Abstract
Objective:
Use of risperidone in preschool-aged children is growing, with rising concerns of adverse metabolic consequences. Longitudinal data on risperidone-related weight gain in preschoolers are scarce. We aimed to evaluate changes in body mass index (BMI) that are associated with risperidone treatment in preschoolers.
Method:
We analyzed naturalistic, longitudinal data on 141 preschool children (112 boys, 29 girls) receiving psychiatric care. Mean patient age at baseline was 5.0 years (SD=0.8) and average follow-up period was 1.3 years (SD=0.8), with >8 mean BMI measurements per patient. We studied the effect of risperidone exposure (n=78) on age-and-sex-standardized BMI (BMI Z-score) implementing mixed models with random subject intercepts to account for repeated measures, covarying for multiple confounders including demographics, stimulant treatment and psychiatric diagnoses. We employed similar models to study dose and duration effects.
Results:
Risperidone treatment was significantly associated with an increase in BMI (effect size of exposure=0.45 SD (SE=0.06), t (949)=7.7, p<0.001) covarying for stimulant exposure and other confounders, independent of treatment indication. Females exhibited stronger effects (risperidone treatment × sex interaction t=2.32, p=0.02)). Risperidone daily dose was associated with increase in BMI (for each additional 1 mg, effect size=0.28 SD (SE=0.07), t(419)=3.76, p<0.001).
Conclusion:
Similar to older populations, risperidone treatment in preschoolers is associated with significant weight gain, with evidence for dose effects. Findings provide critical data that can inform clinicians.
Keywords: Preschool age, risperidone, weight gain, dose response
Introduction
Recently, second-generation antipsychotics (SGAs) have been increasingly prescribed to very young children (Olfson et al., 2010; Patel et al., 2005; Pringsheim et al., 2019), with increasing concern regarding adverse metabolic effects (Kauffman et al., 2017). The annual National Ambulatory Medical Care Survey conducted by the Center for Health Statistics showed that there was a six-fold increase in the number of office visits with children and adolescents obtaining a prescription for antipsychotics from 1993 to 2002 (Olfson et al., 2012). SGAs are prescribed to paediatric patients for a variety of indications, including schizophrenia (Kennedy et al., 2007), mood disorders (Consoli et al., 2007), autism spectrum disorder (ASD) (DeVane et al., 2019) and conduct disorder (Croonenberghs et al., 2005; Gorman et al., 2015). One of the most commonly prescribed SGAs is risperidone a D2, 5HT-2, NE alpha-2 antagonist (Ronsley et al., 2013). Since its introduction, risperidone has been used in a wide range of paediatric populations, above and beyond its use for psychosis and bipolar disorders (Anderson et al., 2007; Cascade et al., 2009). Off-label use of SGAs, most commonly risperidone, occurs frequently in daily practice, and a published survey of child and adolescent psychiatrists revealed that one third of the responding psychiatrists prescribed SGAs for off-label indications (Anderson et al., 2007).
The use of SGAs correlates with weight gain and metabolic syndrome (Bak et al., 2014; Correll et al., 2015; Findling et al., 2010; Parsons et al., 2009; Ronsley et al., 2015; Taylor et al., 2018), posing a major concern for patients, their families and physicians, both in paediatric and adult populations (Correll et al., 2009; Gothelf et al., 2002). Scarce data exist on SGA-related weight gain in early childhood populations (Arango et al., 2014; Kauffman et al., 2017; Martin et al., 2000), especially longitudinal studies. The only data in preschool-aged children receiving SGA exists from a limited number of studies in children with ASD, where risperidone is indicated to treat irritability (Aman et al., 2015; Yoon et al., 2016). To our knowledge, no longitudinal data exists on preschool-aged children receiving SGAs off-label for heterogeneous psychiatric presentations beyond ASD.
In the current study, we aimed to investigate risperidone-related weight gain in a clinical population of very young children (mean age 5 years at beginning of follow-up) who were treated and routinely monitored for weight gain in a psychiatric preschool ward. We hypothesized that similar to older populations, risperidone use would be associated with weight gain in preschool age, regardless of treatment indication, with a moderating effect for stimulant use. We also tested sex effects and conducted exploratory analyses to study effects of duration and dosage on weight gain.
Methods
Participants and setting
The cohort included preschoolers who were admitted to the Preschoolers’ Department at Geha Mental Health Center (GMHC), a regional mental health center in Israel with a catchment area of approximately 900,000 inhabitants. The department provides assessment, diagnostic services and intervention programmes for children between 3 and 6 years of age. Children are referred to the Preschoolers’ Department for a variety of presentations, including aggression and attention difficulties. Referred children receive a diagnostic evaluation by a senior child psychiatrist. The evaluation includes a semi-structured interview of the child’s parents, a set of parents’ and teachers’ questionnaires and a psychiatric examination. While children with mild to moderate symptoms and functional impairment are treated on an outpatient basis, children with severe maladjustment are assigned to be treated by a multidisciplinary team in a day-hospitalization setting at the Preschool Unit. Patients do not spend the night on the department but participate in daytime programming on a daily basis. The Preschoolers’ Department includes a therapeutic kindergarten. Each child is assigned to an individually tailored therapeutic program for at least 3 months and up to 3 years.
