OBJECTIVES:
Older age is a key risk factor for adverse outcomes in critically ill patients with COVID-19. However, few studies have investigated whether preexisting comorbidities and acute physiologic ICU factors modify the association between age and death.
DESIGN:
Multicenter cohort study.
SETTING:
ICUs at 68 hospitals across the United States.
PATIENTS:
A total of 5,037 critically ill adults with COVID-19 admitted to ICUs between March 1, 2020, and July 1, 2020.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
The primary exposure was age, modeled as a continuous variable. The primary outcome was 28-day inhospital mortality. Multivariable logistic regression tested the association between age and death. Effect modification by the number of risk factors was assessed through a multiplicative interaction term in the logistic regression model. Among the 5,037 patients included (mean age, 60.9 yr [± 14.7], 3,179 [63.1%] male), 1,786 (35.4%) died within 28 days. Age had a nonlinear association with 28-day mortality (p for nonlinearity <0.001) after adjustment for covariates that included demographics, preexisting comorbidities, acute physiologic ICU factors, number of ICU beds, and treatments for COVID-19. The number of preexisting comorbidities and acute physiologic ICU factors modified the association between age and 28-day mortality (p for interaction <0.001), but this effect modification was modest as age still had an exponential relationship with death in subgroups stratified by the number of risk factors.
CONCLUSIONS:
In a large population of critically ill patients with COVID-19, age had an independent exponential association with death. The number of preexisting comorbidities and acute physiologic ICU factors modified the association between age and death, but age still had an exponential association with death in subgroups according to the number of risk factors present. Additional studies are needed to identify the mechanisms underpinning why older age confers an increased risk of death in critically ill patients with COVID-19.
Keywords: age, COVID-19, critical care, death, risk factors
Since the outbreak of the severe acute respiratory syndrome coronavirus 2 in December of 2019 in Wuhan, China, more than 219 million people worldwide have developed COVID-19, and more than 6 million people have died as of August 9, 2022 (1). Prior investigations identified several risk factors associated with adverse clinical outcomes in patients with COVID-19. Among these risk factors, older age consistently remains one of the strongest risk factors associated with adverse clinical outcomes in patients with COVID-19 (2–8).
Previous studies have demonstrated that older age is associated with more severe illness from COVID-19, including a higher risk of hospitalization, need for ICU admission, and death (9–15). Fewer studies demonstrated these associations in critically ill patients with COVID-19, a setting in which other patient characteristics and acute severity-of-illness might contribute to a greater degree of an individual’s risk of death (3, 7, 16, 17). Furthermore, whether the number of preexisting comorbidities and acute physiologic ICU factors modifies the association between age and death in critically ill patients with COVID-19 is not well-described. Enhanced understanding of the association of age with mortality in the context of risk factors among critically ill patients may serve to generate hypotheses of potential mechanisms underlying the association between age and death.
Since multiple prior studies evaluated age in categories (18–20), which may not fully capture its association with death, our first aim was to determine whether age had an independent linear or nonlinear association with death. Our second aim was to determine whether the number of preexisting comorbidities and acute physiologic ICU factors serves as an effector modifier of the association between age and death. We used data from a large multicenter cohort study of critically ill patients with COVID-19 admitted to ICUs across the United States to investigate these aims. We hypothesized that age has a nonlinear association with death and that the association between age and death differs according to the number of preexisting comorbidities and acute physiologic ICU factors present within critically ill patients with COVID-19.
MATERIALS AND METHODS
Study Design, Oversight, and Patient Population
We used data from the Study of the Treatment and Outcomes in Critically Ill Patients With COVID-19 (16), a multicenter cohort study that enrolled consecutive adults (≥18 yr old) with laboratory confirmed COVID-19 admitted to ICUs at 68 geographically diverse hospitals across the United States. A complete list of participating sites is provided in the Supplementary Appendix (http://links.lww.com/CCX/B52). We included patients admitted to the ICU during their index admission between March 1, 2020, and July 1, 2020. We followed patients until the first of hospital discharge, death, or August 1, 2020, the date when the study database for the current analysis was locked (16). STOP-COVID was approved with a waiver of informed consent by the Institutional Review Board at Mass General Brigham (protocol number 2007000003) and Northwestern University (March 31, 2020) and is in accordance with the principles of the Declaration of Helsinki.
Data Collection
Study personnel at each site collected data by detailed chart review and used a standardized case report form to enter data into a secure online database (research electronic data capture [REDCap]) (21). Patient-level data included baseline demographic information, coexisting conditions, symptoms, medications before hospital admission, vital signs on ICU admission, and daily data for the 14 days after ICU admission on physiologic and laboratory values, pharmacologic and nonpharmacologic treatments administered, and organ injury and support, as previously described (16). Vital status was collected up to the time of hospital discharge. All data were validated through a series of automated verifications using REDCap’s data quality module.
Exposure and Outcomes
The primary exposure was age, treated as a continuous variable. We also evaluated age in categories: less than 65, 65–79, and greater than or equal to 80 years as performed in prior studies (16, 18–20). The primary outcome was 28-day inhospital mortality. The secondary outcome was 90-day mortality. Patients discharged alive from the hospital before 28 or 90 days were considered to be alive at 28 or 90 days, respectively. The validity of this assumption was tested in a subset of patients, as previously described (16).
Statistical Analysis
Descriptive statistics were summarized as counts with percentages for categorical variables and mean ± sd or median with interquartile range for continuous variables. We used chi-square tests to compare frequency distributions of categorical variables by age group. For evaluations between continuous variables and age group, we used analysis of variance (for normally distributed variables) and Kruskal-Wallis tests (for nonnormally distributed variables).
