PURPOSE
To verify whether the intensification of the upfront chemotherapy backbone with a modified schedule of modified fluorouracil, leucovorin, oxaliplatin, and irinotecan (mFOLFOXIRI) increases the activity of fluorouracil, leucovorin, and oxaliplatin when both regimens are combined with panitumumab as initial treatment for RAS and BRAF wild-type (wt) metastatic colorectal cancer (mCRC).
METHODS
TRIPLETE was a prospective, open-label, phase III trial in which previously untreated patients with unresectable RAS and BRAF wt mCRC were randomly assigned 1:1 to modified FOLFOX/panitumumab (control group) or mFOLFOXIRI/panitumumab (experimental group) up to 12 cycles, followed by fluorouracil/-leucovorin/panitumumab until disease progression. The primary end point was objective response rate (ORR) according to RECIST 1.1. Hypothesizing an ORR of 60% in the control group, 432 cases provided 90% power to a two-sided chi-square test for heterogeneity with a two-sided alpha error of .05 to detect ≥ 15% differences between arms (ClinicalTrials.gov identifier: NCT03231722).
RESULTS
From September 2017 to September 2021, 435 patients were enrolled (control group/experimental group: 217/218) in 57 Italian sites. One hundred sixty (73%) patients treated with mFOLFOXIRI plus panitumumab and 165 (76%) patients treated with modified FOLFOX plus panitumumab achieved RECIST response (odds ratio 0.87, 95% CI, 0.56 to 1.34, P = .526). No differences in early tumor shrinkage rate (57%/58%, P = .878) and deepness of response (median: 48%/47%, P = .845) were reported, nor in R0 resection rate (25%/29%, P = .317). No significant difference between arms was reported in terms of progression-free survival (median progression-free survival: 12.7 in the experimental group v 12.3 months in the control group, hazard ratio: 0.88, 95% CI, 0.70 to 1.11, P = .277).
CONCLUSION
The intensification of the upfront chemotherapy backbone in combination with panitumumab does not provide additional benefit in terms of treatment activity at the price of increased gastrointestinal toxicity in patients with RAS and BRAF wt mCRC.
INTRODUCTION
The combination of two cytotoxic drugs, fluorouracil, leucovorin, and oxaliplatin (FOLFOX) or fluorouracil, leucovorin, and irinotecan (FOLFIRI), with an anti–epidermal growth factor receptor (EGFR) antibody (cetuximab or panitumumab) is an upfront option for patients with unresectable RAS and BRAF wild-type (wt) metastatic colorectal cancer (mCRC).1,2
CONTEXT
Key Objective
Does modified fluorouracil, leucovorin, oxaliplatin, and irinotecan (mFOLFOXIRI) plus panitumumab provide higher activity than fluorouracil, leucovorin, and oxaliplatin (FOLFOX) plus panitumumab in patients with unresectable RAS and BRAF wild-type metastatic colorectal cancer? The phase III randomized TRIPLETE study aims at answering this question.
Knowledge Generated
The intensification of the upfront chemotherapy backbone with mFOLFOXIRI in combination with panitumumab does not provide any benefit in ORR and progression-free survival compared with FOLFOX and panitumumab in RAS and BRAF wild-type metastatic colorectal cancer and is associated with increased gastrointestinal toxicity. FOLFOX plus panitumumab allows achieving remarkable activity and efficacy results, thus supporting patients' selection according to the primary tumor side and RAS and BRAF mutational status to optimize the efficacy of anti–epidermal growth factor receptor (EGFR)-based first-line treatments.
Relevance
Our results do not support the use of the triplet in combination with anti-EGFRs and highlight that patients' selection according to RAS and BRAF mutational status and primary tumor location may optimize the efficacy of anti–EGFR-based first-line treatments.
An intensified upfront chemotherapy backbone, the triplet fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI), in combination with bevacizumab significantly improved response rate, progression-free survival (PFS), and overall survival (OS) compared with chemotherapy doublets (FOLFOX or FOLFIRI) plus bevacizumab at the price of increased chemotherapy-related toxicities.3
Several early trials investigated the combination of FOLFOXIRI with anti-EGFR antibodies and reported promising results in terms of treatment activity and conversion to resectability, with high rates of gastrointestinal toxicities.4,5 More recently, modified schedules of FOLFOXIRI with reduced doses of irinotecan and/or fluorouracil (FU) were combined with anti-EGFR monoclonal antibodies in phase II trials, confirming remarkable activity results and a tolerable safety profile with lower rates of adverse events.6-8 In the phase II randomized VOLFI study, the association of panitumumab with a modified schedule of FOLFOXIRI allowed achieving a higher objective response rate (ORR) than FOLFOXIRI alone (87% v 61%, P = .004) in patients with previously untreated RAS wt mCRC, with no significant PFS difference.9
Owing to its remarkable ability in inducing tumor shrinkage, the choice of the triplet plus an anti-EGFR might be particularly appealing when cytoreduction is an immediate and highly relevant objective of the upfront therapy (ie, in the case of potentially resectable or symptomatic disease).
However, the added value of intensifying the first-line chemotherapy backbone when combined with a targeted agent in a molecularly selected population of patients with mCRC has never been established. Drawing from this background, the TRIPLETE study was conducted with the main objective of comparing the modified triplet (modified fluorouracil, leucovorin, oxaliplatin, and irinotecan [mFOLFOXIRI]) with the doublet FOLFOX, both in combination with panitumumab, as initial treatment of patients with unresectable RAS and BRAF wt mCRC.
METHODS
Study Design and Participants
TRIPLETE is a prospective, open-label, multicenter, randomized phase III study that included patients with mCRC recruited from 57 Oncology Units in Italy (Data Supplement, online only). Eligible patients, stratified according to Eastern Cooperative Oncology Group performance status (ECOG PS; 0-1 v 2), primary tumor location (right [from cecum to transverse colon] versus left [from splenic flexure to rectum]), and liver-only metastases (yes v no) were randomly assigned by minimization to receive FOLFOX plus panitumumab (control group) or mFOLFOXIRI plus panitumumab (experimental group) in a 1:1 ratio.