Patients on the Preschoolers’ Department are routinely monitored for weight gain and growth as part of standard practice. Throughout the study period, patients were measured on the same digital weight and height scale (DETECTO cardinal model 750, Cardinal Scale MFG. Co., Webb City, MO, US) and by the same nurse (co-author IS), after shoes and jackets were removed. Importantly, administration of psychotropic medication (risperidone and stimulants) is done on the Preschoolers’ Department by clinical nursing staff.
In the current analysis, we included all preschoolers hospitalized between 2008 and 2018 that underwent repeated (at least two) measurements of body mass index (BMI) measured during hospitalization. We excluded patients with severe eating disorders and extreme low weight (>3 SD below expected BMI Z-score) at baseline.
The study was approved by the Geha Mental Health Center Institutional Review Board. Owing to the chart review retrospective nature of the study, the need for obtaining informed assent/consent from participants and their parents was waived.
Variables
Data were retrospectively collected from the patients’ electronic health record. Exposures included administration of psychotropic medications. Outcome measure included change in BMI over time. In contrast to adults, the BMI in children changes according to age and gender (Martínez-Ortega et al., 2013). It is therefore critical to account for developmental effects when monitoring changes in weight in this population (Correll et al., 2006). In the present study we used age- and sex-standardized BMI (BMI z) to account for developmental effects. BMI values were normalized to patients’ age and sex, using the Children’s Hospital of Philadelphia (CHOP) BMI Z-score calculator (https://zscore.research.chop.edu/).
We collected baseline information on each child including: age at admission, sex and final psychiatric diagnoses at the time of discharge based on the International Classification of Diseases (ICD) 10th edition. Additionally, we collected sociodemographic data on the parents, including marital status, country of birth and years of education.
Only children treated with risperidone for more than 2 weeks during the hospitalization period were considered risperidone-treated patients (n=78). Information regarding dose and duration of treatment was also recorded.
To allow specificity of findings to risperidone, we excluded from the analysis five children who received other SGAs (olanzapine (n=2) and quetiapine (n=3)). Stimulants included methylphenidate, a dopamine and norepinephrine reuptake inhibitor, or amphetamine, a dopamine and norepinephrine reuptake inhibitor and dopamine releaser. Participants were not treated with other psychotropic drugs that are associated with weight gain like valproate and lithium.
Statistical analysis
To assess the association of risperidone treatment with change in BMI Z-score, we implemented mixed models with random subject intercepts to account for repeated measures. In all models, effect size of risperidone exposure on change in BMI Z-score is represented by standardized beta values.
A major strength of the mixed model framework is that it benefits from data of all time-points. Thus for each BMI measurement point, the model includes data on multiple factors that could have potentially confounded the relationship between risperidone and BMI, including effects of sex (Martínez-Ortega et al., 2013) and effects of baseline BMI (Calarge et al., 2009; Gebhardt et al., 2009).
In addition, the mixed model accounts for all baseline characteristics, including use of risperidone at baseline and its dose (that was reported for n=21).
The first model included age and sex as covariates, plus an interaction between sex and risperidone use. The second model included the same variables as the first, but with the addition of stimulant use as well as mother education and patient birth weight as covariates. The third model included the same variables as the second model, but with the addition of clinical diagnoses as covariates.
Next, in the subgroup of children who received risperidone treatment (n=78), we examined the association of change in BMI Z-score with treatment duration and dose. These analyses – also mixed models accounting for repeated measures – included BMI Z-score as the dependent variable, treatment duration or daily dose in milligrams (mg) as the primary independent variable, and the interaction of sex with duration or with dose. Age, stimulant use, mother’s education and patient birth weight were included as covariates.
Results
Of the 151 patients admitted to the preschool ward during the study period, 148 (98%) patients met inclusion criteria (three patients had fewer than two BMI measurements). Two patients (one boy and one girl) with a primary diagnosis of eating disorder were excluded from the final analyses due to extremely low baseline BMI Z-score (−5.02 and −3.47, respectively). The final sample was composed of 141 preschoolers (112 boys and 29 girls) with a mean baseline age of 5.0 years (SD=0.8) and mean follow-up period of 1.3 years (SD=0.8), and participants had mean 8.3 (SD=5.4) BMI measurements during the study period. Demographic and clinical characteristics are detailed in Table 1. The sample was overall homogeneous in terms of sociodemographics, with most participants from two-parent families, with an average of over 12 years of mean parental education and non-immigration status (Israel born). Pregnancy characteristics were typical with mean 38 weeks of gestation and mean birthweight of 3 kg. Clinically, the most common psychiatric diagnoses were: attention deficit hyperactivity disorder (ADHD) (76.7%); mixed specific developmental disorder (35.6%); oppositional defiant disorder (30.8%); and autism (16.4%), with most patients having more than one psychiatric diagnosis (n=84, 57.5%). A minority of the children were on risperidone at baseline (n=21, 14.9% of sample), and n=11 (7.5%) on stimulants. Baseline standardized BMI Z-score was 0.07, as expected.
