Abstract
Background:
Repeated cocaine administration changes histone acetylation and methylation on Lys residues and Deoxyribonucleic acid (DNA) within the nucleus accumbens (NAc). Recently Nestler’s group explored histone Arg (R) methylation in reward processing models. Damez-Werno et al. (2016) reported that during human investigations and animal self-administration experiments, the histone mark protein-R-methyltransferase-6 (PRMT6) and asymmetric dimethylation of R2 on histone H3 (H3R2me2a) decreased in the rodent and cocaine-dependent human NAc. Overexpression of PRMT6 in D2-MSNs in all NAc neurons increased cocaine seeking, whereas PRMT6 overexpression in D1-MSNs protects against cocaine-seeking.
Hypothesis:
The hypothesis is that dopaminylation (H3R2me2a binding) occurs in psychostimulant use disorder (PSU), and the binding inhibitor Srcin1, like the major DRD2 A2 allelic polymorphism, protects against psychostimulant seeking behavior by normalizing nucleus accumbens (NAc) dopamine expression.
Discussion:
Numerous publications confirmed the association between the DRD2 Taq A1 allele (30–40 lower D2 receptor numbers) and severe cocaine dependence. Lepack et al. (2020) found that acute cocaine increases dopamine in NAc synapses, and results in histone H3 glutamine 5 dopaminylation (H3Q5dop) and consequent inhibition of D2 expression. The inhibition increases with chronic cocaine use and accompanies cocaine withdrawal. They also found that the Src kinase signaling inhibitor 1 (Srcin1 or p140CAP) during cocaine withdrawal reduced H3R2me2a binding. Consequently, this inhibited dopaminylation induced a “homeostatic brake.”
Conclusion:
The decrease in Src signaling in NAc D2-MSNs, (like the DRD2 Taq A2 allele, a well-known genetic mechanism protective against SUD) normalizes the NAc dopamine expression and decreases cocaine reward and motivation to self-administer cocaine. The Srcin1 may be an important therapeutic target.
Keywords: Src, p140CAP, psychostimulants, cocaine, histone arginine (R) methylation, medium spiny neurons, DRD2 gene
1. INTRODUCTION
In 2013 Blum’s group asked the question related to why people drink alcohol or get involved in high rates of drug-seeking behaviors. Other compelling questions surround the millions who seek out high-risk novelty situations are as follows: “Why are millions paying the price of their indiscretions in our jails, in hospitals, in wheelchairs, and are lying dead in our cemeteries. What price must we pay for pleasure-seeking or just plain getting “HIGH”? Maybe the answer lies within our brain. Maybe it is in our genome” [1].
In 2020, Americans faced the worst opioid epidemic, with as many as 185 people dying daily from opioid-related events [2]. There is robust evidence that cognitive, emotional, and behavioral disturbances observed in psychiatric illnesses, including Reward Deficiency Syndrome (RDS), connect with functional deficits in neurological networks [3–8].
Blum et al. identified Reward Deficiency Syndrome (RDS) in 1995 as a putative predictor of impulsive and addictive behaviors [9–12]. Many behavioral phenotypes, including neuropsychiatric disorders, aggression [13], alcoholism, and other rewarding and addictive behaviors [14], are associated with the DRD2 TaqI A1 allele. The DRD1 and DRD2 genes are both involved in reward mechanisms [15]. The net effect of biochemical actions in mesolimbic structures leads to rewarding phenomena when dopamine (DA) levels increase in synaptic spaces; within the nucleus accumbens, where DA interacts with DRD1 and DRD2 and other subtypes of receptors [16, 17]. Positron emission tomography (PET) studies have reported lower DRD2 availability in psychostimulant and alcohol-dependent and obese individuals than control participants [18–20].
Importantly, Noble, Blum, and colleagues [21] had also observed that the A1 and B1 minor alleles of the gene were associated with cocaine use disorder (CUD). These findings suggest that the DRD2 gene, located on q22-q23 region of chromosome 11, conferred susceptibility to psychostimulant use disorder (PUD). These results agreed with Gold’s group’s early reports that had suggested that the D2 agonist, bromocriptine, might have therapeutic potential against cocaine abuse and dependence [22]. However, unlike the DRD2 Taq A1 allele with a reduced number of D2 receptors, the DRD2 Taq A2 allele, the major variant, has a normal complement of D2 receptors that could be protective against psychostimulant abuse.
1.1. Hypothesis
Hypothesizing that dopaminylation H3R2me2a binding) occurs in CUD and during withdrawal, inhibitor Srcin1 or p140Cap blocks H3R2me2a binding and normalizes dopamine expression at the Nucleus Accumbens (NAc) like the major DRD2 Taq A2 allelic polymorphism, and both mechanisms are protective against psychostimulant seeking behavior.
