OBJECTIVES:
To determine whether the early serologic response in COVID-19 critical illness is associated with hospital mortality. To evaluate if time-to-seroconversion differs by receipt of dexamethasone therapy.
DESIGN:
Patients were prospectively enrolled within 24 hours of ICU admission from two University of Washington Hospitals. Plasma was collected on enrollment and on days 3, 7, 10, and 14.
SETTING:
ICUs between March 2020 and April 2021.
PATIENTS:
Consecutive adults with COVID-19 admitted to an ICU.
MEASUREMENTS AND MAIN RESULTS:
We measured longitudinal total antispike protein antibody levels (anti-S abs) and total antinucleocapsid antibody levels (anti-N ab) using a U.S. Food and Drug Administration-authorized Roche instrument. We evaluated whether detectable anti-S abs on ICU admission were associated with host factors, initial disease severity, and hospital mortality. We evaluated whether dexamethasone therapy was associated with time-to-seroconversion. Among 93 unvaccinated participants, 47 (51%) had detectable anti-S abs on ICU admission. There was no difference in Acute Physiology and Chronic Health Evaluation II score or time between first positive severe acute respiratory syndrome coronavirus-2 PCR and ICU admission in those with detectable versus undetectable anti-S abs. Adjusting for age, body mass index, and sex, patients with detectable anti-S abs had a lower risk of inhospital death (hazard ratio, 0.40; 95% CI, 0.17–0.94; p = 0.04). Among 21 patients with undetectable anti-S abs on ICU admission and serial measurements available, time-to-seroconversion was not significantly affected by receipt of dexamethasone therapy.
CONCLUSIONS:
In COVID-19 critical illness, a significant proportion of patients do not have detectable antibodies at ICU admission, and this is independent of severity of illness. Detectable anti-S abs were associated with lower risk of inhospital death. Despite concern that corticosteroids may impair an appropriate antiviral serologic response, early antibody kinetics were not significantly affected by administration of dexamethasone; however, CIs were wide and require further study.
Keywords: adaptive immunity, COVID 19 critical illness, severe acute respiratory syndrome coronavirus 2 antibody response, viral antibody response
In critically ill patients with COVID-19, the early serologic response to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection may play a critical role in viral clearance (1), but it is uncertain whether timely measurement of SARS-CoV-2-specific antibodies has prognostic or therapeutic relevance (2). To date, few small studies in COVID-19 critical illness have suggested a protective role for early seroconversion (1, 3, 4). However, other reports show no relationship between early anti-SARS-CoV-2 antibody levels and clinical outcomes (5).
The interplay between immunomodulatory therapeutics, specifically dexamethasone, and the serologic response is understudied. Although treatment with dexamethasone is currently considered standard of care for treatment of individuals with severe COVID-19, there is concern that the inhibitory effects of corticosteroids on lymphocytes may impair the serologic response. A single-center study in hospitalized patients found no difference in serologic response following treatment with corticosteroids (6), and, to our knowledge, no report has focused on critically ill populations.
To date, studies in critically ill COVID-19 patients assessing the serologic response have been limited by small sample size, variability in the timing of blood collection, and use of non-U.S. Food and Drug Administration (FDA)-approved research assays for antibody measurements. In this study, we leveraged longitudinal serologic measurements performed on the FDA-approved Roche Cobas Analyzer instrument (7) in a well phenotyped, prospectively enrolled, critically ill COVID-19 cohort to address these important questions. The primary aim of the study was to determine whether total anti-spike protein antibody levels were associated with disease severity or hospital mortality. A secondary aim was to evaluate whether dexamethasone therapy may influence time-to-seroconversion.
MATERIALS AND METHODS
Study Design and Data Source
We prospectively enrolled 93 consecutive COVID-19 patients aged greater than or equal to 18 years admitted to an ICU at two University of Washington (UW) Hospitals. Patients were enrolled from April 2, 2020, to May 14, 2021. We excluded patients transferred from outside hospitals from this analysis in order to capture early seroconversion (detectable anti-S abs) at the time of critical illness onset. We excluded patients who received at least one dose of SARS-CoV-2 vaccine (n = 3). The UW institutional review board (IRB) approved the study (UW IRB 9763; study: COVID-19 Host Response and Clinical Outcomes; date: March 17, 2020); the study was conducted in accordance with the ethical standards of the UW IRB and the Helsinki Declaration of 1975, and patients were unrolled under a waiver of informed consent, which was approved by the UW IRB. Additional details of study enrollment have been previously published (8).
