PURPOSE
Combining standard of care (pertuzumab-trastuzumab [PH], chemotherapy) with cancer immunotherapy may potentiate antitumor immunity, cytotoxic activity, and patient outcomes in high-risk, human epidermal growth factor receptor 2 (HER2)–positive early breast cancer. We report the phase III IMpassion050 primary analysis of neoadjuvant atezolizumab, PH, and chemotherapy in these patients.
METHODS
Patients with a primary tumor of > 2 cm and histologically confirmed, positive lymph node status (T2-4, N1-3, M0) were randomly assigned 1:1 to atezolizumab/placebo with dose-dense doxorubicin/cyclophosphamide, followed by paclitaxel, and PH. After surgery, patients were to continue atezolizumab/placebo and PH (total: 1 year of HER2-targeted therapy); those with residual disease could switch to ado-trastuzumab emtansine with atezolizumab/placebo. Coprimary efficacy end points were pathologic complete response (pCR; ypT0/is ypN0) rates in intention-to-treat (ITT) and programmed cell death-ligand 1 (PD-L1)–positive populations.
RESULTS
At clinical cutoff (February 5, 2021), pCR rates in the placebo and atezolizumab groups in the ITT populations were 62.7% (n = 143/228) and 62.4% (n = 141/226), respectively (difference –0.33%; 95% CI, –9.2 to 8.6; P = .9551). The pCR rates in the placebo and atezolizumab groups in patients with PD-L1–positive tumors were 72.5% (n = 79/109) and 64.2% (n = 70/109), respectively (difference –8.26%; 95% CI, –20.6 to 4.0; P = .1846). Grade 3-4 and serious adverse events were more frequent in the atezolizumab versus placebo group. Five grade 5 adverse events occurred (four neoadjuvant, one adjuvant; two assigned to study treatment), all with atezolizumab. Overall, the safety profile was consistent with that of atezolizumab in other combination studies.
CONCLUSION
Atezolizumab with neoadjuvant dose-dense doxorubicin/cyclophosphamide–paclitaxel and PH for high-risk, HER2-positive early breast cancer did not increase pCR rates versus placebo in the ITT or PD-L1–positive populations. PH and chemotherapy remains standard of care; longer follow-up may help to inform the long-term impact of atezolizumab.
INTRODUCTION
Human epidermal growth factor receptor 2 (HER2), overexpressed in approximately 15%-20% of breast carcinomas, conferred a more aggressive phenotype and poorer patient outcomes.1,2 However, the HER2-targeted therapies pertuzumab and trastuzumab (PH) have substantially improved patient prognosis in early and metastatic disease.3,4 In high-risk (tumor > 2 cm/node-positive), HER2-positive early breast cancer (EBC), neoadjuvant/adjuvant PH (total of 1 year) and chemotherapy is standard of care (SOC).5-9 Pathologic complete response (pCR) is associated with significantly improved long-term outcomes in HER2-positive breast cancer (BC).10,11 Patients with residual disease in the breast and/or axilla or at an initially advanced, clinical stage are at increased risk of recurrence or death.10 For patients with residual disease following neoadjuvant therapy, ado-trastuzumab emtansine is approved on the basis of KATHERINE (ClinicalTrials.gov identifier: NCT01772472).5-9 However, despite the use of ado-trastuzumab emtansine in patients with residual disease, > 10% experience relapse after approximately 3.5 years, emphasizing the need to improve pCR rates and outcomes for high-risk, HER2-positive EBC.9
CONTEXT
Key Objective
Can addition of atezolizumab to neoadjuvant standard of care (pertuzumab and trastuzumab [PH], and chemotherapy) improve outcomes in high-risk, human epidermal growth factor receptor 2–positive early breast cancer? To our knowledge, IMpassion050 was the first study to assess this question.
Knowledge Generated
Compared with placebo plus PH and chemotherapy, atezolizumab plus PH and chemotherapy was not superior with regards to pathologic complete response rate, both in the intention-to-treat and programmed cell death-ligand 1–positive population. The overall safety profile was consistent with that observed in other combination studies of atezolizumab.
Relevance
These findings highlight the validity of PH and chemotherapy in human epidermal growth factor receptor 2–positive early breast cancer, but longer follow-up of IMpassion050 is required to inform the long-term role of cancer immunotherapy, such as atezolizumab, in this setting.
Spearheading the antitumor responses of the adaptive immune system,12 tumor-infiltrating lymphocytes (TILs) and activated T cells have been associated with a better prognosis in HER2-positive EBC.13-15 Furthermore, long-term efficacy of dual HER2-blockade suggests that the immune system contributes substantially to the therapeutic effects of monoclonal antibodies,4,16 with preclinical proof of concept that combining HER2-targeted therapy with cancer immunotherapy may result in greater efficacy.17-19
Programmed cell death-ligand 1 (PD-L1) is an immune checkpoint protein that can downregulate the antitumor immune response by impairing T-cell function.12 Atezolizumab, a monoclonal antibody that selectively targets PD-L1 to prevent interaction with its receptors programmed death-1 and B7.1,20 is approved for the treatment of various solid tumors.
