Background:
Inhibiting SDH (succinate dehydrogenase), with the competitive inhibitor malonate, has shown promise in ameliorating ischemia/reperfusion injury. However, key for translation to the clinic is understanding the mechanism of malonate entry into cells to enable inhibition of SDH, its mitochondrial target, as malonate itself poorly permeates cellular membranes. The possibility of malonate selectively entering the at-risk heart tissue on reperfusion, however, remains unexplored.
Methods:
C57BL/6J mice, C2C12 and H9c2 myoblasts, and HeLa cells were used to elucidate the mechanism of selective malonate uptake into the ischemic heart upon reperfusion. Cells were treated with malonate while varying pH or together with transport inhibitors. Mouse hearts were either perfused ex vivo (Langendorff) or subjected to in vivo left anterior descending coronary artery ligation as models of ischemia/reperfusion injury. Succinate and malonate levels were assessed by liquid chromatography-tandem mass spectrometry LC-MS/MS, in vivo by mass spectrometry imaging, and infarct size by TTC (2,3,5-triphenyl-2H-tetrazolium chloride) staining.
Results:
Malonate was robustly protective against cardiac ischemia/reperfusion injury, but only if administered at reperfusion and not when infused before ischemia. The extent of malonate uptake into the heart was proportional to the duration of ischemia. Malonate entry into cardiomyocytes in vivo and in vitro was dramatically increased at the low pH (≈6.5) associated with ischemia. This increased uptake of malonate was blocked by selective inhibition of MCT1 (monocarboxylate transporter 1). Reperfusion of the ischemic heart region with malonate led to selective SDH inhibition in the at-risk region. Acid-formulation greatly enhances the cardioprotective potency of malonate.
Conclusions:
Cardioprotection by malonate is dependent on its entry into cardiomyocytes. This is facilitated by the local decrease in pH that occurs during ischemia, leading to its selective uptake upon reperfusion into the at-risk tissue, via MCT1. Thus, malonate’s preferential uptake in reperfused tissue means it is an at-risk tissue-selective drug that protects against cardiac ischemia/reperfusion injury.
Keywords: ischemia, mitochondria, myocardial infarction, reactive oxygen species, reperfusion
Novelty and Significance.
What Is Known?
Extensive succinate accumulation during ischemia and its subsequent rapid oxidation on reperfusion drives ischemia/reperfusion injury.
Preventing succinate accumulation during ischemia reduces damage on reperfusion.
Inhibiting succinate dehydrogenase with malonate is protective against ischemia/reperfusion injury, although its mechanism of entry into cardiomyocytes is undefined.
What New Information Does This Article Contribute?
The cardioprotective effect of malonate is dependent on its selective uptake into cardiomyocytes on reperfusion after an ischemic period.
Malonate entry into cardiomyocytes upon reperfusion is facilitated by the lowered pH and lactate exchange, which selectively drives malonate into cardiomyocytes as a monoanion via the monocarboxylate transporter MCT1 (monocarboxylate transporter 1). This is the first time malonate has been shown to be a substrate for MCT1.
Malonate selectively enters the at-risk tissue, sparing the nonischemic area on reperfusion. Thus, malonate is the first example of an at-risk tissue-selective, cardioprotective drug.
Determining the molecular basis for selective malonate entry via MCT1 into the ischemic heart upon reperfusion is a significant step toward treating cardiac ischemia/reperfusion injury. Malonate is cardioprotective in small and large animal models and MCT1 is highly expressed in the human heart. Furthermore, this mechanism can be exploited to increase malonate potency using an acidic formulation. The next step is to assess malonate as a treatment for cardiac ischemia/reperfusion injury in patients.
Meet the First Author, see p 475
Editorial, see p 542
Myocardial infarction (MI) and the consequential heart failure is a leading cause of mortality.1,2 Early reperfusion of the ischemic myocardium by primary percutaneous coronary intervention is essential to minimize cardiomyocyte death.3,4 However, paradoxically, the reintroduction of oxygen into the cardiac tissue accelerates cardiomyocyte death by ischemia/reperfusion (IR) injury thereby increasing tissue injury.5–9 Although patients now often survive their initial infarct, IR injury is a major driver of post-MI heart failure.1,5,10,11 Pharmacological interventions to reduce IR injury have remained elusive, likely as a result of a poor understanding of the pathology.12–16 Recent research on mitochondrial metabolic changes during IR injury has opened up new therapeutic opportunities.1,5,10,11,13,15,17
During ischemia, the mitochondrial metabolite succinate accumulates dramatically in ischemic tissue, reaching millimolar concentrations.18–20 The accumulation of succinate and degradation of purine nucleotides are now seen as hallmarks of ischemia conserved in many species, including humans.18,19,21 During reperfusion, the accumulated succinate is rapidly oxidized by the mitochondrial respiratory chain enzyme SDH (succinate dehydrogenase), driving reactive oxygen species (ROS) production by reverse electron transport (RET) at mitochondrial complex I.13,18,22,23 The production of ROS by RET upon reperfusion induces the mitochondrial permeability transition pore opening, initiating the significant cell death associated with IR injury. 13,18,22
This mechanism of RET-derived ROS driving IR injury was supported recently by the finding that a mouse model carrying a mutation (mtDNA [mitochondrial DNA] ND6 G14600A leading to ND6 P25L substitution) that inactivates RET is protected from cardiac IR injury.22 Blocking succinate accumulation using dimethyl malonate ester, a prodrug of the SDH inhibitor malonate, before ischemia was also cardioprotective.18,24,25 However, dimethyl malonate was not protective when infused at the clinically relevant point of reperfusion, due to its slow release of malonate; this could be circumvented using more rapidly hydrolyzed malonate ester prodrugs.25 The cell-permeable malonate esters were developed because malonate was assumed to be cell membrane impermeant. Therefore, it was surprising that malonate itself, administered as disodium malonate (DSM), was protective against cardiac IR injury in mouse ex vivo and pig in vivo.26,27 Malonate is a dicarboxylate carrying 2 negative charges at physiological pH (Figure 1A) and has poor membrane permeability. This is highlighted by the need for high concentrations and extended incubation times to see malonate-dependent effects in vitro.28 Therefore, the mechanism of DSM cardioprotection and thus its potential for translation to the clinic, is unclear (Figure 1B).
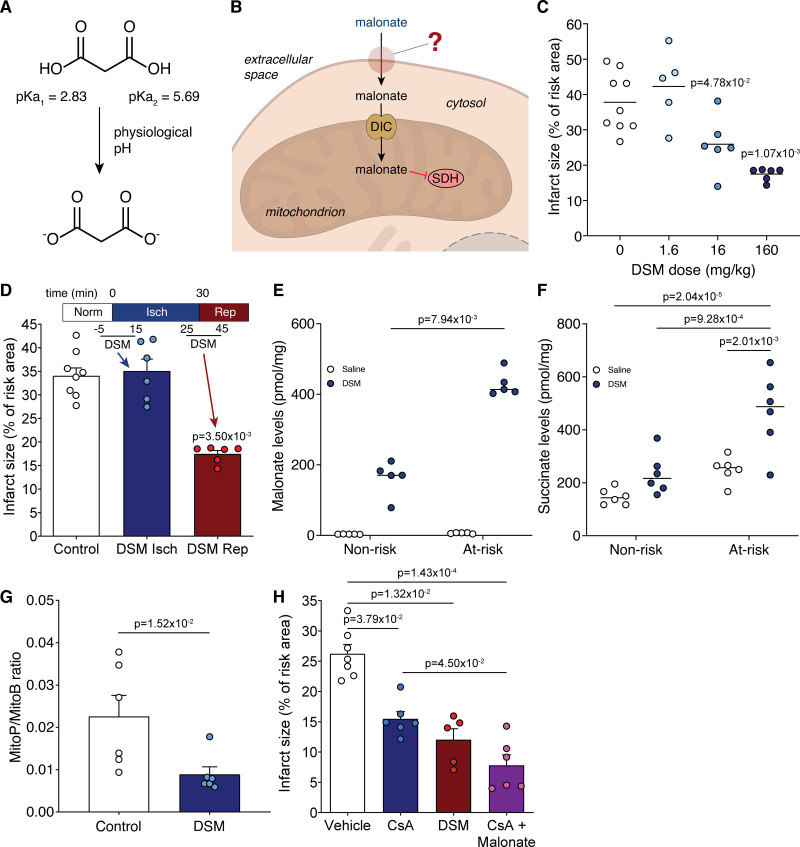
Figure 1.