We used multivariable logistic regression to determine the association of age with death. We fitted a series of hierarchically adjusted models with prespecified covariates on the basis of clinical knowledge and our prior work (16). Model 1 was unadjusted; model 2 was adjusted for sex, race, current smoking status, body mass index (calculated as weight in kilograms divided by height in meters squared, and categorized as ≤25, 25–29.9, 30–34.9, 35–39.9, and ≥40), hypertension, diabetes mellitus, chronic obstructive pulmonary disease, coronary artery disease, congestive heart failure, and active malignancy; model 3 included all model 2 variables as well as symptom duration prior to ICU admission (≤3 vs >3 days), number of ICU beds at each hospital prior to the COVID-19 pandemic (<50, 50–99, and ≥100 beds), shock (simultaneous receipt of ≥2 vasopressors/inotropes), lymphocyte count (<1,000/uL vs ≥1,000/uL), degree of hypoxemia and respiratory support (categorized as no receipt of invasive mechanical ventilation, or receipt of invasive mechanical ventilation with a ratio of Pao2 to Fio2 ≥300, 200–299, 100–199, and <100), and the renal, liver, and coagulation components of the Sequential Organ Failure Assessment (SOFA) score (22); model 4 included all model 3 variables as well as treatments for COVID-19: remdesivir, tocilizumab, and corticosteroids. Acute physiologic ICU factors were assessed during the first 2 days following ICU admission, with the worst value used. The Supplemental Methods (http://links.lww.com/CCX/B52) provide additional details for the multivariable modeling strategy, including definitions of covariates. We examined the possible nonlinear relation between age and death with restricted cubic splines. Tests for nonlinearity used the likelihood ratio test, comparing each model with only the linear term to the analogous model with the linear and cubic-spline terms (23, 24). To evaluate for unmeasured confounding, we calculated an E-value based on the methodology of VanderWeele and Ding (25). This estimates what the odds ratio would have to be for any unmeasured confounder to overcome the observed association of age with death in this study.
To determine the number of risk factors for death present for each individual, we first identified preexisting comorbidities and acute physiologic ICU factors that had a significant association with death in univariate logistic regression models. Next, we summed the number of risk factors present for each individual. We tested for interaction between age and the number of risk factors in multivariable logistic regression models with the use of a multiplicative interaction term.
To account for missing data, we used multiple imputation, for which we used a multiple regression procedure in IVEware 2.0 (26). We generated five imputed datasets and imputed values for missing data on the basis of the observed data with the assumption that the data were missing at random. Imputations were created through a sequence of multiple regression models (27). We combined the test results across the imputed datasets using the rules of Rubin (28). Statistical analyses were performed using the SAS software, Version 9.4 (SAS Institute, Cary, NC). All statistical tests were 2-sided, and p < 0.05 was considered significant.
RESULTS
Baseline Characteristics
Supplemental Table 1 (http://links.lww.com/CCX/B52) summarizes baseline characteristics of STOP-COVID patients (n = 5,037) by age categories. The mean age was 60.9 years (sd ± 14.7), and 3,179 (63.1%) were men. Compared with younger patients, older patients were more likely to be White, current smokers, and to have a lower body mass index. Older patients had a higher prevalence of congestive heart failure, coronary artery disease, chronic obstructive pulmonary disease, hypertension, active cancer, chronic kidney disease, and end-stage kidney disease compared with younger patients. On ICU admission, older patients were more likely to have higher renal and coagulation SOFA scores and to receive vasopressors and invasive mechanical ventilation compared with younger patients. Older patients were less likely to receive treatments such as remdesivir, corticosteroids, or tocilizumab within 2 days following ICU admission compared with younger patients. Older patients were more likely to be admitted to hospitals with less than 50 ICU beds.
Association of Age With Death
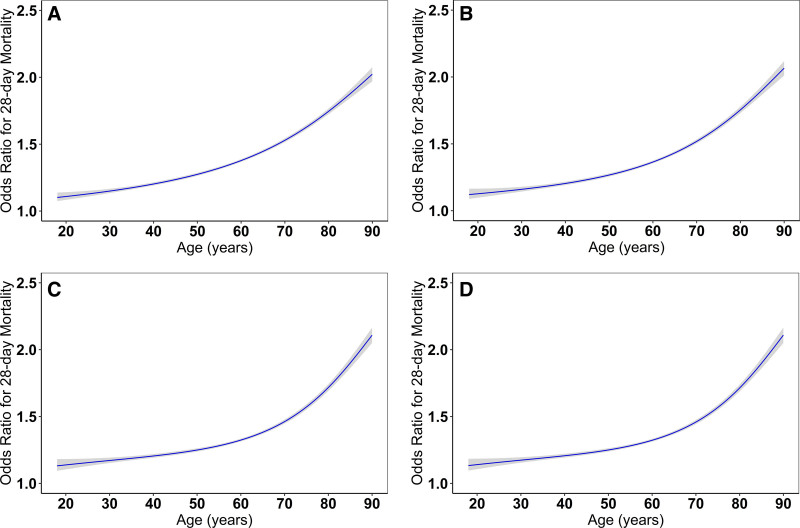
Within 28 days of ICU admission, 1,786 patients (35.5%) died, and 2,017 patients (40.0%) died within 90 days of admission. Figure 1 shows the unadjusted and sequentially multivariable-adjusted restricted cubic-spline models between age and 28-day mortality. Age had a nonlinear association with 28-day mortality in each restricted cubic-spline models (p for nonlinear association <0.001), which had a better fit than modeling age as a linear term (Fig. 1A–D). In the fully adjusted model, the Akaike Information Criterion (AIC) for age modeled as a continuous variable and as a spline was 26,778 and 26,643 (p value for AIC difference < 0.001), respectively. The association between age and 28-day mortality remained significant even after accounting for demographics, preexisting comorbidities, acute physiology ICU factors, number of ICU beds, and treatments for COVID-19 (Fig. 1; and Supplemental Table 2, http://links.lww.com/CCX/B52). The results for 90-day mortality were similar (Supplemental Fig. 1, http://links.lww.com/CCX/B52; and Supplemental Table 2, http://links.lww.com/CCX/B52).
Figure 1.
Nonlinear association between age and 28-day mortality. A, The model is unadjusted. Age-linear akaike information criterion (AIC): 30,713 versus age-spline AIC: 30,686, likelihood ratio test p value for AIC difference: < 0.001. B, This model is further adjusted for demographic characteristics, including male sex, and presence of hypertension, diabetes mellitus, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, and active cancer. Age-linear AIC: 30,218 versus Age-spline AIC: 30,160, likelihood ratio test p value for AIC difference: < 0.001. C, This model is further adjusted for acute ICU physiologic factors, including symptom onset less than or equal to 3 d prior to ICU admission, lymphocyte count less than 1,000/uL, degree of hypoxemia and respiratory support, shock, SOFA coagulation greater than 0, SOFA liver greater than 0, and SOFA renal greater than 0, and the number of ICU beds. Age-linear AIC 26,913 versus Age-spline AIC: 26,783, likelihood ratio test p value for AIC difference: < 0.001. D, This model is further adjusted for COVID-19 treatments, including: remdesivir, tocilizumab, and corticosteroids. Age-linear AIC: 26,778, Age-spline AIC 26,643, likelihood ratio test p value for AIC difference: < 0.001. SOFA = Sequential Organ Failure Assessment.