IMpassion050 (ClinicalTrials.gov identifier: NCT03726879), a double-blind, randomized, placebo-controlled study, evaluated the efficacy and safety of neoadjuvant atezolizumab/placebo, in combination with PH and chemotherapy, for high-risk, HER2-positive EBC. We report the primary analysis.
METHODS
Study Design and Patients
Patients were ≥ 18 years old with a primary tumor of > 2 cm and histologically confirmed, positive lymph node status (T2-4, N1-3, M0), an Eastern Cooperative Oncology Group performance status of 0/1, and a left ventricular ejection fraction of ≥ 55%. Key exclusion criteria were prior history of invasive BC, stage IV BC, prior systemic therapy for BC, or prior anthracyclines or taxanes for any malignancy. HER2-positivity, PD-L1 status, hormone receptor status, and PIK3CA mutation status were assessed centrally.
Eligible patients were randomly assigned 1:1 using a permuted-block method to receive intravenous (IV) atezolizumab or placebo, with neoadjuvant dose-dense doxorubicin and cyclophosphamide, followed by paclitaxel and PH (ddAC-PacPH; Data Supplement, online only). Random assignment was stratified by tumor stage at diagnosis (T2 v T3-4), hormone receptor status (estrogen receptor–positive and/or progesterone receptor–positive v estrogen receptor–/progesterone receptor–negative; enrollment of patients with hormone receptor–positive disease was capped at 50%), and PD-L1 status (PD-L1–stained tumor-infiltrating immune cells [IC] covering ≥ 1% of the tumor area [IC 1/2/3] v < 1% [IC 0]).
On June 4, 2019, the Protocol (online only) was amended to be powered for the primary end point of pCR in the PD-L1–positive population, in addition to the intention-to-treat (ITT) population, because of the potential predictive value of PD-L1 expression for clinical benefit with atezolizumab.21,22 The target sample size was thus increased from 224 to 453 patients.
Study Oversight
IMpassion050 was designed by the senior academic authors and representatives of the sponsor (F. Hoffmann-La Roche Ltd, Basel, Switzerland). Data were collected by the sponsor and analyzed in collaboration with the senior academic authors, who vouched for the completeness and accuracy of the data and analyses, and for the fidelity of the study to the protocol. IMpassion050 was performed in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Protocol approval was obtained from an independent ethics committee for every site. Every patient provided written informed consent.
Study Procedures
During the neoadjuvant phase, patients received, once every 2 weeks for four cycles, IV atezolizumab 840 mg or placebo, and IV doxorubicin 60 mg/m2 and cyclophosphamide 600 mg/m2 (ddAC) and myeloid growth factor support according to local guidelines. This was followed by four cycles of atezolizumab/placebo (1,200 mg IV once every 3 weeks), paclitaxel (80 mg/m2 IV once weekly), trastuzumab (8 mg/kg IV loading dose, followed by 6 mg/kg IV once every 3 weeks), and pertuzumab (840 mg IV loading dose, followed by 420 mg IV once every 3 weeks). In the adjuvant phase, patients continued atezolizumab/placebo with PH to complete 1 year of HER2-targeted therapy. Patients with residual disease at surgery could switch to ado-trastuzumab emtansine (3.6 mg/kg IV once every 3 weeks for 14 cycles), while maintaining atezolizumab/placebo. After review of unblinded safety and efficacy on January 26, 2021, the independent Data Monitoring Committee (iDMC) recommended stopping randomized atezolizumab/placebo treatment because of an unfavorable benefit-risk profile, with patients continuing SOC through the completion of their adjuvant treatment per study protocol. To reflect this, the protocol and informed consent form were subsequently amended (see the Data Supplement for major changes to the protocol from version to version).
HER2-positive status was assessed with US Food and Drug Administration–approved tests, either as an immunohistochemistry (IHC) 3+ score (PATHWAY anti-HER2/neu [4B5] assay; Ventana Medical Systems, Inc, Tucson, AZ), or as a HER2 gene amplification (ratio ≥ 2) by in situ hybridization (ISH; INFORM HER2 dual ISH assay; Ventana Medical Systems, Inc). PD-L1 status was assessed by IHC (VENTANA SP142 antibody test; Ventana Medical Systems, Inc). PIK3CA mutation status was assessed using the cobas PIK3CA Mutation Test (Roche Molecular Diagnostics, Pleasanton, CA) and cobas 4800 System (Roche Molecular Diagnostics), as described previously.23
Study End Points
The coprimary end points were pCR rates (ypT0/is ypN0) in the ITT and PD-L1–positive populations. Secondary end points included pCR in patients with PD-L1–negative tumors, event-free survival (EFS), and safety. EFS was defined as the time from random assignment to the first documented disease recurrence, unequivocal tumor progression determined by the treating investigator, or death from any cause, whichever occurred first. Severity of adverse events (AEs) was graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events version 5.0.24
Statistical Analysis
The ITT population comprised all randomly assigned patients, whether or not the assigned study treatment was received. The safety population comprised patients who received any amount of any study drug.