2. UNDERSTANDING THE ROLE OF PROTEIN R METHYLTRANSFERASE (PRMT) PSYCHOSTIMU-LANT ABUSE
Cocaine increases dopamine neurotransmission from the ventral tegmental area (VTA) to reward-relevant brain regions. This well-known cocaine action is central to its addictive properties. Recently a role for serotonin (5-HT) in developing 5-HTergic neurons has been described whereby 5-HT located in the nucleus of these neurons covalently attached to histone proteins—specifically on H3 (H3Q5)—to regulate gene expression through a called serotonylation [23]. This mechanism has been generalized to other brain monoamines, such as dopamine. paminylation is a process in which a transglutaminase 2 protein can directly attach dopamine molecules to histone proteins [24]. The process of dopaminylation (H3Q5dop) and its role in cocaine abuse is the primary subject of this review and hypothesis.
Repeated exposure to cocaine regulates transcriptional events within the nucleus accumbens (NAc) via epigenetic mechanisms, that change the expression of the individual’s Deoxyribonucleic Acid (DNA) according to individual genetics [25]. Changes in epigenetic markers that include histone acetylation and methylation on Lys (K) residues, as well as DNA methylation, accompany the use of psychostimulants such as cocaine and methamphetamine [26]. Specifically, methamphetamine use disorder (MAUD) is a biopsychosocial disorder accompanied by multiple relapses even after prolonged abstinence. These multiple relapses are attributed to the long-lasting, maladaptive epigenetic changesin the brain.
Cadet’s group has used multiple biochemical and molecular approaches, including chromatin immunoprecipitation (ChIP) And quantitative PCR assays to demonstrate epigenetic changes in the NAc -rat and dorsal striatum of rats exposed to methamphetamine [27]. For example, methamphetamine increased the amount of phosphorylated (pCREB) bound at the promoter of several genes related to drug-seeking behaviors. Methamphetamine also initiated DNA hypomethylation at sites near the Crh transcription start site (TSS) and intragenic Avp sequences and DNA hydroxymethylation at the Crh TSS and intragenic Avp sites. Itis further noteworthy that methamphetamine increased the protein expression of ten-eleven-translocation enzymes that catalyze DNA hydroxymethylation. Methamphetamine increased TET1 binding at the Crh promoter and increased TET3 binding at Avp intragenic regions. Cadet’s group has also shown that chronic methamphetamine self-administration can result in DNA hydroxymethylation of potassium channels in the nucleus accumbens [28] necessary for regulating DA release.
Reports in the literature also demonstrated the functional role of histone Arg (R) methylation [28] in contrast to lysine(K) methylation. The methylation of R residues catalyzed by the protein R methyltransferase (PRMT) enzymes generates methylated R states with differentially diverse functional consequences, including monomethyl arginine (MMA) and dimethylarginine (DMA) residues [29]. In chromatin architecture, alterations are induced by R methylation; they either relax or condense its structure to create binding sites for regulatory proteins that contain specialized binding domains. These histone tails are targets for PRMTs and can affect gene expression [30, 31].
Frankel et al. [32] suggest that PRMT6 in mammalian cells may controlasymmetric dimethylation of R2 on histone H3 (H3R2me2a). Further, others have shown that H3R2me2a 5 mark may be repressive in nature due to its ability to counter the activation function of the nearby H3K4me3 mark [33]. Interestingly, Feng et al. [34] previously demonstrated an enrichment of the H3K4me3 mark that was pronounced at focal gene promoters in the rodent NAc after repeated cocaine exposure. Similarly, Kennedy et al. [35] showed that Class I HDAC inhibition blocks duced plasticity by targeted changes in histone methylation, providing more acetyl groups, and induced repressive histone methylation antagonizing cocaine-induced behaviors. Mostly, this effect via a chromatin-mediated suppression of GABAA receptor subunit expression results in an inhibitory tone on NAc neurons in the ventral tegmental area (VTA) and will increase dopamine release into the synapse. Indeed, this chromatin-mediated suppression of GABAA receptor subunit expression disinhibits the GABAA break on NAc dopamine release to overcome the chronic cocaine-induced loss of dopamine function [36].