The odds ratio for the association between age (modeled continuously per 10 yr) and death was 1.51 (95% CI, 1.42–1.60). The E-value (odds ratio) for the point estimate of age (modeled continuously per 10 yr) was 1.76. Using the suggested language of VanderWeele and Ding (25), the observed odds ratio of 1.51 for the association between age (per 10 yr) and death could be explained by an unmeasured confounder that was associated with both the exposure (age) and the outcome (death) with an odds ratio of 1.76, above and beyond the measured confounders, but a weak confounder could not do so.
Preexisting Comorbidities and Acute Physiologic ICU Factors Associated With Death
To determine whether the association of age with death is modified according to the number of risk factors present, we identified preexisting comorbidities and acute physiologic ICU factors associated with 28-day mortality in univariate logistic regression models (Supplemental Table 3, http://links.lww.com/CCX/B52). Male sex, hypertension, diabetes mellitus, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, active cancer, symptom onset less than or equal to 3 days prior to ICU admission, lymphocyte count less than 1,000/uL, lower Pao2 to Fio2 ratio, shock, higher coagulation, liver, and renal SOFA scores, and admission to a hospital with fewer ICU beds were each associated with a higher odds of death (Supplemental Table 4, http://links.lww.com/CCX/B52). Supplemental Figure 2 (http://links.lww.com/CCX/B52) shows the distribution of the number of preexisting comorbidities and acute physiologic ICU factors associated with 28-day mortality in the STOP-COVID participants.
Effect Modification of Age With 28-day Mortality by Number of Preexisting Comorbidities and Acute Physiologic ICU Factors
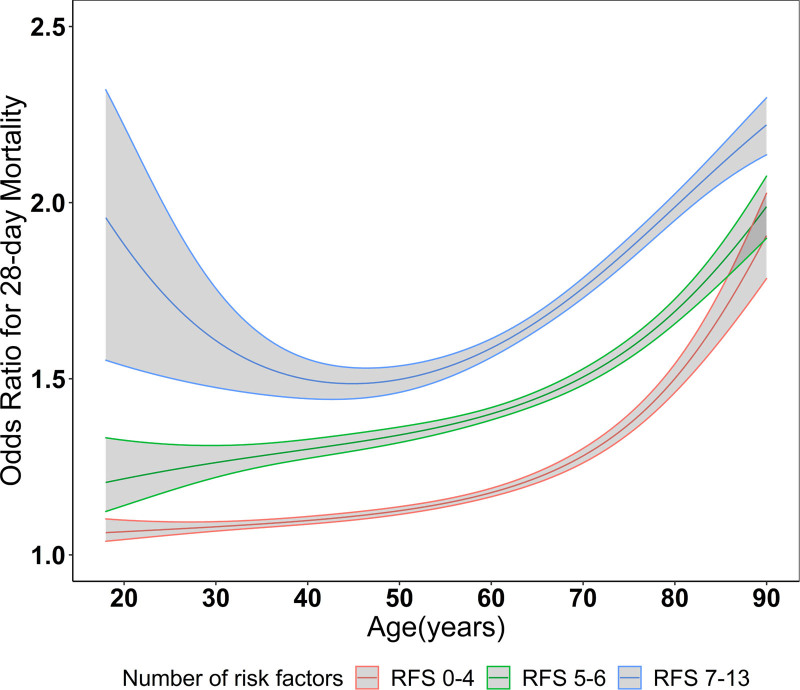
The number of preexisting comorbidities and acute physiologic ICU factors modified the association between age and death (p for interaction < 0.001). Figure 2 shows the association of age with death stratified by tertiles of the number of preexisting comorbidities and acute physiologic ICU factors: 0–4, 5–6, and 7–13 risk factors, which corresponded to 35.9%%, 35.9%, and 28.2% of the study population, respectively. Although the number of preexisting comorbidities and acute physiologic ICU factors modified the association between age and death, this effect modification was modest as age had a nonlinear association with 28-day mortality in each of the risk factor subgroups. Supplemental Table 5 (http://links.lww.com/CCX/B52) shows the association between ages modeled in categories with 28-day mortality in each of the risk factor subgroups. Patients who were in the older age categories had a higher odds of death compared with patients who were less than 65 years old, but the magnitude of association between older age and death was lower with increasing risk factor categories.
Figure 2.
Nonlinear association between age and 28-day mortality stratified by number of risk factors. Nonlinear association of age (continuous) with 28-day mortality stratified by categories of the number of significant preexisting comorbidities and acute physiologic ICU factors. p for nonlinearity in each group less than 0.001. Risk factors: male sex, hypertension, diabetes mellitus, coronary artery disease, congestive heart failure, chronic obstructive pulmonary disease, active cancer, symptom onset less than or equal to 3 d, lymphocyte count less than 1,000/µL, invasive mechanical ventilation, shock, SOFA Coagulation Score greater than 0, SOFA Renal Score greater than 0, SOFA Liver Score greater than 0, and number of ICU beds less than 100. Models are further adjusted for body mass index (in categories), White versus non-White, current smoker, remdesivir, tocilizumab, and corticosteroids. SOFA = Sequential Organ Failure Assessment.
DISCUSSION
In this multicenter cohort study of over 5,000 critically ill patients with COVID-19 admitted to ICUs across the United States, we identified an exponential association between age and death even after multivariable adjustment for demographics, preexisting comorbidities, acute physiologic ICU factors, number of ICU beds, and treatments for COVID-19. We found that the relationship between age and death is modified by the number of preexisting comorbidities and acute physiologic ICU factors present, but this effect modification was modest, and age still had an exponential association with death in subgroups based on the number of risk factors. Collectively, our findings demonstrate that age is an important risk factor for death in critically ill patients with COVID-19, even in subgroups of individuals who have a fewer or greater number of preexisting comorbidities or acute physiologic ICU factors. Future studies are warranted to identify mechanisms that may explain why older age is associated with a significantly increased risk of death in patients with COVID-19.
In our first STOP-COVID study that included 2,215 patients, we identified a graded association between age and 28-day mortality (16). In this study, we found that age has an exponential association with death. These findings are consistent with prior population-based studies of adults at risk for COVID-19 (20). and adults who tested positive for COVID-19 (29). Although these prior studies demonstrated a similar nonlinear association between age and death, our study extends these findings to critically ill patients from a large number of hospitals across the United States. Importantly, our findings were independent of demographics, preexisting comorbidities, and acute physiologic ICU factors, number of ICU beds, and treatments for COVID-19.