Table 1.
Sociodemographic and clinical characteristics of study population.
| Sample size | N=141 | |
|---|---|---|
| Demographics | Mean (n) | SD (%) |
| Age, years | 5 | 0.8 |
| Mother education, years | 13.2 | 2.6 |
| Father education, years | 12.4 | 3 |
| Sex | ||
| Male | 112 | 79.4 |
| Female | 29 | 20.6 |
| Parents’ marital status | ||
| Married | 103 | 73 |
| Divorced | 27 | 19.1 |
| Single parent | 11 | 7.8 |
| Parents’ place of birth | ||
| Father Israel born | 111 | 78.7 |
| Mother Israel born | 110 | 78 |
| Baseline medication | ||
| On risperidone | 21 | 14.9 |
| On methylphenidate | 10 | 7.1 |
| Baseline growth | ||
| Weight, kg | 194 | 4.1 |
| Height, cm | 110 | 7.5 |
| BMI Z-scorea | 0.1 | 1.4 |
| Follow-up characteristics | ||
| Duration, years | 1.3 | 0.8 |
| Number of BMI measurements | 8.3 | 5.4 |
| Clinical diagnoses | ||
| Hyperkinetic disorder (ADHD) | 108 | 76.6 |
| Mixed specific developmental disorders | 50 | 35.5 |
| Oppositional defiant disorder | 45 | 30.8 |
| Autism | 23 | 16.3 |
| Anxiety disorders | 17 | 12.1 |
| Post-traumatic stress disorder | 6 | 4.3 |
| Mood disorders | 4 | 2.8 |
ADHD: attention deficit hyperactivity disorder; BMI: body mass index; SD: standard deviation.
Age- and sex-standardized body mass index.
Association of risperidone exposure and change in BMI Z-score
Throughout the study period, 83 patients were treated with SGAs. The majority of them were treated with risperidone (n=78, 94% of SGA-exposed patients), three with quetiapine and two with olanzapine. Owing to the small sample of non-risperidone SGAs, only risperidone-treated children were included in the final analyses. Exposure to risperidone was associated with significant increase in BMI (repeated measures-mixed model effect size was 0.42 (SE=0.06), t (1081)=5.78, p<0.001), covarying for sex and age at risperidone exposure. Addition of stimulant exposure (n=79, 54% of sample) did not confound the association of risperidone with BMI score (effect size=−0.24, model covaried for maternal education and patient birthweight). As expected, exposure to stimulants was associated with decrease in BMI score (effect size=−0.24). The risperidone-related BMI increase was independent of psychiatric diagnoses (effect size for risperidone=0.45, similar to previous models). Full model statistics are presented in Table 2.
Table 2.
Association of risperidone treatment with change in BMI.
| Model a |
Model b |
Model c |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Effect size | Std error | t | p-value | Effect size | Std error | t | p-value | Effect size | Std error | t | p-value | |
| Risperidone | 0.42 | 0.06 | 7.31 | <0.001 | 0.46 | 0.06 | 7.78 | <0.001 | 0.45 | 0.06 | 7.7 | <0.001 |
| Sex | 0.22 | 0.25 | 0.87 | 0.384 | 0.33 | 0.27 | 1.25 | 0.213 | 0.36 | 0.29 | 1.3 | 0.21 |
| Stimulantsa | −0.24 | 0.05 | −4.51 | <0.001 | −0.24 | 0.05 | −4.55 | <0.001 | ||||
| Risperidone × sex interaction | 2.57 | 0.01 | 2.26 | 0.024 | 2.32 | 0.02 | ||||||
Model a covaries for age.
Model b covaries for age, mother education, patient birth weight and stimulant use.
Model c includes all covariates from model b with the addition of clinical diagnoses.
Stimulants include methylphenidate (n=70) and amphetamines (n=9).
Sex effects on SGA-associated BMI Z-score increase
The study cohort included 112 boys and 29 girls, allowing investigation of sex effects in association with risperidone BMI Z-score increase. While risperidone was associated with increase in BMI Z-score in both sexes, the increase in girls was more pronounced (Figure 1, risperidone × sex interaction t (1076)=2.56, p=0.011). This interaction remained significant in models covarying for stimulant use, demographics and psychiatric diagnoses (Table 2).
Figure 1: Association of risperidone use and BMI across sexes.
