 3B].
Figure 3.
Corticosteroid-free symptomatic remission through Week 152 for all ustekinumab IV induction responders who were randomised in the maintenance study [intent-to-treat population with missing data and treatment failure rules applied]: overall population [A], and biologic treatment history subgroups [B]; data are displayed according to the patients’ randomised treatment group. AE, adverse event; IV, intravenous; LTE, long-term extension; q8w, every 8 weeks; q12w, every 12 weeks; SC, subcutaneous; UC, ulcerative colitis.a. Patients who had both stool frequency and rectal bleeding subscores missing at a visit were considered not to be in symptomatic remission for that visit.
b. Data are displayed by randomised group at maintenance Week 0 regardless of whether patients had a dose adjustment during the LTE. Between Weeks 56 and 152, 60 patients in the q12w group received dose adjustment to q8w.
c. Patients who had a prohibited change in UC medication, an ostomy or colectomy, or used a rescue medication after clinical flare, or discontinued study agent due to lack of therapeutic effect or due to an AE of worsening of UC prior to the Week 44 visit were considered not to be in symptomatic remission.
d. Patients who had a missing value in corticosteroid use at a visit had their last observation carried forward.
Of the patients in symptomatic remission at Week 152, 94.6% [88/93] and 98.0% [97/99] of patients in the ustekinumab q12w and q8w groups, respectively, were in corticosteroid-free symptomatic remission. This result was similar between the subgroups of biologic-naïve patients (96.7% [59/61] and 98.1% [53/54]) and patients with a history of biologic failure (89.7% [26/29] and 97.6% [40/41]).
3.2.2. Randomised patients who were treated in the LTE [with treatment failure and missing data nonresponder imputation analysis applied]
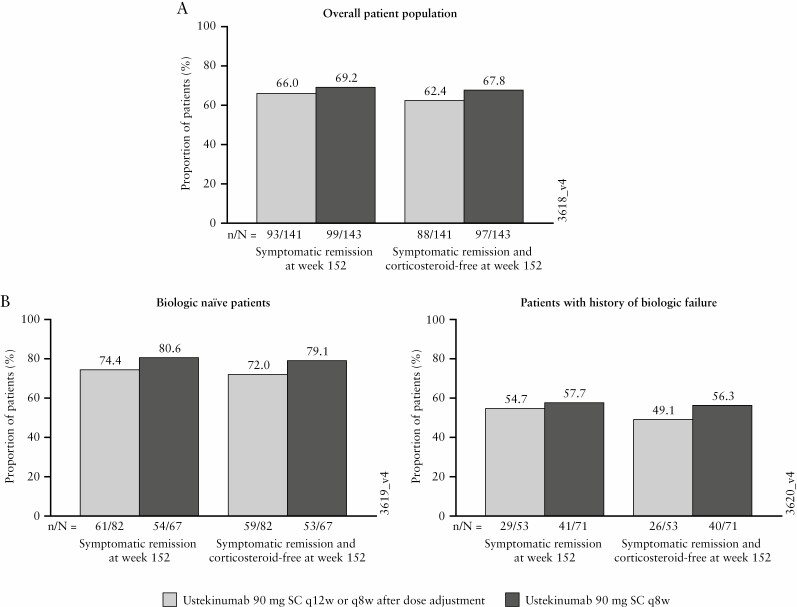
For randomised patients who were treated in the LTE after completion of the maintenance study, 83.0% of patients in the ustekinumab q12w group and 83.2% of patients in the ustekinumab q8w group were in symptomatic remission at Week 44.9 Symptomatic remission rates ranged from 78.7% and 83.2% at Week 92 to 66.0% and 69.2% at Week 152 for patients in the q12w and q8w groups, respectively [Supplementary Figure S1A]. Of the patients in clinical remission at Week 44 who entered the long-term extension, 78.5% [51/65] in the q12w group and 74.7% [56/75] in the q8w group were in symptomatic remission at Week 152. During the LTE, symptomatic remission rates were generally maintained among biologic-naïve patients but slowly decreased for those with a history of biologic failure [Supplementary Figure S1B]. At Week 152, 66.0% and 69.2% of patients in the ustekinumab q12w and q8w groups, respectively, were in symptomatic remission [Figure 4], and nearly all of these patients (q12w group, 88/93 [94.6%]; q8w group, 97/99 [98.0%]) were in corticosteroid-free symptomatic remission. This trend was observed consistently for the overall population [Figure 4A] and by biologic treatment history subgroup [Figure 4B].
Figure 4.
Symptomatic remission and corticosteroid-free symptomatic remission at Week 152 for all ustekinumab IV induction responders who were randomised in the maintenance study and treated in the LTE [with missing data and treatment failure rules applied]: overall population [A], and biologic treatment history subgroups [B]; data are displayed according to the patients’ randomised treatment group. AE, adverse event; IV, intravenous; LTE, long-term extension; q8w, every 8 weeks; q12w, every 12 weeks; SC, subcutaneous; UC, ulcerative colitis.a. Randomised group at maintenance Week 0 regardless of whether patients had a dose adjustment during the LTE.
b. Patients who had a missing value in corticosteroid use at a time point had their last available value carried forward to that time point.
c. Patients who had both stool frequency and rectal bleeding subscores missing at a visit were considered not to be in symptomatic remission for that visit.
d. Patients who had an ostomy or colectomy, or discontinued study agent due to lack of therapeutic effect or due to an AE of worsening of UC prior to the designated visit were considered not to be in symptomatic remission.
3.2.2.1.UC symptoms
Mean absolute stool numbers in the ustekinumab q12w and q8w groups were 2.4 and 2.3 stools per day, respectively, at Week 44, 2.7 and 2.2 at Week 92, and 3.0 and 2.4 at Week 152 [Supplementary Table S3]. Proportions of patients in the ustekinumab q12w and q8w groups who reported no rectal bleeding were 97.2% and 90.2%, respectively, at Week 44, 86.5% and 88.8% at Week 92, and 70.9% and 74.8% at Week 152 [Supplementary Table S3].
3.2.2.2.Corticosteroid use in the LTE
Among patients who were receiving corticosteroids at maintenance baseline, completed the maintenance study, and were treated in the LTE with ustekinumab, 89.7% [61/68] and 91.5% [65/71] of patients in the ustekinumab q12w and q8w groups, respectively, had discontinued corticosteroids by Week 44. These rates were maintained through the LTE with 88.2% [60/68] and 94.4% [67/71] of patients remaining corticosteroid-free at Week 152, respectively.