A prior study identified that the number of risk factors modified the association of age with COVID-19 mortality. The investigators found a higher magnitude of association between older age and death in patients with more risk factors compared with patients with fewer risk factors (20). Importantly, the prior study included participants in a large population-based cohort at risk for COVID-19 with a limited number of risk factors obtained years prior to the diagnosis of COVID-19. We found that the number of preexisting comorbidities and acute physiologic ICU factors modified the association between age and death. While we detected effect modification, this appeared to be modest since the association between age and death remained exponential in the same direction within each of the risk factor subgroups. Although we found a slightly lower magnitude of association between older age and death with increasing risk factor categories, these findings may suggest that the effect of age on death is slightly attenuated in populations enriched with a greater severity of illness. Our findings demonstrate that there may be subtle differences in the magnitudes of the association between age and death based on the number of risk factors present in an individual, but age still remained a strong risk factor for death.
The pathophysiologic link between age and death among patients with COVID-19 remains unclear, but a variety of mechanisms have been proposed. Aging induces a hypersecretory cellular state that leads to the release of inflammatory and tissue repair mediators, predisposing aged cells to damage (30). “Inflammaging,” a chronic low-grade inflammation that occurs in response to endogenous signals during aging in the absence of infection (31), may further drive immune dysfunction and impaired antiviral responses. In older patients, CD3+ T-cells that mediate the antiviral immune response may increase production of interleukin 6 (32), neutrophils demonstrate impaired tissue migration (33), and B-cells show impaired antibody responses in older patients (34).
Although aging may have direct effects on the immune system, aging may encompass risk factors, known and unknown, that indirectly increase mortality in critically ill patients. Elderly patients are more likely to have preexisting comorbid conditions, but a number of comorbidities may remain underdiagnosed due to a subclinical presentation or a desire to reduce the burden of excessive testing (35–37). Prior studies demonstrated that elderly patients may have socioeconomic barriers to healthcare, which may delay their presentation until their health deteriorates significantly (38, 39). We previously reported that hospitals with fewer ICU beds were a risk factor for increased mortality (16), and our current data demonstrated that older patients were more likely to be treated in hospitals with fewer ICU beds. The reason why older patients were more likely to be treated in hospitals with fewer ICU beds remains unclear, but potential barriers may include distance from larger hospitals or transportation (40, 41). Limited resources and hospital strain likely enhanced these barriers during the pandemic (42, 43). Additionally, older patients who are treated in hospitals with fewer ICU beds may be less likely to be transferred to a larger medical center due to a perception of a lower likelihood of survival by healthcare providers or treatment allocation strategies favoring younger patients during times of treatment scarcity.
Although we demonstrated age is a strong independent risk factor for death after adjustment for a number of risk factors, unmeasured or undiagnosed risk factors may further explain the association between age and death. Our E-value analysis demonstrated that an unmeasured confounder of sufficient magnitude could attenuate the association between age and death. Our multivariable-adjusted analyses demonstrated that most, but not all, of the covariates included in this study were under the threshold of the E-value, and therefore, we cannot exclude the possibility of an unmeasured covariate or a combination of unmeasured covariates that could explain the association of age with death. We must acknowledge that an important unmeasured confounder in this analysis is frailty (44–48), which has the potential to have a sufficiently high magnitude of association with both age and death. Prior investigations demonstrated that frailty was a powerful predictor of mortality in patients with COVID-19, with hazard ratios for death ranging from 2.5 to 4.4 and 1.5 to 2.7 in the COVID-19 in Older People (COPE) (18) and COMET (19) studies, respectively. Future studies in critically ill patients with COVID-19 should attempt to capture data on variables such as frailty, to comprehensively evaluate associations between risk factors and death in critically ill patients with COVID-19.
Strengths of this study include the use of a large cohort of geographically diverse, critically ill patients with COVID-19 across the United States. Data were obtained by detailed chart review rather than reliance on administrative or billing codes, allowing us to capture granular and reliable data. Our study also has limitations. Our models do not account for varying degrees of strain on the availability of resources across hospitals, which may affect clinical outcomes. Patients were followed for a maximum of 90 inhospital days in the current analyses, and some of the 90-day survivors may have died after 90 days. However, our prior analyses from the same cohort found that 28- and 90-day mortality rates differed only to a small extent, occurring in 34.5% and 39.6% of patients, respectively (49). Although we included a large number of critically ill patients with COVID-19, there were fewer patients in the younger subgroups, which may have limited our ability to detect a significant association with death. Although we adjusted for therapies against COVID-19, older patients may have been less likely to receive these treatments or other interventions (e.g., extracorporeal membrane oxygenation). We did not assign relative weights for each risk factor when constructing the number of preexisting and acute physiologic ICU factors for our effect modification analyses. The patients included in this study were admitted to ICUs prior to the advent of vaccines and antiviral treatments for COVID-19, which may reduce the generalizability of the results. Nevertheless, our findings still underscore the age-associated risks among the unvaccinated.
In conclusion, age had independent, exponential association with death, and this relationship was modified according to the number of preexisting comorbidities and acute physiologic ICU factors present in a large population of critically ill patients with COVID-19. However, the effect modification was modest as age still had an exponential associated with death in subgroups based on the number of preexisting comorbidities and acute physiologic ICU factors. Our finding of an association between age and COVID-19 mortality independent of risk factors should motivate further investigation into the direct and indirect impacts of age on patient health. Future clinical trials in critically ill patients with COVID-19 should assess whether treatment efficacy differs according to age and risk factor profile.