Malonate is cardioprotective only when given at reperfusion. A, Malonate predominantly carries 2 negatively charged dicarboxylates at physiological pH. B, Schematic of the barriers to effective malonate delivery. C, Infarct size in murine LAD (left anterior descending coronary artery) ligation model with infusion of varying doses of disodium malonate (DSM; 0, 1.6, 16 or 160 mg/kg; n=9, 5, 6, 6 respectively) infused at reperfusion after 30 min ischemia, quantified by TTC (2,3,5-triphenyl-2H-tetrazolium chloride) staining. D, Infarct size in murine LAD model with infusion of DSM (160 mg/kg) at ischemia or at reperfusion (mean±SEM, n=8 (Control), 6 (DSM isch and DSM rep) biological replicates. E and F, Levels of malonate (E) and succinate (F) in nonrisk and at-risk tissue after 1 min reperfusion with either saline or DSM (160 mg/kg) after 30 min ischemia (mean±SEM, n=5 [E], 6 [F] biological replicates). Statistics: Kruskal-Wallis with Dunn post hoc test (C–E), 2-way ANOVA with Tukey post hoc test (F). G, MitoP/B ratio in at-risk heart tissue from LAD model after 30 min ischemia and 15 min reperfusion with either saline (Ctl) or DSM (160 mg/kg) infusion (mean±SEM, n=6 biological replicates, statistics: unpaired, 2-tailed Mann-Whitney U test) H, Infarct size in murine LAD model with infusion of vehicle (ethanol/Cremphor EL in saline)±CsA (cyclosporin A; 10 mg/kg) or DSM (160 mg/kg) at reperfusion (mean±SEM, n=7 [vehicle], 6 [CsA, CsA+malonate], 5 [DSM] biological replicates, statistics: Kruskal-Wallis with Dunn post hoc test). DIC indicates mitochondrial dicarboxylate carrier (SLC25A10); and SDH, succinate dehydrogenase.
Here, we set out to understand the mechanism by which DSM is cardioprotective. We found that under physiological pH (≈7.4), very high concentrations of malonate were required for SDH inhibition in cells. However, at lower pH malonate uptake into cells and the heart was considerably increased. Furthermore, malonate uptake into the heart increased with duration of ischemia, suggesting that prior exposure to ischemic conditions cumulatively enhances malonate uptake upon subsequent reperfusion. This was confirmed in vivo, as DSM was only protective when administered at reperfusion and not when given before the onset of ischemia. Finally, we showed that malonate entry into the heart upon reperfusion is selective for the ischemic area at risk due to the low pH and is mediated by the MCT1 (monocarboxylate transporter 1). This novel mechanism of targeting ischemic tissue upon reperfusion makes DSM an attractive option for the clinical treatment of IR injury.
Methods
Data Availability
Detailed methods and Major Resources Table can be found in the Supplemental Material. Data will be made available upon reasonable request, by contacting a corresponding author.
Results
Malonate Is Protective in an MI Model and Is Taken Up Selectively into the Ischemic Region of the Heart
DSM is protective against cardiac IR injury, but its mechanism of protection remains uncertain. To address this gap in our knowledge we first tested the therapeutic range of DSM in IR injury. We infused DSM at a range of concentrations (1.6–160 mg/kg equivalent to ≈11-1100 µmol/kg) at reperfusion in an in vivo murine LAD (left anterior descending coronary artery) ligation model of MI. DSM infusion during this clinically relevant reperfusion period led to a dose-dependent decrease in infarct size (Figure 1C), with 160 and 16 mg/kg showing robust cardioprotection. However, when DSM was infused before the onset of ischemia it was not protective (Figure 1D), even at 160 mg/kg which gave the smallest infarct when administered at reperfusion. This contrasted with the cell-permeable dimethyl malonate, which can diffuse into the tissue and generate malonate, preventing succinate accumulation and thereby reducing IR injury.18,25 This suggests that either DSM is minimally taken up by tissues during normoxia and is only taken up by tissues after ischemia or that DSM protects against acute IR injury by an extracellular mechanism.
We next measured malonate uptake into the ischemic and healthy heart tissue upon reperfusion in the LAD MI model. This showed that malonate was indeed taken up into cells within the infarct region to a level of ≈400 pmol/mg tissue and to a far greater extent than into cells in the healthy tissue (Figure 1E). Furthermore, reperfusing with malonate slowed succinate oxidation within the infarct region with succinate remaining significantly elevated in the infarct region following 1-minute reperfusion, compared with control reperfusion with saline (Figure 1F). Limiting succinate oxidation at reperfusion with malonate also blunted ROS production in the at-risk tissue (Figure 1G). We next assessed how SDH inhibition compared to direct inhibition of the mitochondrial permeability transition pore with CsA (cyclosporin A). Here we found that the cardioprotection from malonate was additive to cyclosporin A alone (Figure 1H), hence targeting the upstream mechanism of permeability transition pore opening is an increasingly attractive option for IR injury treatment.
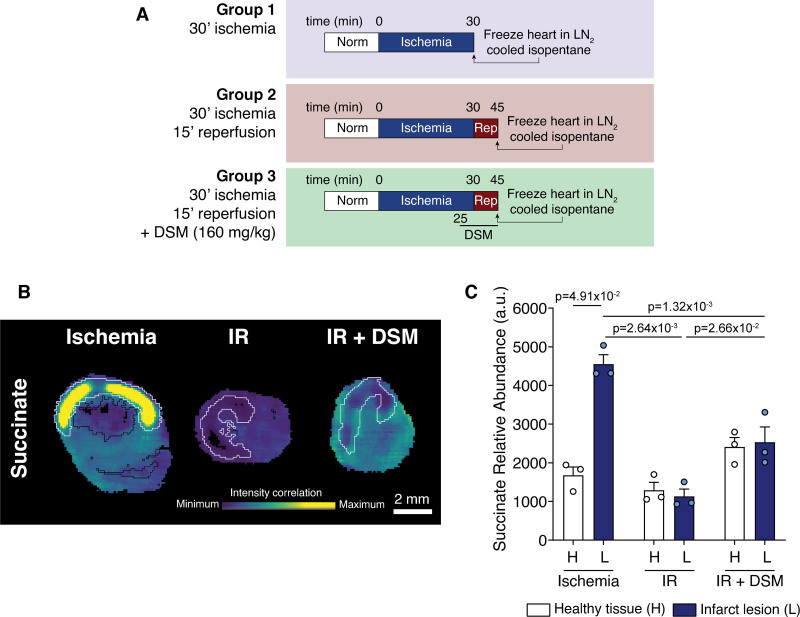
To better understand the metabolic differences between the healthy and infarcted tissue upon malonate treatment, we investigated the in vivo MI model using mass spectrometry imaging. To do this, we subjected hearts to either 30-minute ischemia, 30-minute ischemia before reperfusing the heart for 15-minute, or 30-minute ischemia before reperfusing with DSM for 15 minutes (to mimic the cardioprotective malonate infusion) before snap freezing and processing for mass spectrometry imaging (Figure 2A). Infarct lesions were demarcated using hematoxylin and eosin (H&E), silver infarct staining and metabolite principal component analysis to differentiate the risk and nonrisk regions. mass spectrometry imaging coupled with the demarcated risk areas showed striking changes in the levels of succinate in the infarct region. During ischemia, succinate was significantly elevated in the at-risk tissue, with considerable succinate accumulation in the core of the infarct (Figure 2B and 2C). After 15-minute reperfusion, the succinate levels in the infarct lesion returned to healthy tissue levels, due to succinate oxidation and efflux.21 When malonate was infused at the time of reperfusion, succinate levels remained higher in all regions of the heart than in the nonmalonate treated heart, consistent with the prevention of its oxidation by SDH inhibition by malonate.
Figure 2.
Localization of succinate accumulation in heart tissue identified by mass spectrometry imaging. A, Outline of experimental groups for mass spectrometry imaging. B, Representative images of succinate abundance detected by mass spectrometry imaging in myocardial sections. Black outer line indicates the edge of the tissue slice, white inner line indicates infarct region. C, Quantification of succinate (C) identified by MSI (mean±SEM, n=3 biological replicates, statistics: Friedman paired test for healthy vs lesion and Kruskal-Wallis with Dunn post hoc test between conditions). au indicates arbitrary units; DSM, disodium malonate; H, healthy tissue; IR, ischemia/reperfusion; L, infarct lesion; and rep, reperfusion.
We conclude that malonate is indeed taken up by the heart, but this is greatly enhanced in ischemic region upon reperfusion. Therefore, malonate provides protection against cardiac IR injury in vivo over a range of concentrations following its selective entry into the infarct region, where it slows the oxidation of succinate during reperfusion, preventing the production of RET-derived ROS.
