Abstract
Commonly used pain therapeutics, such as opioid medications, exert dangerous side effects and lack effectiveness in treating some types of pain. Ketamine is also used to treat pain, but side effects limit its widespread use. (2R,6R)-hydroxynorketamine (HNK) is a ketamine metabolite that potentially shares some beneficial behavioral effects of its parent drug without causing significant side effects. This study compared the profile and potential mechanisms mediating the antinociception activity of ketamine and (2R,6R)-HNK in C57BL/6J mice. Additionally, this study compared the reversal of mechanical allodynia by (2R,6R)-HNK with gabapentin in a model of neuropathic pain. Unlike the near-immediate and short-lived antinociception caused by ketamine, (2R,6R)-HNK produced late-developing antinociception 24 hours following administration. Pharmacological blockade of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors with 2,3-dioxo-6-nitro-7-sulfamoyl-benzo[f]quinoxaline (NBQX) prevented the initiation and expressionof (2R,6R)-HNK antinociception, suggesting the involvement of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor-dependent glutamatergic mechanisms in the pain reduction-like responses. Blockade of opioid receptors with naltrexone partially prevented the antinociceptive effect of ketamine but was ineffective against (2R,6R)-HNK. Furthermore, (2R,6R)-HNK did not produce dystaxia, even when tested at doses five times greater than those needed to produce antinociception, indicating a superior safety profile for (2R,6R)-HNK over ketamine. Additionally, (2R,6R)-HNK reversed mechanical allodynia in a spared nerve injury model of neuropathic pain with similar short-term efficacy to gabapentin (within 4 hours) while outperforming gabapentin 24 hours after administration. These findings support the further study of (2R,6R)-HNK as a potentially valuable agent for treating different types of pain and establish certain advantages of (2R,6R)-HNK treatment over ketamine and gabapentin in corresponding assays for pain.
SIGNIFICANCE STATEMENT
The ketamine metabolite (2R,6R)-HNK produced antinociception in male and female mice 24 hours after administration via activation of AMPA receptors. The effects of (2R,6R)-HNK differed in time course and mechanism and presented a better safety profile than ketamine. (2R,6R)-HNK also reversed allodynia in SNI-operated animals within 4 hours of treatment onset, with a duration of effect lasting longer than gabapentin. Taken together, (2R,6R)-HNK demonstrates the potential for development as a non-opioid analgesic drug.
Introduction
An estimated 20.4% of adults in the U.S. suffer from a pain condition. For many adults, pain is sufficient to cause significant limitations in their work capability, social life, or ability to provide self-care (Dahlhamer et al., 2018). Inadequately treating pain over time increases the risk of developing psychological disorders, such as depression (Fine, 2011). Finding effective pain treatments has proven difficult for many suffering from pain conditions. Ketamine is an anesthetic medication that has been in use since 1970 and is known to effectively treat both acute pain (Schwenk et al., 2018) and pain conditions that are chronic and difficult to treat (Eide et al., 1994; Kvarnström et al., 2003; Sigtermans et al., 2009; Cohen et al., 2018). However, clinical treatment of pain with ketamine or its use by patients at home is limited because the drug causes a hallucinogenic state of dissociation, induces behavioral impairment through sedation, and is diverted for recreational abuse. Ketamine is not approved for treating pain by the Food and Drug Administration.
More recently, ketamine has demonstrated effectiveness in providing rapid onset and prolonged relief from treatment-resistant depression (Berman et al., 2000; Ibrahim et al., 2011; Zarate et al., 2012). Based on a compelling body of evidence, the FDA approved the use of the S enantiomer (esketamine) for the indication of treatment-resistant depression in 2019. This breakthrough in depression treatment helped drive a new investigation of ketamine metabolites. Available preclinical data suggest one of the ketamine metabolites, (2R,6R)-hydroxynorketamine (HNK), may share ketamine’s therapeutic effects in rodent models of stress exposure (Zanos et al., 2016; Chou et al., 2018), while lacking the liabilities for abuse potential and dissociative cognition seen with the parent drug (Zanos et al., 2016; Pham et al., 2018; Lumsden et al., 2019).
Given ketamine’s efficacy in treating pain conditions, it was natural to question whether (2R,6R)-HNK shares analgesic properties with the parent compound. A study recently demonstrated that a single (2R,6R)-HNK dose (10 mg//kg) produced a reversal of allodynia in models of postoperative pain, neuropathic pain, and chronic regional pain syndrome-type pain for up to 24 hours following treatment in female mice (Kroin et al., 2019). These studies supported the supposition that (2R,6R)-HNK could be a putative analgesic agent. Still, the time course of treatment onset, ideal doses to treat acute and chronic pain conditions, mechanism of action, and the impact of sex on these outcomes are unknown. Moreover, the antinociceptive effects of (2R,6R)-HNK have not been examined in animals with no existing pain condition. To replicate the initial findings and provide more information on the antinociceptive effects of (2R,6R)-HNK, a series of studies were conducted in healthy mice to determine if (2R,6R)-HNK produces antinociception, to identify the optimal dose and temporal pattern for (2R,6R)-HNK mediated pain reduction, to evaluate sex differences, and to compare the side effect profile and mechanism of action between (2R,6R)-HNK and ketamine. Mechanism of action experiments targeted α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and opioid receptors due to the identified association between (2R,6R)-HNK and increases in AMPA receptor (AMPAR)-dependent glutamatergic signaling and receptor expression (Zanos et al., 2016; Pham et al., 2018), as well as the implication of opioid receptor dependency for some of ketamine’s therapeutic effects. The effects of (2R,6R)-HNK were then examined in the SNI model of neuropathic pain, comparing the time course for reversing allodynia by two doses of (2R,6R)-HNK with two doses of the common neuropathic pain treatment gabapentin.
The results showed that (2R,6R)-HNK produced delayed antinociception lasting more than 24 hours in male and female mice. Unlike ketamine, (2R,6R)-HNK antinociception did not involve opioid receptor activation but was initiated and sustained by the activation of AMPARs. Additionally, (2R,6R)-HNK reversed mechanical allodynia in SNI-treated mice with a similar short-term onset but lasted longer than gabapentin. Finally, (2R,6R)-HNK did not produce the dystaxic effects associated with ketamine treatment. These findings support the further development of (2R,6R)-HNK as a potential analgesic agent for treating different types of pain.
Materials and Methods
Animals
Male and female C57BL/6J mice (Jackson Laboratory; Bar Harbor, ME), aged 8–15 weeks, were housed with four to five animals per cage, maintained on a standard 12-hour light/dark cycle (lights on at 06:00), and provided with food and water ad libitum. Each experiment was conducted with separate cohorts of animals. The animals were randomly assigned to treatment groups for all experiments. Measurements from mice were excluded from experiments if their baseline response extended beyond mean group values by two standard deviations. All experiments were carried out following the National Institutes of Health (NIH) guidelines for the care and use of laboratory animals and with approval from the Uniformed Services University of the Health Sciences Institutional Animal Care and Use Committee.
Drugs
The (2R,6R)-HNK and (2S,6S)-HNK used for these experiments were obtained from the National Center for Advancing Translational Sciences (NCATS; Bethesda, MD), which was synthesized as previously described (Morris et al., 2017). (R/S) ketamine was purchased from Mylan Pharmaceuticals (Canonsburg, PA; #67457010810), while gabapentin was obtained from Acros Organics through Fisher Scientific (#458020010). The AMPAR antagonist 2,3-dioxo-6-nitro-7-sulfamoyl-benzo[f]quinoxaline (NBQX) disodium salt (2,3-dioxo-6-nitro-7-sulfamoyl-benzo[f]quinoxaline, Tocris, #1044) and the opioid receptor antagonist naltrexone hydrochloride (Millipore Sigma, MO; #N3136) were used in the pharmacological mechanism of action studies. Physiologic saline (0.9% NaCl; Quality Biologic, MD; #114-055-721) was used as the control and to dilute medications. Doses were calculated according to the molecular weight of the base. All drugs were administered via the intraperitoneal route at a 10 ml/kg volume.