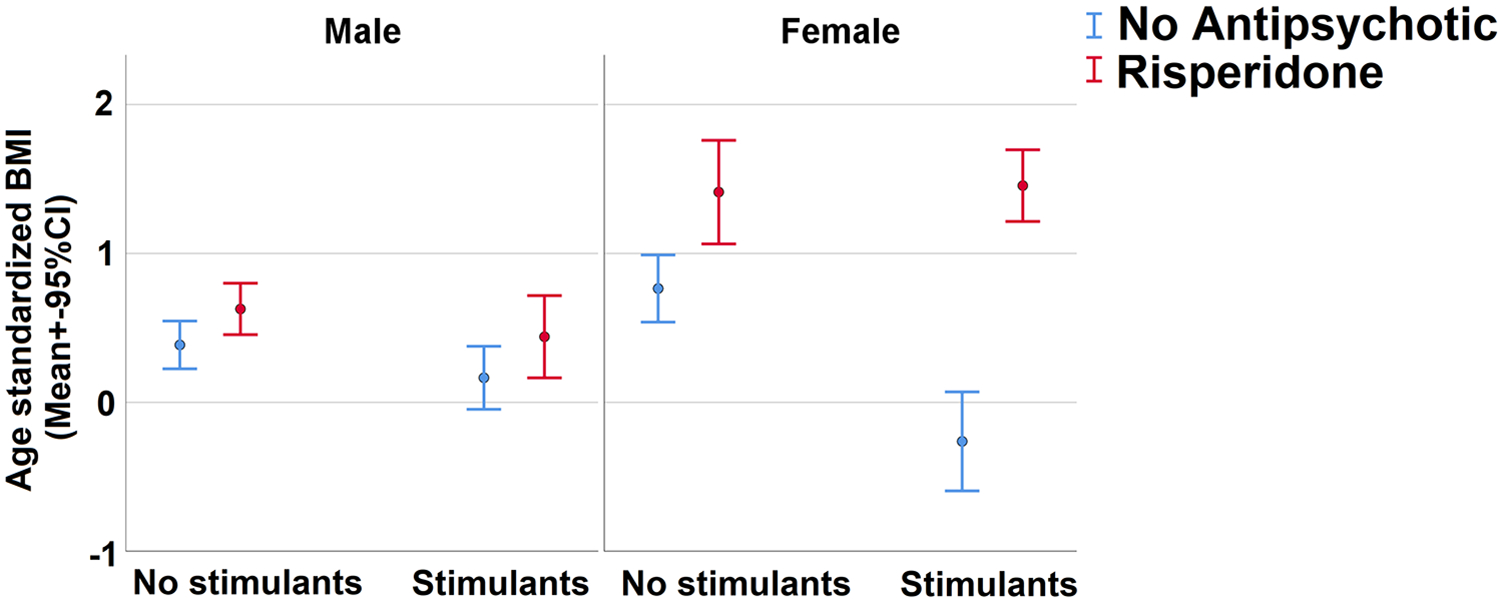
Sex differences stratified by stimulant use. Sample included 117 males (mean 7.7 BMI measurements per patient) and 29 females (mean 10.6 BMI measurements per patient). BMI=Body mass index.
Dose and duration associations of risperidone and change in BMI Z-score
We investigated effects of dose and duration of treatment on BMI Z-scores within the population that received SGA. Since the majority of children received risperidone (n=78, 94% of children receiving SGA), we limited the analyses of dose and duration effects to risperidone. We employed two repeated measures-mixed models (one for dose and one for duration effects), covarying for age, sex, stimulant use, maternal education and patient birth weight.
Among the children receiving risperidone, the mean daily dose was 1.01 mg (SD=0.57, range 0.125–5 mg). We found a significant main effect for risperidone dose in association with BMI Z-score increase (for each additional 1 mg, effect size=0.28 (SE=0.07), t(419)=3.76, p<0.001). Dose effect was slightly stronger in females (Figure 2, dose × sex interaction, t=2.012, p=0.045). Evaluation of duration effect revealed no significant main effect for duration of risperidone treatment on increase in BMI Z-score (for each additional week on risperidone, p=0.24).
Figure 2: Association of risperidone dose with BMI.