Main inclusion criteria were as follows: histologically confirmed colorectal adenocarcinoma; RAS (KRAS and NRAS exons 2, 3, and 4) and BRAF codon 600 wt status of primary tumor and/or related metastasis assessed by local laboratory; age between 18 and 75 years; an ECOG PS of 0-2 if age ≤ 70 years or 0 if age 71-75 years; unresectable and measurable metastatic disease according to RECIST version 1.110; and adequate bone marrow, hepatic, and renal function. Main exclusion criteria were as follows: any previous treatment for metastatic disease; adjuvant treatment with oxaliplatin; adjuvant treatment with fluoropyrimidine monotherapy completed < 6 months before relapse; and peripheral neuropathy of grade 2 or higher according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0.11
Modified FOLFOX plus panitumumab consisted of a 30- to 60-minute intravenous infusion of panitumumab at 6 mg/kg, followed by a 120-minute infusion of oxaliplatin at 85 mg/m2 given concurrently with leucovorin at 200 mg/m2, followed by bolus infusion of FU at 400 mg/m2, followed by a 48-hour continuous infusion of FU at 2,400 mg/m2, starting on day 1. Cycles were repeated once every 14 days. mFOLFOXIRI plus panitumumab was administered as a 30- to 60-minute intravenous infusion of panitumumab at 6 mg/kg, followed by a 60-minute infusion of irinotecan at 150 mg/m2, followed by a 120-minute infusion of oxaliplatin at 85 mg/m2 given concurrently with leucovorin at 200 mg/m2, followed by a 48-hour continuous infusion of FU at 2,400 mg/m2, starting on day 1. Cycles were repeated once every 14 days. In both groups, treatment was administered up to 12 cycles, followed by maintenance with FU/leucovorin and panitumumab every 14 days, until progressive disease, patient's refusal, unacceptable adverse events, or consent withdrawal.
All tumor assessments were based on investigator-reported measurements and were performed according to RECIST 1.1 by means of computed tomography scans repeated every 8 weeks.10 The assessment of surgical resectability by an experienced and dedicated local multidisciplinary team was recommended at every tumor assessment. In the case of surgical radical resection of residual metastases, postoperative therapy with the same preoperative regimen was planned up to 12 cycles.
Adverse events were graded according to the NCI-CTCAE version 4.0.11 Treatment modifications were allowed according to the study protocol. The use of granulocyte colony-stimulating factor was not recommended as primary prophylaxis.
The Protocol (online only) was approved by the local ethics committees at participating centers, and the study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. All patients provided their written informed consent to study procedures before enrollment. The study Protocol is available in the Data Supplement.
The trial is registered with ClinicalTrials.gov identifier: NCT03231722.
Study End Points
The primary end point was objective response rate (ORR) defined as the percentage of patients achieving a complete or partial response, according to RECIST 1.1 criteria, during the whole treatment, including both the induction and the maintenance phases, on the basis of investigator-reported measurements.
Secondary end points included safety, PFS (defined as the time from random assignment to the first documentation of disease progression, according to RECIST version 1.1,10 or death from any cause, whichever occurred first; patients who were alive and progression-free at the time of the analysis were censored at the date of the last tumor assessment; no censoring for secondary surgery or treatment interruption because of any cause was made), early tumor shrinkage (ETS) rate (defined as the percentage of patients achieving a ≥ 20% decrease in the sum of the diameters of the RECIST target lesions after 8 weeks from treatment start compared with baseline), deepness of response (DoR; defined as the relative change in the sum of the longest diameters of the RECIST target lesions at the nadir, in the absence of new lesions or progression of nontarget lesions, compared with baseline), R0 resection rate (defined as the proportion of patients undergoing secondary resection of metastases with no macroscopic or microscopic residual tumor), and OS (defined as the time from random assignment to death because of any cause, not yet mature at the time of the analysis).
Statistical Analysis
The primary analysis of ORR and all efficacy analyses were performed in the intention-to-treat population, including all randomly assigned patients. Adverse events were assessed in the safety population, including patients who received at least one dose of the study treatment.
The chi-square test for heterogeneity and the odds ratio (OR) with 95% CIs were used to compare the ORR between treatment groups. Under the assumption of an ORR in the control group equal to 60%,12,13 a sample size of 432 cases, randomly assigned in a 1:1 ratio, provided approximately 90% power to a two-sided chi-square test for heterogeneity at the 0.05 significance level, to detect ≥ 15% differences in ORR between arms.
R0 resection rate of metastases, ETS, and DoR in the two groups were compared with a chi-square test or Mann-Whitney test when appropriate; ORs and 95% CIs were estimated with a logistic regression model.
The median period of follow-up was calculated according to the reverse Kaplan-Meier method. Distribution of time-to-event variables for PFS was estimated using the Kaplan-Meier product limit method. The log-rank test was used as primary analysis for treatment groups' comparison. Hazard ratios (HRs) with 95% CIs were estimated with a Cox proportional hazards model. The proportional hazards assumption was graphically assessed by a log(−log) plot (Data Supplement). Since it was violated, the 26-month restricted mean survival time for each treatment group and the between-group difference were reported as post hoc analysis.
Stratified analyses of ORR and PFS were also performed.
Exploratory subgroup analyses were conducted by interaction tests to determine the consistency of the treatment effect according to key baseline characteristics.
All statistical tests were two-sided, and P values ≤.05 were deemed significant. Statistical analyses were performed using SAS version 9.4 and R version 4.1.1.