Supplementary Material
APPENDIX. STOP-COVID INVESTIGATORS
Baylor College of Medicine: Carl P. Walther*, Samaya J. Anumudu
Baylor University Medical Center: Justin Arunthamakun*, Kathleen F. Kopecky, Gregory P. Milligan, Peter A. McCullough, Thuy-Duyen Nguyen
Beth Israel Deaconess Medical Center: Shahzad Shaefi*, Megan L. Krajewski, Sidharth Shankar, Ameeka Pannu, Juan D. Valencia
Boston Medical Center: Sushrut S. Waikar*, Zoe A. Kibbelaar
Cook County Health: Ambarish M. Athavale*, Peter Hart, Shristi Upadhyay, Ishaan Vohra, Ajiboye Oyintayo
Cooper University Healthcare: Adam Green*, Jean-Sebastien Rachoin, Christa A. Schorr, Lisa Shea
Duke University Medical Center: Daniel L. Edmonston*, Christopher L. Mosher
Hackensack Meridian Health Mountainside Medical Center: Alexandre M. Shehata*, Zaza Cohen, Valerie Allusson, Gabriela Bambrick-Santoyo, Noor ul aain Bhatti, Bijal Mehta, Aquino Williams
Hackensack Meridian Health Hackensack University Medical Center: Samantha K. Brenner*, Patricia Walters, Ronaldo C. Go, Keith M. Rose
Harvard T. H. Chan School of Public Health: Miguel A. Hernán
Harvard University: Amy M. Zhou, Ethan C. Kim, Rebecca Lisk
Icahn School of Medicine at Mount Sinai: Lili Chan*, Kusum S. Mathews*, Steven G. Coca, Deena R. Altman, Aparna Saha, Howard Soh, Huei Hsun Wen, Sonali Bose, Emily A. Leven, Jing G. Wang, Gohar Mosoyan, Girish N. Nadkarni, Pattharawin Pattharanitima, Emily J. Gallagher
Indiana University School of Medicine/Indiana University Health: Allon N. Friedman*, John Guirguis, Rajat Kapoor, Christopher Meshberger, Katherine J. Kelly
Johns Hopkins Hospital: Chirag R. Parikh*, Brian T. Garibaldi, Celia P. Corona-Villalobos, Yumeng Wen, Steven Menez, Rubab F. Malik, Carmen Elena Cervantes, Samir C. Gautam
Kings County Hospital Center: Mary C. Mallappallil*, Jie Ouyang, Sabu John, Ernie Yap, Yohannes Melaku, Ibrahim Mohamed, Siddhartha Bajracharya, Isha Puri, Mariah Thaxton, Jyotsna Bhattacharya, John Wagner, Leon Boudourakis
Loma Linda University: H. Bryant Nguyen*, Afshin Ahoubim
Mayo Clinic, Arizona: Leslie F. Thomas*, Dheeraj Reddy Sirganagari
Mayo Clinic, Florida: Pramod K. Guru*
Mayo Clinic, Rochester: Kianoush Kashani* and Shahrzad Tehranian
Medical College of Wisconsin: Yan Zhou,* Paul A. Bergl, Jesus Rodriguez, Jatan A. Shah, Mrigank S. Gupta
MedStar Georgetown University Hospital: Princy N. Kumar*, Deepa G. Lazarous, Seble G. Kassaye
Montefiore Medical Center/Albert Einstein College of Medicine: Michal L. Melamed*, Tanya S. Johns, Ryan Mocerino, Kalyan Prudhvi, Denzel Zhu, Rebecca V. Levy, Yorg Azzi, Molly Fisher, Milagros Yunes, Kaltrina Sedaliu, Ladan Golestaneh, Maureen Brogan, Neelja Kumar, Michael Chang, Jyotsana Thakkar
New York-Presbyterian Queens Hospital: Ritesh Raichoudhury*, Akshay Athreya, Mohamed Farag
New York-Presbyterian/Weill Cornell Medical Center: Edward J. Schenck*, Soo Jung Cho, Maria Plataki, Sergio L. Alvarez-Mulett, Luis G. Gomez-Escobar, Di Pan, Stefi Lee, Jamuna Krishnan, William Whalen
New York University Langone Hospital: David Charytan*, Ashley Macina, Sobaata Chaudhry, Benjamin Wu, Frank Modersitzki
Northwestern Memorial Hospital: Northwestern University Feinberg School of Medicine - Anand Srivastava*, Alexander S. Leidner, Carlos Martinez, Jacqueline M. Kruser, Richard G. Wunderink, Alexander J. Hodakowski
Ochsner Medical Center: Juan Carlos Q. Velez*, Eboni G. Price-Haywood, Luis A. Matute-Trochez, Anna E. Hasty, Muner MB. Mohamed
Oregon Health and Science University Hospital: Rupali S. Avasare*, David Zonies*
Partners Healthcare: Brigham and Women’s Hospital, Brigham and Women’s Faulkner Hospital, Massachusetts General Hospital, and Newton Wellesley Hospital - David E. Leaf*, Shruti Gupta*, Meghan E. Sise, Erik T. Newman, Samah Abu Omar, Kapil K. Pokharel, Shreyak Sharma, Harkarandeep Singh, Simon Correa, Tanveer Shaukat, Omer Kamal, Wei Wang, Heather Yang, Jeffery O. Boateng, Meghan Lee, Ian A. Strohbehn, Jiahua Li, Ariel L. Mueller
ProMedica Health System: Roberta E. Redfern,* Nicholas S. Cairl, Gabriel Naimy, Abeer Abu-Saif, Danyell Hall, Laura Bickley
Renown Health: Chris Rowan*, Farah Madhani-Lovely*, Vivian S. Cruz, Kristen M. Hess, Alanna L. Jacobs
Rush University Medical Center: Vasil Peev*, Jochen Reiser, John J. Byun, Andrew Vissing, Esha M. Kapania, Zoe Post, Nilam P. Patel, Joy-Marie Hermes
Rutgers/New Jersey Medical School: Anne K. Sutherland*, Amee Patrawalla, Diana G. Finkel, Barbara A. Danek, Sowminya Arikapudi, Jeffrey M. Paer, Peter Cangialosi, Mark Liotta
Rutgers/Robert Wood Johnson Medical School: Jared Radbel*, Sonika Puri, Jag Sunderram, Matthew T. Scharf, Ayesha Ahmed, Ilya Berim, Jayanth S. Vatson
Stanford Healthcare: Stanford University School of Medicine—Shuchi Anand*, Joseph E. Levitt, Pablo Garcia
Temple University Hospital: Suzanne M. Boyle*, Rui Song
Thomas Jefferson University Hospital: Jingjing Zhang*, Sang Hoon Woo, Xiaoying Deng, Goni Katz-Greenberg, Katharine Senter
Tulane Medical Center: Moh’d A. Sharshir*, Vadym V. Rusnak
United Health Services Hospitals: Muhammad Imran Ali
University of Colorado Anschutz Medical Campus: Anip Bansal*, Amber S. Podoll, Michel Chonchol, Sunita Sharma, Ellen L. Burnham, David J. Douin
University Hospitals Cleveland Medical Center: Arash Rashidi*, Rana Hejal
University of Alabama-Birmingham Hospital: Eric Judd*, Laura Latta, Ashita Tolwani
University of California-Davis Medical Center: Timothy E. Albertson*, Jason Y. Adams
University of California-Los Angeles Medical Center: Ronald Reagan-UCLA Medical Center - Steven Y. Chang*, Rebecca M. Beutler; UCLA Medical Center, Santa Monica—Carl E. Schulze
University of California-San Diego Medical Center: Etienne Macedo*, Harin Rhee
University of California-San Francisco Medical Center: Kathleen D. Liu*, Vasantha K. Jotwani
University of Chicago Medical Center: Jay L. Koyner*
University of Florida Health-Gainesville: Chintan V. Shah*
University of Florida-Health-Jacksonville: Vishal Jaikaransingh*
University of Illinois Hospital and Health Sciences System: Stephanie M. Toth-Manikowski*, Min J. Joo*, James P. Lash