Clinical data were abstracted from the electronic medical record into standardized case report forms by trained research coordinators. All patients included in this analysis had complete hospital data. Acute Physiology and Chronic Health Evaluation (APACHE) II scores were calculated with variables collected in the first 24 hours of ICU admission. We adjudicated the 8-point National Institutes of Health (NIH) ordinal scale within 24 hours of ICU admission.
Plasma Total Antispike Protein and Antinucleocapsid Protein Antibody Measurements
Plasma was collected using EDTA tubes within 24 hours of ICU admission and then subsequently on hospital days 3, 7, 10, and 14 in those patients who remained hospitalized. The Roche Elecsys anti-SARS-CoV-2-S assay (total anti-spike protein antibody, i.e., anti-S abs), Elecsys Anti-SARS-CoV-2 assay (total antinucleocapsid antibody, i.e., anti-N abs), and Elecsys interleukin (IL)-6 assay are chemiluminescent immunoassays conducted on the Cobas e411 immunoassay analyzer (Roche diagnostics, Indianapolis, IN). All assays are authorized by the FDA under an Emergency Use Authorization (7). Assay coefficients of variation can be found in Supplementary Table 1 (http://links.lww.com/CCX/B53).
Statistical Analysis
We summarized participant characteristics at baseline as mean ± sd or median and interquartile range (IQR) for continuous variables, and as number and frequency for categorical variables. We estimated the risk of inhospital mortality via Cox regression, censoring participants at hospital discharge. To examine the outcome of time-to-seroconversion among individuals with undetectable anti-S ab at ICU admission, we used Cox regression, censoring participants at the time of the last available blood draw. Each regression model was adjusted for potential confounding factors of age, gender, and body mass index (BMI). For all analyses, a two-tailed p value of less than 0.05 was taken as evidence of statistical significance. All statistical analyses were performed in R 4.1.1 (R Project for Statistical Computing, Vienna, Austria).
RESULTS
Among 93 unvaccinated ICU patients with COVID-19, 47 (51%) had detectable anti-S abs at the time of ICU admission. Baseline characteristics are provided in Table 1. We used date of first-positive SARS-CoV-2 PCR as a surrogate for symptoms prompting testing. Median time between first positive SARS-CoV-2 PCR and ICU admission blood sample collection was similar in individuals with detectable anti-S abs (median, 1 d; IQR, 0–2) and individuals with undetectable anti-S abs (median, 1 d; IQR, 0–4) (p = 0.32) (Table 1). We did not observe any differences in age, diabetes mellitus, chronic kidney disease, and cancer diagnosis between those with detectable and undetectable anti-S abs. The distribution of the NIH Ordinal scale was overlapping between groups (p = 0.92) with a similar proportion of patients with detectable and undetectable anti-S abs requiring oxygen therapy via high-flow nasal cannula (ordinal scale score [OSS] = 6) (38% vs 33%) and invasive mechanical ventilation (OSS = 7) (21% vs 24%) at study enrollment. Median APACHE II score was identical in patients with detectable (22, IQR, 18–28) and undetectable (22, IQR, 13.5–26.5) anti-S abs (p = 0.27). Although anti-S abs and anti-N abs were correlated, 11 patients with detectable anti-S abs had undetectable anti-N abs.
TABLE 1.