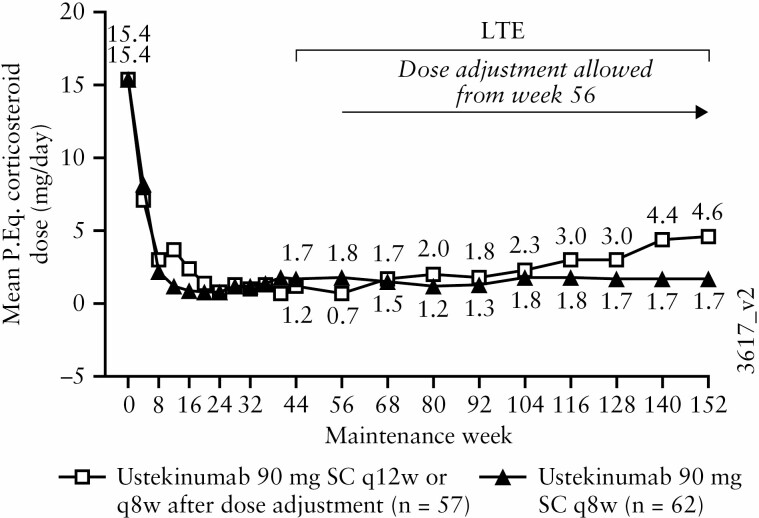
Among patients who were receiving corticosteroids [excluding budesonide and beclomethasone dipropionate] at maintenance baseline, mean prednisone-equivalent doses in the ustekinumab q8w group remained consistent, ranging from 1.7 mg/day at Week 44 to 1.7 mg/day at Week 152 [Figure 5]. During the same period, mean prednisone-equivalent doses increased slightly in the ustekinumab q12w group, ranging from 1.2 mg/day at Week 44 to 4.6 mg/day at Week 152.
Figure 5.
Daily prednisone-equivalent [P.Eq] corticosteroid dose [mg/day] through Week 152 for all ustekinumab IV induction responders randomised in the maintenance study and treated in the LTE who were receiving corticosteroids at maintenance baseline [excluding budesonide and beclomethasone] [with missing data and treatment failure rules applied]; data are displayed according to the patients’ randomised maintenance treatment group. AE, adverse event; IV, intravenous; LTE, long-term extension; PEq, prednisone equivalent; q8w, every 8 weeks; q12w, every 12 weeks; SC, subcutaneous; UC, ulcerative colitis.a. Patients who had an ostomy or colectomy, or discontinued study agent due to lack of therapeutic effect or due to an AE of worsening of UC, or were dose adjusted [only occurred from Week 56 onward] prior to the Week 152 visit had their Week 0 value of the induction study carried forward from the time of the event onward.
b. Analysis of patients who were receiving corticosteroid at baseline. Corticosteroid includes prednisone only; excludes budesonide and beclomethasone dipropionate.
c. Data are displayed by randomised group at maintenance Week 0 regardless of whether patients had a dose adjustment during the LTE. Between Weeks 56 and 152, 20 patients in the q12w group received dose adjustment to q8w.
d. Patients who had a missing value in corticosteroid use at a time point had their last available value carried forward to that time point.
3.2.2.3.IBDQ
Among randomised patients in IBDQ remission at maintenance baseline who were treated in the LTE, 92.0%, 88.5%, and 74.7% of patients in the ustekinumab q12w group, and 87.8%, 87.8%, and 74.4% of patients in the ustekinumab q8w group, continued to be in IBDQ remission at Weeks 44, 92, and 152, respectively [Supplementary Figure S2, nonresponder imputation for missing data and treatment failures]. An as observed case analysis, using nonresponder imputation for treatment failures only, showed IBDQ remission was similarly maintained at high rates [Supplementary Figure S3].
3.2.2.4.Biomarkers
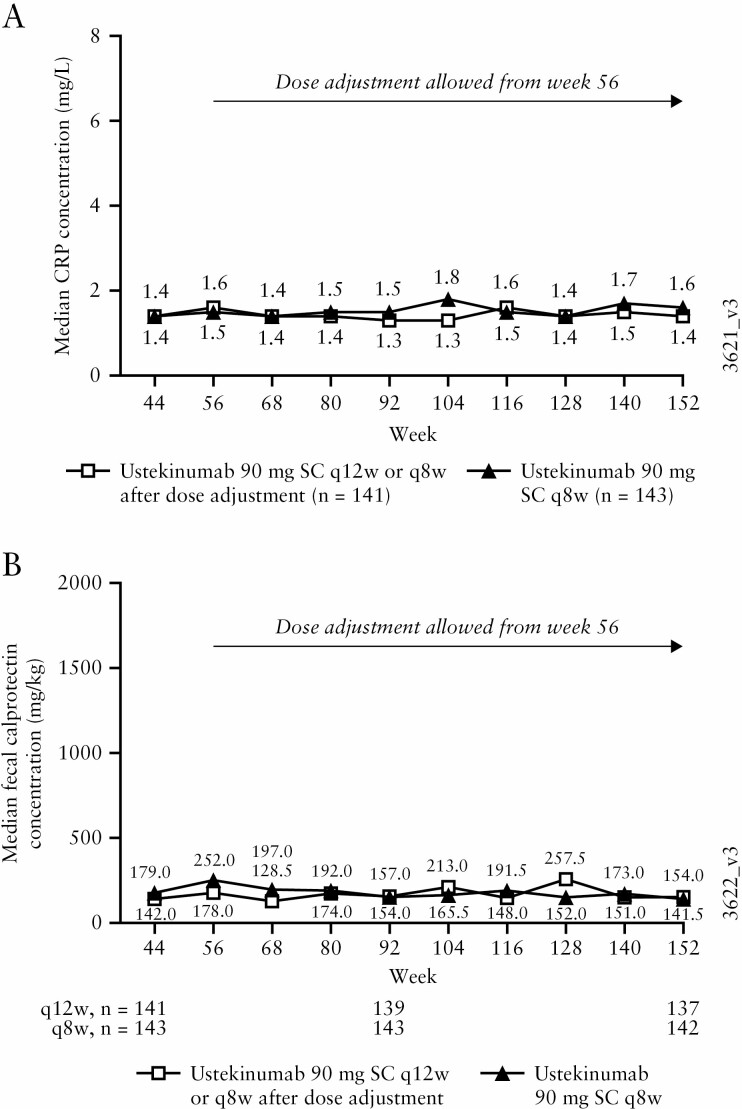
Median serum CRP and faecal calprotectin remained low from Weeks 44 through 152 in the combined q12w and q8w ustekinumab groups [Figure 6]. These values were lower than the median induction baseline values for the combined ustekinumab group of CRP 3.7 mg/L and faecal calprotectin 1404 mg/kg.