Further, the proposition is that long-term cocaine abuse is due to its remarkable inhibition of presynaptic dopamine transporters that prevent the reabsorption of dopamine required for the next action potential and subsequently further decreases new dopamine synthesis. This lack of dopamine transported from the mitochondria (not the synaptic cleft) to the pre-neuronal dopamine storage vesicles compromises the enzyme tyrosine hydroxylase (rate-limiting for pamine synthesis) required to catalyze more synthesis of dopamine to overcome a “homeostatic break” [37]. Specifically, Trulson et al. [37] found that chronic administration of cocaine (for10 consecutive days of 10 mg/kg, IP, every 12 hours) generally decreased tyrosine hydroxylase staining of axons and terminal boutons in the rat frontal cortex and NAc terminals, which originate from the midbrain VTA. treatment also produced a long-lasting depletion of tyrosine hydroxylase immunoreactivity in the VTA when examined 60 days following the final cocaine injection. The authors concluded that: “these data demonstrated that chronic administration of cocaine produces a long-term, if not permanent, loss of tyrosine hydroxylase enzyme in both the bodies of the midbrain ventral tegmental area as well as in the nerve terminals in post-synaptic target regions of forebrain.”
Damez-Werno et al. [29] were the first to report that after repeated cocaine exposure, PRMT6 and its associated histone mark, asymmetric dimethylation of R2 on histone H3 (H3R2me2a), decreased in the NAc of mice and rats as well as in the NAc of cocaine-addicted humans. PRMTs regulate multiple biological processes that include DNA transcription, mRNA splicing, and piRNA biogenesis; however, little is known about the regulation of Prmt translation from mRNA into protein. Specifically, they revealed that cocaine-induced PRMT6 down-regulation occurs selectively in NAc medium spiny neurons expressing dopamine D2 receptors (D2-MSNs) and serves to protect against cocaine-induced behavioral abnormalities like anhedonia. Moreover, cocaine-induced PRMT6 up-regulation occurs selectively in NAc medium spiny neurons expressing dopamine D1 receptors (D1-MSNs). Therefore, the function of PRMT6 is consistent with opposing the activation of D2 and D1 receptor containing neurons in the NAc to dampen the final output from the ventral striatum. This group also found that Src kinase signaling inhibitor 1 (Srcin1 or p140Cap) is a key target for this chromatin modification. Srcin1 induction in the NAc after cocaine exposure, which is associated with reduced Src signaling, decreases cocaine reward and self-administration. To induce homeostatic feedback and prevent cocaine-induced abnormalities, one would expect a requirement that enables the normal expression of both D1 and D2-MSNs. However, albeit currently unexplained, the regulatory role of PRMT6 as induced by long-term cocaine abuse seems to selectively down-regulate D2-MSNs relative to D1-MSNs.
2.1. To Be Or Not Be D2
Dopamine is the neurotransmitter involved in motivation and reward behavior. When dopamine is chemically attached to histone proteins, cells switch different genes on and off to significantly affect cocaine vulnerability and relapse. Damez-Werno et al., as stated, persuasively demonstrated the downregulation of PRMT6 in the NAc of mice and rats treated repeatedly with cocaine. They also demonstrated this effect in cocaine-addicted postmortem humans. Cocaine down-regulation of PRMT6 seems to be specific toD2 MSNs in this brain region. Accordingly, Nestler’s group also suggested that this effect constitutes a homeostatic response and reinforces the hypothesis that this D2 MSN subtype is necessary to mediate and oppose cocaine-addictive behavior [29]. Elucidation of the role of PRMT6 in the NAc revealed that cocaine-induced decreases in H3R2me2a and increases in H3K4me3. Specifically, with chronic cocaine, there is an induction of the inhibitory geneSrcin1. Coincidental, with the induction of theSrcin1 by cocaine, is the suppression of the Src signaling pathway. Such resultant suppression mimics the similar suppression as observed with PRMT6/H3R2me2a along with subsequent opposition to the rewarding effects of cocaine, including self-administration of the drug. Previous work had demonstrated that H3R2 methylation by PRMT6 is prevented by H3K4me3, confirming H3R2me2a’s transcriptional repressive role [32]. From the neuro-psychopharmacological viewpoint, D1-MSNs vs. D2-MSNs have opposite roles in drug addiction. D1-MSNs promote both reward and sensitizing responses to psychostimulants, while D2-MSNs dampens these behaviors [37]. Thus, after cocaine exposure, increased PRMT6 levels in D1-MSNs and decreased PRMT6 levels in D2-MSNs may form a homeostatic response toward a common outcome, both opposing cocaine-addictive behaviors. In support of this hypothesis, Srcin1 overexpression in the NAc exerts anti-addictive properties by opposing the rewarding responsesto cocaine. What is even more remarkable, this action of Srcin1 (p140Cap), like that of PRMT6, appears to be specific to D2-MSNs.