Antinociception in Healthy Animals
Antinociception was assessed in normal healthy mice by measuring paw withdrawal latencies on a Thermal Analgesia Meter (Ugo Basile, Stoelting Co.; Wood Dale, IL) set to 50°C, as described previously (Jacobson et al., 2020). After placement on the hot plate, the latency for the mouse to either jump or lick a hind paw was measured. The animal was immediately removed from the hot plate upon response.
The antinociceptive effects of ketamine 10 and 30 mg/kg, (2R,6R)-HNK 10 mg/kg, and (2S,6S)-HNK 10 mg/kg with saline control were examined in healthy male mice (N = 10–22, 10 for ketamine 30 mg/kg, 21 for ketamine 10 mg/kg, 20 for saline control, 22 for both HNK groups). Two animals (one from the control group and one from the ketamine 10 mg/kg group) were excluded for baseline values beyond two standard deviations from the group mean. The effect of (2R,6R)-HNK (10 mg/kg) on nociception was confirmed in a second experiment that used both males (N = 30) and females (N = 36). Dose-response curves were generated from latencies of male (N = 10–12 per dose) and female (N = 16–17 per dose) mice tested 24 hours following administration of (2R,6R)-HNK at quarter log doses beginning with 3 mg/kg (10°.5) for male or 6 mg/kg (10°.75) for female mice and ending with 100 mg/kg (102), comparing each dose to saline control. Treatment groups in the male mice consisted of 12 animals for the saline group, 11 animals for the 6 and 18 mg/kg groups, and 10 animals for all other groups. Two female animals, one from the saline group and one from the 18 mg/kg group, were excluded for baseline values that exceeded two standard deviations from the group mean.
To test the contribution of opioid receptors in mediating the initiation of (2R,6R)-HNK’s effect on nociception, the nonselective opioid receptor antagonist naltrexone (1 mg/kg) was administered 30 minutes before (2R,6R)-HNK treatment (N = 12) and the animals underwent testing 24 hours following treatment. The 1 mg/kg dose was selected because it blocked the antinociceptive effects of morphine in the hot plate test (data not shown). To test the contribution of opioid receptors in mediating the expression of (2R,6R)-HNK’s behavioral effects, naltrexone 1 mg/kg was administered 24 hours following (2R,6R)-HNK treatment (N = 12) and 30 minutes before behavioral testing.
The contribution of AMPAR in the initiation of (2R,6R)-HNK′s delayed antinociception effect was tested by administering the antagonist NBQX 10 mg/kg 30 minutes before (2R,6R)-HNK treatment (Dalgaard et al., 1994; Karasawa et al., 2005; Zanos et al., 2016) with the animals (N = 12) undergoing testing 24 hours following (2R,6R)-HNK. Testing for the contribution of AMPAR to the sustained expression of (2R,6R)-HNK’s effect was done by administering NBQX 10 mg/kg 24 hours following (2R,6R)-HNK (N = 11–12), with hot plate testing 30 minutes later. One animal from the saline/saline group was excluded for a baseline measurement beyond two standard deviations from the group mean. NBQX 10 mg/kg, naltrexone 1 mg/kg, or saline pretreatment 30 minutes before ketamine 10 mg/kg or saline treatment were administered to test the contribution of both opioid and AMPAR in mediating ketamine′s antinociception effect (N = 10). In this case, the animals were tested on the hot plate 10 minutes following ketamine or saline treatment.
Spared Nerve Injury
Surgery
Spared nerve injury (SNI) surgery was performed to model a neuropathic pain condition in male mice aged 8–12 weeks. The model was initially developed in rats (Decosterd and Woolf, 2000) but is also used in mice (Shields et al., 2003; Bourquin et al., 2006). Briefly, mice underwent axotomy of the tibial and peroneal branches of the sciatic nerve, while carefully preserving the sural branch under aseptic conditions and 2–2.5% isoflurane anesthesia. The sciatic nerve branches were gently dissected to minimize stretching of the sural nerve. A 6-0 silk suture was tied tightly around the tibial and peroneal branches just distal to the bifurcation of the sural nerve from the sciatic nerve bundle. A second 6-0 silk suture was tightly applied 1.5 mm distal to the first, and a 1 mm section of the adjoined tibial and peroneal branches was removed. Following surgery, the incision was closed with sutures, and the mice were returned to their home cages after recovery from anesthesia. The total time required for the procedure was 15 minutes or less for each animal. The animals were given 11 days to recover from surgery before further experimentation. All animals exhibited normal food intake, grooming, and regular movements other than favoring the injured limb before post-surgery experimentation.
Mechanosensitivity (von Frey)
Paw withdrawal threshold was measured in SNI-treated mice by examining the responses to lateral-plantar stimulation with von Frey aesthesiometer monofilaments (Stoelting Co.; Wood Dale, IL; 58011) using the classic up-down method (Dixon, 1980; Chaplan et al., 1994). The animals were placed in small plastic enclosures atop a wire mesh platform. Monofilaments (beginning with 1.0 g f) were gently applied with sufficient force only to bend the filament slightly. The monofilament was removed after one second of application. A positive response was defined as licking or rapid paw withdrawal upon stimulation. The subsequent monofilament applied was either the next higher or lower force dependent upon the previous response. The animals were given 3–4 minutes between each application. Once a change in response from the previous monofilament applications was recorded, four additional monofilaments were applied, moving up or down according to the prior response. The paw withdrawal threshold was defined as the 50% threshold in grams force according to the following formula: where Xf was the value in log units of the final monofilament used, k was the tabular value for the response pattern (Chaplan et al., 1994), and δ was the average log unit increment between monofilaments (0.224).
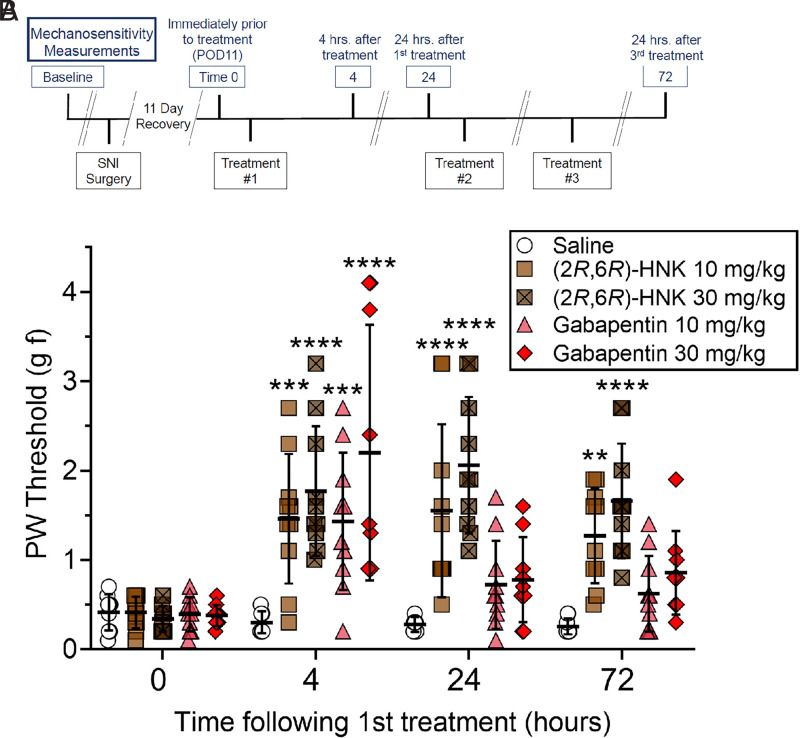
The mechanical allodynia reversing effects of (2R,6R)-HNK (10 and 30 mg/kg) and gabapentin (10 and 30 mg/kg) were compared with saline injection in the SNI operated male mice (N = 9–10, 9 for the saline and gabapentin 30 mg/kg groups and 10 for all other groups). Gabapentin is a calcium channel blocker commonly used to treat neuropathic type pain in humans. In mice, gabapentin has been shown to suppress inflammatory edema (Dias et al., 2014), reverse pain-depressed burrowing behavior (Shepherd et al., 2018), produce antinociception in healthy animals (Kilic et al., 2012), and reverse SNI-induced allodynia (Shepherd and Mohapatra, 2018) at doses as small as 1 mg/kg and up to 30 mg/kg, depending upon the effect. In this study, the SNI mice received once-daily treatment for three days with mechanosensitivity testing immediately preceding treatment on day two and at the same time on day four (24 hours following the third and final treatment, see Fig. 4A).