Figure 6.
Median CRP [mg/L] [A] and faecal calprotectin [mg/kg] concentrations [B] during the LTE for all ustekinumab IV induction responders who were randomised in the maintenance study and treated in the LTE; data are displayed according to the patients’ randomised treatment group. AE, adverse event; CRP, C-reactive protein; IV, intravenous; LTE, long-term extension; q8w, every 8 weeks; q12w, every 12 weeks; SC, subcutaneous; UC, ulcerative colitis.a. Patients who had an ostomy or colectomy, or discontinued study agent due to lack of therapeutic effect or due to an AE of worsening of UC prior to the Week 152 visit had their Week 0 value of the induction study carried forward from the time of the event onward.
b. Patients who had a missing CRP or faecal calprotectin value at the designated analysis time point had their last observation carried forward.
3.2.3. Observed case analysis with and without treatment failure rules applied
Among patients with data available for analysis at each visit [modified observed case analysis, with treatment failure rules applied], the proportions of patients in symptomatic remission were sustained from Week 44 (83.0% [117/141] and 83.2% [119/143]) through Week 152 (81.6% [62/76] and 81.7% [76/93]) in the ustekinumab q12w and q8w groups, respectively [Supplementary Figure S4A]. In this analysis, symptomatic remission was generally sustained for biologic-naïve patients in both dose groups and in patients with a history of biologic failure in the ustekinumab q8w group [Supplementary Figure S4B].
Likewise, similar sustained symptomatic remission rates were found in patients with data available for analysis at each visit without treatment failure rules applied, in which the proportions of patients in symptomatic remission were 83.0% ([117/141] and 83.9% [120/143] at Week 44 and 85.7% [96/112] and 82.6% [100/121]) at Week 152 in the ustekinumab q12w and q8w groups, respectively [Supplementary Figure S5A]. Symptomatic remission was generally sustained in both ustekinumab groups for biologic-naïve patients and patients with a history of biologic failure [Supplementary Figure S5B].
3.2.4. Ustekinumab dose adjustment before treatment unblinding [with treatment failure and missing data nonresponder imputation analysis applied]
Patients in the q12w group could receive dose adjustment to q8w starting at Week 56 if their UC disease activity worsened, based upon the clinical opinion of the investigator. Dose adjustment was conducted in a blinded fashion until the maintenance study analysis was complete and the full study was unblinded; therefore, patients randomised to q8w dosing underwent a ‘sham’ dose adjustment. Overall, 60 [42.6%] patients adjusted from q12w to q8w, and 40 [28.0%] sham-adjusted from q8w to q8w. For this analysis, 34 patients in the q12w group and 36 patients in the q8w group underwent dose adjustment [or sham dose adjustment] before treatment unblinding and had data for ≥16 weeks following dose adjustment. We found 58.8% of patients who adjusted from q12w to q8w and 63.9% who sham dose adjusted from q8w to q8w were in symptomatic remission at the first visit ≥16 weeks after dose adjustment [Supplementary Table S4].
3.3.Immunogenicity
Through Week 156 of the LTE, 5.5% [22/400] of patients who received ustekinumab in maintenance and continued on ustekinumab in the LTE were positive for ADAs at any visit. This is the total rate among patients who were Week 8 responders to ustekinumab IV induction and randomised to ustekinumab SC maintenance, and those who were Week 16 responders who received SC maintenance thereafter. Of the 22 patients who were positive for ADA, only five patients [22.7%] were positive for neutralising antibodies.
3.4.Safety
From maintenance Week 0 to Week 156, patients who received ustekinumab [q12w and 18w combined] had 1236.4 patient-years of follow-up, and patients who received placebo had 304.0 patient-years of follow-up. Incidences of key AEs, SAEs, and serious infections per 100 patient-years of follow-up for combined ustekinumab vs placebo were AEs: 244.41 vs 285.81, SAEs: 8.01 vs 10.52, and serious infections: 2.43 vs 3.29 [Table 2]. Ulcerative colitis worsening, nasopharyngitis, and upper respiratory tract infection were the most frequently reported AEs [those reported at a rate of five or more events per 100 patient-years of follow-up in the q12w and q8w combined ustekinumab group] through Week 156 and were also the most common AEs through Week 96.9 Non-melanoma skin carcinomas were numerically higher for ustekinumab vs placebo (0.73 [0.33, 1.38] vs 0.33 [0.01, 1.83]), with overlapping confidence intervals [Table 2].
Table 2.
Summary of key safety findings per 100 patient-years of follow-up from Week 0 of maintenance through Week 156: patients who were treated in the LTE.
| Ustekinumab | |||||
|---|---|---|---|---|---|
| Placebo SCa [N = 188] |
90 mg SC q12wb [N = 141] |
90 mg SC q8wc [N = 376] |
Combinedd [N = 457] |
All ustekinumabe [N = 516] |
|
| Mean duration of follow-up [weeks] | 84.1 | 124.0 | 124.5 | 140.7 | 126.4 |
| Patient-years of follow-up | 304.0 | 336.1 | 900.3 | 1236.4 | 1254.3 |
| Number of events per 100 patient-years of follow-up [95% CI]f | |||||
| Any AE | 285.81[267.12, 305.46] | 224.34 [208.61, 240.94] |
251.90[241.64, 262.49] | 244.41 [235.77, 253.28] |
246.36[237.75, 255.20] |
| Infectionsg | 85.51 [75.43, 96.56] |
74.98 [66.01, 84.83] |
76.53 [70.92, 82.46] |
76.11 [71.32, 81.13] |
76.62 [71.85, 81.62] |
| AEs leading to d/c of study agent | 5.26 [3.01, 8.55] |
2.08 [0.84, 4.29] |
2.89 [1.89, 4.23] |
2.67 [1.84, 3.75] |
2.63 [1.81, 3.69] |
| Serious adverse events | 10.52 [7.20, 14.86] |
6.84 [4.34, 10.27] |
8.44 [6.65, 10.57] |
8.01 [6.51, 9.75] |
7.89 [6.42, 9.61] |
| Serious infectionsg | 3.29 [1.58, 6.05] |
2.98 [1.43, 5.47] |
2.22 [1.36, 3.43] |
2.43 [1.64, 3.46] |
2.39 [1.61, 3.41] |
| All malignancies | 0.66 [0.08, 2.38] |
0.89 [0.18, 2.61] |
0.67 [0.24, 1.45] |
0.73 [0.33, 1.38] |
0.72 [0.33, 1.36] |
| Excluding nonmelanoma skin cancer | 0.33 [0.01, 1.83] |
0.00 [0.00, 0.89] |
0.00 [0.00, 0.33] |
0.00 [0.00, 0.24] |
0.00 [0.00, 0.24] |
| Nonmelanoma skin cancer | 0.33 [0.01, 1.83] |
0.89 [0.18, 2.61] |
0.67 [0.24, 1.45] |
0.73 [0.33, 1.38] |
0.72 [0.33, 1.36] |
| Death | 0.00 [0.00, 0.99] |
0.00 [0.00, 0.89] |
0.11 [0.00, 0.62] |
0.08 [0.00, 0.45] |
0.08 [0.00, 0.44] |
AE, adverse event; CI, confidence interval; d/c, discontinuation; IV, intravenous; LTE, long-term extension; q8w, every 8 weeks; q12w, every 12 weeks; SC, subcutaneous
aIncludes 1] data from Maintenance Week 8 onward for patients who were in clinical response to ustekinumab IV induction dosing and were randomised to placebo SC on entry into the maintenance study, up to the dose adjustment for patients who had dose adjustment during LTE; and 2] data from Week 0 of maintenance for patients who were in clinical response to placebo IV induction dosing and received placebo SC on entry into the maintenance study.