Malonate Uptake into Cells Is Inefficient at pH 7.4
To explore the mechanism of malonate uptake in vitro, we incubated C2C12 and H9c2 myoblasts with DSM and measured intracellular malonate levels by LC-MS/MS (liquid chromatography-tandem mass spectrometry). Incubation with DSM for 15 minutes at pH 7.4, led to a dose-dependent increase in intracellular malonate (Figure S1A and S1D). Intracellular succinate also accumulated in a malonate-dependent manner (Figure S1B and S1E), confirming that once malonate enters the cell it is rapidly transported into mitochondria and inhibits SDH. However, incubating cells with 1 mmol/L DSM led to variable malonate uptake and little SDH inhibition, indicating that malonate uptake across biological membranes is inefficient. Moreover, the intracellular levels of malonate achieved by 250 µmol/L of the malonate ester prodrug diacetoxymethyl malonate, are 80-fold more than that generated by incubation with 5 mmol/L DSM.25 As DSM was protective in the LAD model when infused at 16 mg/kg, corresponding to a maximum possible blood malonate concentration of ≈1.8 mmol/L (assuming a blood volume of 1.5 mL in a 25 g mouse),29 this compares to the dose range tested in cells (furthermore, far more malonate is available for uptake in vitro than in vivo due to the large reservoir in the incubation medium). Even so, the intracellular malonate levels were far lower with DSM compared to diacetoxymethyl malonate.25
The kinetics of cell uptake upon incubation with 5 mmol/L DSM showed time-dependent uptake, although extended incubation times were required for succinate elevation (Figure S1C and S1F). This is likely due to the initial concentrations of malonate entering the cell being insufficient to inhibit SDH. These data are consistent with the lack of protection by DSM delivered before ischemia in vivo, as well as its limited uptake into normoxic tissues. Together these suggest that exposure to ischemia may facilitate malonate entry into the heart.
Malonate Uptake into Cells and the Heart Can Be Modulated by pH
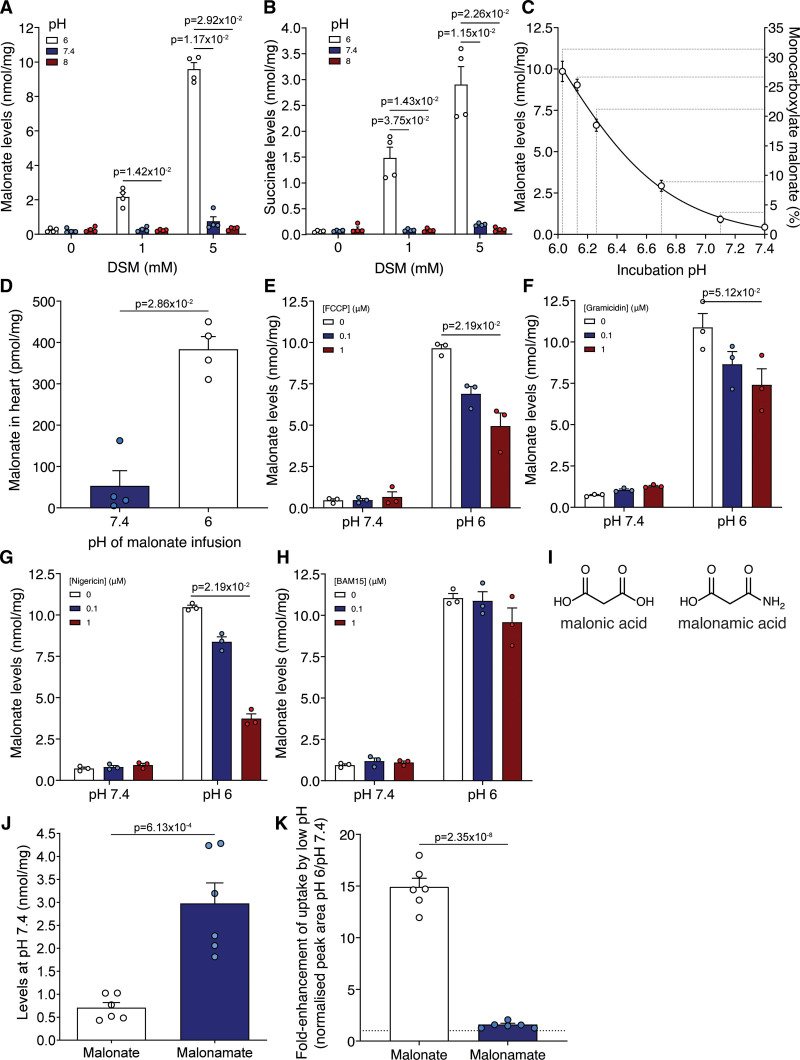
Malonate is a dicarboxylate at physiological pH (Figure 1A, pKa=2.83 and 5.69), suggesting that the pH decrease in ischemic tissue9,11,30 may enhance malonate uptake into the heart during early reperfusion, by increasing the concentration of its monocarboxylic form. Incubating cells with DSM at either pH 6, 7.4, or 8 for 15 minutes, led to large differences in malonate uptake (Figure 3A and Figure S2A). At pH 6, the levels of malonate in the cell were significantly higher than at pH 7.4 or 8, thus malonate uptake is favored by acidic pH. Succinate levels mirrored those of malonate, with greater succinate accumulation as a result of increased malonate-dependent SDH inhibition at low pH (Figure 3B and Figure S2B). Low pH alone had no effect on succinate levels (Figure 3B and Figure S2B), suggesting that it was due to malonate entry into the cells followed by SDH inhibition.
Figure 3.
Malonate uptake is enhanced at low pH. A and B, C2C12 cells were incubated with disodium malonate (DSM; 0, 1, or 5 mmol/L) for 15 min at either pH 6, 7.4, or 8 before measuring intracellular malonate (A) and succinate (B) by LC-MS/MS (liquid chromatography-tandem mass spectrometry) (mean±SEM, n=4 biological replicates, statistics: Kruskal-Wallis with Dunn post hoc test). C, Malonate levels in C2C12 cells after incubation with DSM (5 mmol/L) for 15 min at various (patho)physiological pH (mean±SEM, n=3 biological replicates). D, Malonate levels in murine isolated Langendorff-perfused hearts treated with 5 mmol/L DSM infused at either pH 7.4 or 6 for 5 min (mean±SEM, n=4 biological replicates, statistics: unpaired, 2-tailed Mann-Whitney U test). E to H, C2C12 cells were incubated with DSM (5 mmol/L) for 15 min at either pH 6 or 7.4 in the presence of FCCP (carbonyl cyanide-p-trifluoromethoxyphenylhydrazone) (E), gramicidin (F), nigericin (G), or BAM15 (N5,N6-bis(2-Fluorophenyl)-[1,2,5]oxadiazolo[3,4-b]pyrazine-5,6-diamine) (H) before measuring intracellular malonate by LC-MS/MS (mean±SEM, n=3 biological replicates, statistics: Kruskal-Wallis with Dunn post hoc test). I, Structures of malonic acid and malonamic acid. J, Quantification of levels of malonate and malonamate in C2C12 cells after incubation (5 mmol/L DSM or disodium malonamate; 15 min) at pH 7.4 (n=6 biological replicates). K, C2C12 cells were incubated with either DSM or disodium malonamate (both 5 mmol/L) at pH 6 or 7.4 for 15 min and the intracellular levels measured by LC-MS/MS. (mean±SEM of the fold-enhancement of uptake at pH 6 vs pH 7.4, n=6 biological replicates). Statistics for J and K: unpaired, 2-tailed Student t test).
Incubating cells with malonate over a range of pH values between pH 7.4 and 6 clearly showed the pH-dependent uptake of malonate, in line with the increase in monocarboxylate malonate proportion (Figure 3C). This was mirrored by succinate levels (Figure S2C). Therefore, even a small drop in pH can lead to a substantial increase in malonate uptake, and thus is likely to be relevant for its entry into ischemic tissue upon reperfusion.
As the cultured cells used are noncontractile and substantially differ in their properties from in situ contracting cardiomyocytes,15 importantly, we next assessed whether decreasing the pH also enhanced malonate uptake into the ex vivo perfused Langendorff heart. Malonate was infused into the heart at either pH 7.4 or 6 for 5 minutes, before briefly flushing at pH 7.4 to remove nonmyocardial malonate. Remarkably, the levels of malonate were significantly elevated when infused at pH 6 compared to 7.4, confirming that the in vitro results translate to the heart (Figure 3D). We conclude that low pH conditions facilitate the entry of malonate into cardiomyocytes.
Malonate Uptake Can Be Perturbed by Modulating the Plasma Membrane H+ Gradient
As pH modulated malonate entry into cells in vitro and in the heart, we next probed the factors affecting malonate uptake into cells. Malonate entry into cells at either pH 7.4 or 6 was blocked at 4 °C and was associated with negligible succinate levels (Figure S2D and S2E). That a decrease in extracellular pH increased malonate entry into cells, suggested uptake driven by the proton gradient. Therefore, we next abolished the plasma membrane proton gradient using ionophores21 which prevented the cellular uptake of malonate (Figure 3E–3G and Figure S3A) and subsequent increase in succinate levels (Figure S3B through S3E). However, the uncoupler BAM15, which is selective for the mitochondrial inner membrane over the plasma membrane,31 had little effect, consistent with the plasma membrane proton gradient driving malonate uptake (Figure 3H and Figure S3F).