RESULTS
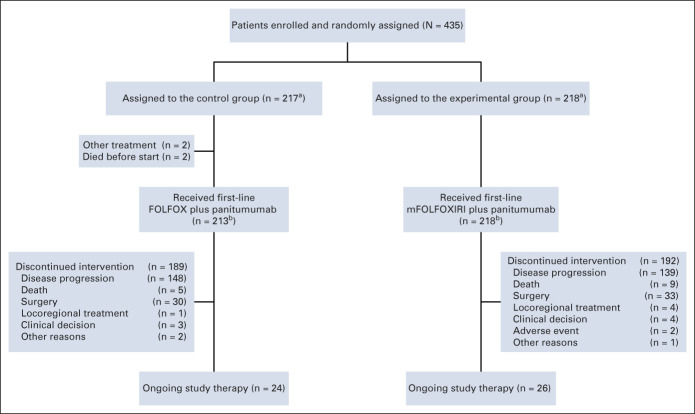
From September 2017 to September 2021, 435 patients were randomly assigned to the control (n = 217) or the experimental group (n = 218; Fig 1) and were included in the intention-to-treat population. Four hundred thirty-one patients (213 in the control group and 218 in the experimental group) received at least one dose of the study treatment and were included in the safety population (Fig 1).
FIG 1.
CONSORT diagram. aPatients included in the intention-to-treat population. bPatients included in the safety population. FOLFOX, fluorouracil, leucovorin, and oxaliplatin; mFOLFOXIRI, modified fluorouracil, leucovorin, oxaliplatin, and irinotecan.
The cutoff date for the present analysis was March 7, 2022.
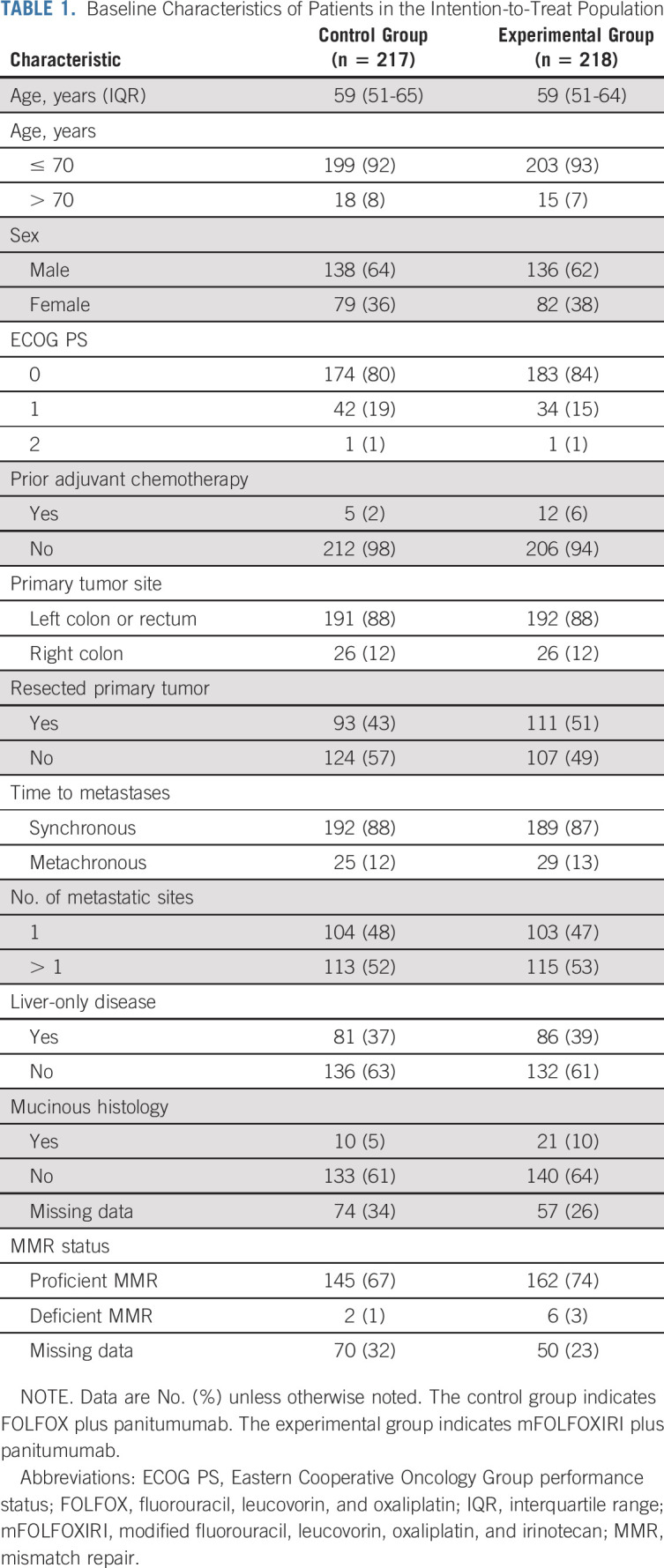
Patient- and tumor-related characteristics at baseline were well balanced between groups (Table 1). All patients were White, and the median age was 59 (interquartile range [IQR], 51-65) years. Most patients had an ECOG PS of 0 (82%), presented with synchronous metastases (88%) and a left-sided primary tumor (88%). Overall, 52% of patients had multiple metastatic sites and 38% showed liver-only disease (Table 1).
TABLE 1.