bIncludes data from Maintenance Week 0 through Week 156, or up to the dose adjustment if patients had a dose adjustment during the LTE, for patients who were in clinical response to ustekinumab IV induction dosing and were randomised to ustekinumab 90 mg SC q12w on entry into the maintenance study.
cIncludes: 1] patients who were in clinical response to ustekinumab IV induction dosing and were randomised to receive ustekinumab 90 mg SC q8w on entry into the maintenance study, with data from Maintenance Week 0 through Week 156; 2] patients who were in clinical response to ustekinumab IV induction dosing, randomised to receive placebo SC or ustekinumab 90 mg SC q12w on entry into the maintenance study, and had a dose adjustment to ustekinumab SC 90 mg q8w, with data from the time of dose adjustment onward; 3] patients who were not in clinical response to ustekinumab at induction Week 8 but were in clinical response at induction Week 16 after an SC administration of ustekinumab at Induction Week 8 and received ustekinumab 90 mg SC q8w on entry into the maintenance study with data from Maintenance Week 0 through Week 156.
dIncludes 56 patients who dose-adjusted from placebo, and one patient in non-randomised population who incorrectly received ustekinumab.
eIncludes: 1] data from maintenance Week 0 to maintenance Week 8 for patients who were in clinical response to ustekinumab IV induction dosing and received placebo SC on entry into the maintenance study; 2] data from the first ustekinumab dose through Week 156 for patients who were treated with ustekinumab 90 mg SC [q12w or q8w] on entry into the maintenance study or for patients who were in clinical response to ustekinumab IV induction dosing and were randomised to placebo SC on entry into the maintenance study and had a dose adjustment during the long-term extension with data from the time of dose adjustment onward.
fConfidence intervals based on an exact method assuming that the observed number of events follows a Poisson distribution.
gInfection as assessed by the investigator.
Between Weeks 96 and 156, one ustekinumab-treated patient [q12w group] with prior and family history of basal cell carcinoma [BCC], no concomitant immunomodulator therapy, and a family history of fair skin, reported two BCCs.
One patient in the 90 mg q8w ustekinumab group, receiving concomitant 6-mercaptopurine, experienced concurrent SAEs of neutropenic sepsis and oral herpes simplex between Weeks 96 and 156 which was considered a potential opportunistic infection. The dose of 6-mercaptopurine was reduced and the patient recovered and did not discontinue ustekinumab.
Between Weeks 96 and 156, no new deaths or major adverse cardiovascular events occurred. Throughout the maintenance study and long-term extension, there was no reported posterior reversible encephalopathy syndrome [PRES, previously referred to as reversible posterior leukoencephalopathy syndrome] or tuberculosis infections.
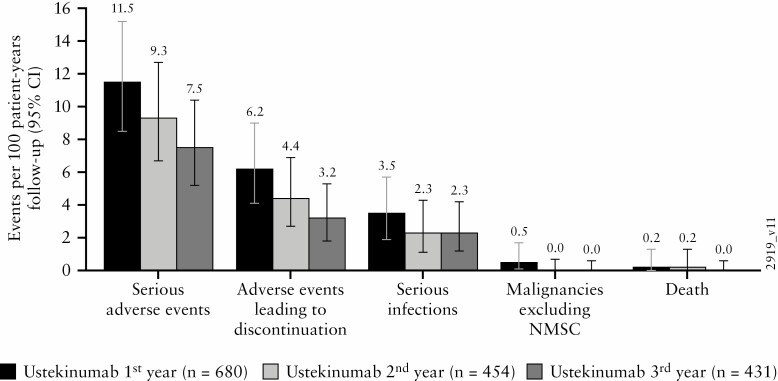
With ustekinumab treatment from Week 96 through Week 156, no increase in the rates of serious AEs, serious infections, deaths, AEs leading to discontinuation, and malignancies [excluding nonmelanoma skin cancer] were observed relative to prior years [Figure 7].
Figure 7.
Key safety events per 100 patient-years of exposure during the first, second, and third year of ustekinumab maintenance therapy. AE, adverse events; CI, confidence interval; IV, intravenous; LTE, long-term extension; NMSC, nonmelanoma skin cancer; q8w, every 8 weeks; q12w, every 12 weeks; SC, subcutaneous.a. Number of AEs per 100 patient-years of follow-up and 95% confidence interval [rates by each year of follow-up] in the pooled ustekinumab ulcerative colitis safety cohort. Confidence intervals based on an exact method assuming that the observed number of events follows a Poisson distribution.
b. Infection as assessed by the investigator.
c. All ustekinumab [first year] includes: 1] patients who received ustekinumab SC [q8w or q12w] in this maintenance study; 2] patients who were in clinical response to ustekinumab IV induction dosing and received placebo SC on entry into this maintenance study; and 3] data from Week 0 to Week 8 for patients who were in clinical response to ustekinumab IV induction dosing and were randomised to placebo SC on entry into this maintenance study.