We next used nonspecific transport inhibitors to assess whether we could block malonate uptake at low pH. DIDS (4,4’-Diisothiocyano-2,2’-stilbenedisulfonic acid), an irreversible inhibitor of chloride/bicarbonate exchange that inhibits malonate uptake into erythrocytes,32,33 led to a dose-dependent inhibition of malonate uptake, although succinate remained high (Figure S3G and S3H). Thus, malonate uptake into cardiomyocytes at low pH is driven by a proton gradient through a transporter-dependent process.
Malonate’s pKa’s are 2.83 and 5.69, so at pH 6.4, ≈16% of the malonate would be in its monocarboxylate form. To determine whether the uptake of malonate was dependent on its protonation to a monocarboxylate form, we assessed the uptake of a compound that mimics the monocarboxylate malonate. In 3-amino-3-oxopropionate (malonamate; Figure 3I), one carboxylic acid has been replaced with a neutral amido group leaving a single carboxylic acid of pKa ≈4.75. Thus, at pH 7.4 malonamate resembles the monocarboxylate form of malonate. This led to more of malonamate being taken up into cells at pH 7.4 than malonate (Figure 3J). Lowering the pH to 6 led to ≈15-fold increase in malonate uptake while malonamate uptake changed negligibly (Figure 3K) because it will remain as a monocarboxylate across this pH range.
Together, these data support transport of the monoanionic form of malonate, under the conditions that occur during early reperfusion. Therefore, a pH gradient can drive the uptake of malonate via protonation and transport in its monocarboxylate form.
Malonate Uptake Under Reperfusion Conditions Is Dependent on MCT1
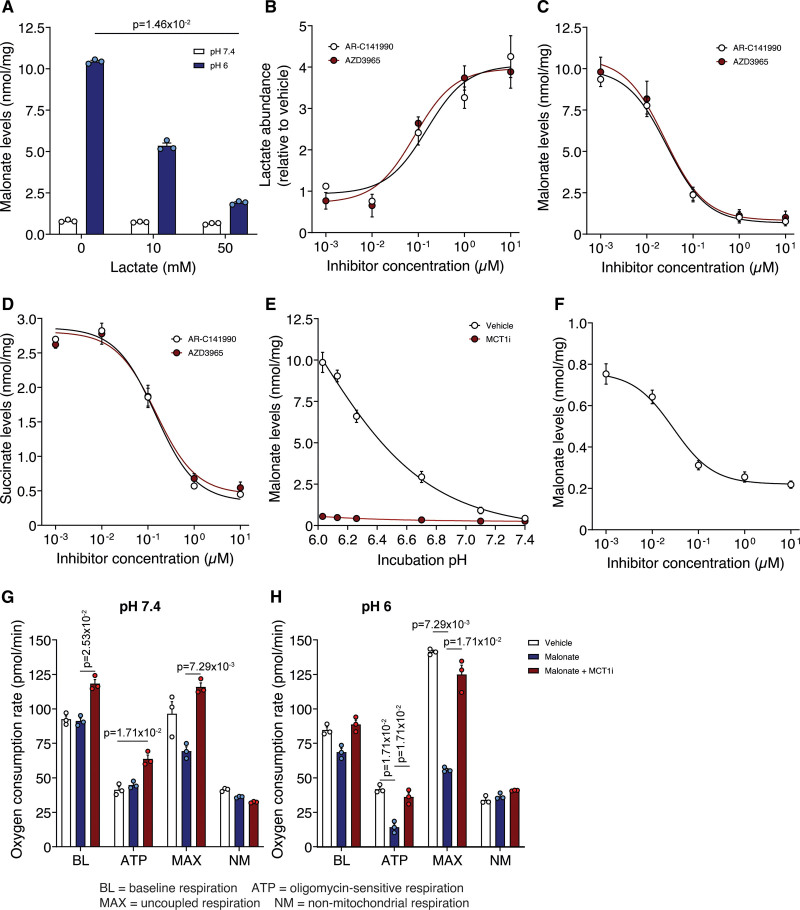
The inhibitory effect of DIDS, which also inhibits MCT1,34 raised the possibility of the uptake of the protonated form of malonate being catalyzed by a monocarboxylate transporter (MCT; SLC16 (solute carrier family member 16)). MCT1 (SLC16A1) is the principal transporter of lactate, which is carried in symport with a proton, both of which are elevated during ischemia, making MCT1 an attractive candidate transporter for malonate uptake upon reperfusion. Additionally, it was recently shown that MCT1 mediates the efflux of succinate from the ischemic heart during reperfusion and from exercising muscle.21,35 As MCT1 is a lactate transporter, we first assessed whether high concentrations of lactate in the incubation medium compete with malonate. Excess lactate led to a concentration-dependent decrease in malonate uptake into the cell and a corresponding decrease in succinate accumulation (Figure 4A and Figure S4A). This suggested that at a low pH, malonate uptake into the cells is via MCT1, which is highly expressed in cardiomyocytes.36
Figure 4.
Inhibition of MCT1 (monocarboxylate transporter 1) prevents the enhanced uptake of malonate at lowered pH. A, Malonate uptake (5 mmol/L disodium malonate [DSM], 15 min) in C2C12 cells in the presence of lactate (0, 10 or 50 mmol/L). B, Lactate levels in C2C12 cells after 15 min treatment with varying concentrations of MCT1 inhibitors. C and D, Effect of MCT1 inhibition by AR-C141990 or AZD3965 on malonate (5 mmol/L DSM, 15 min) uptake (C) at pH 6 and subsequent succinate levels (D). E, C2C12 cells were incubated with DSM (5 mmol/L) for 15 min at various pH±10 µmol/L AR-C141990. F, MCT1 inhibition by AR-C141990 on malonate (5 mmol/L DSM, 15 min) uptake at pH 7.4 (A to F, mean±SEM, n=3 biological replicates, statistics: (A) Kruskal-Wallis with Dunn post hoc test). G and H, Effect of malonate (5 mmol/L DSM) on cellular oxygen consumption at pH 7.4 (G) or 6 (H) ±MCT1 inhibitor (10 µmol/L AR-C141990; data presented as nonmitochondrial respiration normalized mean oxygen consumption rate (OCR)±SEM of 3 biological replicates (n=12–16 technical replicates per biological replicate), statistics: Kruskal-Wallis with Dunn post hoc test). ATP indicates OCR in the presence of 1.5 µmol/L oligomycin; BL, baseline OCR; MAX, OCR in the presence of 1 µmol/L FCCP (carbonyl cyanide-p-trifluoromethoxyphenylhydrazone); and NM, OCR in the presence of 4 µg/mL rotenone and 10 µmol/L antimycin A.
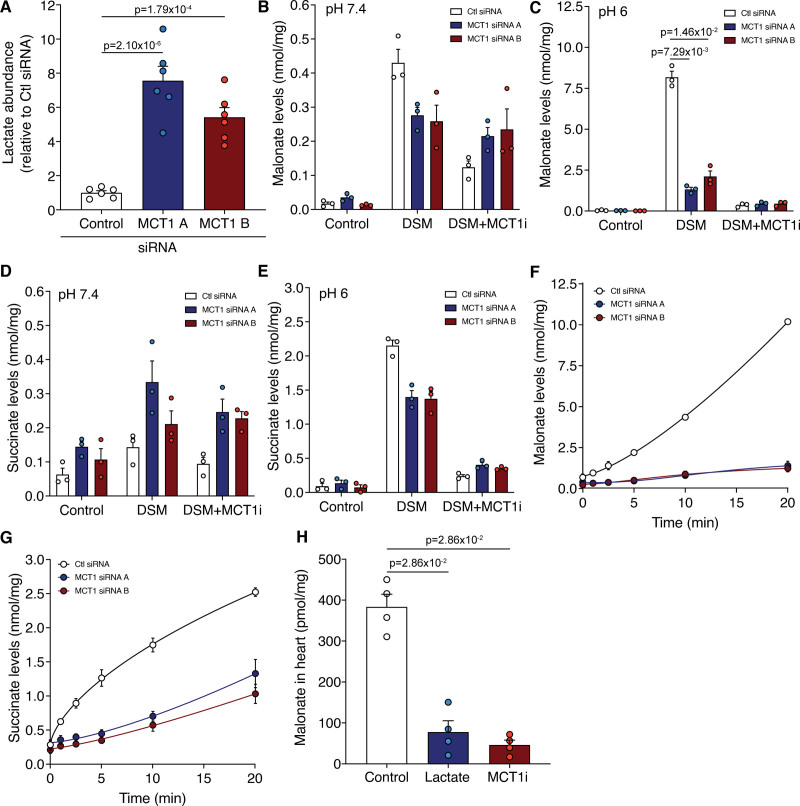
To confirm that MCT1 was the malonate transporter, we assessed the potent and selective MCT1 inhibitors, AR-C141990 and AZD3965.21,35,37,38 When cells were incubated with these MCT1 inhibitors, they led to a dramatic dose-dependent increase in lactate levels within the cell, consistent with preventing lactate efflux via MCT1 (Figure 4B). MCT1 inhibition by either AR-C141990 or AZD3965 led to a profound dose-dependent decrease in malonate uptake (Figure 4C). Inhibition of malonate uptake by MCT1 inhibition led to a corresponding decrease in succinate accumulation within cells (Figure 4D). Additionally, a time course of malonate uptake at low pH, showed that MCT1 inhibition largely abolished the increase in malonate over time, along with the corresponding increase in succinate (Figure S4B and S4C).