University of Kentucky Medical Center: Javier A. Neyra*, Nourhan Chaaban, Madona Elias, Yahya Ahmad
University Medical Center of Southern Nevada: Alfredo Iardino, Elizabeth H. Au, Jill H. Sharma
University of Miami Health System: Marie Anne Sosa*, Sabrina Taldone, Gabriel Contreras, David De La Zerda, Hayley B. Gershengorn, Bhavarth Shukla, Alessia Fornoni, Tanira Ferreira
University of Michigan: Salim S. Hayek*, Pennelope Blakely, Hanna Berlin, Tariq U. Azam, Husam Shadid, Michael Pan, Patrick O’ Hayer, Chelsea Meloche, Rafey Feroze, Rayan Kaakati, Danny Perry, Abbas Bitar, Elizabeth Anderson, Kishan J. Padalia, John P. Donnelly, Andrew J. Admon
University of North Carolina School of Medicine: Jennifer E. Flythe*, Matthew J. Tugman, Emily H. Chang
University of Oklahoma Health Sciences Center: Brent R. Brown*
University of Pennsylvania Health System: Amanda K. Leonberg-Yoo*, Ryan C. Spiardi, Todd A. Miano, Meaghan S. Roche, Charles R. Vasquez
University of Pittsburgh Medical Center: Amar D. Bansal*, Natalie C. Ernecoff, Sanjana Kapoor, Siddharth Verma, Huiwen Chen
University of Tennessee Health Science Center and Memphis VA Medical Center/Methodist
University Hospital—Csaba P. Kovesdy*, Miklos Z. Molnar*, Ambreen Azhar
University of Texas Southwestern Medical Center and Parkland Health and Hospital System: S. Susan Hedayati*, Mridula V. Nadamuni, Shani Shastri, Duwayne L. Willett
University of Vermont Larner College of Medicine: Samuel A.P. Short
University of Virginia Health System: Amanda D. Renaghan*, Kyle B. Enfield
University of Washington Medical Center: Pavan K. Bhatraju*, A. Bilal Malik
Vanderbilt University Medical Center: Matthew W. Semler
Washington University in St. Louis/Barnes Jewish Hospital: Anitha Vijayan*, Christina Mariyam Joy, Tingting Li, Seth Goldberg, Patricia F. Kao
Wellforce Health System: Lowell General Hospital - Greg L. Schumaker*, Tufts Medical Center - Nitender Goyal*, Anthony J. Faugno, Greg L. Schumaker, Caroline M. Hsu, Asma Tariq, Leah Meyer, Ravi K. Kshirsagar, Daniel E. Weiner, Aju Jose
Westchester Medical Center: Marta Christov*, Jennifer Griffiths, Sanjeev Gupta, Aromma Kapoor
Yale School of Medicine: Perry Wilson,* Tanima Arora, Ugochukwu Ugwuowo
*Site Principal Investigator
Footnotes
Drs. Leaf and Srivastava contributed equally to this work.
A full list of STOP-COVID investigators is provided in the Appendix.
Dr. Srivastava reports personal fees from Horizon Therapeutics, PLC, AstraZeneca, Bayer, CVS Caremark, and Tate & Latham (medicolegal consulting). Dr. Gupta receives research funding from GE HealthCare and BTG International. Ms. Cho received research funding from Northwestern University’s Summer Internship Grant Program (SIGP). Dr. Gupta is supported by National Institute of Health (NIH) grant K23DK125672. Dr. Mehta is supported by the NIH grant K23HL150236. Dr. Leaf is supported by NIH grants R01HL144566 and R01DK125786. Dr. Srivastava is supported by NIH grant K23DK120811 and core resources from the George M. O’Brien Kidney Research Center at Northwestern University (NU-GoKIDNEY) P30DK114857. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Contributor Information
Collaborators: Carl P. Walther, Samaya J. Anumudu, Justin Arunthamakun, Kathleen F. Kopecky, Gregory P. Milligan, Peter A. McCullough, Thuy-Duyen Nguyen, Shahzad Shaefi, Megan L. Krajewski, Sidharth Shankar, Ameeka Pannu, Juan D. Valencia, Sushrut S. Waikar, Zoe A. Kibbelaar, Ambarish M. Athavale, Peter Hart, Shristi Upadhyay, Ishaan Vohra, Ajiboye Oyintayo, Adam Green, Jean-Sebastien Rachoin, Christa A. Schorr, Lisa Shea, Daniel L. Edmonston, Christopher L. Mosher, Alexandre M. Shehata, Zaza Cohen, Valerie Allusson, Gabriela Bambrick-Santoyo, Noor ul aain Bhatti, Bijal Mehta, Aquino Williams, Samantha K. Brenner, Patricia Walters, Ronaldo C. Go, Keith M. Rose, Miguel A. Hernán, Amy M. Zhou, Ethan C. Kim, Rebecca Lisk, Lili Chan, Kusum S. Mathews, Steven G. Coca, Deena R. Altman, Aparna Saha, Howard Soh, Huei Hsun Wen, Sonali Bose, Emily A. Leven, Jing G. Wang, Gohar Mosoyan, Girish N. Nadkarni, Pattharawin Pattharanitima, Emily J. Gallagher, Allon N. Friedman, John Guirguis, Rajat Kapoor, Christopher Meshberger, Katherine J. Kelly, Chirag R. Parikh, Brian T. Garibaldi, Celia P. Corona-Villalobos, Yumeng Wen, Steven Menez, Rubab F. Malik, Carmen Elena Cervantes, Samir C. Gautam, Mary C. Mallappallil, Jie Ouyang, Sabu John, Ernie Yap, Yohannes Melaku, Ibrahim Mohamed, Siddhartha Bajracharya, Isha Puri, Mariah Thaxton, Jyotsna Bhattacharya, John Wagner, Leon Boudourakis, H. Bryant Nguyen, Afshin Ahoubim, Leslie F. Thomas, Dheeraj Reddy Sirganagari, Pramod K. Guru, Kianoush Kashani, Shahrzad Tehranian, Yan Zhou, Paul A. Bergl, Jesus Rodriguez, Jatan A. Shah, Mrigank S. Gupta, Princy N. Kumar, Deepa G. Lazarous, Seble G. Kassaye, Michal L. Melamed, Tanya S. Johns, Ryan Mocerino, Kalyan Prudhvi, Denzel Zhu, Rebecca V. Levy, Yorg Azzi, Molly Fisher, Milagros Yunes, Kaltrina Sedaliu, Ladan Golestaneh, Maureen Brogan, Neelja Kumar, Michael Chang, Jyotsana Thakkar, Ritesh Raichoudhury, Akshay Athreya, Mohamed Farag, Edward J. Schenck, Soo Jung Cho, Maria Plataki, Sergio L. Alvarez-Mulett, Luis G. Gomez-Escobar, Di Pan, Stefi Lee, Jamuna Krishnan, William Whalen, David Charytan, Ashley Macina, Sobaata Chaudhry, Benjamin Wu, Frank Modersitzki, Anand Srivastava, Alexander S. Leidner, Carlos Martinez, Jacqueline M. Kruser, Richard G. Wunderink, Alexander J. Hodakowski, Juan Carlos Q. Velez, Eboni G. Price-Haywood, Luis A. Matute-Trochez, Anna E. Hasty, Muner MB. Mohamed, Rupali S. Avasare, David Zonies, David E. Leaf, Shruti Gupta, Meghan E. Sise, Erik T. Newman, Samah Abu Omar, Kapil K. Pokharel, Shreyak Sharma, Harkarandeep Singh, Simon Correa, Tanveer Shaukat, Omer Kamal, Wei Wang, Heather Yang, Jeffery O. Boateng, Meghan Lee, Ian A. Strohbehn, Jiahua Li, Ariel L. Mueller, Roberta E. Redfern, Nicholas S. Cairl, Gabriel Naimy, Abeer Abu-Saif, Danyell Hall, Laura Bickley, Chris Rowan, Farah Madhani-Lovely, Vivian