d. All ustekinumab-treated in the LTE [second year] includes: 1] data from Week 44 through Week 96 for patients who were in clinical response to ustekinumab IV induction dosing and were randomised to ustekinumab 90 mg SC q12w on entry into the maintenance study; 2] patients who were in clinical response to ustekinumab IV induction dosing and were randomised to receive ustekinumab 90 mg SC q8w on entry into the maintenance study, with data from Week 44 through Week 96; 3] patients who were in clinical response to ustekinumab IV induction dosing, randomised to receive placebo SC on entry into the maintenance study, and had a dose adjustment prior to Week 96 to ustekinumab 90 mg SC q8w, with data from the time of dose adjustment onward; and 4] patients who were not in clinical response to ustekinumab at induction Week 8 but were in clinical response at induction Week 16 after an SC administration of ustekinumab at induction Week 8 and received ustekinumab 90 mg SC q8w on entry into the maintenance study with data from Week 44 through Week 96.
e. All ustekinumab in the LTE [third year] includes: 1] data from Week 96 through Week 156 for patients who were in clinical response to ustekinumab IV induction dosing and were randomised to ustekinumab 90 mg SC q12w on entry into the maintenance study; 2] patients who were in clinical response to ustekinumab IV induction dosing and were randomised to receive ustekinumab 90 mg SC q8w on entry into the maintenance study, with data from Week 96 through Week 156; 3] patients who were in clinical response to ustekinumab IV induction dosing, randomised to receive placebo SC on entry into the maintenance study, and had a dose adjustment at or after Week 96 to ustekinumab 90 mg SC q8w, with data from the time of dose adjustment onward; and 4] patients who were not in clinical response to ustekinumab at induction Week 8 but were in clinical response at induction Week 16 after an SC administration of ustekinumab at induction Week 8 and received ustekinumab 90 mg SC q8w on entry into the maintenance study with data from Week 96 through Week 156.
4. Discussion
Patients with UC may face a lifetime of therapy based on disease severity and extent, and the choice of therapy is based on shared decision making with their provider.12,13 To make treatment decisions in adults with moderate to severe UC, it is important to weigh efficacy of induction and maintenance of clinical remission, safety, and tolerability.14
Durability of response to biologic therapy in patients with UC is important because of the need for long-term therapy. In anti TNF-α treated patients, UC patients required dose escalation more often and more quickly than patients with Crohn’s disease [CD].15,16 Overall, 30-40% of patients discontinue biologic therapy over time due to side effects or failure to achieve clinical benefit.15–18 Generally loss of response and need for dose adjustment occur more often in UC patients given adalimumab compared with infliximab.16 Furthermore, the clinical remission rate for adalimumab at Week 52 was 22%,19 corroborated in the first head-to-head study of vedolizumab and adalimumab,20 and without improvement with dose intensification.21 In VARSITY, adalimumab-treated patients [82/147, 55.8%] discontinued because of lack of efficacy at a higher rate than vedolizumab-treated patients [41/96, 42.7%].20 Persistence rates for ustekinumab in CD were significantly greater than those for anti-TNFα agents and vedolizumab (hazard ratio 1.79 [95% CI: 1.32, 2.38]). Unlike for anti TNF-α agents where persistence declined without thiopurine or methotrexate combination treatment, the persistence of ustekinumab was maintained on monotherapy.22 In a Groupe d’Etude Thérapeutique des Affections Inflammatoires du tube Digestif [GETAID] cohort of 103 consecutive patients with ulcerative colitis with inadequate response to immunosuppressants, anti-TNF antagonists, or vedolizumab, more than one-half of patients were still receiving ustekinumab and one-third were in steroid-free clinical remission at 1 year.23 Thus, the sustainability of medical therapy should be taken into account when choosing among available therapies.
Here we present data for the sustainability of ustekinumab through Week 156 of the UNIFI study. Data through Week 156 of the UNIFI study showed a discontinuation rate of 20% among ustekinumab-treated patients with UC, which is lower than the 30% reported through Week 156 among ustekinumab-treated patients with CD,24 with reasons primarily related to lack of efficacy or UC worsening. Biologic-naïve patients had lower discontinuation rates than patients with a history of biologic failure. These results provide an indirect comparison of the high level of persistency in patients with IBD receiving ustekinumab relative to those receiving TNF-α antagonists.
We assessed efficacy in all patients who responded to IV ustekinumab induction therapy and were randomised to ustekinumab q12w or q8w at maintenance baseline [Week 0]. In this intent-to-treat analysis, patients who discontinued the study or did not enter the LTE for any reason were considered to not be in symptomatic remission at all subsequent time points through Week 152. Using this conservative approach, more than half of all patients randomised to ustekinumab maintenance were in symptomatic remission at Week 152. Among patients who entered the LTE at Week 44, approximately two-thirds were in symptomatic remission at Week 152. Among patients with data available at Week 152, more than 80% were in symptomatic remission. The rest of the patients who were not in symptomatic remission continued to receive ustekinumab therapy, presumably because the patient and provider felt they had ongoing benefit. Not surprisingly, symptomatic remission rates were greater in biologic-naïve patients than in patients with a history of biologic failure, but durable symptomatic remission through the LTE was demonstrated in both subgroups.
Most patients achieved and maintained symptomatic remission without concomitant corticosteroid use. Overall, more than 90% of patients in symptomatic remission at Week 152 were corticosteroid free. Of the patients who were receiving corticosteroids at maintenance baseline and entered the long-term extension, approximately 90% were not receiving corticosteroids at Week 152.
It is important to determine whether patients had evidence of systemic inflammation and/or mucosal inflammation, because in this long-term extension study, protocolised colonoscopies after Week 44 were only done for patients who had endoscopy data at Week 44. CRP and faecal calprotectin concentrations that were reduced after IV ustekinumab induction were maintained at low levels through the LTE. Additionally, ustekinumab therapy sustained IBDQ remission levels achieved after induction.
As previously observed, nasopharyngitis, upper respiratory tract infection, and UC worsening remained the most frequently reported AEs.6,9 AE and SAE rates per 100 patient-years of follow-up at Week 156 for combined ustekinumab vs placebo were similar. The rates of key AEs did not increase with additional ustekinumab exposure but appeared to decrease over time, which may be due to patients discontinuing from the study, patients’ fatigue in reporting non-severe AEs, and/or patients generally doing well as measured by the sustained efficacy we report during the LTE. Nonmelanoma skin cancer [NMSC] rates were numerically higher for ustekinumab, but with overlapping confidence intervals. All NMSC cases reported through Week 156 occurred in patients with confounding factors [e.g., family history, fair skin, smoker/ex-smoker, concomitant immunosuppressant use].6,9 No other solid tumours have been reported during the LTE. No new deaths or major adverse cardiovascular events occurred between Weeks 96 to 156. One death and three major adverse cardiovascular events occurred between Weeks 44 and 96, as previously reported.9 The safety profile of ustekinumab through Week 156 is consistent with previous observations across indications in controlled ustekinumab studies.