MCT1 inhibition blocked the pH-dependent increase in malonate uptake (Figure 4E) and in parallel prevented succinate accumulation (Figure 4D). The MCT1 inhibitor led to a dose-dependent inhibition of malonate uptake even at pH 7.4 (Figure 4F). Therefore, malonate can be transported by MCT1 at pH 7.4, but this is greatly enhanced at lower pH due to the increased proportion of malonate in its monocarboxylate form.
We next assessed the impact of the malonate taken up into cells on mitochondrial function by measuring respiration. Lowering pH itself had little effect; however, addition of malonate severely reduced cellular respiration (Figure 4G and H and Figure S4E and S4F) and this effect was rescued by MCT1 inhibition (Figure 4G and H and Figure S4E and S4F). MCT1 inhibition led to a small increase in oxygen consumption at baseline, which may be due to lactate accumulation increasing the NADH/NAD+ ratio driving mitochondrial respiration.39
To confirm the pharmacological effects of MCT1 inhibition on malonate uptake genetically, we knocked down (KD) MCT1 using siRNA in both HeLa and C2C12 cells (Figure S5A through S5D). Consequently, lactate levels in MCT1 KD cells were significantly elevated compared to the control siRNA (Figure 5A). When MCT1 KD cells were incubated with malonate at low pH, malonate uptake was dramatically blocked (Figure 5B through 5E and Figure S5C), confirming MCT1 is directly responsible for malonate transport at low pH. Residual malonate uptake in the MCT1 KD cells was inhibited by cotreatment with an MCT1 inhibitor, further confirming its MCT1 dependence (Figure 5F and Figure S5F). Additionally, succinate was elevated in the MCT1 KD cells compared with control siRNA cells, a result not seen with acute pharmacological MCT1 inhibition (Figure 5D). This suggests that MCT1 may also play an important role in normoxic succinate metabolism and homeostasis and not just during myocardial reperfusion and intense exercise.21,35 Intriguingly, the succinate levels after malonate treatment over time differed between the 2 cell types, with succinate levels remaining low in C2C12 KD but elevated in HeLa MCT1 KDs compared to controls, suggesting a reliance on MCT1 for succinate efflux (Figure 5G and Figure S5G).
Figure 5.
Genetic knockdown of MCT1 (monocarboxylate transporter 1) prevents the uptake of malonate. A, Relative lactate levels in C2C12 cells treated with control or MCT1 siRNA (mean±SEM of lactate levels relative to control, n=6 biological replicates, statistics: 1-way ANOVA with Bonferroni post hoc test). B to E, Incubation of malonate (5 mmol/L disodium malonate [DSM]) in MCT1 KD cells at pH 7.4 (B and D) or 6 (C and E) for 15 min±MCT1i (10 µmol/L, MCT1 inhibitor AR-C141990). Levels of malonate (B and C) and succinate (D and E) quantified by LC-MS/MS (liquid chromatography-tandem mass spectrometry) (mean±SEM, n=3 biological replicates, statistics: Kruskal-Wallis with Dunn post hoc test). F and G, Time course of malonate uptake (5 mmol/L DSM) at pH 6 in MCT1 KD cells (F) and corresponding succinate levels (G; mean±SEM, n=3 biological replicates). H, Malonate levels in murine Langendorff hearts perfused at pH 6 for 5 min with 5 mmol/L DSM±lactate (50 mmol/L) or MCT1 inhibitor (10 µmol/L AR-C141990; MCT1i; mean±SEM, n=4, statistics: unpaired, 2-tailed Mann-Whitney U test vs control).
As HeLa cells constitutively express MCT4, which is under the control of HIF (hypoxia-inducible factor)-1α and may be implicated in the transport of metabolites in IR, we KD MCT4 independently of MCT1 (Figure S5D and S5E). MCT4 KD had little effect on malonate uptake, with malonate and succinate levels mirroring those of control siRNA-treated cells (Figure S5F and S5G). Thus, MCT1 is the main driver of malonate uptake at lowered pH.
Overall, diminishing MCT1 activity, either by pharmacological inhibition or genetic knockdown, impairs malonate uptake at both acidic and normal physiological pH. In addition, this is the first evidence that MCT1 can transport a 3-carbon chain length dicarboxylate, which may also have implications in normal physiology and other pathologies.
Finally, we assessed if MCT1 was responsible for malonate uptake at lowered pH in the heart. Malonate uptake into the Langendorff-perfused heart was blocked by either excess lactate, or the MCT1 inhibitor AR-C141990, in the perfusion medium (Figure 5H). Together these findings confirm that MCT1 is responsible for the low pH uptake of malonate, both in cells and in the intact heart.
Ischemic Conditions Are Sufficient to Drive Malonate Uptake into the Heart Upon Reperfusion
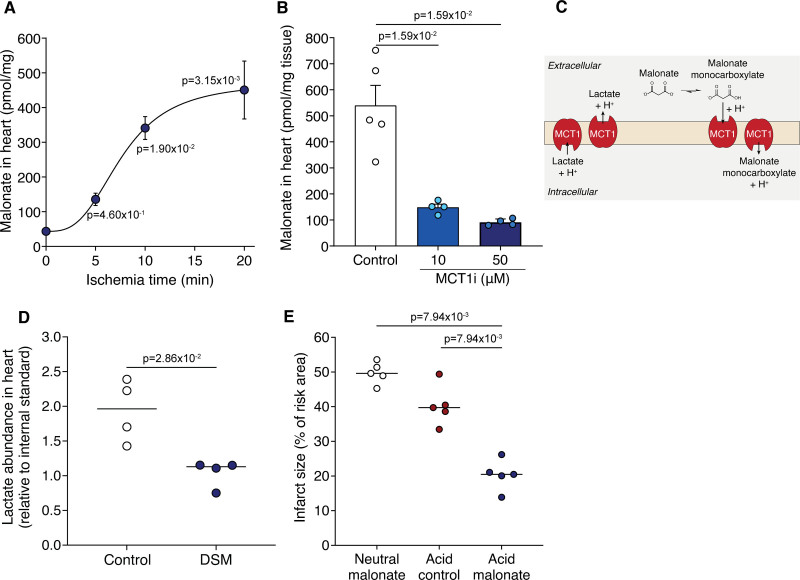
That low pH enhanced malonate entry into heart cells was consistent with the protection afforded by DSM in IR injury being due to the local decrease in pH during ischemia and initial reperfusion. To test this hypothesis, Langendorff-perfused hearts were held ischemic for various times before DSM was infused for 5 minutes, followed by flushing, and measurement of malonate. The malonate levels in heart tissue were dependent on ischemia-time, with the highest occurring after 20 minutes ischemia and being ≈10-fold greater than in the normoxic heart (Figure 6A). This malonate uptake into the ischemic Langendorff-perfused heart was dramatically reduced by the MCT1 inhibitor AR-C141990 (Figure 6B). This confirmed that malonate uptake into the ischemic heart upon reperfusion is a selective process, driven by the low pH and facilitated by the MCT1.
Figure 6.
Ischemia drives malonate protonation, uptake, and cardioprotection. A, Langendorff-perfused murine hearts were held ischemic for either 0, 5, 10, or 20 min and reperfused with 5 mmol/L disodium malonate (DSM; pH 7.4) before malonate levels measured in the heart by LC-MS/MS (liquid chromatography-tandem mass spectrometry) (mean±SEM, n=4 (control, 5 min) or 6 (10 and 20 min) biological replicates, statistics: Kruskal-Wallis with Dunn post hoc test). B, Malonate levels in murine Langendorff hearts exposed to 20 min ischemia and reperfused with 5 mmol/L malonate (pH 7.4)±MCT1 (monocarboxylate transporter 1) inhibitor (10 or 50 µmol/L AR-C141990; mean±SEM, n=4–5 biological replicates, statistical significance assessed by unpaired, 2-tailed Mann-Whitney U test vs control). C, Model of potential lactate and malonate exchange during reperfusion. D, Lactate levels in the Langendorff heart after 20 min ischemia and 1 min reperfusion±5 mmol/L DSM (mean, n=4 biological replicates, statistics: 2-tailed, unpaired Mann-Whitney U test). E, Infarct size in murine LAD (left anterior descending coronary artery) ligation MI model with 100 µl bolus of 8 mg/kg DSM, pH 4 acid control or 8 mg/kg pH 4 formulated malonate at reperfusion after 30 min ischemia (mean, n=5 biological replicates, statistics: 2-tailed, unpaired Mann-Whitney U test vs acid malonate).