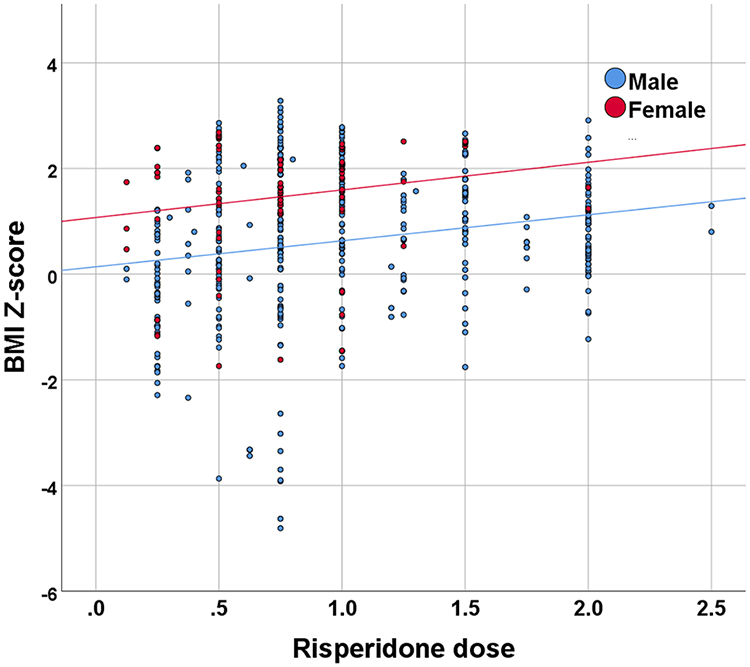
Risperidone dose effects in preschoolers (n=78). Sample included 66 males and 12 females (mean 6.5 and 8.3 BMI measurements per patient on risperidone, respectively). BMI=Body mass index.
Discussion
The metabolic consequences of SGA treatment pose a major health concern, especially in young populations. Here we describe the association of increase in BMI Z-score with risperidone exposure in preschoolers in a dose-dependent manner for risperidone treatment. Risperidone-related BMI Z-score increase was independent of multiple confounders including demographics, concomitant stimulant treatment and the child’s diagnoses. To our knowledge this study is the first to longitudinally evaluate association of risperidone treatment with change in BMI in preschoolers (mean age at beginning of follow-up was 5). In addition, as the population received daily care for the entire follow-up period, we were able to longitudinally follow (1.3 years) a relatively large sample composed of 141 children (29 girls), with multiple BMI measurements (more than eight on average), across a dynamic period with frequent dosage changes. This allowed a unique opportunity to study sex effects and treatment dose and duration effects, while not having the confounding effect of adherence that is a problem in outpatient settings (i.e., in our study all children received risperidone at the hospital in the presence of a nurse).
To our knowledge, there is only one study that reported SGA-associated metabolic effects in preschoolers (Kauffman et al., 2017), which focused on the implementation of metabolic monitoring and follow-up on a group of 40 preschool (nine girls) outpatients. This study had limited baseline weight monitoring as only 23 out of the 40 children had baseline weight measurements prior to risperidone prescription. The only literature to report more careful metabolic follow-up in children treated with SGA comes from older populations (6.9–14 years), mostly focusing on children and adolescents with ASD and disruptive behaviours (Aman et al., 2015; Arango et al., 2014; Martin et al., 2000; Pozzi et al., 2019; Reyes et al., 2006; Scahill et al., 2016; Yoon et al., 2016). Our findings add to the literature concerning SGA-induced weight gain in older populations (Correll et al., 2015; Gothelf et al., 2002; Ratzoni et al., 2002; Spertus et al., 2018) and add to the scarce data reporting SGA-related weight gain in young children with autism (Aman et al., 2015; Yoon et al., 2016).
Most preschoolers who are treated with SGAs are boys (Baird et al., 2006; Croonenberghs et al., 2005; Demmer et al., 2017; Johnson et al., 2007). This is primarily because the most common indications for SGA treatment in childhood is ASD, and the most common off-label indication is externalizing behaviour – both predominantly affect boys of preschool age. Thus, our sample (29 girls and 112 boys) provided a unique opportunity to study sex effects in SGA-related weight gain. We found that female sex was associated with greater risperidone-related BMI Z-score increase, and that this BMI Z-score increase in females had greater sensitivity to dose (evaluated only for risperidone). Little data exist on sex differences in SGA-related weight gain. A recent meta-analysis of adult samples reported no difference in weight gain between male and female patients treated with SGAs (Schoretsanitis et al., 2018), and a similar result was reported in adolescent patients treated with risperidone (Kelly et al., 1998), and in children and adolescents with early-onset schizophrenia spectrum disorders (Taylor et al., 2018). In contrast, a single study reported that male sex is a risk factor for weight gain in adolescents treated with risperidone and olanzapine (Ratzoni et al., 2002). It is possible that there is a developmental aspect of the sex effects in risperidone-induced weight gain, which may explain the discrepancy of our findings with existing literature. More research is needed to better understand the sex-specific developmental effects of SGA use in children and adolescents.
Dose effects of SGA-related weight gain have been described in adult populations (Simon et al., 2009; Spertus et al., 2018). Data on dose effects in the paediatric population are scarce. The Second-Generation Antipsychotic Treatment Indications, Effectiveness and Tolerability in Youth (SATIETY) study reported that risperidone treatment in children (n=168) in doses higher than 1.5 mg per day is associated with weight gain in a dose-dependent manner (Correll et al., 2015). We report here that risperidone is associated with a dose-dependent increase in BMI Z-score. Notably, the vast majority of our patients received doses lower than 1.5 mg per day (mean risperidone dose in our sample was 1.01 mg per day). It is possible that the longer follow-up (1.3 years in our study and 12 weeks in SATIETY) with multiple BMI measurements in our study allowed us to detect the risperidone dose effects associated with BMI Z-score increase. In summary, our findings support the notion that paediatric risperidone treatment is associated with BMI increase in a dose-dependent manner.