The LTE study design does have limitations. Investigators selected patients who, in their opinion, might benefit from continued treatment, which may limit the generalisability of the results of analyses based solely on the cohort of patients treated in the LTE. Unlike the rigorously controlled maintenance study where concomitant UC medications, except for oral corticosteroids, were required to remain stable through Week 44, patients could change concomitant medications at any time in the LTE, mimicking real-world practice. Regarding the dose-adjustment results, it should be noted that the decision to dose-adjust was based on the investigator’s clinical judgement of a patient’s disease activity. The decision to make a dose adjustment was not based on protocol-specified criteria, e.g., clinical flare based on partial Mayo score or ustekinumab serum concentrations. Therefore, the interpretability of these data is limited as many of the patients who underwent a dose adjustment were in symptomatic remission at the time.
In summary, patients with moderately-to-severely active UC treated with ustekinumab 90 mg SC q12w or q8w maintained symptomatic remission through the third year of maintenance treatment. The safety profile observed for ustekinumab in the third year of maintenance treatment was consistent with that reported through the first 2 years and with the established ustekinumab safety profile; no new safety signals were identified.
Supplementary Material
Acknowledgements
The authors thank the patients, investigators, and study personnel who made the UNIFI study possible; and Omoniyi Adedokun who analysed serum data for the immunogenicity results. Under the direction of the authors and in accordance with Good Publication Practices, James P. Barrett of Janssen Scientific Affairs, LLC, provided writing and editorial assistance.
Contributor Information
Maria T Abreu, Division of Gastroenterology, Department of Medicine, University of Miami Miller School of Medicine, Miami, FL, USA.
David S Rowbotham, Department of Gastroenterology & Hepatology, Auckland City Hospital, Auckland, New Zealand.
Silvio Danese, Gastroenterology and Endoscopy, IRCCS Ospedale San Raffaele and University Vita-Salute San Raffaele, Milano, Italy.
William J Sandborn, Division of Gastroenterology, University of California San Diego, La Jolla, CA, USA.
Ye Miao, Janssen Research & Development LLC., Immunology, Spring House, PA, USA.
Hongyan Zhang, Janssen Research & Development LLC., Immunology, Spring House, PA, USA.
Ilia Tikhonov, Janssen Research & Development LLC., Immunology, Spring House, PA, USA.
Remo Panaccione, Inflammatory Bowel Disease Unit, Division of Gastroenterology and Hepatology, University of Calgary, Calgary, AB, Canada.
Tadakazu Hisamatsu, Department of Internal Medicine, Kyorin University School of Medicine, Tokyo, Japan.
Ellen J Scherl, Jill Roberts Center for Inflammatory Bowel Disease, New York Presbyterian Weill Cornell Medical College, New York, NY, USA.
Rupert W Leong, Gastroenterology and Liver Services Concord Hospital and Department of Gastroenterology Macquarie University Hospital, Sydney, NSW, Australia.
Ramesh P Arasaradnam, Department of Gastroenterology, University Hospital Coventry & Warwickshire NHS Trust, Coventry, UK.
Waqqas Afif, Division of Gastroenterology, McGill University Health Centre, Montreal, QC, Canada.
Laurent Peyrin-Biroulet, University of Lorraine, CHRU-Nancy, Department of Gastroenterology and Nutrition, Genetics, and Environmental Risk Exposure, Nancy, France.
Bruce E Sands, Dr. Henry D. Janowitz Division of Gastroenterology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Colleen Marano, Janssen Research & Development LLC., Immunology, Spring House, PA, USA.
Funding
This work was supported by Janssen Research & Development, LLC. The data sharing policy of Janssen Pharmaceutical Companies of Johnson & Johnson is available at https://www.janssen.com/clinical-trials/transparency. As noted on this site, requests for access to the study data can be submitted through the Yale Open Data Access [YODA] Project site at [http://yoda.yale.edu].
Conflict of Interest
MTA reports having received grant support from Prometheus Bioscience, Takeda, and Pfizer; consulting fees from Janssen Research & Development, LLC., Prometheus Bioscience, Takeda, Focus Medical Communications, Pfizer, Boehringer Ingelheim Pharmaceuticals, Gilead, Imedex, Cornerstone Health, Landos Biophama, UCB Biopharma SRL, Eli Lilly, Bristol Myers Squibb, Arena Pharmaceuticals, and Cosmo Biopharma. DSR reports research funding from and/or has served as a speaker, consultant, and/or advisory board member for AbbVie; Janssen Research & Development, LLC., Baxter; Pharmaco; bioCSL; Given imaging; Emerge Health, and Hospira. SD reports receiving consulting fees from AbbVie, Alimentiv, Allergan, Amgen, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Eli Lilly, Enthera, Ferring Pharmaceuticals, Gilead, Hospira, Inotrem, Janssen Research & Development, LLC., Johnson & Johnson, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB, and Vifor; and reports lecture fees from AbbVie, Amgen, Ferring Pharmaceuticals, Gilead, Janssen, Mylan, Pfizer, and Takeda. WJS reports grants from AbbVie, Aviva, Arena Pharmaceuticals, Boehringer Ingelheim, Celgene, Genentech, Gilead Sciences, Glaxo-Smith-Kline, Janssen Research & Development, LLC., Eli Lilly, Pfizer, Prometheus Biosciences, Seres Therapeutics, Shire, Takeda, and TheraVance Biopharma; consulting fees from AbbVie, Abivax, Admirx, Alfa sigma, Alimentiv [previously Robarts Clinical Trials, owned by Alimentiv Health Trust], Alivio Therapeutics, Allakos, Allergan, Amgen, Applied Molecular Transport, Arena Pharmaceuticals, Avexegen Therapeutics, Bausch Health [Salix], BeiGene, Bellatrix Pharmaceuticals, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Meyers