During ischemia, the pH of ischemic tissue lowers but in addition, lactate accumulates and upon reperfusion can be transported out of cardiomyocytes by MCT1. This could enhance malonate uptake, as the MCT1 may then act as a lactate-H+/monocarboxylate malonate-H+ exchanger (Figure 6C). To assess this, we measured lactate levels in malonate-perfused IR Langendorff hearts. We found that compared to reperfusion alone, lactate levels decreased in the malonate-treated hearts (Figure 6D). Furthermore, malonate treatment decreased lactate levels in cells that had been treated with the mitochondrial inhibitor antimycin A to enhance lactate production (Figure S6A). This suggests that extracellular malonate and a proton exchange for intracellular lactate, help drive malonate entry into cardiomyocytes upon reperfusion and contribute to its at-risk tissue-selective effect.
Therefore, malonate uptake into the heart upon reperfusion is dependent on the tissue having first undergone a period of ischemia, leading to a drop in pH and an accumulation of lactate.
Low pH Formulation Improves Cardioprotection by Malonate
The mechanism of malonate uptake into cardiac tissue suggested that lowering the pH of the malonate infusion would increase the proportion in the monocarboxylate form and thereby increase its potency as a cardioprotective agent. To assess this, we used a malonate dose that was not protective (8 mg/kg) when administered at a neutral pH in the in vivo LAD MI model. When this dose of malonate was reformulated at pH 4 (a pH currently used in Food and Drug Administration–approved parenteral formulations)40 and administered as a bolus, this conferred significant protection that was not due to the low pH alone (Figure 6E and Figure S6B). Furthermore, although neutral malonate administered before ischemia was not protective, acidified malonate drove its uptake into cardiomyocytes and significantly reduced infarct size when infused before ischemia (Figure S6C).
Discussion
No medicine is currently available that can be given at reperfusion to prevent cardiac IR injury.1,6 Drugs that prevent cardiac IR injury should both reduce MI damage and the subsequent development of heart failure.1,12 Targeting succinate metabolism has been shown to be a promising therapeutic approach. Inhibiting SDH using the reversible, competitive inhibitor malonate reduces infarct size in small and large animal models of cardiac IR injury, despite its mechanism of entry into heart tissue being unknown.26,27
Here, we found that malonate uptake into cells and the heart at pH 7.4 was inefficient. However, the uptake of malonate into cells was dramatically enhanced at the lower levels of pH that occur during ischemia and by lactate accumulation within cells. Thus, ischemia provides an environment that will protonate malonate and thereby enable its uptake by the MCT1 transporter. Interestingly, this transporter undergoes a trans-acceleration phenomenon, whereby its transition from the outward-facing to the inward-facing conformation occurs more rapidly in the presence of a trans-substrate.41,42 In this case, the uptake of malonate on the extracellular side of the plasma membrane may be accelerated by lactate efflux. In the LAD MI model of IR injury, malonate is readily available at the point of reperfusion, thus a proportion of the malonate would be protonated and accessible for transport by MCT1. This enables malonate entry into the heart in an at-risk tissue-selective manner.
Additionally, as well as the inhibition of SDH, accelerating lactate efflux from the heart may also play a role in the reduction of IR injury. By shifting the equilibrium to facilitate anaerobic ATP production, this may promote the early restoration of ionic gradients through ATP-dependent transporters.43,44 Furthermore, facilitating lactate efflux may promote the extrusion of protons from the intracellular environment, thus reducing the activity of H+ transporters such as the Na+-H+ exchanger, though further investigation into these mechanisms is warranted.43,45
This is the first account of an ischemia-selective cardioprotective agent, utilizing the pathological differences between risk and nonrisk tissue to drive uptake. As MCT1 is highly expressed in the hearts of mice, rats, pigs, and humans (Figure S6D) and the characteristics of ischemia are conserved between these species,19,24 these drivers for malonate uptake and the plasma membrane transporter may facilitate malonate cardioprotection in humans. In addition to its place in the treatment of IR injury in MI, the mechanism of ischemia-enhanced delivery of malonate may provide a novel treatment option for IR injury under many other circumstances.
There is much interest in targeted drug delivery and the ability to engage the intended site while reducing off-target effects. Here, we have shown that malonate is not only a cardioprotective agent, but it does this while its entry into the rest of the heart is limited. Thus, malonate is a potent and ischemic tissue-targeted drug, explaining how large doses can be delivered acutely with minimal toxic effects, which is likely to be important bearing in mind the many comorbidities associated with MI.46 Furthermore, as malonate can be efficiently metabolised28,47 and has the potential to promote cardiomyocyte regeneration.48
Malonate is robustly protective in acute IR injury, though further work is now required to understand the tractability of malonate treatment in chronic IR injury models; in particular, conducting a double-blind chronic large animal IR injury study.49 This would provide the greatest insight into the cardioprotection capabilities of malonate post-MI and its effect on the development of heart failure and define its potential for translation to the clinic.
Conclusions
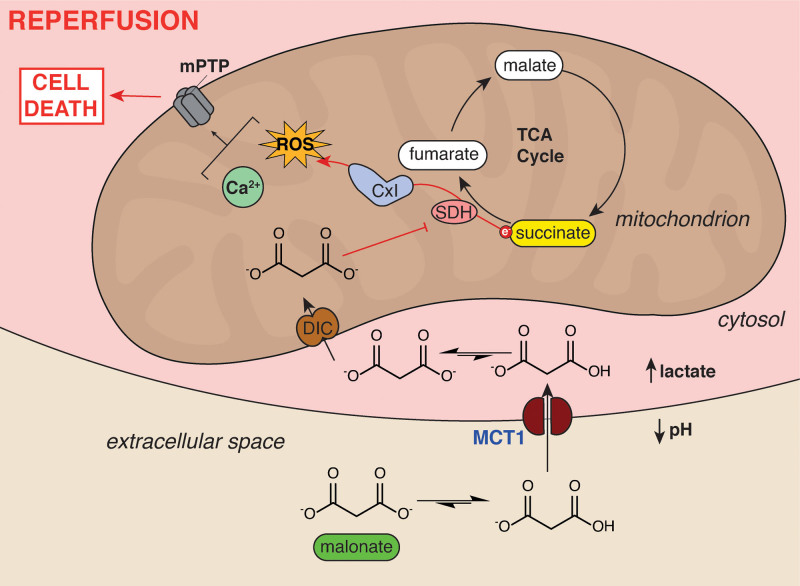
We have shown that malonate is an ischemia-selective drug, due to the lowered pH and lactate accumulation of ischemic tissue driving its uptake via the MCT1 (Figure 7). Furthermore, we show that a low pH formulation of malonate enhances its therapeutic potency. Malonate is the first at-risk tissue-selective cardioprotective drug and represents a significant step toward the treatment of IR injury.
Figure 7.
Schematic of ischemia-dependent malonate uptake via MCT1 (monocarboxylate transporter 1). The accumulation of lactate and protons in ischemic tissue and equilibration with the extracellular space facilitates protonation of malonate to its monocarboxylate form. This enables it to be an MCT1 substrate and enter cardiomyocytes upon reperfusion. Here, the malonate is transported into mitochondria by the mitochondrial dicarboxylate carrier where it can subsequently go on to inhibit SDH (succinate dehydrogenase). SDH inhibition reduces succinate oxidation, reactive oxygen species (ROS) production by reverse electron transport (RET) through complex I and opening of the mitochondrial permeability transition pore, thereby reducing cell death from ischemia/reperfusion (IR) injury. CxI indicates complex I; DIC, mitochondrial dicarboxylate carrier (SLC25A10); mPTP, mitochondrial permeability transition pore; and TCA, tricarboxylic acid.
Article Information
Acknowledgments
The authors thank Stephen Large, Fouad Taghavi, and Margaret M. Huang (Department of Surgery, University of Cambridge) for obtaining human heart tissue and Benjamin Thackray (Department of Medicine, University of Cambridge) for assistance with initial experiments.
Sources of Funding
This work was supported by the British Heart Foundation (PG/20/10025 to T. Krieg); the Medical Research Council (MC_UU_00015/3 to M.P. Murphy and MR/P000320/1 to T. Krieg), the Wellcome Trust (220257/Z/20/Z to M.P. Murphy, 221604/Z/20/Z to D. Aksentijevic), Barts Charity (MRC0215 to D. Aksentijevic).