These findings indicate a novel role of histone R methylation as a key regulator in the induction of Srcin1, an Src signaling repressor that opposes cocaine action. This work further suggests, in particular, the importance of D2 –MSNs in anti-cocaine effects [21] and provides additional supporting evidence to tag DRD2 function as a therapeutic target for the treatment of cocaine addiction. These findings relate to the possibility that vulnerability to relapse during periods of attempted cocaine abstinence results from the rewiring of brain reward circuitries, particularly VTA dopamine neurons such as D2 receptors [38]. In terms of relapse, especially in Alcohol Use Disorder (AUD), reduced dopamine D2 receptor (D2R) ligand binding has repeatedly been demonstrated in the human striatum. The attenuated D2R binding reflects reduced D2R density [18], which drives craving and relapse.
Moreover, long-term voluntary alcohol drinking significantly reduced mRNA levels of the long D2R isoform in the NAc [24]. Notably, alcohol intake reduced the striatal density of D2R-D2R homoreceptor complexes and increased the density of NAc shell A2AR (adenosine) -D2R heteroreceptor complexes, and decreased the density of sigma1R-D2R heteroreceptor complexes in the dorsal striatum. Understandably, Feltmann et al. [39] suggested that both reduced striatal D2R levels and reduced D2R protomer affinity within the striatal A2AR-D2R complex might underlie reduced D2R radioligand binding in humans with AUD. These intriguing results support the Blum group [40] hypothesis of a hypodopaminergic system in AUD and propose that the D2R heteroreceptor complex is a potential novel treatment target. With that stated, there is strong evidence that DRD2 A1 allele may induce relapse to not only alcoholism [41] but cocaine [42]. As previously stated, distinct populations of D1-and D2-dopamine receptor-expressing medium spiny neurons (D1-/D2-MSNs) comprise the nucleus accumbens, and activity in D1-MSNs promotes, whereas activity in D2-MSNs inhibits motivated behaviors. Heinsbroek et al. [42] demonstrated that both cell types sent GABAergic projections to the ventral pallidum and promoted cue-induced reinstatement of cocaine-seeking via the ventral pallidum differentially. After cocaine self-administration, selectively D2, but not D1, lose synaptic plasticity to the ventral pallidum’s inputs. The selective impairment in D2 afferents may promote D1 inputs’ influence to drive relapse to cocaine seeking.
Lepack et al. [24] also found a critical role in cocaine-induced transcriptional plasticity for the midbrain Transglutaminase a protein, directly attaches dopamine molecules to histone proteins. This process histone paminylation or H3Q5dop occurred during periods of caine withdrawal when rodents showed an accumulation of H3Q5dop in the VTA. Lepack et al. reduced cocaine-seeking behavior, attenuated dopamine release in the nucleus accumbens, and reversed cocaine-mediated gene expression changes by reducing H3Q5dop in the VTA during cocaine withdrawal (see Fig. 1). As stated earlier, while the relatively new concept of histone and the formidable work by Jayanthi et al. from NIDA [27] concerning Methamphetamine induced TET1- and TET3-Dependent DNA hydroxymethylation of Crh and Avp Genes in the rat NAc may suggest a generalized molecular epigenetic DNA hydroxymethylation and subsequent regulation at possibly the transcriptional 3’ loci as first found by Blum et al. [43], concerning the polymorphisms in the DRD2 gene.
Fig. (1). Dopaminylation in Cocaine Use and Withdrawal.
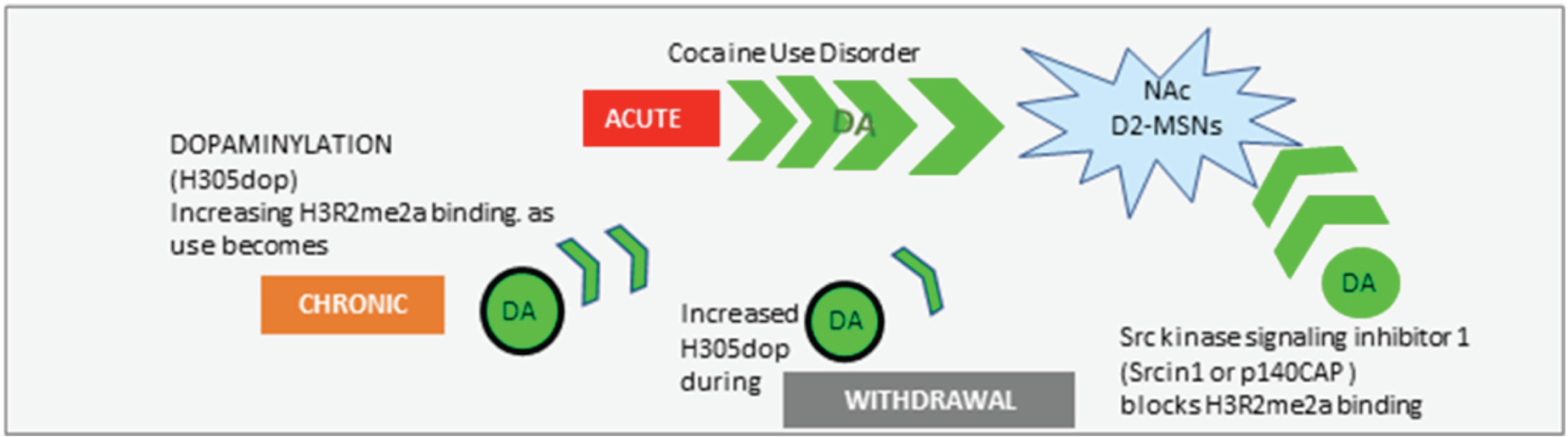
Illustrates how histone arginine dopaminylation that occurs in psychostimulant abuse is combated by H3R2me2a binding inhibitor Srcin1 and during withdrawal and normalizes dopamine expression at the Nucleus Accumbens (NAc) and like the major DRD2 A2 allelic polymorphism is protective against psychostimulant seeking behavior. DA = Dopamine.