Squibb, Celgene, Celltrion, Cellularity, Conatus, Cosmo Pharmaceuticals, Equillium, Escalier Biosciences, Ferring, Forbion, Genentech/Roche, Gilead Sciences, Glenmark Pharmaceuticals, Gossamer Bio, Immunic [Vital Therapies], Incyte, Index Pharmaceuticals, Intact Therapeutics, Janssen, Kyowa Kirin Pharmaceutical Research, Kyverna Therapeutics, Landos Biopharma, Eli Lilly, Miraca Life Sciences, Nivalis Therapeutics, Novartis, Nutrition Science Partners, Oppilan Pharma [acquired by Ventyx Biosciences], Otsuka, Pandion Therapeutics, Paul Hastings, Pfizer, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonists Therapeutics, Provention Bio, Reistone Biopharma, Ritter Pharmaceuticals, Seres Therapeutics, Shanghai Pharma Biotherapeutics, Shire, Shoreline Biosciences, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Surrozen, Takeda, Theravance Biopharma, Thetis Pharmaceuticals, Tigenix, Tillotts Pharma, UCB Pharma, Vendata Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivelix Pharmaceuticals, Vivreon Biosciences, and Zealand Pharma; and stock or stock options from Allakos, BeiGene, Gossamer Bio, Oppilan Pharma [acquired by Ventyx Biosciences], Prometheus Biosciences, Prometheus Laboratories, Progenity, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences, and Vivreon Biosciences; and employment at Shoreline Biosciences. Spouse has received from; Iveric Bio consultancy, stock options; Oppilan Pharma [acquired by Ventyx Biosciences]: consultancy, stock options; Progenity: stock; Prometheus Biosciences: employment, stock, stock options; Prometheus Laboratories: stock, stock options, consultancy; Ventyx Biosciences: stock, stock options; and Vimalan Biosciences: stock, stock options. RP has received consulting fees from AbbVie, Abbott, Alimentiv [formerly Robarts], Amgen, Arena, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim Celgene, Celltrion, Cosmos Pharmaceuticals, Eisai, Elan, Eli Lilly, Ferring, Galapagos, Genentech, Gilead Sciences, Glaxo-Smith-Kline, Janssen, Merck, Mylan, Oppilan Pharma, Pandion Therapeutics, Pfizer, Progenity, Protagonist Therapeutics, Roche, Satisfai Health, Sandoz, Schering-Plough, Shire, Sublimity Therapeutics, Theravance, UCB, and Takeda; speaker fees from AbbVie, Arena, Celgene, Eli Lilly, Ferring, Gilead Sciences, Janssen Research & Development, LLC., Merck, Pfizer, Roche, Sandoz, Shire, and Takeda; research/educational support from AbbVie, Ferring, Janssen, Pfizer, and Takeda; and has served on an advisory board for AbbVie, Amgen, Arena, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Ferring, Galapagos, Genentech, Gilead Sciences, Glaxo-Smith Kline, Janssen, Merck, Mylan, Oppilan Pharma, Pandion Pharma, Pfizer, Sandoz, Shire, Sublimity Therapeutics, Theravance, and Takeda. TH reports having received grant support from AbbVie, Daiichi-Sankyo, EA Pharma, JIMRO, Mitsubishi Tanabe Pharma Corporation, Mochida Pharmacuetical, Nippon Kayaku, Pfizer, Takeda Pharmaceutical; consulting fees from EA Pharma, Janssen Research & Development, LLC; and lecture fees from AbbVie, EA Pharma, Mitsubishi Tanabe Pharma Corporation, Takeda Pharmaceutical. EJS reports having received grant support from AbbVie, AstraZeneca, Crohn’s and Colitis Foundation, Janssen Research & Development, LLC., New York Crohn’s Foundation, Pfizer, UCB, Genentech, Seres Therapeutics, and Celgene; consulting fees from AbbVie, Abgenomics, Crohn’s and Colitis Foundation, Evidera, GI Health Foundation, Janssen, Protagonist, Seres, Takeda; stock from Gilead; speaker fees from GI Health Foundation, Prime Therapeutics. RWL reports having grant support from NHMRC, Janssen, Shire, Takeda, Gastroenterological Society of Australia, Gutsy Group, Celltrion, and Pfizer; and consulting fees from Janssen Research & Development, LLC, AbbVie, Aspen, BMS, Celgene, Chiesi, Ferring, Glutagen, Hospira, MSD, Novartis, Pfizer, Takeda reports having received grant support from NHMRC, Janssen, Shire, Takeda, Gastroenterological Society of Australia, Gutsy Group, Celltrion, and Pfizer; and consulting fees from Janssen Research & Development, LLC, AbbVie, Aspen, BMS, Celgene, Chiesi, Ferring, Glutagen, Hospira, MSD, Novartis, Pfizer, Takeda. WA reports grant support from Janssen Research & Development, LLC. LP-B reports personal fees from Galapagos, AbbVie, Janssen, Genentech, Ferring, Tillots, Celltrion, Takeda, Pfizer, Index Pharmaceuticals, Sandoz, Celgene, Biogen, Samsung Bioepis, Inotrem, Allergan, MSD, Roche, Arena, Gilead, Amgen, BMS, Vifor, Norgine, Mylan, Eli Lilly, Fresenius Kabi, OSE Immunotherapeutics, Enthera, Theravance, Pandion Therapeutics, Gossamer Bio, Viatris, Thermo Fisher; grants from Abbvie, MSD, Takeda, Fresenius Kabi; stock options from CTMA, Molecular Transport, OSE Immunotherapeutics, Enthera, Theravance. BES reports consulting fees from 4D Pharma, AbbVie, Abivax, Allergan, Amgen, AstraZeneca, Baxalta Bioscience India, Boehringer-Ingelheim, Boston Pharmaceuticals, Capella Bioscience, Celltrion Healthcare, Ferring, Genentech, Gilead Sciences, Glaxo-Smith-Kline, Hoffmann-La Roche, Immunic, InDex Pharmaceuticals, Inotrem, Ironwood Pharmaceuticals, Janssen, Johnson & Johnson, Kallyope, Eli Lilly, Morphic Therapeutic, Oppilan Pharma, OSE Immunotherapeutics, Otsuka, Palatin Technologies, Pfizer, Progenity, Prometheus Laboratories, Prometheus Biosciences IBD, Protagonist Therapeutics, Redhill Biopharma, Rheos Medicines, Salix Pharmaceuticals, Seres Therapeutics, Shire, Sienna Biopharmaceuticals, Surrozen, Takeda, TARGET RWE, USWM Enterprises, Viela Bio, Vivelix Pharmaceuticals; grants and consulting fees from Arena Pharmaceuticals, Celgene, and Theravance Biopharma R&D; consulting fees and stock options from Ventyx Biosciences; and stock options from Vivante Health. YM, HZ, IT, and CM are employees at Janssen Research & Development, LLC, and own stock or stock options.