Relatively strong evidence exists regarding weight gain upon initiation of SGA treatment. The typical trajectory in adult patients treated with SGAs shows a rapid increase in the first 12 weeks of treatment gradually reaching a plateau within 1 year (Correll et al., 2015). Children and adolescent patients show a similar vulnerability to SGA-induced weight gain (Taylor et al., 2018). In both children and adults, drug-naïve patients may be more likely to gain weight compared to chronic patients. Patients younger than 7 years may be more vulnerable than children older than 7 years (Correll et al., 2015; Scahill et al., 2016). In our study, we found no significant effect of duration of treatment on weight gain. We suggest that our findings regarding duration effects should be interpreted with caution. Because our study was a retrospective analysis of naturalistically collected data, it is likely that those who showed rapid weight gain following risperidone initiation were soon taken off the medication. The longitudinal measurements are therefore biased to include children who did not suffer risperidone-related weight gain, and consequently no duration effect emerged. Future prospective studies should aim to clarify the duration effects.
Given the growing use of SGA in young populations for off-label indications (Olfson et al., 2010, 2012; Patel et al., 2005; Pringsheim et al., 2019; Ronsley et al., 2013), our findings have critical clinical implications. First, when considering risk and benefits of SGA treatment in young age groups, and particularly when discussing with parents, physicians often find themselves in a difficult position as they have limited data to rely on. In that sense, our study in preschoolers with longitudinal follow-up fills a much-needed clinical gap. Second, once the decision to prescribe an SGA like risperidone has been made, our data suggest that the dosage is a variable that should be considered when discussing the metabolic risks of treatment. Notably, in the absence of data showing dose response association with weight gain, clinicians find themselves in a situation whereby the metabolic risks of dose increase are relatively unknown compared to the initiation of SGA treatment. Our findings provide initial evidence that keeping dosage as low as possible when treating young children with risperidone is warranted. Third, our findings suggest that special attention should be given to female preschoolers as they may be at particularly high risk for antipsychotic-related weight gain.
To balance the concerns discussed above, it is important to note the context in which data were collected for this study. We included preschoolers who were at the extreme end of the clinical spectrum that resulted in day-hospitalization of children at a very young age. It is evident that for this population, treatment with risperidone was offered after all other psychosocial and educational interventions had taken place, and that the risks of not treating the children were substantial for the long-term developmental trajectory (e.g., severe maladjustment, violence, social rejection). Therefore, we emphasize that risk and benefit consideration concerning SGA treatments should always be made on a case-by-case basis.
Strengths of our study include the large sample size and the long follow-up compared to existing literature in young populations. In addition, the setting in which the data were collected inherently controls for multiple biases and confounders: (1) the children were in a hospital setting on a daily basis, hence the administration of the risperidone was done by the nursing staff resulting in high adherence to the SGA medications; (2) the children included in the study spent the majority of the day in the Preschoolers’ Department and had similar meal options and daily activities for the entire study period; and (3) the children’s BMI measurements were conducted by the same nurse using the same procedure for the entire study period.
Our findings should be interpreted in the context of study limitations. The major limitation is the study’s naturalistic retrospective design, which could result in a bias related to maintenance of SGA treatment based on observed weight gain (i.e., if a child gains weight, he/she will be taken off the drug and will have fewer measurements showing increase in weight, whereas the dataset may be enriched for those who did not gain weight). This type of bias suggests that risperidone-associated weight gain may be more severe than our findings. In addition, the naturalistic design results in inconsistent numbers of BMI measurements (and their time intervals) and varied follow-up durations. We tried to address this limitation through the employment of the repeated measure mixed model that best handles missing values (i.e. no need for listwise deletion). Last, even though we had a large sample relative to the extant literature, the numbers limit the type of analyses that can be performed; specifically, the sample included only 29 girls, limiting our ability to study sex effects, especially in the models studying dose and duration effect of risperidone.
In conclusion, we report the association of risperidone treatment in preschoolers with increase in BMI. Our findings suggest that preschool-aged patients should be routinely evaluated for weight gain and metabolic profile throughout follow-up. Careful consideration should be given to increases in risperidone doses. Parental education regarding the risk for weight gain (and potentially corresponding metabolic complications) and available weight management programmes (Taylor et al., 2017) is warranted.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: RB gratefully acknowledges support from the National Institutes of Mental Health under Award Number K23MH-120437 and from the Lifespan Brain Institute of CHOP and Penn Medicine. JHT gratefully acknowledges support from the National Institutes of Health (KL2TR001879), Brain and Behavior Research Foundation NARSAD Young Investigator Award, and American Academy of Child and Adolescent Psychiatry Pilot Award. The funding organization had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RB serves on the scientific board of ‘Taliaz Health’. RB and MA report stock ownership in ‘Taliaz Health’, with no conflict of interest relevant to this work.