Fig. 4.
The SNI condition produced mechanical allodynia 11 days after surgery. Time 0 represents mechanosensitivity results on day 11 before treatment. Results of a baseline measurement before surgery demonstrated no significant difference between groups (data not shown, average baseline before surgery for all groups was 4.3 ± 1.10 g f). Panel A: The experimental paradigm comprised once-daily treatment of three days and mechanosensitivity testing before daily treatment. Panel B: (2R,6R)-HNK reversed mechanical allodynia associated with the neuropathic pain condition within 4 hours of treatment, and the effect persisted for at least 24 hours. Repeat dosing did not appear to lessen the pain reduction-like effect. Gabapentin reversed mechanical allodynia 4 hours following treatment but did not produce a significant effect 24 hours following the first or third treatment. Vertical bars represent the group means ± S.D. Drug doses are compared with saline at the same time point, **P < 0.01, ***P < 0.001, ****P < 0.0001, N = 9-10 (N = 9 for saline and gabapentin 30 mg/kg groups, N = 10 for all other groups).
Gait Analysis
Gait analysis was used to determine if ketamine or (2R,6R)-HNK produced motor impairments at the doses effective in the pain assays (N = 12 for each group). Gait analysis was performed utilizing the DigiGait treadmill system and Video Imaging Acquisition software (Mouse Specifics, Inc.; Framingham, MA) adapted from the original protocol (Wooley et al., 2005) with speed set to 9 cm/s and data represented as fore and hind paw measurements for each animal (Browne et al., 2022). This system analyzes paw placement and measures spatiotemporal gait characteristics. Following 30 minutes of habituation to the testing room and five minutes of familiarization with the treadmill apparatus, the animals were treated with either ketamine (10 mg/kg or 30 mg/kg), (2R,6R)-HNK (10 mg/kg or 50 mg/kg), or saline. Video sequences of two to five seconds were captured for each animal walking on the treadmill ten minutes after injection. The images were then analyzed using the software. Eight animals were excluded from the study for refusal to walk on the treadmill, one from the ketamine 10 mg/kg, one from the ketamine 30 mg/kg, two from the (2R,6R)-HNK cohort saline control, two from the (2R,6R)-HNK 10 mg/kg, and two from the (2R,6R)-HNK 50 mg/kg groups.
Data Analysis
The data are expressed as scatter plots showing group means ± S.D. A repeated measures two-way ANOVA with Dunnett multiple comparisons was used to determine significant differences between groups comparing ketamine, (2R,6R)-HNK, (2S,6S)-HNK, and vehicle control thermal pain sensitivity over time. A repeated measures two-way ANOVA with Šidák multiple comparisons was used to compare (2R,6R)-HNK against saline control in the time course thermal antinociception experiments for male and female animals. A repeated measures three-way ANOVA with Tukey multiple comparisons was used to analyze a sex effect in the combined data.
A one-way ANOVA with Dunnett multiple comparisons was used to compare thermal nociception responses to different doses of (2R,6R)-HNK 24 hours following treatment. Mechanism of action data were analyzed using a two-way ANOVA with Tukey multiple comparisons. Gait characteristics of groups following ketamine or (2R,6R)-HNK were compared with the respective saline control group using a one-way ANOVA. Repeated measures two-way ANOVA with Tukey multiple comparisons was used to compare two doses of (2R,6R)-HNK, two doses of gabapentin, and saline control in reversing mechanical allodynia in SNI-operated mice. All statistical analyses were performed using GraphPad Prism 9.0.0 for Windows (GraphPad Software; San Diego, CA, www.graphpad.com). Statistical significance was defined as P < 0.05.
Results
(2R,6R)-HNK Produced Antinociception: Time Course, Sex Differences and Dose-Response Curve
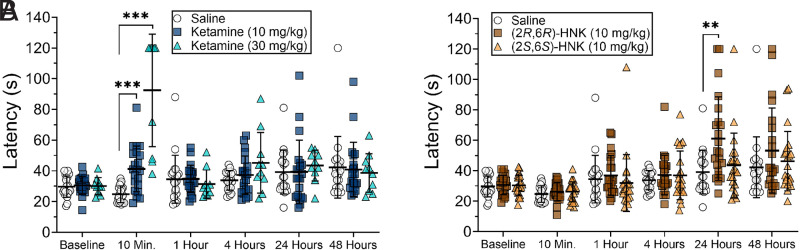
The time course for the effects of ketamine, (2R,6R)-HNK, and (2S,6S)-HNK on the hot plate showed different patterns of antinociceptive response (Fig. 1; time x treatment interaction F(20, 450) = 10.60, P < 0.0001). The groups were analyzed together and shown in separated graphs for clarity, with the same saline group shown in both. Mice that received (2R,6R)-HNK at 10 mg/kg demonstrated increased latency to respond to the heat stimulus 24 hours following injection compared with saline (Fig. 1B, P = 0.0087). Ketamine produced short-lived antinociception with response latencies increasing significantly only at 10 minutes (Fig. 1A, P = 0.0003 for 10 mg/kg and P = 0.0008 for 30 mg/kg). In contrast, (2S,6S)-HNK did not increase the response latency at any time point (Fig. 1B). The same data are shown as %MPE in Supplemental Fig. 1.
Fig. 1.
(2R,6R)-HNK produced delayed antinociception (Panel B), while ketamine produced rapid and short-lived antinociception (Panel A). (2S,6S)-HNK did not produce antinociception at any time point (Panel B). The data were analyzed together using a repeated measures two-way ANOVA but are shown in two separate graphs for clarity. The error bars represent group means ± S.D. Comparisons are made between drug/dose and saline at the same time point: **P < 0.01, ***P < 0.001. N = 20 for saline control, N = 21 for ketamine 10 mg/kg, N = 10 for ketamine 30 mg/kg, and N = 22 for both HNK groups.
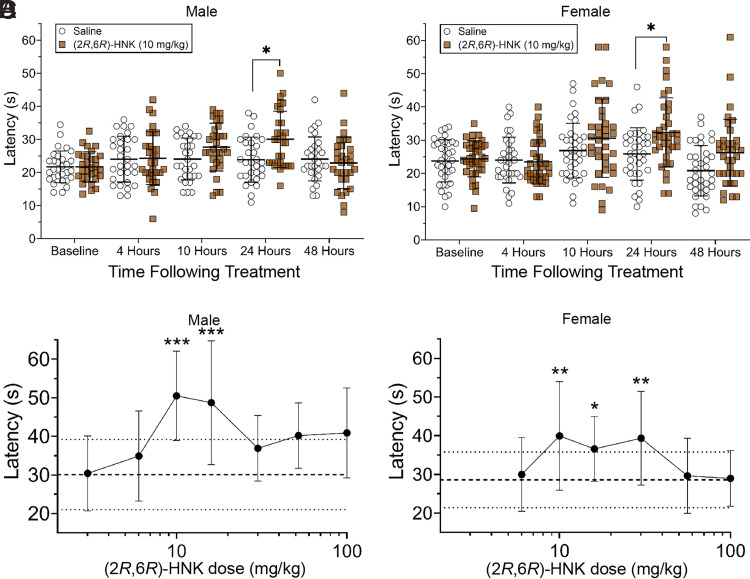
An additional experiment compared the time course for (2R,6R)-HNK in male and female mice. The results of this experiment confirmed that (2R,6R)-HNK increased latency to respond for both male (Fig. 2A; P = 0.0149) and female (Fig. 2B; P = 0.0203) mice 24 hours following treatment when compared with saline control (males: time x treatment interaction F(4, 232) = 4.28, P = 0.0023; females: time x treatment interaction F(4, 280) = 3.884, P = 0.0044). The results of the time-course experiment indicate the onset of (2R,6R)-HNK′s delayed antinociception to be between 10 and 24 hours following administration. A three-way ANOVA of the male and female data, excluding the baseline measurement, revealed a sex effect (F(1, 62) = 5.216, P = 0.0258). However, the post-hoc analysis revealed no significant sex differences at the same treatment and time. The same data are shown as %MPE in Supplemental Fig. 1.