Disclosures
Some authors currently hold a patent on the use of malonate esters in cardiac ischemia/reperfusion (IR) injury (M.P. Murphy and T. Krieg) and have submitted patent applications on the use of malonate in IR injury associated with ischemic stroke (M.P. Murphy and T. Krieg) and the pH-enhancement of malonate described in this article (H.A. Prag, M.P. Murphy, and T. Krieg). The other authors report no conflicts.
Supplemental Materials
Supplemental Methods
Major Resources Table
Figures S1–S6
Supplementary Material
Nonstandard Abbreviations and Acronyms
- CsA
- cyclosporin A
- DSM
- disodium malonate
- HIF
- hypoxia-inducible factor
- IR
- ischemia/reperfusion
- MCT1
- monocarboxylate transporter 1
- MI
- myocardial infarction
- RET
- reverse electron transport
- ROS
- reactive oxygen species
- SDH
- succinate dehydrogenase
T. Krieg and M.P. Murphy are joint senior authors.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.121.320717.
For Sources of Funding and Disclosures, see page 540.
References
- 1.Davidson SM, Ferdinandy P, Andreadou I, Bøtker HE, Heusch G, Ibáñez B, Ovize M, Schulz R, Yellon DM, Hausenloy DJ, et al. ; CARDIOPROTECTION COST Action (CA16225). Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC Review Topic of the Week. J Am Coll Cardiol. 2019;73:89–99. doi: 10.1016/j.jacc.2018.09.086 [DOI] [PubMed] [Google Scholar]
- 2.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667 [DOI] [PubMed] [Google Scholar]
- 3.Murphy E, Steenbergen C. Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury. Physiol Rev. 2008;88:581–609. doi: 10.1152/physrev.00024.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lesnefsky EJ, Chen Q, Tandler B, Hoppel CL. Mitochondrial dysfunction and myocardial ischemia-reperfusion: implications for novel therapies. Annu Rev Pharmacol Toxicol. 2017;57:535–565. doi: 10.1146/annurev-pharmtox-010715-103335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, Bonnefoy-Cudraz E, Guérin P, Elbaz M, Delarche N, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489 [DOI] [PubMed] [Google Scholar]
- 6.Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123:92–100. doi: 10.1172/JCI62874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hausenloy DJ, Botker HE, Engstrom T, Erlinge D, Heusch G, Ibanez B, Kloner RA, Ovize M, Yellon DM, Garcia-Dorado D. Targeting reperfusion injury in patients with ST-segment elevation myocardial infarction: trials and tribulations. Eur Heart J. 2016;ehw145. doi: 10.1093/eurheartj/ehw145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Hüttemann M. Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol. 2013;47:9–23. doi: 10.1007/s12035-012-8344-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229–317. doi: 10.1016/B978-0-12-394309-5.00006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142 [DOI] [PubMed] [Google Scholar]
- 11.Kloner RA, Brown DA, Csete M, Dai W, Downey JM, Gottlieb RA, Hale SL, Shi J. New and revisited approaches to preserving the reperfused myocardium. Nat Rev Cardiol. 2017;14:679–693. doi: 10.1038/nrcardio.2017.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heusch G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nature Rev Cardiol. 2020;17:773–789. doi: 10.1038/s41569-020-0403-y [DOI] [PubMed] [Google Scholar]
- 13.Chouchani ET, Pell VR, James AM, Work LM, Saeb-Parsy K, Frezza C, Krieg T, Murphy MP. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab. 2016;23:254–263. doi: 10.1016/j.cmet.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 14.Heusch G. Critical issues for the translation of cardioprotection. Circ Res. 2017;120:1477–1486. doi: 10.1161/CIRCRESAHA.117.310820 [DOI] [PubMed] [Google Scholar]
- 15.Lecour S, Andreadou I, Bøtker HE, Davidson SM, Heusch G, Ruiz-Meana M, Schulz R, Zuurbier CJ, Ferdinandy P, Hausenloy DJ; on behalf of the European Union-CARDIOPROTECTION COST ACTION CA16225. IMproving Preclinical Assessment of Cardioprotective Therapies (IMPACT) criteria: guidelines of the EU-CARDIOPROTECTION COST Action. Basic Res Cardiol. 2021;116:52. doi: 10.1007/s00395-021-00893-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bøtker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM, Deshwal S, Devaux Y, Di Lisa F, Di Sante M, et al. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol. 2018;113:39. doi: 10.1007/s00395-018-0696-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bøtker HE, Cabrera-Fuentes HA, Ruiz-Meana M, Heusch G, Ovize M. Translational issues for mitoprotective agents as adjunct to reperfusion therapy in patients with ST-segment elevation myocardial infarction. J Cell Mol Med. 2020;24:2717–2729. doi: 10.1111/jcmm.14953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chouchani ET, Pell VR, Gaude E, Aksentijević D, Sundier SY, Robb EL, Logan A, Nadtochiy SM, Ord ENJ, Smith AC, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin JL, Costa ASH, Gruszczyk AV, Beach TE, Allen FM, Prag HA, Hinchy EC, Mahbubani K, Hamed M, Tronci L, et al. Succinate accumulation drives ischaemia-reperfusion injury during organ transplantation. Nat Metab. 2019;1:966–974. doi: 10.1038/s42255-019-0115-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Wang YT, Miller JH, Day MM, Munger JC, Brookes PS. Accumulation of succinate in cardiac ischemia primarily occurs via canonical krebs cycle activity. Cell Rep. 2018;23:2617–2628. doi: 10.1016/j.celrep.2018.04.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prag HA, Gruszczyk AV, Huang MM, Beach TE, Young T, Tronci L, Nikitopoulou E, Mulvey JF, Ascione R, Hadjihambi A, et al. Mechanism of succinate efflux upon reperfusion of the ischaemic heart. Cardiovasc Res. 2021;117:1188–1201. doi: 10.1093/cvr/cvaa148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yin Z, Burger N, Kula-Alwar D, Aksentijević D, Bridges HR, Prag HA, Grba DN, Viscomi C, James AM, Mottahedin A, et al. Structural basis for a complex I mutation that blocks pathological ROS production. Nat Commun. 2021;12:707. doi: 10.1038/s41467-021-20942-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milliken AS, Kulkarni CA, Brookes PS. Acid enhancement of ROS generation by complex-I reverse electron transport is balanced by acid inhibition of complex-II: Relevance for tissue reperfusion injury. Redox Biol. 2020;37:101733. doi: 10.1016/j.redox.2020.101733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kula-Alwar D, Prag HA, Krieg T. Targeting succinate metabolism in ischemia/reperfusion injury. Circulation. 2019;140:1968–1970. doi: 10.1161/CIRCULATIONAHA.119.042791 [DOI] [PubMed] [Google Scholar]
- 25.Prag HA, Pala L, Kula-Alwar D, Mulvey JF, Luping D, Beach TE, Booty LM, Hall AR, Logan A, Sauchanka V, et al. Ester prodrugs of malonate with enhanced intracellular delivery protect against cardiac ischemia-reperfusion injury in vivo. Cardiovasc Drugs Ther. 2022;36:1–13. doi: 10.1007/s10557-020-07033-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valls-Lacalle L, Barba I, Miró-Casas E, Alburquerque-Béjar JJ, Ruiz-Meana M, Fuertes-Agudo M, Rodríguez-Sinovas A, García-Dorado D. Succinate dehydrogenase inhibition with malonate during reperfusion reduces infarct size by preventing mitochondrial permeability transition. Cardiovasc Res. 2016;109:374–384. doi: 10.1093/cvr/cvv279 [DOI] [PubMed] [Google Scholar]
- 27.Valls-Lacalle L, Barba I, Miró-Casas E, Ruiz-Meana M, Rodríguez-Sinovas A, García-Dorado D. Selective inhibition of succinate dehydrogenase in reperfused myocardium with intracoronary malonate reduces infarct size. Sci Rep. 2018;8:2442. doi: 10.1038/s41598-018-20866-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowman CE, Rodriguez S, Selen Alpergin ES, Acoba MG, Zhao L, Hartung T, Claypool SM, Watkins PA, Wolfgang MJ. The mammalian malonyl-CoA synthetase ACSF3 is required for mitochondrial protein malonylation and metabolic efficiency. Cell Chem Biol. 2017;24:673–684.e4. doi: 10.1016/j.chembiol.2017.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poitout-Belissent F, Aulbach A, Tripathi N, Ramaiah L. Reducing blood volume requirements for clinical pathology testing in toxicologic studies-points to consider. Vet Clin Pathol. 2016;45:534–551. doi: 10.1111/vcp.12429 [DOI] [PubMed] [Google Scholar]