S. Cruz, Kristen M. Hess, Alanna L. Jacobs, Vasil Peev, Jochen Reiser, John J. Byun, Andrew Vissing, Esha M. Kapania, Zoe Post, Nilam P. Patel, Joy-Marie Hermes, Anne K. Sutherland, Amee Patrawalla, Diana G. Finkel, Barbara A. Danek, Sowminya Arikapudi, Jeffrey M. Paer, Peter Cangialosi, Mark Liotta, Jared Radbel, Sonika Puri, Jag Sunderram, Matthew T. Scharf, Ayesha Ahmed, Ilya Berim, Jayanth S. Vatson, Shuchi Anand, Joseph E. Levitt, Pablo Garcia, Suzanne M. Boyle, Rui Song, Jingjing Zhang, Sang Hoon Woo, Xiaoying Deng, Goni Katz-Greenberg, Katharine Senter, Moh’d A. Sharshir, Vadym V. Rusnak, Muhammad Imran Ali, Anip Bansal, Amber S. Podoll, Michel Chonchol, Sunita Sharma, Ellen L. Burnham, David J. Douin, Arash Rashidi, Rana Hejal, Eric Judd, Laura Latta, Ashita Tolwani, Timothy E. Albertson, Jason Y. Adams, Steven Y. Chang, Rebecca M. Beutler, Carl E. Schulze, Etienne Macedo, Harin Rhee, Kathleen D. Liu, Vasantha K. Jotwani, Jay L. Koyner, Chintan V. Shah, Vishal Jaikaransingh, Stephanie M. Toth-Manikowski, Min J. Joo, James P. Lash, Javier A. Neyra, Nourhan Chaaban, Madona Elias, Yahya Ahmad, Alfredo Iardino, Elizabeth H. Au, Jill H. Sharma, Marie Anne Sosa, Sabrina Taldone, Gabriel Contreras, David De La Zerda, Hayley B. Gershengorn, Bhavarth Shukla, Alessia Fornoni, Tanira Ferreira, Salim S. Hayek, Pennelope Blakely, Hanna Berlin, Tariq U. Azam, Husam Shadid, Michael Pan, Patrick O’ Hayer, Chelsea Meloche, Rafey Feroze, Rayan Kaakati, Danny Perry, Abbas Bitar, Elizabeth Anderson, Kishan J. Padalia, John P. Donnelly, Andrew J. Admon, Jennifer E. Flythe, Matthew J. Tugman, Emily H. Chang, Brent R. Brown, Amanda K. Leonberg-Yoo, Ryan C. Spiardi, Todd A. Miano, Meaghan S. Roche, Charles R. Vasquez, Amar D. Bansal, Natalie C. Ernecoff, Sanjana Kapoor, Siddharth Verma, Huiwen Chen, Csaba P. Kovesdy, Miklos Z. Molnar, Ambreen Azhar, S. Susan Hedayati, Mridula V. Nadamuni, Shani Shastri, Duwayne L. Willett, Samuel A.P. Short, Amanda D. Renaghan, Kyle B. Enfield, Pavan K. Bhatraju, A. Bilal Malik, Matthew W. Semler, Anitha Vijayan, Christina Mariyam Joy, Tingting Li, Seth Goldberg, Patricia F. Kao, Greg L. Schumaker, Nitender Goyal, Anthony J. Faugno, Greg L. Schumaker, Caroline M. Hsu, Asma Tariq, Leah Meyer, Ravi K. Kshirsagar, Daniel E. Weiner, Aju Jose, Marta Christov, Jennifer Griffiths, Sanjeev Gupta, Aromma Kapoor, Perry Wilson, Tanima Arora, and Ugochukwu Ugwuowo
REFERENCES
- 1.Dong E, Du H, Gardner L: An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020; 20:533–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williamson EJ, Walker AJ, Bhaskaran K, et al. : Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584:430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Greco M, Zanella A, et al. ; COVID-19 Lombardy ICU Network: Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med 2020; 180:1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim L, Garg S, O’Halloran A, et al. : Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET). Clin Infect Dis 2021; 72:e206–e214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yan Y, Yang Y, Wang F, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care 2020;8:e001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch JS, Ng JH, Ross DW, et al. ; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium: Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 2020; 98:209–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cummings MJ, Baldwin MR, Abrams D, et al. : Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet 2020; 395:1763–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou F, Yu T, Du R, et al. : Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020; 395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson S, Hirsch JS, Narasimhan M, et al. ; the Northwell COVID-19 Research Consortium: Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA 2020; 323:2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rashedi J, Mahdavi Poor B, Asgharzadeh V, et al. : Risk factors for COVID-19. Infez Med 2020; 28:469–474 [PubMed] [Google Scholar]
- 11.Ko JY, Danielson ML, Town M, et al. ; COVID-NET Surveillance Team: Risk factors for coronavirus disease 2019 (COVID-19)-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis 2021; 72:e695–e703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenthal N, Cao Z, Gundrum J, et al. : Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open 2020; 3:e2029058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pijls BG, Jolani S, Atherley A, et al. : Demographic risk factors for COVID-19 infection, severity, ICU admission and death: A meta-analysis of 59 studies. BMJ Open 2021; 11:e044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramos-Rincon JM, Buonaiuto V, Ricci M, et al. ; SEMI-COVID-19 Network: Clinical characteristics and risk factors for mortality in very old patients hospitalized with COVID-19 in Spain. J Gerontol A Biol Sci Med Sci 2021; 76:e28–e37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bellan M, Patti G, Hayden E, et al. : Fatality rate and predictors of mortality in an Italian cohort of hospitalized COVID-19 patients. Sci Rep 2020; 10:20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gupta S, Hayek SS, Wang W, et al. ; STOP-COVID Investigators: Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med 2020; 180:1436–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med 2021;47:60–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hewitt J, Carter B, Vilches-Moraga A, et al. ; COPE Study Collaborators: The effect of frailty on survival in patients with COVID-19 (COPE): A multicentre, European, observational cohort study. Lancet Public Health 2020; 5:e444–e451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sablerolles RSG, Lafeber M, van Kempen JAL, et al. ; COMET research team: Association between Clinical Frailty Scale score and hospital mortality in adult patients with COVID-19 (COMET): An international, multicentre, retrospective, observational cohort study. Lancet Healthy Longev 2021; 2:e163–e170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ho FK, Petermann-Rocha F, Gray SR, et al. : Is older age associated with COVID-19 mortality in the absence of other risk factors? General population cohort study of 470,034 participants. PLoS One 2020; 15:e0241824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris PA, Taylor R, Thielke R, et al. : Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincent JL, Moreno R, Takala J, et al. : The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22:707–710 [DOI] [PubMed] [Google Scholar]