Fig. 2.
Male (Panel A) and female (Panel B) time course for latency to respond to hot plate stimulus following (2R,6R)-HNK 10 mg/kg treatment. Comparisons are made between the treatment and saline groups at the same time point: *P < 0.05. N = 30 for males and N = 36 for females. Panel C (males) and Panel D (females) represent the quarter log dose-response for (2R,6R)-HNK mediated antinociception measured 24 hours after treatment. (2R,6R)-HNK produced antinociception at doses of 10 and 18 mg/kg in both sexes, while the 32 mg/kg dose produced antinociception in female mice only. Asterisks indicate significant differences compared with the saline control. *P < 0.05, **P < 0.01, ***P < 0.001. N = 10–12 for males, and N = 17 for females. The error bars represent group means ± S.D. For the dose-response figures, the mean of the saline group is represented by the dashed line, while the ± S.D. are represented by dotted lines.
The response on the hot plate was evaluated at quarter log scale doses of (2R,6R)-HNK 24 hours after administration and revealed an inverted U-shaped dose response curve for both males (Fig. 2C; F(7, 76) = 4.986, P = 0.0001) and females (Fig. 2D; F(6, 110) = 4.971, P = 0.0002). Specifically, males exhibited increased latencies to doses of 10 mg/kg (P = 0.0004) and 18 mg/kg (P = 0.0010). Females displayed increases in latency to (2R,6R)-HNK at doses of 10 mg/kg (P = 0.0028), 18 mg/kg (P = 0.0262), and 32 mg/kg (P = 0.0049). (2R,6R)-HNK at a dose of 30 mg/kg was also tested at 10 minutes, 1, and 4 hours (data not shown) but did not increase latency to respond to the hotplate at any measured time. Additional examination is required to determine if even higher doses of (2R,6R)-HNK can reduce the onset of its antinociception effect.
AMPA but not Opioid Receptors are Involved in the Initiation and Expression of the Antinociceptive Effects of (2R,6R)-HNK
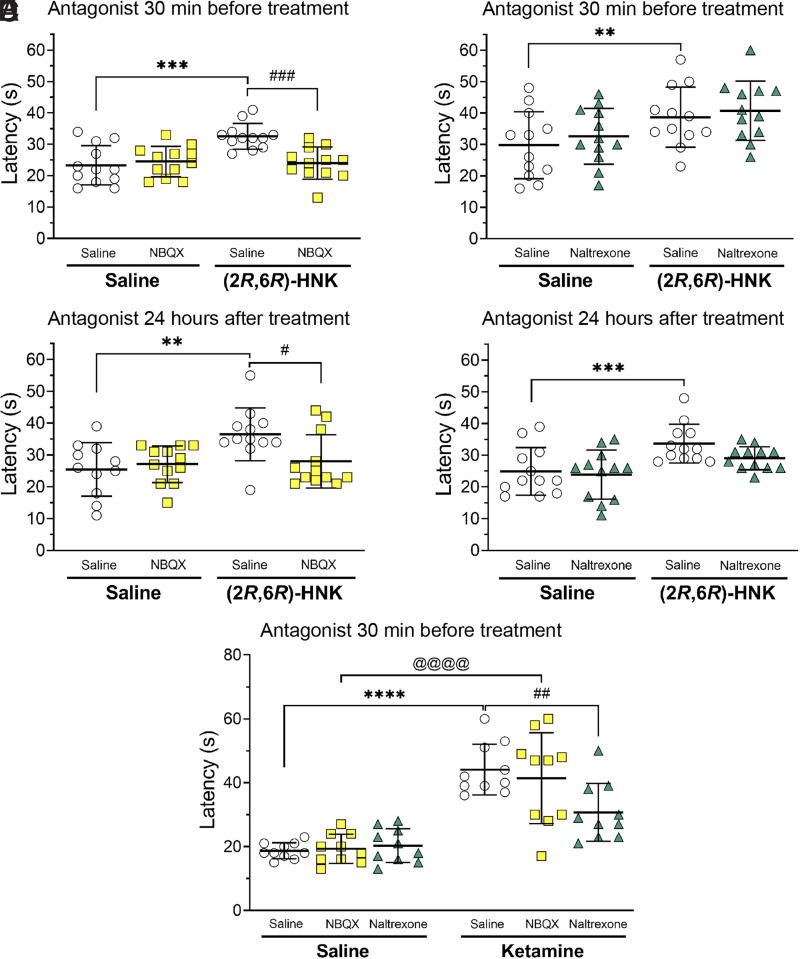
The effect of AMPAR blockade on the initiation of (2R,6R)-HNK antinociception was tested by administering NBQX 30 minutes before injection. Pretreatment with NBQX (10 mg/kg) blocked the (2R,6R)-HNK-generated hot plate effect when tested 24 hours post-injection (Fig. 3A; pretreatment x treatment interaction, F(1, 58) = 4.592, P = 0.0363, saline/saline versus saline/HNK P = 0.0015, saline/HNK versus NBQX/HNK P = 0.0491). The effect of AMPAR blockade on the expression of (2R,6R)-HNK antinociception was tested by administering NBQX 24 hours following treatment and 30 minutes before testing. NBQX given at this time also blocked the (2R,6R)-HNK mediated increase in latency to respond to the hot plate (Fig. 3C; treatment x post-treatment interaction, F(1, 43) = 4.972, P = 0.0310, saline/saline versus HNK/saline P = 0.0077, HNK/saline versus HNK/NBQX P = 0.0496). Altogether, these data suggest that both the initiation and expression of the delayed antinociception effect of (2R,6R)-HNK are dependent on the interaction between (2R,6R)-HNK and AMPAR.
Fig. 3.
The effects of AMPA receptor antagonism with NBQX (10 mg/kg) or opioid receptor antagonism with naltrexone (1 mg/kg) on the initiation or expression of antinociception by (2R,6R)-HNK. The effects of (2R,6R)-HNK (10 mg/kg) were measured 24 hours after injection. Panel A: The NBQX given 30 minutes before (2R,6R)-HNK treatment blocked the initiation of (2R,6R)-HNK antinociception. Panel B: Naltrexone given 30 minutes before (2R,6R)-HNK treatment did not block the initiation of (2R,6R)-HNK antinociception. Panel C: NBQX given 24 hours following (2R,6R)-HNK treatment blocked the expression of (2R,6R)-HNK mediated antinociception. Panel D: Naltrexone given 24 hours following (2R,6R)-HNK treatment did not alter (2R,6R)-HNK antinociception. Panel E: Ketamine (10 mg/kg) mediated antinociception measured 10 minutes following treatment was unaffected by pretreatment with NBQX (10 mg/kg given 30 minutes prior), while naltrexone (1 mg/kg given 30 minutes prior) blocked ketamine antinociception. The error bars represent group means ± S.D. Group comparisons are indicated with horizontal lines. Asterisks represent treatment/saline to saline/saline comparisons: **P < 0.01, ***P < 0.001, and ****P < 0.0001. The # symbol represents treatment/NBQX to treatment/saline comparisons: # P < 0.05, ## P < 0.01, and ### P < 0.001. The @ symbol represents NBQX/saline compared with NBQX/ketamine: @@@@, P < 0.0001. N = 11–12 for the (2R,6R)-HNK experiments, N = 10 for the ketamine experiment.