- 30.El Banani H, Bernard M, Baetz D, Cabanes E, Cozzone P, Lucien A, Feuvray D. Changes in intracellular sodium and pH during ischaemia-reperfusion are attenuated by trimetazidine. Comparison between low- and zero-flow ischaemia. Cardiovasc Res. 2000;47:688–696. doi: 10.1016/s0008-6363(00)00136-x [DOI] [PubMed] [Google Scholar]
- 31.Kenwood BM, Weaver JL, Bajwa A, Poon IK, Byrne FL, Murrow BA, Calderone JA, Huang L, Divakaruni AS, Tomsig JL, et al. Identification of a novel mitochondrial uncoupler that does not depolarize the plasma membrane. Mol Metab. 2014;3:114–123. doi: 10.1016/j.molmet.2013.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajjawi OS, Hider RC. Malonate transport in human red blood cells. Mol Cell Biochem. 1987;75:43–49. doi: 10.1007/BF00231607 [DOI] [PubMed] [Google Scholar]
- 33.Hajjawi OS. Sequential mechanism of the malonate transport system in human red blood cells. Eur J Sci Res. 2010;46:18–27. [Google Scholar]
- 34.Wilson MC, Meredith D, Bunnun C, Sessions RB, Halestrap AP. Studies on the DIDS-binding site of monocarboxylate transporter 1 suggest a homology model of the open conformation and a plausible translocation cycle. J Biol Chem. 2009;284:20011–20021. doi: 10.1074/jbc.M109.014217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reddy A, Bozi LHM, Yaghi OK, Mills EL, Xiao H, Nicholson HE, Paschini M, Paulo JA, Garrity R, Laznik-Bogoslavski D, et al. pH-Gated succinate secretion regulates muscle remodeling in response to exercise. Cell. 2020;183:62–75.e17. doi: 10.1016/j.cell.2020.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jóhannsson E, Lunde PK, Heddle C, Sjaastad I, Thomas MJ, Bergersen L, Halestrap AP, Blackstad TW, Ottersen OP, Sejersted OM. Upregulation of the cardiac monocarboxylate transporter MCT1 in a rat model of congestive heart failure. Circulation. 2001;104:729–734. doi: 10.1161/hc3201.092286 [DOI] [PubMed] [Google Scholar]
- 37.Beloueche-Babari M, Casals Galobart T, Delgado-Goni T, Wantuch S, Parkes HG, Tandy D, Harker JA, Leach MO. Monocarboxylate transporter 1 blockade with AZD3965 inhibits lipid biosynthesis and increases tumour immune cell infiltration. Br J Cancer. 2020;122:895–903. doi: 10.1038/s41416-019-0717-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Påhlman C, Qi Z, Murray CM, Ferguson D, Bundick RV, Donald DK, Ekberg H. Immunosuppressive properties of a series of novel inhibitors of the monocarboxylate transporter MCT-1. Transpl Int. 2013;26:22–29. doi: 10.1111/j.1432-2277.2012.01579.x [DOI] [PubMed] [Google Scholar]
- 39.Benjamin D, Robay D, Hindupur SK, Pohlmann J, Colombi M, El-Shemerly MY, Maira SM, Moroni C, Lane HA, Hall MN. Dual Inhibition of the Lactate Transporters MCT1 and MCT4 Is Synthetic Lethal with Metformin due to NAD+ Depletion in Cancer Cells. Cell Rep. 2018;25:3047–3058.e4. doi: 10.1016/j.celrep.2018.11.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Usach I, Martinez R, Festini T, Peris JE. subcutaneous injection of drugs: literature review of factors influencing pain sensation at the injection site. Adv Ther. 2019;36:2986–2996. doi: 10.1007/s12325-019-01101-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vinnakota KC, Beard DA. Kinetic analysis and design of experiments to identify the catalytic mechanism of the monocarboxylate transporter isoforms 4 and 1. Biophys J. 2011;100:369–380. doi: 10.1016/j.bpj.2010.11.079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994;76:865–873. doi: 10.1016/0092-8674(94)90361-1 [DOI] [PubMed] [Google Scholar]
- 43.Vaughan-Jones RD, Spitzer KW, Swietach P. Intracellular pH regulation in heart. J Mol Cell Cardiol. 2009;46:318–331. doi: 10.1016/j.yjmcc.2008.10.024 [DOI] [PubMed] [Google Scholar]
- 44.de Lazzari F, Prag HA, Gruszczyk AV, Whitworth AJ, Bisaglia M. DJ-1: A promising therapeutic candidate for ischemia-reperfusion injury. Redox Bio. 2021;41:101884. Doi:10.1016/j.redox.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karmazyn M. NHE-1: still a viable therapeutic target. J Mol Cell Cardiol. 2013;61:77–82. doi: 10.1016/j.yjmcc.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 46.Kleinbongard P, Bøtker HE, Ovize M, Hausenloy DJ, Heusch G. Co-morbidities and co-medications as confounders of cardioprotection-does it matter in the clinical setting? Br J Pharmacol. 2020;177:5252–5269. doi: 10.1111/bph.14839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sacksteder KA, Morrell JC, Wanders RJ, Matalon R, Gould SJ. MCD encodes peroxisomal and cytoplasmic forms of malonyl-CoA decarboxylase and is mutated in malonyl-CoA decarboxylase deficiency. J Biol Chem. 1999;274:24461–24468. doi: 10.1074/jbc.274.35.24461 [DOI] [PubMed] [Google Scholar]
- 48.Bae J, Salamon RJ, Brandt EB, Paltzer WG, Zhang Z, Britt EC, Hacker TA, Fan J, Mahmoud AI. Malonate promotes adult cardiomyocyte proliferation and heart regeneration. Circulation. 2021;143:1973–1986. doi: 10.1161/CIRCULATIONAHA.120.049952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lobo-Gonzalez M, Galán-Arriola C, Rossello X, González-Del-Hoyo M, Vilchez JP, Higuero-Verdejo MI, García-Ruiz JM, López-Martín GJ, Sánchez-González J, Oliver E, et al. Metoprolol blunts the time-dependent progression of infarct size. Basic Res Cardiol. 2020;115:55. doi: 10.1007/s00395-020-0812-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prag HA, Kula-Alwar D, Pala L, Caldwell ST, Beach TE, James AM, Saeb-Parsy K, Krieg T, Hartley RC, Murphy MP. Selective delivery of dicarboxylates to mitochondria by conjugation to a lipophilic cation via a cleavable linker. Mol Pharm. 2020;17:3526–3540. doi: 10.1021/acs.molpharmaceut.0c00533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aksentijević D, Karlstaedt A, Basalay MV, O’Brien BA, Sanchez-Tatay D, Eminaga S, Thakker A, Tennant DA, Fuller W, Eykyn TR, et al. Intracellular sodium elevation reprograms cardiac metabolism. Nat Commun. 2020;11:4337. doi: 10.1038/s41467-020-18160-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antonucci S, Mulvey JF, Burger N, Di Sante M, Hall AR, Hinchy EC, Caldwell ST, Gruszczyk AV, Deshwal S, Hartley RC, et al. Selective mitochondrial superoxide generation in vivo is cardioprotective through hormesis. Free Radic Biol Med. 2019;134:678–687. doi: 10.1016/j.freeradbiomed.2019.01.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gramstad L, Gjerløw JA, Hysing ES, Rugstad HE. Interaction of cyclosporin and its solvent, Cremophor, with atracurium and vecuronium. Studies in the cat. Br J Anaesth. 1986;58:1149–1155. doi: 10.1093/bja/58.10.1149 [DOI] [PubMed] [Google Scholar]
- 54.Dannhorn A, Kazanc E, Ling S, Nikula C, Karali E, Serra MP, Vorng JL, Inglese P, Maglennon G, Hamm G, et al. Universal sample preparation unlocking multimodal molecular tissue imaging. Anal Chem. 2020;92:11080–11088. doi: 10.1021/acs.analchem.0c00826 [DOI] [PubMed] [Google Scholar]
- 55.Zille M, Farr TD, Przesdzing I, Müller J, Sommer C, Dirnagl U, Wunder A. Visualizing cell death in experimental focal cerebral ischemia: promises, problems, and perspectives. J Cereb Blood Flow Metab. 2012;32:213–231. doi: 10.1038/jcbfm.2011.150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cochemé HM, Quin C, McQuaker SJ, Cabreiro F, Logan A, Prime TA, Abakumova I, Patel JV, Fearnley IM, James AM, et al. Measurement of H2O2 within living Drosophila during aging using a ratiometric mass spectrometry probe targeted to the mitochondrial matrix. Cell Metab. 2011;13:340–350. doi: 10.1016/j.cmet.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Detailed methods and Major Resources Table can be found in the Supplemental Material. Data will be made available upon reasonable request, by contacting a corresponding author.