Finally, the demonstration by Lepack et al. [24] regarding their seminal finding that Src kinase signaling inhibitor 1 (p140CAP) during cocaine withdrawal reduced H3R2me2a binding was indeed a protective mechanism in downregulating PRMT6 in the NAc. Consequently, inhibited dopaminylation induced a “homeostatic brake,” with a decrease NAc D2-MSNs Src signaling and subsequently reduced cocaine reward and motivation to self-administer cocaine. These findings mimic earlier publications from Blum and Noble’s group [18, 21, 43] in terms of not only cocaine but alcoholism, and Volkow’s group [44] on ADHD, that suggested that DRD2 Taq A2 allele is an inborn protective mechanism.
Recently a study by the Gondré-Lewis group45 [] at Howard University utilized a known model for binge drinking found that alcohol-preferring (P) adult male and female rats significantly mitigated their alcohol intake when treated with a neuro-nutrient, KB220Z, designed to augment DA signaling. Surprisingly, preliminary studies of KB220Z initially found fewer DRD2 punctae in neurons of the NAc. However, corrected for volume and cell number, the NAc shell expressed more DRD2 mRNA per cell than the NAc core. Independent of KB220Z, the study indicated a non-significant trend where KB220Z induced down-regulation of DRD2 mRNA in D2-MSNs.While expected, KB220Z up-regulation of the D2-MSNs, the importance of down-regulation of PRMT6 specific to D2-MSNs now understood to combat drug addiction, future research in our laboratory will attemptto dissect this exciting potential (see Fig. 2).
Fig. (2). Epigenetic and transcriptional effects of chronic cocaine exposure and psychostimulant abuse.

Histone acetylation and methylation on Lys residues result from cocaine and methamphetamine abuse (dull green). After repeated cocaine exposure, Protein-arginine (R)-methyltransferase-6 (PRMT6) and its associated histone decreased in the NAc of mice and rats and the NAc of cocaine-dependent humans due to dimethylation of R2 on histone H3 (H3R2me2a), which was asymmetric. Downregulation of demethylation by PRMT6 selectively occurred in NAc medium spiny neurons (MSNs) expressing dopamine D2 receptors (DRD2) (D2-MSNs), with opposite regulation manifested in D1-MSNs. These changes appeared to protect against cocaine-seeking and associated behavioral abnormalities (dull orange). Acute cocaine increased DA and histone H3 glutamine 5 dopaminylation (H3Q5dop), with a secondary decreased in D2 expression (bright green). Withdrawal from cocaine led to the accumulation of histone H3 glutamine 5 dopaminylation (H3Q5-dop) and subsequent D2 expression inhibition. Administration of the Src kinase signaling inhibitor 1 (p140CAP) during cocaine withdrawal reduced H3R2me2a binding. Inhibited dopaminylation induced a “homeostatic brake” and decreased NAc Src signaling in NAc D2-MSNs accompanied by decreased cocaine reward and motivation to self-administer cocaine (tan). These results are consistent with the idea that the DRD2 Taq A2 allele serves as a genetic protective mechanism (bright yellow).
In terms of psychostimulant induced alterations in brain structure and function, including microglia, a series of experiments involving methamphetamine should also be considered beyond DNA methylation and subsequent regulation of gene expression [46–49].