The nonselective opioid receptor antagonist naltrexone (1 mg/kg) was used to test the involvement of opioid receptors in the initiation and expression of (2R,6R)-HNK (10 mg/kg) antinociception. Administration of naltrexone 30 minutes before (2R,6R)-HNK treatment had no impact on the initiation of antinociception produced by (2R,6R)-HNK when measured 24 hours after treatment (Fig. 3B; HNK/saline treatment effect F(1, 44) = 9.364, P = 0.0038, pretreatment x treatment interaction F(1, 44) = 0.01805, P = 0.8937). Similarly, the administration of naltrexone 24 hours after (2R,6R)-HNK treatment and 30 minutes before hot plate testing failed to alter the expression of (2R,6R)-HNK antinociception (Fig. 3D; HNK treatment effect, F(1, 44) = 13.93, P = 0.0005, HNK treatment x naltrexone administration interaction F(1, 44) = 0.9235, P = 0.3418). These data suggest that any potential interaction (2R,6R)-HNK may have with opioid receptors had no impact on its mediation of antinociception at the initiation of the delayed effect or its expression 24 hours later.
Pretreatment with either saline, NBQX, or naltrexone 30 minutes before ketamine injection was used to test the involvement of AMPA and opioid receptors in the antinociceptive effect of ketamine (10 mg/kg) (Fig. 3E; pretreatment x ketamine treatment interaction, F(2, 54) = 4.634, P = 0.0139). AMPAR antagonism with NBQX had no impact on ketamine-mediated antinociception (P = 0.9763). In contrast, naltrexone pretreatment reversed ketamine mediated antinociception (saline/ketamine versus naltrexone/ketamine P = 0.0072). These results suggest that the mechanism for ketamine′s antinociception differs from that of (2R,6R)-HNK.
(2R,6R)-HNK Reversed Mechanical Allodynia in SNI-Treated Mice with a Longer Duration of Effect than Gabapentin
Two doses of (2R,6R)-HNK (10 and 30 mg/kg) were compared with two doses of gabapentin (10 and 30 mg/kg) for reversal of mechanical allodynia in an SNI model of neuropathic pain. All of the animals exhibited a significantly lower threshold to respond to mechanical stimulation on day 11 after SNI surgery compared with their corresponding baseline values (data not shown, average baseline before surgery for all groups was 4.3 ± 1.10 g f), indicating the presence of mechanical allodynia. There was no significant difference between groups at time 0 (day 11) before treatment. The mice were treated once daily for three days. Mechanosensitivity testing was repeated 4 and 24 hours following the first treatment and again 24 hours following the third treatment (Fig. 4A). Analysis of the results revealed a time x treatment interaction (F(12, 129) = 6.211, P < 0.0001). (2R,6R)-HNK reversed hypersensitivity associated with the SNI condition 4 hours after administration (Fig. 4B, P = 0.0003 for 10 mg/kg and P < 0.0001 for 30 mg/kg). (2R,6R)-HNK’s allodynia-reversing effect persisted for greater than 24 hours after a single dose (P < 0.0001 for 10 mg/kg and P < 0.0001 for 30 mg/kg) and was still present 24 hours following the third treatment (P = 0.0023 for 10 mg/kg and P < 0.0001 for 30 mg/kg). Both doses of gabapentin reversed the hypersensitivity when tested 4 hours after treatment (P = 0.0005 for 10 mg/kg and P < 0.0001 for 30 mg/kg). However, gabapentin failed to maintain a reversal of mechanical allodynia 24 hours following the first or even third drug treatment.
(2R,6R)-HNK Did Not Impair Gait or Induce Loss of Motor Coordination, Unlike Ketamine
Male mice underwent gait analysis 10 minutes following treatment with either ketamine at doses of 10 mg/kg and 30 mg/kg or (2R,6R)-HNK at doses of 10 mg/kg and 50 mg/kg. The animals’ gait characteristics were compared with animals that received saline injections. Animals receiving ketamine exhibited dose-dependent alterations in fore and hind paw gait parameters, including brake/stride ratio, propel/stride ratio, paw area variability at peak stance, propel duration, and brake duration (Table 1). These gait alterations, taken altogether, reflect a reduction in motor coordination. In contrast, animals receiving (2R,6R)-HNK did not exhibit significant changes in any of these parameters even at 50 mg/kg, which is five times greater than the dose needed to produce antinociception or analgesia.
TABLE 1.
Gait analysis following the administration of ketamine and (2R,6R)-HNK. Computer analysis of gait form was performed 10 minutes following ketamine or (2R,6R)-HNK injection and assessed a variety of motor parameters using the DigiGait apparatus. The data shown are group means ± S.D. Two separate cohorts, one for each medication, testing two doses against saline control were analyzed using a one-way ANOVA. N = 11–12 for the ketamine cohort and N = 10 for the (2R,6R)-HNK cohort.
| Parameter | Treatment | Saline | 10 mg/kg (for Ket) 10 mg/kg (for HNK) | 30 mg/kg (for Ket) 50 mg/kg (for HNK) | Treatment effect | |
|---|---|---|---|---|---|---|
| Brake/Stride Ratio (%) | Ketamine | Forepaw | 26.15 ± 7.35 | 29.06 ± 6.52 | 34.89 ± 9.27 | F(2, 65) = 7.433, P = 0.0012 |
| Hindpaw | 13.35 ± 5.41 | 17.81 ± 6.56 | 19.69 ± 7.43 | F(2, 65) = 5.849, P = 0.0046 | ||
| (2R,6R)-HNK | Forepaw | 33.44 ± 6.91 | 32.92 ± 6.17 | 30.36 ± 7.68 | n.s. | |
| Hindpaw | 15.36 ± 6.35 | 16.50 ± 7.59 | 16.29 ± 5.99 | n.s. | ||
| Propel/Stride Ratio (%) | Ketamine | Forepaw | 43.55 ± 7.13 | 39.62 ± 7.16 | 37.09 ± 9.91 | F(2, 65) = 3.689, P = 0.0304 |
| Hindpaw | 62.92 ± 7.32 | 60.46 ± 6.61 | 55.47 ± 8.83 | F(2, 65) = 5.612, P = 0.0056 | ||
| (2R,6R)-HNK | Forepaw | 37.88 ± 5.65 | 36.41 ± 6.36 | 38.89 ± 5.87 | n.s. | |
| Hindpaw | 62.00 ± 7.98 | 60.99 ± 8.42 | 61.59 ± 6.95 | n.s. | ||
| Paw Area Variability at Peak Stance (cm2) | Ketamine | Forepaw | 0.026 ± 0.008 | 0.036 ± 0.017 | 0.048 ± 0.022 | F(2, 65) = 9.973, P = 0.0002 |
| Hindpaw | 0.054 ± 0.026 | 0.057 ± 0.025 | 0.095 ± 0.045 | F(2, 65) = 10.89, P < 0.0001 | ||
| (2R,6R)-HNK | Forepaw | 0.036 ± 0.021 | 0.034 ± 0.016 | 0.031 ± 0.018 | n.s. | |
| Hindpaw | 0.050 ± 0.017 | 0.048 ± 0.023 | 0.039 ± .016 | n.s. | ||
| Propel Duration (seconds) | Ketamine | Forepaw | 0.177 ± 0.040 | 0.175 ± 0.046 | 0.142 ± 0.048 | F(2, 65) = 4.240, P = 0.0186 |
| Hindpaw | 0.250 ± 0.037 | 0.259 ± 0.051 | 0.224 ± 0.052 | F(2, 65) = 3.235, P = 0.0458 | ||
| (2R,6R)-HNK | Forepaw | 0.147 ± 0.030 | 0.144 ± 0.042 | 0.151 ± 0.025 | n.s. | |
| Hindpaw | 0.250 ± 0.052 | 0.254 ± 0.050 | 0.253 ± 0.051 | n.s. | ||
| Brake Duration (seconds) | Ketamine | Forepaw | 0.105 ± 0.029 | 0.127 ± 0.031 | 0.132 ± 0.038 | F(2, 65) = 4.577, P = 0.0138 |
| Hindpaw | 0.053 ± 0.024 | 0.076 ± 0.030 | 0.077 ± 0.028 | F(2, 65) = 5.602, P = 0.0057 | ||
| (2R,6R)-HNK | Forepaw | 0.130 ± 0.033 | 0.127 ± 0.026 | 0.120 ± 0.040 | n.s. | |
| Hindpaw | 0.061 ± 0.022 | 0.067 ± 0.029 | 0.065 ± 0.022 | n.s. |
Discussion
In this study, the ketamine metabolite (2R,6R)-HNK produced an increase in the threshold for healthy mice to respond to an aversive heat stimulus (Fig. 1 and 2) and reversed SNI-induced mechanical allodynia (Fig. 4). Antinociception on the hot plate was produced in healthy animals without a pre-existing pain condition and is a standard screen for analgesic drugs (Piercey and Schroeder, 1981). Ketamine showed some key differences from (2R,6R)-HNK. Ketamine-mediated antinociception was rapid (onset <10 minutes) and lasted less than 1 hour. In contrast, (2R,6R)-HNK produced delayed antinociception measured 24 hours after injection when the drug has already been eliminated from the body (Zanos et al., 2016). Moreover, the pain-reducing effects of ketamine were mediated partially through opioid receptors, while those of (2R,6R)-HNK were AMPAR dependent and opioid receptor independent (Fig. 3). The persistent duration of (2R,6R)-HNK analgesia was also seen in effects measured with the SNI model. (2R,6R)-HNK produced an acute and prolonged increase in threshold to respond to mechanical stimulation with a duration of action exceeding that of gabapentin, a drug used to treat neuropathic pain, even after repeated dosing.