Baseline Characteristics of Patients in the Intention-to-Treat Population
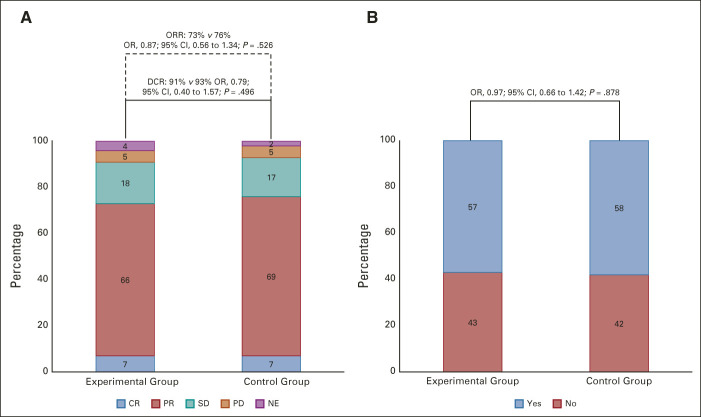
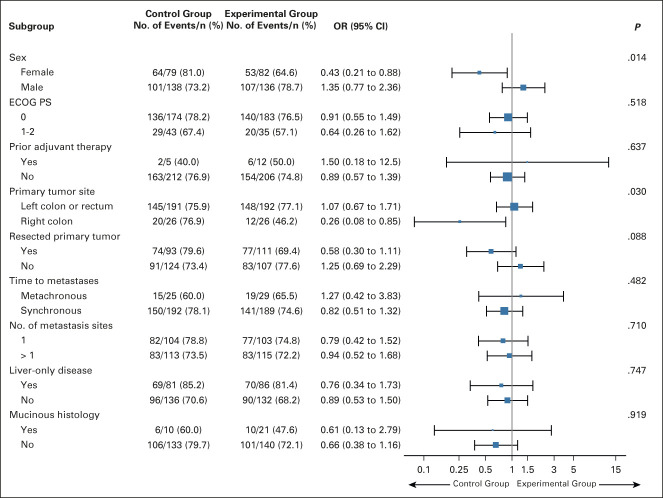
One hundred sixty (73%) of 218 patients in the experimental group achieved response versus 165 (76%) of 217 patients in the control group (OR: 0.87 [95% CI, 0.56 to 1.34]; P = .526). Fifteen (7%) complete responses and 145 (66%) partial responses were observed in the experimental group versus 15 (7%) complete and 150 (69%) partial responses in the control group (Fig 2A). After adjustment for stratification factors, no difference between arms was observed (OR: 0.83 [95% CI, 0.53 to 1.29]; stratified P = .407). The subgroup analyses revealed no significant interaction effect between treatment arms and clinical factors at baseline except for sex (P for interaction = .014) and primary tumor site (P for interaction = .03; Fig 3).
FIG 2.
Response parameters according to the treatment arm: (A) ORR and disease control rate and (B) early tumor shrinkage. The control group indicates FOLFOX plus panitumumab. The experimental group indicates mFOLFOXIRI plus panitumumab. CR, complete response; DCR, disease control rate; FOLFOX, fluorouracil, leucovorin, and oxaliplatin; mFOLFOXIRI, modified fluorouracil, leucovorin, oxaliplatin, and irinotecan; NE, not evaluable; OR, odds ratio; ORR, objective response rate; PD, progression disease; PR, partial response; SD, stable disease.
FIG 3.
Subgroup analyses of the objective response rate according to clinical characteristics of the intention-to-treat population. The control group indicates FOLFOX plus panitumumab; the experimental group indicates mFOLFOXIRI plus panitumumab. ECOG PS, Eastern Cooperative Oncology Group performance status; FOLFOX, fluorouracil, leucovorin, and oxaliplatin; mFOLFOXIRI, modified fluorouracil, leucovorin, oxaliplatin, and irinotecan; OR, odds ratio.
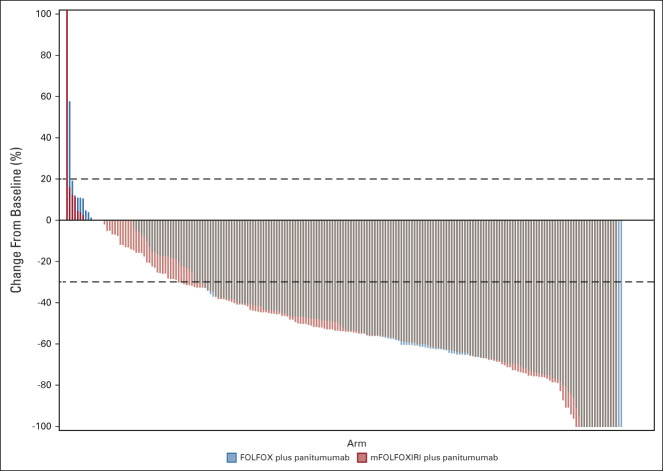
Two hundred fifty-one (58%) patients achieved ETS with no difference between groups (57% in the experimental group v 58% in the control group; OR 0.97 [95% CI, 0.66 to 1.42]; P = .878; Fig 2B). Similar results were shown for median DoR that was 48% (IQR: 32%-60%) in the experimental group and 47% (IQR: 30%-62%) in the control group (P = .845; Fig 4).
FIG 4.
Deepness of response. The control group indicates FOLFOX plus panitumumab. The experimental group indicates mFOLFOXIRI plus panitumumab. FOLFOX, fluorouracil, leucovorin, and oxaliplatin; mFOLFOXIRI, modified fluorouracil, leucovorin, oxaliplatin, and irinotecan.
R0 resection rate was not significantly different between the two treatment groups both in the overall population (54 [25%] in the experimental arm v 63 [29%] in the control arm, OR 0.81 [95% CI, 0.53 to 1.23]; P = .317) and in the liver-only subgroup (36 [42%] of 86 v 35 [43%] of 81, OR 0.95 [95% CI, 0.51 to 1.75]; P = .860).
At a median follow-up of 26.5 (IQR: 13.7-35.9) months, 305 (70%) events of disease progression occurred (148 [68%] of 218 patients in the experimental group and 157 [72%] of 217 patients in the control group). The median PFS was 12.7 months (95% CI, 11.1 to 15.5) in the experimental group and 12.3 months (95% CI, 11.1 to 14.3) in the control group (HR, 0.88, 95% CI, 0.70 to 1.11; log-rank test P = .277; Fig 5). As the proportional hazards assumption was violated (Data Supplement), the 26-month restricted mean survival time was post hoc calculated and it was 14.3 months (95% CI, 13.1 to 15.4) in the experimental group and 13.6 months (95% CI, 12.6 to 14.7) in the control group, with a difference of 0.62 months (95% CI, –0.99 to 2.22; P = .451). After adjustment for stratification factors, no difference between arms was observed in terms of PFS (HR, 0.89, 95% CI, 0.71 to 1.11; stratified log-rank test P = .369). No significant interaction effect was shown between treatment arms and analyzed subgroups (Data Supplement).