CONCLUSION
In conclusion, the histone-DNA spool to enable environmentally environmentally regulated alterations in gene expression. Due to this functional rewiring of the VTA reward circuitry, histone dopaminylation drives heightened vulnerability in chronic CUD. The buildup of H3Q5dop in the VTA can increase cocaine-seeking behavior. In contrast, reducing H3Q5dop in rats undergoing cocaine withdrawal significantly reversed gene expression changes and reduced caine-seeking behavior [24]. These findings also clearly establish a neurotransmission-independent role for neuronal dopamine in relapse-related transcriptional plasticity in the VTA [24]. Thus, histone arginine dopaminylation occurs in psychostimulant abuse, and the H3R2me2a binding inhibitor Srcin1 or p140Cap normalizes dopamine expression at the NAc, which mimics DRD2 A2 allelic polymorphic protection against psychostimulant seeking behavior.
Understanding these complex neurogenetic and novel epigenetic mechanisms concerning psychostimulant and alcohol use disorders, we continue to promote the need for DA homeostasis in addiction treatment and prevention. These activities will require studies using genetic addiction risk assessment with a nutrigenomic precision behavioral management that occasionally seeks epigenetic repair, encouraging dopamine homeostasis to prevent drug and non-drug seeking behaviors [42].
ACKNOWLEDGEMENTS
We would like to thank Margaret A Madigan for expert edits and development of Fig 1. in collaberation with KB.
FUNDING
KB and MGL are recipients of R41 MD012318/MD/NIMHD NIH HHS/United States; RDB is the recipient of I01 CX000479/CX/CSRD VA/United States; PKT is the recipient of R01 AA011034/AA/NIAAA NIH HHS/United States.
Footnotes
CONFLICT OF INTEREST
KB and RB are executives of the Geneus Health LLC and are members of the Board of Directors. KB owns units in the Geneus Health LLC. RDB, PKT, DB, and MGL are unpaid members of the Geneus Health Scientific Advisory Board. MSG is an unpaid honorary member of the Geneus Health Scientific Advisory Board. There are no other conflicts to report.
REFERENCES
- [1].Blum K, Giordano J, Morse S, Bowirrat A, Madigan M, Downs W, et al. Understanding the high mind Humans are still evolving genetically. IIOAB-India 2010; 1(2): 1-14-4. [Google Scholar]
- [2].Volkow ND, Blanco C. The changing opioid crisis: development, challenges and opportunities. Mol Psychiatry 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gold MS, Blum K, Febo M, et al. Molecular role of dopamine in anhedonia linked to reward deficiency syndrome (RDS) and anti-reward systems. Front Biosci (Schol Ed) 2018; 10: 309–25. 10.2741/s518 [DOI] [PubMed] [Google Scholar]
- [4].Borsook D, Linnman C, Faria V, Strassman AM, Becerra L, Elman I. Reward deficiency and anti-reward in pain chronification. Neurosci Biobehav Rev 2016; 68: 282–97. 10.1016/j.neubiorev.2016.05.033 [DOI] [PubMed] [Google Scholar]
- [5].Bowirrat A, Oscar-Berman M. Relationship between dopaminergic neurotransmission, alcoholism, and Reward Deficiency syndrome. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics 2005; 132b(1): 29–37. 10.1002/ajmg.b.30080 [DOI] [PubMed] [Google Scholar]
- [6].Filippi A, Mueller T, Driever W. vglut2 and gad expression reveal distinct patterns of dual GABAergic versus glutamatergic cotransmitter phenotypes of dopaminergic and noradrenergic neurons in the zebrafish brain. J Comp Neurol 2014; 522(9): 2019–37. 10.1002/cne.23524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Valentino RJ, Koroshetz W, Volkow ND. Neurobiology of the Opioid Epidemic: Basic and Translational Perspectives. Psychiatry 2020; 87(1): 2–3. 10.1016/j.biopsych.2019.09.003 [DOI] [PubMed] [Google Scholar]
- [8].Browne CJ, Godino A, Salery M, Nestler EJ. Epigenetic Mechanisms of Opioid Addiction. Biol Psychiatry 2020; 87(1): 22–33. 10.1016/j.biopsych.2019.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Blum K, Chen AL, Chen TJ, et al. Activation instead of blocking mesolimbic dopaminergic reward circuitry is a preferred modality in the long term treatment of reward deficiency syndrome (RDS): a commentary. Theor Biol Med Model 2008; 5: 24. 10.1186/1742-4682-5-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res 2000; 126: 325–41. 10.1016/S0079-6123(00)26022-6 [DOI] [PubMed] [Google Scholar]