An earlier publication demonstrated that (2R,6R)-HNK could reverse mechanical allodynia associated with acute and chronic pain conditions in mice (Kroin et al., 2019). The reduction in mechanical allodynia was evident within 1 hour, persisted for 24 hours after a single administration, and continued up to 72 hours after once-daily repeated administration. The onset of the antinociception by (2R,6R)-HNK described in this study occurred between 10 and 24 hours. Still, it was active beyond the expected physical presence of the drug, considering that the half-life of (2R,6R)-HNK in the brain is approximately 1 hour (Zanos et al., 2016), and plasma half-life is 0.2–0.8 hours in mice (Highland et al., 2019). These data suggest that persistent changes in neuroplasticity, presently unidentified, induced by (2R,6R)-HNK may underlie the protracted antinociception effect.
(2S,6S)-HNK is another ketamine metabolite receiving attention for its potential role in ketamine′s antidepressant behavioral effects. This metabolite would be derived in racemic form from RS-ketamine or selectively from S-ketamine, an antidepressant approved by the FDA in the form of a nasal spray (Spravato). Several studies have found that (2R,6R)-HNK was more potent and effective than (2S,6S)-HNK on tests in rodents for antidepressant activity (Zanos et al., 2016; Highland et al., 2021). However, a recent study demonstrated that (2S,6S)-HNK, and not (2R,6R)-HNK, effectively reversed depression-like behavior in a corticosterone-induced mouse model of depression (Yokoyama et al., 2020). In these experiments, (2S,6S)-HNK at doses of 10 mg/kg (Fig. 1B) and 30 mg/kg (data not shown) did not produce antinociception. Although additional doses could be tested, (2S,6S)-HNK did not appear to produce the analgesia-like effects produced by (2R,6R)-HNK and racemic ketamine.
This study is the first to investigate a dose-response relationship for (2R,6R)-HNK-mediated reduction of pain behavior. The inverted U-shaped dose response for antinociception was consistent between male and female mice. (2R,6R)-HNK also showed an inverted U-shaped dose-response to reduce escape failures in an inescapable shock paradigm (Zanos et al., 2019). The loss of antinociception as the (2R,6R)-HNK dose increased to and above 30 mg/kg in this study does not likely reflect behavioral competition because (2R,6R)-HNK does not alter motor coordination or locomotor activity (Zanos et al., 2016). Instead, the descending limb may reflect competing downstream effects of (2R,6R)-HNK at higher doses that are yet unidentified. Interestingly, some of ketamine′s behavioral and cellular therapeutic effects have also shown an inverted U-shaped dose response. Ketamine induced mechanistic target of rapamycin activation in the rat prefrontal cortex at moderate doses, while low or high doses produced no effect (Li et al., 2010).
The mechanism underlying the initiation of delayed antinociception of (2R,6R)-HNK was investigated using antagonists for AMPA and opioid receptors. (2R,6R)-HNK has been reported to increase AMPAR-dependent synaptic transmission by increasing glutamate release and AMPAR expression (Zanos et al., 2016; Pham et al., 2018). AMPAR antagonism with NBQX has been shown to prevent the sustained antidepression-like effects in mice by ketamine and (2R,6R)-HNK 24 hours after injection (Koike and Chaki, 2014; Zanos et al., 2016), demonstrating that some persistent behavioral effects of these drugs appear to be mediated by the AMPAR. AMPAR glutamatergic signaling also is known to play a vital role in pain transmission. Correlations have been identified between increased AMPAR subunit composition within the anterior cingulate cortex and the facilitation of pain signal transmission (Xu et al., 2008) as well as AMPAR subunit expression in the spinal cord and a stress-induced transition from acute to chronic pain (Li et al., 2014). AMPARs have also been implicated in pain-reduction in rodents, where AMPAR-positive allosteric modulators (AMPAkines) reduced mechanical allodynia in rats with induced neuropathic and inflammatory type pains (Le et al., 2014). The ability of NBQX to block the behavioral expression of (2R,6R)-HNK antinociception 24 hours after injection is consistent with (2R,6R)-HNK producing a persistent increase in AMPAR signal transmission (Zanos et al., 2016; Pham et al., 2018). The results of this study suggest that (2R,6R)-HNK-induced antinociception requires AMPA receptor activation in both the initiation and expression of the delayed antinociceptive effect. This aligns with previous data in which AMPAR blockade inhibited (2R,6R)-HNK mediated behavioral effects in rodent models of stress and increased gamma power measured via quantitative electroencephalography (Zanos et al., 2016).
The involvement of opioid receptors has also been implicated in ketamine′s antidepressant effects. Pretreatment with the nonselective opioid receptor antagonist naltrexone blocked improvements in depression scores associated with ketamine infusion therapy in humans (Williams et al., 2018), signifying that the acute antidepressant effects of ketamine are at least partially dependent upon opioid receptor activation. Opioid receptor antagonism has also been shown to block the antinociceptive effects of ketamine on the tail-flick test in mice (Pacheco et al., 2014). In this study, ketamine antinociception was also blocked by naltrexone pretreatment but not AMPAR antagonism. In contrast, (2R,6R)-HNK antinociception was not blocked by naltrexone pretreatment, while AMPAR antagonism blocked the antinociception, signifying that the drugs use different mechanisms to induce pain reduction effects. These results align with previous data in which naltrexone did not block (2R,6R)-HNK mediated reversal of mechanical allodynia in a model of postoperative pain (Kroin et al., 2019).
Kroin et al. (2019) demonstrated that (2R,6R)-HNK 10 mg/kg reversed mechanical allodynia associated with a model of neuropathic pain in female mice. (2R,6R)-HNK’s allodynia-reversing effect was confirmed in male SNI-treated mice using multiple doses and compared the results to gabapentin treatment (Fig. 4). Acutely, (2R,6R)-HNK reversed the allodynia 4 hours after administration at 10 and 30 mg/kg. The onset of analgesia for (2R,6R)-HNK in the SNI model was more rapid than the antinociceptive effects in healthy animals and may reflect changes in behavioral sensitivity when testing in the presence of spontaneous pain. Nevertheless, (2R,6R)-HNK demonstrated a sustained analgesic effect 24 hours after treatment, whereas gabapentin did not. Although similar to gabapentin when tested at 4 hours, the effects of (2R,6R)-HNK persisted longer than gabapentin following cessation of a once-daily for three days dosing regimen, indicating that (2R,6R)-HNK exerts a longer duration of effect compared with gabapentin and is not subject to tolerance under these conditions. Additional research is needed to examine whether (2R,6R)-HNK’s allodynia reversing effect in this model of neuropathic pain, or other pain models, is dependent on AMPAR.