Baseline Characteristics
| Parameter | Total (n = 93) | Undetectable Total S Antibody; ≤ 0.8 (n = 46) | Detectable Total S Antibody; >0.8 (n = 47) | p |
|---|---|---|---|---|
| Antinucleocapsid antibody ≤ 1 | 48 (52) | 37 (80) | 11 (23) | < 0.001 |
| Age, median (IQR) | 59.0 (47.0–67.0) | 60.5 (50.0–69.5) | 55.0 (45.0–65.5) | 0.27 |
| Male, n (%) | 66 (71) | 32 (70) | 34 (72) | 0.95 |
| Body mass index, kg/m2, median (IQR) | 27.2 (23.9–33.1) | 27.3 (23.6–34.1) | 27.2 (24.4–31.6) | 0.20 |
| Time PCR to V1, median (IQR) | 1.0 (0.0–4.0) | 1.0 (0.0–2.0) | 1.0 (0.0–4.0) | 0.32 |
| Race, n (%) | ||||
| American Indian | 5 (5) | 3 (7) | 2 (4) | 0.10 |
| Asian | 13 (14) | 6 (13) | 7 (15) | |
| Black/African American | 16 (17) | 12 (26) | 4 (9) | |
| Pacific Islander | 1 (1) | 1 (2) | 0 (0) | |
| White | 56 (60) | 23 (50) | 33 (70) | |
| Unknown | 2 (2) | 1 (2) | 1 (2) | |
| Ethnicity, n (%) | ||||
| Hispanic/LatinX | 34 (37) | 11 (24) | 23 (49) | 0.92 |
| Comorbidities, n (%) | ||||
| Asthma | 9 (10) | 7 (15) | 2 (4) | 0.15 |
| Chronic kidney disease | 20 (22) | 11 (24) | 9 (19) | 0.76 |
| Diabetes mellitus | 30 (32) | 15 (33) | 15 (32) | 0.99 |
| Hypertension | 9 (10) | 3 (7) | 6 (13) | 0.50 |
| Alcohol abuse | 16 (17) | 9 (20) | 7 (15) | 0.75 |
| Cerebrovascular disease | 10 (11) | 6 (13) | 4 (9) | 0.71 |
| Coronary artery disease | 12 (13) | 6 (13) | 6 (13) | 0.99 |
| Active solid cancer | 7 (8) | 4 (9) | 3 (6) | 0.98 |
| Lymphoma | 1 (1) | 0 (0) | 1 (2) | 0.99 |
| Leukemia | 1 (1) | 0 (0) | 1 (2) | 0.99 |
| Solid organ transplant | 4 (4) | 1 (2) | 3 (6) | 0.62 |
| HIV | 3 (3) | 2 (4) | 1 (2) | 0.98 |
| Cirrhosis | 9 (10) | 3 (7) | 6 (13) | 0.50 |
| 8-Point Ordinal Scale Score, n (%) | ||||
| 4 (hospitalization) | 19 (20) | 10 (22) | 9 (19) | 0.92 |
| 5 (any supplemental oxygen) | 20 (22) | 10 (22) | 10 (21) | |
| 6 (high-flow nasal cannula or noninvasive positive pressure ventilation) | 33 (35) | 15 (33) | 18 (38) | |
| 7 (invasive mechanical ventilation or ECMO) | 21 (23) | 11 (24) | 10 (21) | |
| Acute Physiology and Chronic Health Evaluation II, median (IQR) | 22.0 (15.0–28.0) | 22.0 (18.0–28.8) | 22.0 (13.5–26.5) | 0.27 |
| Treatments during hospitalization, n (%) | ||||
| Corticosteroids | 57 (61) | 30 (65) | 27 (57) | 0.58 |
| Remdesivir | 52 (56) | 28 (61) | 24 (51) | 0.46 |
| Tocilizumab | 4 (4) | 0 (0) | 4 (9) | 0.13 |
ECMO = extracorporeal membrane oxygenation, IQR = interquartile range, PCR = polymerase chain reaction.