All the authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Aman M, Rettiganti M, Nagaraja HN, et al. (2015) Tolerability, safety, and benefits of risperidone in children and adolescents with autism: 21-month follow-up after 8-week placebo-controlled trial. J Child Adolesc Psychopharmacol 25: 482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson GM, Scahill L, McCracken JT, et al. (2007) Effects of short- and long-term risperidone treatment on prolactin levels in children with autism. Biol Psychiatry 61: 545–550. [DOI] [PubMed] [Google Scholar]
- Arango C, Giráldez M, Merchán-Naranjo J, et al. (2014) Second-generation antipsychotic use in children and adolescents: A six-month prospective cohort study in drug-naïve patients. J Am Acad Child Adolesc Psychiatry 53: 1179–1190.e1–4. [DOI] [PubMed] [Google Scholar]
- Baird G, Simonoff E, Pickles A, et al. (2006) Prevalence of disorders of the autism spectrum in a population cohort of children in South Thames: The Special Needs and Autism Project (SNAP). Lancet 368: 210–215. [DOI] [PubMed] [Google Scholar]
- Bak M, Fransen A, Janssen J, et al. (2014) Almost all antipsychotics result in weight gain: A meta-analysis. PLoS One 9: e94112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calarge CA, Acion L, Kuperman S, et al. (2009) Weight gain and metabolic abnormalities during extended risperidone treatment in children and adolescents. J Child Adolesc Psychopharmacol 19: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascade E, Kalali A, Findling R (2009) Use of antipsychotics in children. Psychiatry (Edgmont (Pa. : Township)) 6: 21–23. [PMC free article] [PubMed] [Google Scholar]
- Consoli A, Deniau E, Huynh C, et al. (2007) Treatments in child and adolescent bipolar disorders. Eur Child Adolesc Psychiatry 16: 187–198. [DOI] [PubMed] [Google Scholar]
- Correll CU, Detraux J, De Lepeleire J, et al. (2015) Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry 14: 119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Manu P, Olshanskiy V, et al. (2009) Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 302: 1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Penzner JB, Parikh UH, et al. (2006) Recognizing and monitoring adverse events of second-generation antipsychotics in children and adolescents. Child Adolesc Psychiatr Clin N Am 15: 177–206. [DOI] [PubMed] [Google Scholar]
- Croonenberghs J, Fegert JM, Findling RL, et al. (2005) Risperidone in children with disruptive behavior disorders and subaverage intelligence: A 1-year, open-label study of 504 patients. J Am Acad Child Adolesc Psychiatry 44: 64–72. [DOI] [PubMed] [Google Scholar]
- Demmer DH, Hooley M, Sheen J, et al. (2017) Sex differences in the prevalence of oppositional defiant disorder during middle childhood: A meta-analysis. J Abnorm Child Psychol 45: 313–325. [DOI] [PubMed] [Google Scholar]
- DeVane CL, Charles JM, Abramson RK, et al. (2019) Pharmacotherapy of autism spectrum disorder: Results from the randomized BAART clinical trial. Pharmacotherapy 39: 626–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findling RL, Johnson JL, McClellan J, et al. (2010) Double-blind maintenance safety and effectiveness findings from the Treatment of Early-Onset Schizophrenia Spectrum (TEOSS) study. J Am Acad Child Adolesc Psychiatry 49: 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt S, Haberhausen M, Heinzel-Gutenbrunner M, et al. (2009) Antipsychotic-induced body weight gain: Predictors and a systematic categorization of the long-term weight course. J Psychiatr Res 43: 620–626. [DOI] [PubMed] [Google Scholar]
- Gorman DA, Gardner DM, Murphy AL, et al. (2015) Canadian guidelines on pharmacotherapy for disruptive and aggressive behaviour in children and adolescents with attention-deficit hyperactivity disorder, oppositional defiant disorder, or conduct disorder. Can J Psychiatry 60: 62–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gothelf D, Falk B, Singer P, et al. (2002) Weight gain associated with increased food intake and low habitual activity levels in male adolescent schizophrenic inpatients treated with olanzapine. Am J Psychiatry 159: 1055–1057. [DOI] [PubMed] [Google Scholar]
- Johnson CP, Myers SM, Lipkin PH, et al. (2007) Identification and evaluation of children with autism spectrum disorders. Pediatrics 120: 1183–1215. [DOI] [PubMed] [Google Scholar]
- Kauffman YS, Delate T, Botts S (2017) Metabolic monitoring in children 5 years of age and younger prescribed second-generation antipsychotic medications. Ment Health Clin 7: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly DL, Comely RR, Love RC, et al. (1998) Weight gain in adolescents treated with risperidone and conventional antipsychotics over six months. J Child Adolesc Psychopharmacol 8: 151–159. [DOI] [PubMed] [Google Scholar]