Identifying novel analgesics that lack the side effects common with opioids and ketamine is critical to identifying more effective long-term pain treatments. Ketamine induces transient alterations in motor coordination (Irifune et al., 1995; Razoux et al., 2007), produces dissociative cognition, and demonstrates considerable abuse potential. Careful assessment of motor impairment can differentiate between actual analgesia behavior and analgesia masked by motor dysfunction. Here treatment-induced motor impairment following ketamine or (2R,6R)-HNK administration was evaluated by analyzing a sensitive computerized assay of gait characteristics. Treadmill videography has been used preclinically to measure spatiotemporal gait alterations resulting from pathologic conditions (Berryman et al., 2009; Zhan et al., 2019; Kwok et al., 2020) and may be sensitive enough to identify gait alterations even before the impairment becomes clinically significant. Subsequent analysis identified ketamine-induced dystaxia in several gait parameters (Table 1). (2R,6R)-HNK did not produce changes in gait, even at doses considerably higher than required to achieve a pain reduction-like effect. Based on the present and published data, (2R,6R)-HNK is unlikely to induce motor incoordination, impair sensorimotor gaiting, or possess rewarding effects (Zanos et al., 2016).
In conclusion, these data indicate that (2R,6R)-HNK can produce antinociception and analgesia in animals, with a duration of action that exceeds its physical presence. (2R,6R)-HNK likely initiates and sustains this effect through persistent activation of AMPAR, a different mechanism than other common pain therapeutics. In addition, (2R,6R)-HNK does not produce the side effects that limit the clinical utility of other strong analgesic medications. Further investigation into the mechanism underlying (2R,6R)-HNK’s antinociceptive effects and exploration of its efficacy in different pain conditions may prove valuable toward developing novel therapeutics for treating pain conditions and discerning pathophysiological alterations that may be normalized by (2R,6R)-HNK treatment in spontaneous pain conditions.
Acknowledgments
The authors thank Dr. Mumeko C. Tsuda and Ms. Laura Tucker for their assistance with the assays used in this research and the resources made available to us through the Preclinical Behavior and Modeling Core at Uniformed Services University of the Health Sciences. The authors appreciate Dr. Yumin Zhang’s help with training in developing the SNI pain model. The authors are grateful to Dr. Craig J. Thomas and Dr. Patrick Morris of the National Center for Advancing Translational Sciences for help in obtaining (2R,6R)-hydroxynorketamine and for helpful comments on this manuscript. The information or content and conclusions do not necessarily represent the official position or policy of, nor should any official endorsement be inferred by, the TriService Nursing Research Program, the Uniformed Services University of the Health Sciences, the Department of Defense, or the U.S. Government.
Abbreviations
- (2R,6R)-HNK
(2R,6R)-hydroxynorketamine
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor
- HNK
hydroxynorketamine
- NBQX
2,3-dioxo-6-nitro-7-sulfamoyl-benzo[f]quinoxaline
- SNI
spared nerve injury
Authorship Contributions
Participated in research design: Yost, Browne, Lucki.
Conducted experiments: Yost, Wulf, Browne.
Performed data analysis: Yost, Wulf, Browne, Lucki.
Wrote or contributed to the writing of the manuscript: Yost, Browne, Lucki.
Footnotes
This research was sponsored by the TriService Nursing Research Program, Uniformed Services University of the Health Sciences (Grant NA20-A02GR), and CDMRP (Award W81XWH-21-2-011). The information or content and conclusions do not necessarily represent the official position or policy of, nor should any official endorsement be inferred by, the TriService Nursing Research Program, Uniformed Services University of the Health Sciences, the Department of Defense, or the U.S. Government.
This work was previously presented as a poster presentation at the following workshops: Military Health System Research Symposium, Sept 2020, venue canceled (abstract only); Uniformed Services University Research Days Graduate Student Colloquium, May 2021, Bethesda, MD; Military Health System Research Symposium, Sept 2020, venue canceled (abstract only); Annual Meeting of the American College of Neuropsychopharmacology, Dec 2021, San Juan, Puerto Rico; ASPET Annual Meeting at EB22, Apr 2022, Philadelphia, PA.
No author has an actual or perceived conflict of interest with the contents of this article.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354. [DOI] [PubMed] [Google Scholar]
- Berryman ER, Harris RL, Moalli M, Bagi CM (2009) Digigait quantitation of gait dynamics in rat rheumatoid arthritis model. J Musculoskelet Neuronal Interact 9:89–98. [PubMed] [Google Scholar]
- Bourquin A-F, Süveges M, Pertin M, Gilliard N, Sardy S, Davison AC, Spahn DR, Decosterd I (2006) Assessment and analysis of mechanical allodynia-like behavior induced by spared nerve injury (SNI) in the mouse. Pain 122:14. e1–14. [DOI] [PubMed] [Google Scholar]
- Browne CA, Wulf HA, Jacobson ML, Oyola MG, Wu TJ, Lucki I (2022) Long-term increase in sensitivity to ketamine’s behavioral effects in mice exposed to mild blast induced traumatic brain injury. Exp Neurol 350:113963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53:55–63. [DOI] [PubMed] [Google Scholar]
- Chou D, Peng HY, Lin TB, Lai CY, Hsieh MC, Wen YC, Lee AS, Wang HH, Yang PS, Chen GD, et al. (2018) (2R,6R)-hydroxynorketamine rescues chronic stress-induced depression-like behavior through its actions in the midbrain periaqueductal gray. Neuropharmacology 139:1–12. [DOI] [PubMed] [Google Scholar]
- Cohen SP, Bhatia A, Buvanendran A, Schwenk ES, Wasan AD, Hurley RW, Viscusi ER, Narouze S, Davis FN, Ritchie EC, et al. (2018) Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Chronic Pain From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med 43:521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, Kerns R, Von Korff M, Porter L, Helmick C (2018) Prevalence of Chronic Pain and High-Impact Chronic Pain Among Adults - United States, 2016. MMWR Morb Mortal Wkly Rep 67:1001–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgaard L, Hjortkjaer RK, Regnier B, Nordholm L (1994) Pharmacokinetics of the neuroprotective glutamate antagonist NBQX (6-nitro-7-sulfamoyl-benzo(f)quinoxaline-2,3-dione) in mice, rats, and dogs. Interactions with probenecid. Drug Metab Dispos 22:289–293. [PubMed] [Google Scholar]
- Decosterd I, Woolf CJ (2000) Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain 87:149–158. [DOI] [PubMed] [Google Scholar]
- Dias JM, de Brito TV, de Aguiar Magalhães D, da Silva Santos PW, Batista JA, do Nascimento Dias EG, de Barros Fernandes H, Damasceno SRB, Silva RO, Aragão KS, et al. (2014) Gabapentin, a synthetic analogue of gamma aminobutyric acid, reverses systemic acute inflammation and oxidative stress in mice. Inflammation 37:1826–1836. [DOI] [PubMed] [Google Scholar]
- Dixon WJ (1980) Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol 20:441–462. [DOI] [PubMed] [Google Scholar]
- Eide PK, Jørum E, Stubhaug A, Bremnes J, Breivik H (1994) Relief of post-herpetic neuralgia with the N-methyl-D-aspartic acid receptor antagonist ketamine: a double-blind, cross-over comparison with morphine and placebo. Pain 58:347–354. [DOI] [PubMed] [Google Scholar]
- Fine PG (2011) Long-term consequences of chronic pain: mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Med 12:996–1004. [DOI] [PubMed] [Google Scholar]