- [11].Blum K, Chen AL, Oscar-Berman M, et al. Generational association studies of dopaminergic genes in reward deficiency syndrome (RDS) subjects: selecting appropriate phenotypes for reward dependence behaviors. Int J Environ Res Public Health 2011; 8(12): 4425–59. 10.3390/ijerph8124425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Febo M, Blum K, Badgaiyan RD, et al. Dopamine homeostasis: brain functional connectivity in reward deficiency syndrome. Front Biosci 2017; 22: 669–91. 10.2741/4509 [DOI] [PubMed] [Google Scholar]
- [13].Rosell DR, Siever LJ. The neurobiology of aggression and violence. CNS Spectr 2015; 20(3): 254–79. 10.1017/S109285291500019X [DOI] [PubMed] [Google Scholar]
- [14].Mahna D, Puri S, Sharma S. DNA methylation signatures: Biomarkers of drug and alcohol abuse. Mutat Res 2018; 777: 19–28. 10.1016/j.mrrev.2018.06.002 [DOI] [PubMed] [Google Scholar]
- [15].D’Aquila PS, Elia D, Galistu A. Role of dopamine D1-like and D2-like receptors in the activation of ingestive behaviour in thirsty rats licking for water. Psychopharmacology (Berl) 2019; 236(12): 3497–512. 10.1007/s00213-019-05317-w [DOI] [PubMed] [Google Scholar]
- [16].Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell 2015; 162(4): 712–25. 10.1016/j.cell.2015.07.046 [DOI] [PubMed] [Google Scholar]
- [17].Yamamoto K, Fontaine R, Pasqualini C, Vernier P. Classification of Dopamine Receptor Genes in Vertebrates: Nine Subtypes in Osteichthyes. Brain Behav Evol 2015; 86(3–4): 164–75. 10.1159/000441550 [DOI] [PubMed] [Google Scholar]
- [18].Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry 1991; 48(7): 648–54. 10.1001/archpsyc.1991.01810310066012 [DOI] [PubMed] [Google Scholar]
- [19].Volkow ND, Chang L, Wang GJ, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 2001; 158(12): 2015–21. 10.1176/appi.ajp.158.12.2015 [DOI] [PubMed] [Google Scholar]
- [20].Volkow ND, Wang GJ, Telang F, et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 2008; 42(4): 1537–43. 10.1016/j.neuroimage.2008.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Noble EP, Blum K, Khalsa ME, et al. Allelic association of the D2 dopamine receptor gene with cocaine dependence. Drug Alcohol Depend 1993; 33(3): 271–85. 10.1016/0376-8716(93)90113-5 [DOI] [PubMed] [Google Scholar]
- [22].Dackis CA, Gold MS. Bromocriptine as treatment of cocaine abuse. Lancet 1985; 1(8438): 1151–2. 10.1016/S0140-6736(85)92448-12860349 [DOI] [PubMed] [Google Scholar]
- [23].Muma NA, Mi Z. Serotonylation and Transamidation of Other Monoamines. ACS Chem Neurosci 2015; 6(7): 961–9. 10.1021/cn500329r [DOI] [PubMed] [Google Scholar]
- [24].Lepack AE, Werner CT, Stewart AF, et al. Dopaminylation of histone H3 in ventral tegmental area regulates cocaine seeking. Science 2020; 368(6487): 197–201. 10.1126/science.aaw8806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci 2011; 12(11): 623–37. 10.1038/nrn3111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Maze I, Covington HE III, Dietz DM, et al. Essential role of the histone methyltransferase G9a in cocaine-induced plasticity. Science 2010; 327(5962): 213–6. 10.1126/science.1179438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jayanthi S, Gonzalez B, McCoy MT, Ladenheim B, Bisagno V, Cadet JL. Methamphetamine Induces TET1- and TET3-Dependent DNA Hydroxymethylation of Crh and Avp Genes in the Rat Nucleus Accumbens. Mol Neurobiol 2018; 55(6): 5154–66. 10.1007/s12035-017-0750-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cadet JL, Brannock C, Krasnova IN, et al. Genome-wide DNA hydroxymethylation identifies potassium channels in the nucleus accumbens as discriminators of methamphetamine addiction and abstinence. Mol Psychiatry 2017; 22(8): 1196–204. 10.1038/mp.2016.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Damez-Werno DM, Sun H, Scobie KN, et al. Histone arginine methylation in cocaine action in the nucleus accumbens. Proc Natl Acad Sci USA 2016; 113(34): 9623–8. 10.1073/pnas.1605045113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gary JD, Clarke S. RNA and protein interactions modulated by protein arginine methylation. Prog Nucleic Acid Res Mol Biol 1998; 61: 65–131. 10.1016/S0079-6603(08)60825-9 [DOI] [PubMed] [Google Scholar]