- 23.Orsini N, Li R, Wolk A, et al. : Meta-analysis for linear and nonlinear dose-response relations: Examples, an evaluation of approximations, and software. Am J Epidemiol 2012; 175:66–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desquilbet L, Mariotti F: Dose-response analyses using restricted cubic spline functions in public health research. Stat Med 2010; 29:1037–1057 [DOI] [PubMed] [Google Scholar]
- 25.VanderWeele TJ, Ding P: Sensitivity analysis in observational research: Introducing the E-value. Ann Intern Med 2017; 167:268–274 [DOI] [PubMed] [Google Scholar]
- 26.Raghunathan T, Solenberger P, Van Hoewyk J. IVEware: Imputation and Variance Estimation Software, Ann Arbor, MI, University of Michigan, Institute for Social Research, Survey Research Center, 2000 [Google Scholar]
- 27.Raghunathan T, Lepkowski J, Van Hoewyk J. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol 2001; 27:85–95 [Google Scholar]
- 28.Rubin D: Multiple Imputation for Nonresponse in Surveys. New York, John Wiley and Sons, 2004 [Google Scholar]
- 29.See how age and illnesses change the risk of dying from covid-19. The Economist 2021, 2021. Available at: https://www.economist.com/graphic-detail/covid-pandemic-mortality-risk-estimator?. Accessed September 1, 2021
- 30.Kumari R, Jat P: Mechanisms of cellular senescence: Cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol 2021; 9:645593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franceschi C, Garagnani P, Parini P, et al. : Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 2018; 14:576–590 [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Sanoff HK, Cho H, et al. : Expression of p16(INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell 2009; 8:439–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adrover JM, Nicolás-Ávila JA, Hidalgo A: Aging: A temporal dimension for neutrophils. Trends Immunol 2016; 37:334–345 [DOI] [PubMed] [Google Scholar]
- 34.Frasca D, Romero M, Diaz A, et al. : A molecular mechanism for TNF-α-mediated downregulation of B cell responses. J Immunol 2012; 188:279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bellia V, Pistelli R, Catalano F, et al. : Quality control of spirometry in the elderly. The SA.R.A. study. Salute Respiration nell’Anziano = Respiratory Health in the Elderly. Am J Respir Crit Care Med 2000; 161:1094–1100 [DOI] [PubMed] [Google Scholar]
- 36.DeSantis CE, Miller KD, Dale W, et al. : Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J Clin 2019; 69:452–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hancock HC, Close H, Mason JM, et al. : High prevalence of undetected heart failure in long-term care residents: Findings from the Heart Failure in Care Homes (HFinCH) study. Eur J Heart Fail 2013; 15:158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baum SA, Rubenstein LZ: Old people in the emergency room: Age-related differences in emergency department use and care. J Am Geriatr Soc 1987; 35:398–404 [DOI] [PubMed] [Google Scholar]
- 39.Fitzpatrick AL, Powe NR, Cooper LS, et al. : Barriers to health care access among the elderly and who perceives them. Am J Public Health 2004; 94:1788–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valley TS, Sjoding MW, Ryan AM, et al. : Association of intensive care unit admission with mortality among older patients with pneumonia. JAMA 2015; 314:1272–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fitzpatrick AL, Powe NR, Cooper LS, et al. : Barriers to health care access among the elderly and who perceives them. Am J Public Health 2004; 94:1788–1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Churpek MM, Gupta S, Spicer AB, et al. Hospital-level variation in death for critically ill patients with COVID-19. Am J Respir Crit Care Med 2021;204:403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.French G, Hulse M, Nguyen D, et al. : Impact of hospital strain on excess deaths during the COVID-19 pandemic - United States, July 2020-July 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1613–1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Montgomery C, Stelfox H, Norris C, et al. : Association between preoperative frailty and outcomes among adults undergoing cardiac surgery: A prospective cohort study. CMAJ Open 2021; 9:E777–E787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferrante LE, Murphy TE, Leo-Summers LS, et al. : The combined effects of frailty and cognitive impairment on post-ICU disability among older ICU survivors. Am J Respir Crit Care Med 2019; 200:107–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brummel NE, Bell SP, Girard TD, et al. : Frailty and subsequent disability and mortality among patients with critical illness. Am J Respir Crit Care Med 2017; 196:64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hope AA, Hsieh SJ, Petti A, et al. : Assessing the usefulness and validity of frailty markers in critically ill adults. Ann Am Thorac Soc 2017; 14:952–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zampieri FG, Iwashyna TJ, Viglianti EM, et al. ; ORCHESTRA Study Investigators: Association of frailty with short-term outcomes, organ support and resource use in critically ill patients. Intensive Care Med 2018; 44:1512–1520 [DOI] [PubMed] [Google Scholar]
- 49.Short SAP, Gupta S, Brenner SK, et al. ; STOP-COVID Investigators: D-dimer and death in critically ill patients with coronavirus disease 2019. Crit Care Med 2021; 49:e500–e511 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.