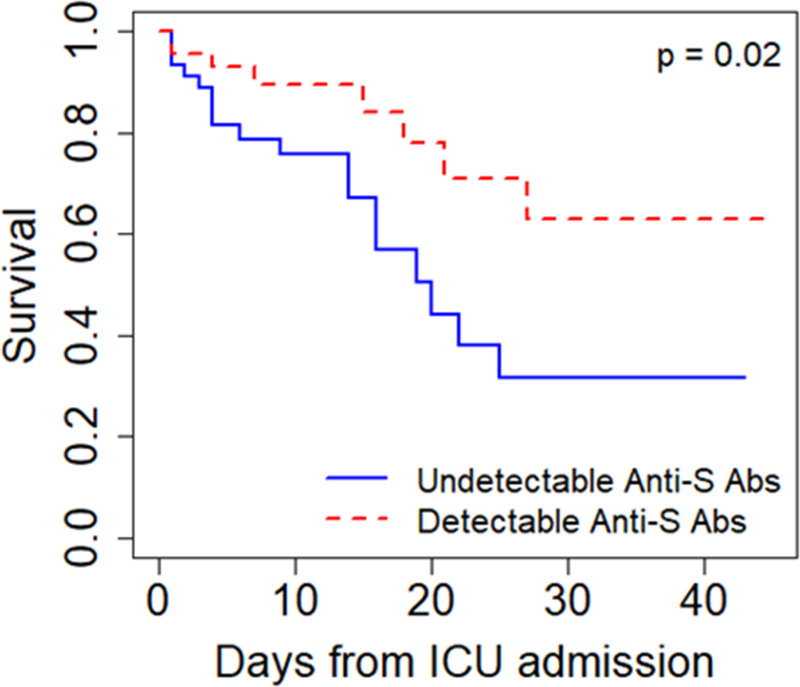
In a Cox regression model adjusting for age, sex, and BMI, patients with detectable anti-S abs at the time of ICU arrival had a lower risk of death (adjusted hazard ratio [aHR], 0.40; 95% CI, 0.17–0.94; p = 0.04) (Fig. 1; and Table S2, http://links.lww.com/CCX/B53). Detectable anti-N abs were not significantly associated with reduced risk of death (aHR, 0.47 [0.21–1.08]; p = 0.07) (Table S2, http://links.lww.com/CCX/B53). ICU admission IL-6 levels did not significantly differ in those with (median, 56 pg/mL; IQR, 18–192) and without (median, 72 pg/mL; IQR, 31–299) detectable anti-S abs on ICU admission (p = 0.37).
Figure 1.
Undetectable anti-S antibodies at the time of ICU admission are associated with increased risk of death in COVID-19. Kaplan-Meier curves for hospital mortality stratified by patients with and without detectable antispike protein antibody levels (anti-S abs) at admission. The curves demonstrate that patients with detectable anti-S abs had a lower hospital mortality rate compared with patients with undetectable anti-S abs.
Among 32 patients with undetectable anti-S abs and longitudinal antibody measurements, we found that 24 patients (75%) subsequently developed detectable anti-S abs. Median time-to-seroconversion was 4.2 days (95% CI, 3.1–7.0 d) after ICU admission. In those with undetectable anti-S abs on ICU admission and with available subsequent longitudinal antibody measurements, we evaluated whether time to detectable anti-S abs (time-to-seroconversion) differed by exposure to standard dose dexamethasone (6 mg daily) for COVID-19 respiratory failure. We excluded those who were treated with convalescent plasma from this analysis to minimize confounding (n = 11). Among the remaining patients (n = 21), time-to-seroconversion did not differ significantly by dexamethasone treatment (aHR, 2.42; 95% CI, 0.57–10.37; p = 0.23) (Table S3, http://links.lww.com/CCX/B53).
DISCUSSION
In critically ill patients with COVID-19, the early SARS-CoV-2-specific antibody response is an important component of the host antiviral response. We found that the early SARS-CoV-2-specific antibody response is highly heterogeneous and not significantly related to patient comorbidities, initial severity of illness, or proinflammatory mediator levels. However, the death rate was lower in those with detectable anti-S abs on ICU admission. Prior work in COVID-19 critical illness suggests a possible protective role for early seroconversion (1, 3, 4), and our data are supportive of this conclusion. There are two arms within the antiviral adaptive immune response: humoral immunity and cell-mediated immunity. Our study suggests a protective role for an early humoral response within a coordinated adaptive response. We hypothesize that the humoral response may improve viral clearance and disease resolution, and this may be independent of initial proinflammatory responses. However, it should be noted that alternative pathogenic and protective mechanisms, including those related to cell-mediated immunity, are also very likely contributing to outcomes in COVID-19 critical illness.
In addition to having potential prognostic value, measurement of SARS-CoV-2 antibodies may guide the use of therapeutics including monoclonal antibodies. Recently, two randomized control trials demonstrated that monoclonal antibody therapy does not reduce mortality in hospitalized patients. However, in subgroup analyses, patients with low or absent neutralizing antibodies upon hospital admission benefitted from monoclonal antibody therapy (9, 10). In our analysis, we demonstrate that a sizable proportion of COVID-19 ICU patients have undetectable SARS-CoV-2-specific antibodies and, in turn, may potentially benefit from monoclonal antibody therapy.