- Highland JN, Zanos P, Riggs LM, Georgiou P, Clark SM, Morris PJ, Moaddel R, Thomas CJ, Zarate CA, Pereira EFR, Gould TD(2021) Hydroxynorketamines: Pharmacology and Potential Therapeutic Applications. Pharmacol Rev 73:763–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highland JN, Morris PJ, Zanos P, Lovett J, Ghosh S, Wang AQ, Zarate CA Jr, Thomas CJ, Moaddel R, Gould TD (2019) Mouse, rat, and dog bioavailability and mouse oral antidepressant efficacy of (2R,6R)-hydroxynorketamine. J Psychopharmacol 33:12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, Mallinger AG, Zarate CA Jr (2011) Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog Neuropsychopharmacol Biol Psychiatry 35:1155–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irifune M, Shimizu T, Nomoto M, Fukuda T (1995) Involvement of N-methyl-D-aspartate (NMDA) receptors in noncompetitive NMDA receptor antagonist-induced hyperlocomotion in mice. Pharmacol Biochem Behav 51:291–296. [DOI] [PubMed] [Google Scholar]
- Jacobson ML, Wulf HA, Browne CA, Lucki I (2020) The kappa opioid receptor antagonist aticaprant reverses behavioral effects from unpredictable chronic mild stress in male mice. Psychopharmacology (Berl) 237:3715–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa J, Shimazaki T, Kawashima N, Chaki S (2005) AMPA receptor stimulation mediates the antidepressant-like effect of a group II metabotropic glutamate receptor antagonist. Brain Res 1042:92–98. [DOI] [PubMed] [Google Scholar]
- Kilic FS, Sirmagul B, Yildirim E, Oner S, Erol K (2012) Antinociceptive effects of gabapentin & its mechanism of action in experimental animal studies. Indian J Med Res 135:630–635. [PMC free article] [PubMed] [Google Scholar]
- Koike H, Chaki S (2014) Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behav Brain Res 271:111–115. [DOI] [PubMed] [Google Scholar]
- Kroin JS, Das V, Moric M, Buvanendran A (2019) Efficacy of the ketamine metabolite (2R,6R)-hydroxynorketamine in mice models of pain. Reg Anesth Pain Med 44:111–117. [DOI] [PubMed] [Google Scholar]
- Kvarnström A, Karlsten R, Quiding H, Emanuelsson BM, Gordh T (2003) The effectiveness of intravenous ketamine and lidocaine on peripheral neuropathic pain. Acta Anaesthesiol Scand 47:868–877. [DOI] [PubMed] [Google Scholar]
- Kwok A, Rosas S, Bateman TA, Livingston E, Smith TL, Moore J, Zawieja DC, Hampton T, Mao XW, Delp MD, et al. (2020) Altered rodent gait characteristics after ∼35 days in orbit aboard the International Space Station. Life Sci Space Res (Amst) 24:9–17. [DOI] [PubMed] [Google Scholar]
- Le AM, Lee M, Su C, Zou A, Wang J (2014) AMPAkines have novel analgesic properties in rat models of persistent neuropathic and inflammatory pain. Anesthesiology 121:1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Yang Y, Liu S, Fang H, Zhang Y, Furmanski O, Skinner J, Xing Y, Johns RA, Huganir RL, et al. (2014) Stress induces pain transition by potentiation of AMPA receptor phosphorylation. J Neurosci 34:13737–13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu R-J, Banasr M, Dwyer JM, Iwata M, Li X-Y, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964 American Association for the Advancement of Science. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden EW, Troppoli TA, Myers SJ, Zanos P, Aracava Y, Kehr J, Lovett J, Kim S, Wang FH, Schmidt S, et al. (2019) Antidepressant-relevant concentrations of the ketamine metabolite (2R,6R)-hydroxynorketamine do not block NMDA receptor function. Proc Natl Acad Sci USA 116:5160–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris PJ, Moaddel R, Zanos P, Moore CE, Gould TD, Zarate CA Jr, Thomas CJ (2017) Synthesis and N-Methyl-d-aspartate (NMDA) Receptor Activity of Ketamine Metabolites. Org Lett 19:4572–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco DdaF, Romero TRL, Duarte IDG (2014) Central antinociception induced by ketamine is mediated by endogenous opioids and μ- and δ-opioid receptors. Brain Res 1562:69–75. [DOI] [PubMed] [Google Scholar]
- Pham TH, Defaix C, Xu X, Deng SX, Fabresse N, Alvarez JC, Landry DW, Brachman RA, Denny CA, Gardier AM (2018) Common Neurotransmission Recruited in (R,S)-Ketamine and (2R,6R)-Hydroxynorketamine-Induced Sustained Antidepressant-like Effects. Biol Psychiatry 84:e3–e6. [DOI] [PubMed] [Google Scholar]
- Piercey MF, Schroeder LA (1981) Spinal and Supraspinal sites for morphine and nefopam analgesia in the mouse. Eur J Pharmacol 74:135–140. [DOI] [PubMed] [Google Scholar]
- Razoux F, Garcia R, Léna I (2007) Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens. Neuropsychopharmacology 32:719–727. [DOI] [PubMed] [Google Scholar]
- Schwenk ES, Viscusi ER, Buvanendran A, Hurley RW, Wasan AD, Narouze S, Bhatia A, Davis FN, Hooten WM, Cohen SP (2018) Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management From the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg Anesth Pain Med 43:456–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AJ, Cloud ME, Cao Y-Q, Mohapatra DP (2018) Deficits in Burrowing Behaviors are Associated with Mouse Models of Neuropathic but not Inflammatory Pain or Migraine. Front Behav Neurosci 12:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd AJ, Mohapatra DP (2018) Pharmacological validation of voluntary gait and mechanical sensitivity assays associated with inflammatory and neuropathic pain in mice. Neuropharmacology 130:18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SD, Eckert WA 3rd, Basbaum AI (2003) Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain 4:465–470. [DOI] [PubMed] [Google Scholar]
- Sigtermans MJ, van Hilten JJ, Bauer MCR, Arbous SM, Marinus J, Sarton EY, Dahan A (2009) Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain 145:304–311. [DOI] [PubMed] [Google Scholar]
- Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, Hawkins J, Birnbaum J, Lyons DM, Rodriguez CI, et al. (2018) Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry 175:1205–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooley CM, Sher RB, Kale A, Frankel WN, Cox GA, Seburn KL (2005) Gait analysis detects early changes in transgenic SOD1(G93A) mice. Muscle Nerve 32:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wu L-J, Wang H, Zhang X, Vadakkan KI, Kim SS, Steenland HW, Zhuo M (2008) Presynaptic and postsynaptic amplifications of neuropathic pain in the anterior cingulate cortex. J Neurosci 28:7445–7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama R, Higuchi M, Tanabe W, Tsukada S, Naito M, Yamaguchi T, Chen L, Kasai A, Seiriki K, Nakazawa T, et al. (2020) (S)-norketamine and (2S,6S)-hydroxynorketamine exert potent antidepressant-like effects in a chronic corticosterone-induced mouse model of depression. Pharmacol Biochem Behav 191:172876. [DOI] [PubMed] [Google Scholar]
- Zanos P, Highland JN, Liu X, Troppoli TA, Georgiou P, Lovett J, Morris PJ, Stewart BW, Thomas CJ, Thompson SM, et al. (2019) (R)-Ketamine exerts antidepressant actions partly via conversion to (2R,6R)-hydroxynorketamine, while causing adverse effects at sub-anaesthetic doses. Br J Pharmacol 176:2573–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, et al. (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA (2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry 71:939–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan J, Yakimov V, Rühling S, Fischbach F, Nikolova E, Joost S, Kaddatz H, Greiner T, Frenz J, Holzmann C, et al. (2019) High Speed Ventral Plane Videography as a Convenient Tool to Quantify Motor Deficits during Preclinical Experimental Autoimmune Encephalomyelitis. Cells 8:1439. [DOI] [PMC free article] [PubMed] [Google Scholar]