Specific Author Contributions
YM, HZ, and CM participated in the conception and design of the study, participated in acquisition/collection of data, analysis and interpretation of data, and drafted/revised the manuscript for important intellectual content. IT, MTA, SD, WJS, RP, TH, EJS, RWL, RPA, WA, LP-B, and BES participated in conception and design of the study, analysis and interpretation of the data, and drafted/revised the manuscript for important intellectual content. All authors approved the final version of the manuscript for submission, including the authorship list.
Some of the data displayed in this article were presented at Digestive Disease Week 2021 [virtual] and European Crohn’s and Colitis Organisation 2021 [virtual].
References
- 1. Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med 2011;365:1713–25. [DOI] [PubMed] [Google Scholar]
- 2. Rutgeerts P, Sandborn WJ, Feagan BG, et al. . Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 2005;353:2462–76. [DOI] [PubMed] [Google Scholar]
- 3. Sandborn WJ, Feagan BG, Marano C, et al. ; PURSUIT-SC Study Group. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:85–95; quiz e14–5. [DOI] [PubMed] [Google Scholar]
- 4. Sandborn WJ, Feagan BG, Marano C, et al. ; PURSUIT-Maintenance Study Group. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2014;146:96–109.e1. [DOI] [PubMed] [Google Scholar]
- 5. Reinisch W, Sandborn WJ, Hommes DW, et al. . Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomized controlled trial. Gut 2011;60:780–7. [DOI] [PubMed] [Google Scholar]
- 6. Sands BE, Sandborn WJ, Panaccione R, et al. ; UNIFI Study Group. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2019;381:1201–14. [DOI] [PubMed] [Google Scholar]
- 7. Feagan BG, Rutgeerts P, Sands BE, et al. ; GEMINI 1 Study Group. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2013;369:699–710. [DOI] [PubMed] [Google Scholar]
- 8. Sandborn WJ, Su C, Sands BE, et al. ; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017;376:1723–36. [DOI] [PubMed] [Google Scholar]
- 9. Panaccione R, Danese S, Sandborn WJ, et al. . Ustekinumab is effective and safe for ulcerative colitis through 2 years of maintenance therapy [published Corrigenda in: Aliment Pharmacol Ther 2021;53:1057–60 and Aliment Pharmacol Ther 2021;54:1221–2]. Aliment Pharmacol Ther 2020;52:1658–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Irvine EJ, Feagan B, Rochon J, et al. . Quality of life: a valid and reliable measure of therapeutic efficacy in the treatment of inflammatory bowel disease. Canadian Crohn’s Relapse Prevention Trial Study Group. Gastroenterology 1994;106:287–96. [DOI] [PubMed] [Google Scholar]
- 11. Hlavaty T, Persoons P, Vermeire S, et al. . Evaluation of short-term responsiveness and cutoff values of inflammatory bowel disease questionnaire in Crohn’s disease. Inflamm Bowel Dis 2006;12:199–204. [DOI] [PubMed] [Google Scholar]
- 12. Stange EF, Travis SP, Vermeire S, et al. ; European Crohn’s and Colitis Organisation [ECCO]. European evidence-based consensus on the diagnosis and management of ulcerative colitis: definitions and diagnosis. J Crohns Colitis 2008;2:1–23. [DOI] [PubMed] [Google Scholar]
- 13. Travis SP, Stange EF, Lémann M, et al. ; European Crohn’s and Colitis Organisation [ECCO]. European evidence-based consensus on the management of ulcerative colitis: current management. J Crohns Colitis 2008;2:24–62. [DOI] [PubMed] [Google Scholar]
- 14. Feuerstein JD, Isaacs KL, Schneider Y, Siddique SM, Falck-Ytter Y, Singh S; AGA Institute Clinical Guidelines Committee. AGA Clinical Practice Guidelines on the management of moderate to severe ulcerative colitis. Gastroenterology 2020;158:1450–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Donnell S, Stempak JM, Steinhart AH, Silverberg MS.. Higher rates of dose optimisation for infliximab responders in ulcerative colitis than in Crohn’s disease. J Crohns Colitis 2015;10:830–6. [DOI] [PubMed] [Google Scholar]
- 16. Bastida G, Marín–Jiménez I, Forés A, et al. . Treatment patterns and intensification within 5 years of follow-up of the first-line anti-TNF α used for the treatment of IBD: results from the VERNE study. Dig Liver Dis 2021;. doi: 10.1016/j.dld.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 17. Gibson PR, Feagan BG, Sandborn WJ, et al. . Maintenance of efficacy and continuing safety of golimumab for active ulcerative colitis: PURSUIT-SC maintenance study extension through 1 year. Clin Transl Gastroenterol 2016;7:e168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loftus EV Jr, Colombel JF, Feagan BG, et al. . Long-term efficacy of vedolizumab for ulcerative colitis. J Crohns Colitis 2017;11:400–11. [DOI] [PubMed] [Google Scholar]
- 19. Sandborn WJ, van Assche G, Reinisch W, et al. . Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology 2012;142:257–65.e1–3. [DOI] [PubMed] [Google Scholar]
- 20. Sands BE, Peyrin-Biroulet L, Loftus EV Jr, et al. ; VARSITY Study Group. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med 2019;381:1215–26. [DOI] [PubMed] [Google Scholar]
- 21. Colombel JF, Panes J, D’Haens G, et al. . Higher vs. standard adalimumab maintenance regimens in patients with moderately to severely active ulcerative colitis: results from the SERENE–UC maintenance study. J Crohns Colitis 2020;14:S001 [abstract OP01]. [Google Scholar]
- 22. Ko Y, Paramsothy S, Yau Y, Leong RW. Superior treatment persistence with ustekinumab in Crohn’s disease and vedolizumab in ulcerative colitis compared with anti-TNF biological agents: real-world registry data from the Persistence Australian National IBD Cohort [PANIC] study. Aliment Pharmacol Ther 2021;54:292–301. [DOI] [PubMed] [Google Scholar]
- 23. Fumery M, Filippi J, Abitbol V, et al. . Effectiveness and safety of ustekinumab maintenance therapy in 103 patients with ulcerative colitis: a GETAID cohort study. Aliment Pharmacol Ther 2021;54:944–51. [DOI] [PubMed] [Google Scholar]
- 24. Hanauer SB, Sandborn WJ, Feagan BG, et al. . IM-UNITI: three-year efficacy, safety, and immunogenicity of ustekinumab treatment of Crohn’s disease. J Crohns Colitis 2020;14:23–32. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.