It has been hypothesized that corticosteroid treatment for COVID-19 may inhibit short-term seroconversion related to its inhibition of helper T cell function-mediated B-cell humoral response negatively impacting viral clearance. In a single study in hospitalized, among 38 patients with undetectable anti-S IgG upon study entry, time from symptom onset to detectable anti-S IgG did not differ by receipt of dexamethasone. This study was not conducted in an ICU population limiting generalizability to critical illness, and plasma measurements were only collected every 7 days limiting granularity in this time-to-seroconversion analysis (6). Here, we extend this analysis to COVID-19 critical illness, and we similarly find that dexamethasone treatment is not associated with time-to-seroconversion.
Our study has a number of limitations. First, the FDA-approved Roche assays measure a combination of IgG and IgM anti-SARS-CoV-2 abs, limiting inference on antibody subpopulations. Second, in our study, patients were unvaccinated limiting generalizability to fully vaccinated patients. However, currently, only 66% of the adult U.S. population is fully vaccinated, and the rate of critical illness among vaccinated patients is very low (usafacts.gov). Finally, although our findings are consistent with prior work, we acknowledge that our study, due to sample size limitation, was underpowered to make definitive conclusions about the effect of dexamethasone on time-to-seroconversion.
In COVID-19 critical illness, there is significant heterogeneity in the early SARS-CoV-2-specific antibody response. Presence of early SARS-CoV-2 antibodies is linked to reduced risk of death, suggesting a protective role for a coordinated adaptive immune response, and, notably, treatment with dexamethasone may not impact virus-specific antibody kinetics.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by the National Institutes of Health: National Heart, Lung, and Blood Institute (NHLBI) K23 HL144916 (to Dr. Morrell), National Institute of Diabetes and Digestive and Kidney Diseases K23 DK116967 (to Dr. Bhatraju), and NHLBI T32 HL007287 (to Dr. Mabrey), and Investigator-initiated Study from Roche Diagnostics (to Drs. Wurfel, Liles, and Bhatraju).
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Martin-Vicente M, Almansa R, Martínez I, et al. : Low anti-SARS-CoV-2 S antibody levels predict increased mortality and dissemination of viral components in the blood of critical COVID-19 patients. J Intern Med 2022; 291:232–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Post N, Eddy D, Huntley C, et al. : Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS One 2020; 15:e0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fourati S, Hue S, Pawlotsky JM, et al. : SARS-CoV-2 viral loads and serum IgA/IgG immune responses in critically ill COVID-19 patients. Intensive Care Med 2020; 46:1781–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asif S, Frithiof R, Lipcsey M, et al. : Weak anti-SARS-CoV-2 antibody response is associated with mortality in a Swedish cohort of COVID-19 patients in critical care. Crit Care 2020; 24:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun B, Feng Y, Mo X, et al. : Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg Microbes Infect 2020; 9:940–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mühlemann B, Thibeault C, Hillus D, et al. ; Pa-COVID-19 collaborative study group: Impact of dexamethasone on SARS-CoV-2 concentration kinetics and antibody response in hospitalized COVID-19 patients: Results from a prospective observational study. Clin Microbiol Infect 2021; 27:1520.e7–1520.e10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perkmann T, Perkmann-Nagele N, Koller T, et al. : Anti-spike protein assays to determine SARS-CoV-2 antibody levels: A head-to-head comparison of five quantitative assays. Microbiol Spectr 2021; 9:e0024721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatraju PK, Morrell ED, Zelnick L, et al. : Comparison of host endothelial, epithelial and inflammatory response in ICU patients with and without COVID-19: A prospective observational cohort study. Crit Care 2021; 25:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ACTIV-3/TICO Bamlanivimab Study Group Lundgren JD, Grund B, et al. : Responses to a neutralizing monoclonal antibody for hospitalized patients with COVID-19 according to baseline antibody and antigen levels: A randomized controlled trial. Ann Intern Med 2022;175:234–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.RECOVERY Collaborative Group: Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2022; 399:665-676 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.