FIG 5.
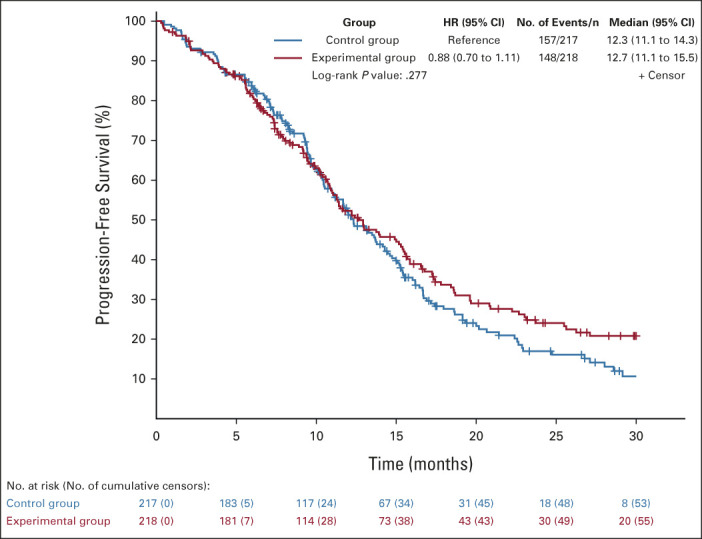
Kaplan-Meier estimates of progression-free survival in the intention-to-treat population. The control group indicates FOLFOX plus panitumumab; the experimental group indicates mFOLFOXIRI plus panitumumab. FOLFOX, fluorouracil, leucovorin, and oxaliplatin; HR, hazard ratio; mFOLFOXIRI, modified fluorouracil, leucovorin, oxaliplatin, and irinotecan.
The median number of cycles administered per patient as induction treatment was 9 (IQR, 6-12) in both arms. The median relative dose intensity was 81% for FOLFOX plus panitumumab (IQR: 70%-92%) and 75% for mFOLFOXIRI plus panitumumab (IQR, 63%-86%). In the control group, the median relative dose intensities of fluorouracil continuous infusion, fluorouracil bolus infusion, and oxaliplatin were 82%, 79%, and 82%, respectively. In the experimental group, the median relative dose intensities of fluorouracil, irinotecan, and oxaliplatin were 75%, 72%, and 76%, respectively. One hundred fifty-seven (72%) and 133 (62%) patients required at least one dose reduction in the experimental and in the control group, respectively. Treatment was delayed because of any reason in 194 (89%) and 175 (82%) patients in the experimental and control arm, respectively.
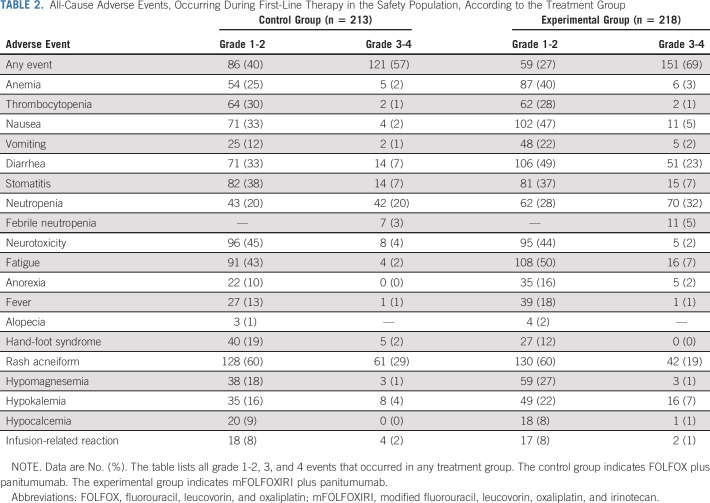
Adverse events are shown in Table 2. Grade 3-4 adverse events were reported in 151 (69%) of 218 patients in the experimental group and in 121 (57%) of 213 patients in the control group. The most frequent all-cause grade 3-4 events were neutropenia (70 [32%] in the experimental group v 42 [20%] in the control group), diarrhea (51 [23%] v 14 [7%]), rash acneiform (42 [19%] v 61 [29%]), stomatitis (15 [7%] v 14 [7%]), hypokalemia (16 [7%] v 8 [4%]), and fatigue (16 [7%] v 4 [2%]).
TABLE 2.
All-Cause Adverse Events, Occurring During First-Line Therapy in the Safety Population, According to the Treatment Group
Serious adverse events occurred in 72 (33%) and 44 (21%) patients in the experimental and control group, respectively. Three deaths because of treatment-related adverse events (sepsis in one patient and diarrhea in two patients) were reported in the experimental group (1%) versus none in the control group.
DISCUSSION
The TRIPLETE study did not meet its primary end point, failing to demonstrate improved activity with a modified schedule of FOLFOXIRI compared with FOLFOX when combined with the anti-EGFR monoclonal antibody panitumumab as initial therapy of patients with unresectable RAS and BRAF wt mCRC.