- Kennedy E, Kumar A, Datta SS (2007) Antipsychotic medication for childhood-onset schizophrenia. Cochrane Database Syst Rev 2007: CD004027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Landau J, Leebens P, et al. (2000) Risperidone-associated weight gain in children and adolescents: A retrospective chart review. J Child Adolesc Psychopharmacol 10: 259–268. [DOI] [PubMed] [Google Scholar]
- Martínez-Ortega JM, Funes-Godoy S, Díaz-Atienza F, et al. (2013) Weight gain and increase of body mass index among children and adolescents treated with antipsychotics: A critical review. Eur Child Adolesc Psychiatry 22: 457–479. [DOI] [PubMed] [Google Scholar]
- Olfson M, Blanco C, Liu S-M, et al. (2012) National trends in the office-based treatment of children, adolescents, and adults with antipsychotics. Arch Gen Psychiatry 69: 1247–1256. [DOI] [PubMed] [Google Scholar]
- Olfson M, Crystal S, Huang C, et al. (2010) Trends in antipsychotic drug use by very young, privately insured children. J Am Acad Child Adolesc Psychiatry 49: 13–23. [DOI] [PubMed] [Google Scholar]
- Parsons B, Allison DB, Loebel A, et al. (2009) Weight effects associated with antipsychotics: A comprehensive database analysis. Schizophr Res 110: 103–110. [DOI] [PubMed] [Google Scholar]
- Patel NC, Crismon ML, Hoagwood K, et al. (2005) Trends in the use of typical and atypical antipsychotics in children and adolescents. J Am Acad Child Adolesc Psychiatry 44: 548–556. [DOI] [PubMed] [Google Scholar]
- Pozzi M, Pisano S, Marano G, et al. (2019) Weight-change trajectories of pediatric outpatients treated with risperidone or aripiprazole in a naturalistic setting. J Child Adolesc Psychopharmacol 29: 133–140. [DOI] [PubMed] [Google Scholar]
- Pringsheim T, Stewart DG, Chan P, et al. (2019) The pharmacoepidemiology of psychotropic medication use in Canadian children from 2012 to 2016. J Child Adolesc Psychopharmacol 29: 740–745. [DOI] [PubMed] [Google Scholar]
- Ratzoni G, Gothelf D, Brand-Gothelf A, et al. (2002) Weight gain associated with olanzapine and risperidone in adolescent patients: A comparative prospective study. J Am Acad Child Adolesc Psychiatry 41: 337–343. [DOI] [PubMed] [Google Scholar]
- Reyes M, Buitelaar J, Toren P, et al. (2006) A randomized, double-blind, placebo-controlled study of risperidone maintenance treatment in children and adolescents with disruptive behavior disorders. Am J Psychiatry 163: 402–410. [DOI] [PubMed] [Google Scholar]
- Ronsley R, Nguyen D, Davidson J, et al. (2015) Increased risk of obesity and metabolic dysregulation following 12 months of second-generation antipsychotic treatment in children: A prospective cohort study. Can J Psychiatry 60: 441–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronsley R, Scott D, Warburton WP, et al. (2013) A population-based study of antipsychotic prescription trends in children and adolescents in British Columbia, from 1996 to 2011. Can J Psychiatry 58: 361–369. [DOI] [PubMed] [Google Scholar]
- Scahill L, Jeon S, Boorin SJ, et al. (2016) Weight gain and metabolic consequences of risperidone in young children with autism spectrum disorder. J Am Acad Child Adolesc Psychiatry 55: 415–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoretsanitis G, Drukker M, Van Os J, et al. (2018) No differences in olanzapine- and risperidone-related weight gain between women and men: A meta-analysis of short- and middle-term treatment. Acta Psychiatr Scand 138: 110–122. [DOI] [PubMed] [Google Scholar]
- Simon V, Van Winkel R, De Hert M (2009) Are weight gain and metabolic side effects of atypical antipsychotics dose dependent? A literature review. J Clin Psychiatry 70: 1041–1050. [DOI] [PubMed] [Google Scholar]
- Spertus J, Horvitz-Lennon M, Abing H, et al. (2018) Risk of weight gain for specific antipsychotic drugs: A meta-analysis. NPJ Schizophr 4: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Jakubovski E, Gabriel D, et al. (2018) Predictors and moderators of antipsychotic-related weight gain in the treatment of early-onset schizophrenia spectrum disorders study. J Child Adolesc Psychopharmacol 28: 474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Xu Y, Li F, et al. (2017) Psychosocial predictors and moderators of weight management programme outcomes in ethnically diverse obese youth. Pediatr Obes 12: 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon Y, Wink LK, Pedapati EV, et al. (2016) Weight gain effects of second-generation antipsychotic treatment in autism spectrum disorder. J Child Adolesc Psychopharmacol 26: 822–827. [DOI] [PubMed] [Google Scholar]