- [31].Gayatri S, Bedford MT. Readers of histone methylarginine marks. Biochim Biophys Acta 2014; 1839(8): 702–10. 10.1016/j.bbagrm.2014.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Frankel A, Yadav N, Lee J, Branscombe TL, Clarke S, Bedford MT. The novel human protein arginine N-methyltransferase PRMT6 is a nuclear enzyme displaying unique substrate specifici-ty. J Biol Chem 2002; 277(5): 3537–43. 10.1074/jbc.M108786200 [DOI] [PubMed] [Google Scholar]
- [33].Kirmizis A, Santos-Rosa H, Penkett CJ, et al. Arginine methylation at histone H3R2 controls deposition of H3K4 trimethylation. Nature 2007; 449(7164): 928–32. 10.1038/nature06160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Feng J, Wilkinson M, Liu X, et al. Chronic cocaine-regulated epigenomic changes in mouse nucleus accumbens. Genome Biol 2014; 15(4): R65. 10.1186/gb-2014-15-4-r65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kennedy PJ, Feng J, Robison AJ, et al. Class I HDAC inhibition blocks cocaine-induced plasticity by targeted changes in histone methylation. Nat Neurosci 2013; 16(4): 434–40. 10.1038/nn.3354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Adermark L, Clarke RB, Ericson M, Söderpalm B. Subregion-Specific Modulation of Excitatory Input and Dopaminergic Output in the Striatum by Tonically Activated Glycine and GABA(A) Receptors. Front Syst Neurosci 2011; 5: 85. 10.3389/fnsys.2011.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Trulson ME, Joe JC, Babb S, Raese JD. Chronic cocaine administration depletes tyrosine hydroxylase immunoreactivity in the meso-limbic dopamine system in rat brain: quantitative light microscopic studies. Brain Res Bull 1987; 19(1): 39–45. 10.1016/0361-9230(87)90163-8 [DOI] [PubMed] [Google Scholar]
- [38].Ferguson SM, Eskenazi D, Ishikawa M, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci 2011; 14(1): 22–4. 10.1038/nn.2703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Feltmann K, Borroto-Escuela DO, Rüegg J, et al. Effects of Long-Term Alcohol Drinking on the Dopamine D2 Receptor: Gene Expression and Heteroreceptor Complexes in the Striatum in Rats. Alcohol Clin Exp Res 2018; 42(2): 338–51. 10.1111/acer.13568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fried L, Modestino EJ, Siwicki D, Lott L, Thanos PK, Baron D, et al. Hypodopaminergia and “Precision Behavioral Management” (PBM): It is a Generational Family Affair. Curr Pharm Biotechnol 2019. 10.2174/1389201021666191210112108 [DOI] [PubMed] [Google Scholar]
- [41].Dahlgren A, Wargelius HL, Berglund KJ, et al. Do alcohol-dependent individuals with DRD2 A1 allele have an increased risk of relapse? A pilot study. Alcohol Alcohol 2011; 46(5): 509–13. 10.1093/alcalc/agr045 [DOI] [PubMed] [Google Scholar]
- [42].Heinsbroek JA, Neuhofer DN, Griffin WC III, et al. Loss of Plasticity in the D2-Accumbens Pallidal Pathway Promotes Cocaine Seeking. J Neurosci 2017; 37(4): 757–67. 10.1523/JNEUROSCI.2659-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Blum K, Noble EP, Sheridan PJ, et al. Allelic association of human dopamine D2 receptor gene in alcoholism. JAMA 1990; 263(15): 2055–60. 10.1001/jama.1990.03440150063027 [DOI] [PubMed] [Google Scholar]
- [44].Volkow ND, Wang GJ, Newcorn JH, et al. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry 2011; 16(11): 1147–54. 10.1038/mp.2010.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Blum K, Gondré-Lewis MC, Baron D, et al. Introducing Precision Addiction Management of Reward Deficiency Syndrome, the Construct That Underpins All Addictive Behaviors. Front Psychiatry 2018; 9: 548. 10.3389/fpsyt.2018.00548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Solanki N, Abijo T, Galvao C, Darius P, Blum K, Gondré-Lewis MC. Administration of a putative pro-dopamine regulator, a neuronutrient, mitigates alcohol intake in alcohol-preferring rats. Behav Brain Res 2020; 385112563 10.1016/j.bbr.2020.112563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Clark KH, Wiley CA, Bradberry CW. Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotox Res 2013; 23(2): 174–88. 10.1007/s12640-012-9334-7 [DOI] [PubMed] [Google Scholar]
- [48].Beardsley PM, Hauser KF. Glial modulators as potential treatments of psychostimulant abuse. Adv Pharmacol 2014; 69: 1–69. 10.1016/B978-0-12-420118-7.00001-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pierce RC, Bari AA. The role of neurotrophic factors in psychostimulant-induced behavioral and neuronal plasticity. Rev Neurosci 2001; 12(2): 95–110. 10.1515/REVNEURO.2001.12.2.95 [DOI] [PubMed] [Google Scholar]