In the past few years, the use of anti–EGFR-based first-line regimens in patients with RAS wt mCRC increased worldwide and several post hoc subgroup analyses of controlled trials led to improvement in the selection of patients who are most likely to benefit from this treatment option beyond RAS mutational status. In fact, the limited benefit achieved with the addition of anti-EGFR agents to standard chemotherapy in the BRAF V600–mutant subgroup led to restriction of their upfront use to patients with RAS and BRAF wt mCRC.14,15
Moreover, consistent results from subgroup analyses of randomized trials of chemotherapy plus anti-EGFR versus chemotherapy alone or plus bevacizumab showed a significant interaction between the anti-EGFR effect and the primary tumor location.16,17 A clinically relevant PFS and OS benefit from the upfront use of anti-EGFRs was shown among patients with RAS wt tumors originating from the left side of the colon, thus making FOLFOX or FOLFIRI plus cetuximab or panitumumab a preferred upfront option for these patients.2,18 Conversely, patients with right-sided tumors were mostly excluded from anti–EGFR-based upfront therapies, and this was supported by the acknowledgment of a higher prevalence in these tumors of other rare molecular alterations predictive of intrinsic resistance to anti-EGFRs.19
When the TRIPLETE study was designed, the differential efficacy of anti-EGFR agents according to the primary tumor location was not established, whereas the negative prognostic impact of the right-sidedness was well known,20 so that the primary tumor side was used as a stratification factor to avoid relevant unbalances between arms but not as an eligibility criterion. However, because of the abovementioned evidence, the percentage of patients with left-sided tumors in the study population was as high as 88%. As a consequence, we observed an ORR in the FOLFOX plus panitumumab group of 76% that was significantly higher than that planned in the null hypothesis (60%), on the basis of the results of the pivotal PRIME study.13
Despite acknowledging all the methodological limitations of cross-trials comparisons, our results with FOLFOX plus panitumumab in a large (n = 217) and prospective cohort of clinically and molecularly selected patients seem to favorably compare with those reported among patients with left-sided RAS wt tumors treated with doublets plus anti-EGFRs in other pivotal trials, where ORRs ranged from 64% to 73%.16 The growing amount of evidence about the role of several rare molecular alterations as predictors of intrinsic resistance to anti-EGFRs21 and the widespread NGS panels allowing extensive tumor molecular characterization probably led to an hyperselection of enrolled patients beyond RAS and BRAF mutational status. Centralized translational analyses on both tissue and blood samples to verify this hypothesis are currently ongoing.
Moreover, patients included in our trial were clinically selected for being able to receive an intensified treatment. In fact, 82% had an ECOG PS of 0, and the median age was quite low (59 years).
In our study, although the intensification of the chemotherapy backbone was associated with a very high ORR (73%), even higher among patients with left-sided tumors (77%), it did not provide any advantage in any measure of treatment activity. The ORR in our cohort of 218 patients treated at 57 Italian centers was lower than the 87% ORR reported for 63 RAS wt patients treated at 21 German centers in the mFOLFOXIRI plus panitumumab arm of the phase II randomized VOLFI study.9 The ability to induce a rapid and relevant tumor shrinkage was regarded as the strongest point for the combination of the triplet with an anti-EGFR, and we chose ORR as the primary end point of this phase III trial. Despite acknowledging that prolonging OS is the ultimate goal of systemic treatments in the metastatic setting, the choice of OS as the primary end point of the study would have hampered its feasibility. ORR was preferred because of its reliable association with OS in previous trials investigating anti–EGFR-based regimens, which was not demonstrated for PFS.22-26 In particular, among patients with RAS wt tumors enrolled in the FIRE-3 study comparing FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab, a significant advantage in favor of the anti-EGFR arm was shown in terms of both centrally assessed ORR (72.0% v 56.1%, P = .0029) and OS (32.5 v 26.1 months; HR, 0.75, 95% CI, 0.57 to 0.98; P = .035) with no difference in PFS (8.4 v 9.7 months; HR, 1.08, 95% CI, 0.86 to 1.36; P = .53), thus questioning the reliability of PFS as an appropriate surrogate end point for OS, when evaluating anti–EGFR-based treatments.25 Consistent results were reported in a meta-analysis of three randomized studies comparing upfront doublets plus anti-EGFR or bevacizumab.27 Moreover, achieving cytoreduction is an important objective in several clinical scenarios of mCRC, including not only potentially resectable patients but also those with high tumor load and/or suffering from tumor-related symptoms. In our study, no significant differences were also observed in terms of PFS in the overall study population and in all analyzed subgroups, but median PFS durations > 12 months were reported in both arms.
The lack of a central review of computed tomography scans for the assessment of response is a limitation of our study, but this planned activity will hardly affect the results of the trial given the absence of any signal of difference in favor of the experimental treatment.
Finally, with regard to the safety profile, the triplet was associated with higher occurrence of grade ≥ 3 adverse events and, in particular, of diarrhea (23%), even if reduced doses of irinotecan (150 mg/sqm once every 2 weeks) and FU (2,400 mg/sqm once every 2 weeks) were adopted. Similar results were reported in the VOLFI study, where grade ≥ 3 diarrhea was reported in 25% of patients, with the same dose of irinotecan and a higher dose of FU (3,000 mg/sqm once every 2 weeks).9 Conversely, in our previous MACBETH study, where irinotecan was further reduced to 130 mg/sqm once every 2 weeks and FU was administered at 2,400 mg/sqm once every 2 weeks grade ≥ 3, diarrhea occurred in 18% of patients.7
In conclusion, the intensification of the upfront chemotherapy backbone in combination with panitumumab in patients with RAS and BRAF wt and mostly (88%) left-sided mCRC does not provide any benefit in terms of treatment activity at the price of a non-negligible increase in gastrointestinal toxicity. FOLFOX plus panitumumab allows achieving an ORR as high as 76% with a median PFS of 12.3 months, thus supporting patients' selection according to the primary tumor side and RAS and BRAF mutational status to optimize the efficacy of anti–EGFR-based first-line treatments.
ACKNOWLEDGMENT
The authors are grateful to all participating patients, their families, their caregivers, and the Italian GONO Foundation investigators from the participating Italian sites.
Daniele Rossini
Speakers' Bureau: MSD Oncology
Sara Lonardi
Consulting or Advisory Role: Amgen, Merck Serono, Lilly, Servier, AstraZeneca, Incyte, Daiichi Sankyo, Bristol Myers Squibb, MSD
Speakers' Bureau: Roche, Lilly, Bristol Myers Squibb, Servier, Merck Serono, Pierre Fabre, GlaxoSmithKline, Amgen, Mirati Therapeutics
Research Funding: Amgen, Merck Serono, Bayer (Inst), Roche (Inst), Lilly (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst)
Filippo Pietrantonio
Honoraria: Servier, Bayer, AstraZeneca/MedImmune, Lilly, Sanofi, MSD Oncology, Amgen
Consulting or Advisory Role: Amgen, Servier, MSD Oncology, Organon
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca (Inst)
Federica Morano
Honoraria: Servier, Lilly
Travel, Accommodations, Expenses: Sanofi, Servier
Carmelo Pozzo
Consulting or Advisory Role: Amgen, Servier, Lilly
Vincenzo Formica
Honoraria: Amgen, Servier, Bayer
Massimo Aglietta
Consulting or Advisory Role: Bristol Myers Squibb
Research Funding: AstraZeneca (Inst), PharmaMar
Travel, Accommodations, Expenses: Merck, Tesaro, Bristol Myers Squibb
Roberto Bordonaro
Honoraria: Novartis, AstraZeneca, Sanofi, Amgen, Roche, Pfizer, Janssen-Cilag, Bristol Myers Squibb
Consulting or Advisory Role: Novartis, Bayer, AstraZeneca, Sanofi, Amgen, Roche, Pfizer, Janssen-Cilag, Bristol Myers Squibb
Speakers' Bureau: AstraZeneca, Sanofi, Novartis, Bayer, Amgen, Roche, Pfizer, Janssen-Cilag, Bristol Myers Squibb
Giuseppe Aprile
Consulting or Advisory Role: Amgen, Bristol Myers Squibb/Celgene, Servier, Lilly, Baxter, Merck, MSD
Luca Boni
Patents, Royalties, Other Intellectual Property: International Patent No.. PCT/EP2012/065661
Chiara Cremolini
Honoraria: Roche, Amgen, Bayer, Servier, MSD, Merck, Pierre Fabre, Organon
Consulting or Advisory Role: Roche, Bayer, Amgen, MSD, Pierre Fabre
Speakers' Bureau: Servier, Merck
Research Funding: Merck, Bayer, Roche, Servier
No other potential conflicts of interest were reported.
DISCLAIMER
The corresponding author had full access to all study data and had the final responsibility for the decision to submit for publication. Amgen had no role in the design and conduct of the trial; collection, management, analysis, and interpretation of the data; or the decision to submit the manuscript for publication.
PRIOR PRESENTATION
Presented in part at the 2022 ASCO Annual Meeting, Chicago, IL, June 3-7, 2022.
SUPPORT
Supported by the GONO Foundation. Amgen provided panitumumab in the experimental arm and partial financial support for study conduction.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Daniele Rossini, Roberto Moretto, Gabriella Fontanini, Gianluca Masi, Luca Boni, Chiara Cremolini
Provision of study materials or patients: Lorenzo Antonuzzo, Francesca Bergamo, Emiliano Tamburini, Alessandro Passardi, Sabina Murgioni, Angela Buonadonna, Gabriella Fontanini, Massimo Aglietta, Gianluca Masi
Collection and assembly of data: Daniele Rossini, Carlotta Antoniotti, Sara Lonardi, Filippo Pietrantonio, Roberto Moretto, Lorenzo Antonuzzo, Alessandra Boccaccino, Federica Morano, Marco Brugia, Federica Marmorino, Francesca Bergamo, Giovanni Randon, Beatrice Borelli, Angela Buonadonna, Mirella Giordano, Veronica Conca, Vincenzo Formica, Massimo Aglietta, Roberto Bordonaro, Giuseppe Aprile, Gianluca Masi, Luca Boni, Chiara Cremolini
Data analysis and interpretation: Daniele Rossini, Roberto Moretto, Carmelo Pozzo, Emiliano Tamburini, Alessandro Passardi, Sabina Murgioni, Beatrice Borelli, Gabriella Fontanini, Massimo Aglietta, Roberto Bordonaro, Giuseppe Aprile, Gianluca Masi, Luca Boni, Chiara Cremolini
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Upfront Modified Fluorouracil, Leucovorin, Oxaliplatin, and Irinotecan Plus Panitumumab Versus Fluorouracil, Leucovorin, and Oxaliplatin Plus Panitumumab for Patients With RAS/BRAF Wild-Type Metastatic Colorectal Cancer: The Phase III TRIPLETE Study by GONO
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Daniele Rossini
Speakers' Bureau: MSD Oncology
Sara Lonardi
Consulting or Advisory Role: Amgen, Merck Serono, Lilly, Servier, AstraZeneca, Incyte, Daiichi Sankyo, Bristol Myers Squibb, MSD
Speakers' Bureau: Roche, Lilly, Bristol Myers Squibb, Servier, Merck Serono, Pierre Fabre, GlaxoSmithKline, Amgen, Mirati Therapeutics
Research Funding: Amgen, Merck Serono, Bayer (Inst), Roche (Inst), Lilly (Inst), AstraZeneca (Inst), Bristol Myers Squibb (Inst)
Filippo Pietrantonio
Honoraria: Servier, Bayer, AstraZeneca/MedImmune, Lilly, Sanofi, MSD Oncology, Amgen
Consulting or Advisory Role: Amgen, Servier, MSD Oncology, Organon
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca (Inst)
Federica Morano
Honoraria: Servier, Lilly
Travel, Accommodations, Expenses: Sanofi, Servier
Carmelo Pozzo
Consulting or Advisory Role: Amgen, Servier, Lilly
Vincenzo Formica
Honoraria: Amgen, Servier, Bayer
Massimo Aglietta
Consulting or Advisory Role: Bristol Myers Squibb
Research Funding: AstraZeneca (Inst), PharmaMar
Travel, Accommodations, Expenses: Merck, Tesaro, Bristol Myers Squibb
Roberto Bordonaro
Honoraria: Novartis, AstraZeneca, Sanofi, Amgen, Roche, Pfizer, Janssen-Cilag, Bristol Myers Squibb
Consulting or Advisory Role: Novartis, Bayer, AstraZeneca, Sanofi, Amgen, Roche, Pfizer, Janssen-Cilag, Bristol Myers Squibb
Speakers' Bureau: AstraZeneca, Sanofi, Novartis, Bayer, Amgen, Roche, Pfizer, Janssen-Cilag, Bristol Myers Squibb
Giuseppe Aprile
Consulting or Advisory Role: Amgen, Bristol Myers Squibb/Celgene, Servier, Lilly, Baxter, Merck, MSD
Luca Boni
Patents, Royalties, Other Intellectual Property: International Patent No.. PCT/EP2012/065661
Chiara Cremolini
Honoraria: Roche, Amgen, Bayer, Servier, MSD, Merck, Pierre Fabre, Organon
Consulting or Advisory Role: Roche, Bayer, Amgen, MSD, Pierre Fabre
Speakers' Bureau: Servier, Merck
Research Funding: Merck, Bayer, Roche, Servier
No other potential conflicts of interest were reported.
REFERENCES
- 1.Van Cutsem E, Cervantes A, Adam R, et al. : ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 27:1386-1422, 2016 [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network (NCCN) Guidelines : Colon Cancer Version 1.2022. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf [Google Scholar]
- 3.Cremolini C, Antoniotti C, Stein A, et al. : Individual patient data meta-analysis of FOLFOXIRI plus bevacizumab versus doublets plus bevacizumab as initial therapy of unresectable metastatic colorectal cancer. J Clin Oncol 38:3314-3324, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Garufi C, Torsello A, Tumolo S, et al. : Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer 103:1542-1547, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assenat E, Desseigne F, Thezenas S, et al. : Cetuximab plus FOLFIRINOX (ERBIRINOX) as first-line treatment for unresectable metastatic colorectal cancer: A phase II trial. Oncologist 16:1557-1564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fornaro L, Lonardi S, Masi G, et al. : FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: A phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol 24:2062-2067, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Cremolini C, Antoniotti C, Lonardi S, et al. : Activity and safety of cetuximab plus modified FOLFOXIRI followed by maintenance with cetuximab or bevacizumab for RAS and BRAF wild-type metastatic colorectal cancer: A randomized phase 2 clinical trial. JAMA Oncol 4:529-536, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ychou M, Rivoire M, Thezenas S, et al. : Chemotherapy (doublet or triplet) plus targeted therapy by RAS status as conversion therapy in colorectal cancer patients with initially unresectable liver-only metastases. The UNICANCER PRODIGE-14 randomised clinical trial. Br J Cancer 126:1264-1270, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Modest DP, Martens UM, Riera-Knorrenschild J, et al. : FOLFOXIRI plus panitumumab as first-line treatment of RAS wild-type metastatic colorectal cancer: The randomized, open-label, phase II VOLFI study (AIO KRK0109). J Clin Oncol 37:3401-3411, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 11.National Cancer Institute : Common Terminology Criteria for Adverse Events. Version 4.0, 2010. https://www.eortc.be/services/doc/ct/ctcae_4.03_2010-06-14_quickreference_5x7.pdf [Google Scholar]
- 12.Van Cutsem E, Lenz H-J, Köhne C-H, et al. : Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 33:692-700, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Douillard J-Y, Oliner KS, Siena S, et al. : Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 369:1023-1034, 2013 [DOI] [PubMed] [Google Scholar]
- 14.Rowland A, Dias MM, Wiese MD, et al. : Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br J Cancer 112:1888-1894, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pietrantonio F, Petrelli F, Coinu A, et al. : Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: A meta-analysis. Eur J Cancer 51:587-594, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Arnold D, Lueza B, Douillard JY, et al. : Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol 28:1713-1729, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holch JW, Ricard I, Stintzing S, et al. : The relevance of primary tumour location in patients with metastatic colorectal cancer: A meta-analysis of first-line clinical trials. Eur J Cancer 70:87-98, 2017 [DOI] [PubMed] [Google Scholar]
- 18.Yoshino T, Arnold D, Taniguchi H, et al. : Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol 29:44-70, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Cremolini C, Morano F, Moretto R, et al. : Negative hyper-selection of metastatic colorectal cancer patients for anti-EGFR monoclonal antibodies: The PRESSING case-control study. Ann Oncol 28:3009-3014, 2017 [DOI] [PubMed] [Google Scholar]
- 20.Loupakis F, Yang D, Yau L, et al. : Primary tumor location as a prognostic factor in metastatic colorectal cancer. J Natl Cancer Inst 107:dju427, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morano F, Corallo S, Lonardi S, et al. : Negative hyperselection of patients with RAS and BRAF wild-type metastatic colorectal cancer who received panitumumab-based maintenance therapy. J Clin Oncol 37:3099-3110, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heinemann V, Stintzing S, Modest DP, et al. : Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer 51:1927-1936, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Heinemann V, von Weikersthal LF, Decker T, et al. : FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol 15:1065-1075, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Schwartzberg LS, Rivera F, Karthaus M, et al. : PEAK: A randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol 32:2240-2247, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Stintzing S, Modest DP, Rossius L, et al. : FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): A post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 17:1426-1434, 2016 [DOI] [PubMed] [Google Scholar]
- 26.Rivera F, Karthaus M, Hecht JR, et al. : Final analysis of the randomised PEAK trial: Overall survival and tumour responses during first-line treatment with mFOLFOX6 plus either panitumumab or bevacizumab in patients with metastatic colorectal carcinoma. Int J Colorectal Dis 32:1179-1190, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khattak MA, Martin H, Davidson A, et al. : Role of first-line anti-epidermal growth factor receptor therapy compared with anti-vascular endothelial growth factor therapy in advanced colorectal cancer: A meta-analysis of randomized clinical trials. Clin Colorectal Cancer 14:81-90, 2015 [DOI] [PubMed] [Google Scholar]