The planned sample size was 453 patients (40% predicted to have PD-L1–positive tumors). In the population with PD-L1–positive tumors, this enabled 80% power to detect a pCR improvement from 65% to 83% in the atezolizumab group at a 4.8% significance level (two-sided), assuming a dropout rate of 7%. Patients with a missing pCR assessment were counted as not achieving a pCR. In the ITT population, the sample size enabled 82.8% power to detect an improvement from 54% to 72% at a 0.2% two-sided significance level, assuming a dropout rate of 10%. pCR treatment comparisons were made using Cochran-Mantel-Haenszel tests, stratified according to the factors used at random assignment. CIs for pCR differences between groups were determined using the normal approximation to the binomial distribution. Hazard ratios for EFS were estimated using a Cox proportional hazards model. A predefined unstratified pCR subgroup analysis was conducted for the ITT and PD-L1–positive populations. Safety analyses were descriptive.
RESULTS
Study Population
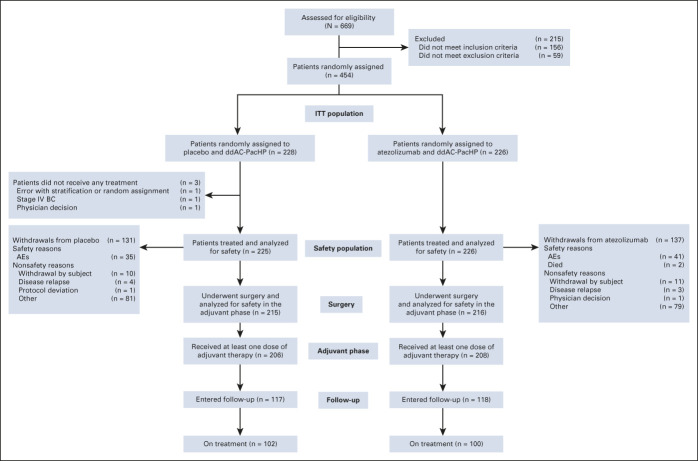
Patients were enrolled from January 2019 to August 2020; 454 patients (228 with placebo and 226 with atezolizumab; Fig 1) were randomly assigned across 73 sites in 12 countries. At clinical cutoff (February 5, 2021), 202 patients were on active treatment (44.5%), 228 were in follow-up (50.2%), 24 had discontinued from the study (5.3%), and three were yet to undergo surgery (Fig 1). Median duration of follow-up was 15.9 (placebo) and 15.7 (atezolizumab) months.
FIG 1.
CONSORT diagram. AE, adverse event; BC, breast cancer; ddAC, dose-dense doxorubicin and cyclophosphamide; H, trastuzumab; ITT, intention-to-treat; IV, intravenous; P, pertuzumab; Pac, paclitaxel.
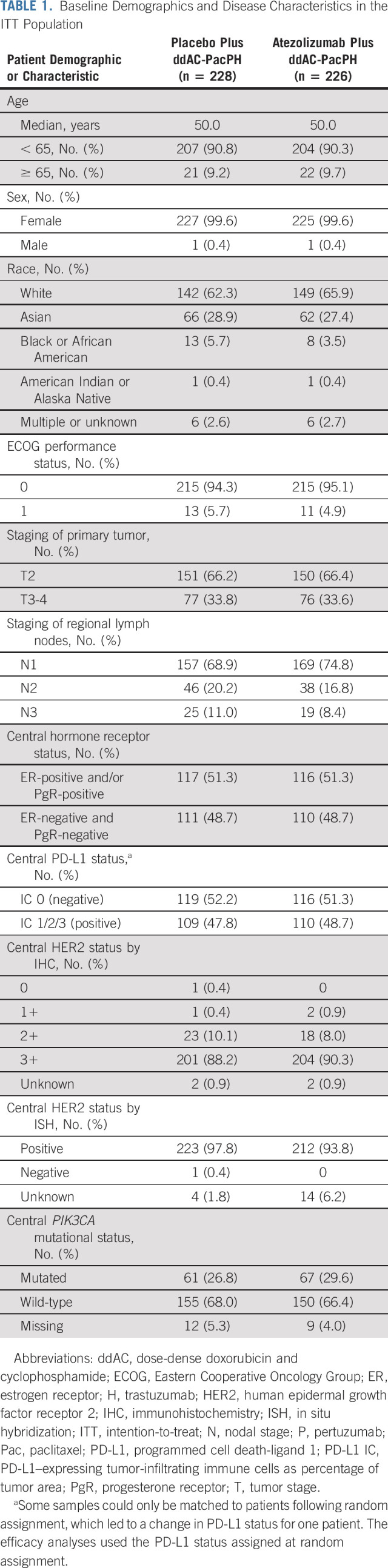
Baseline demographics and disease characteristics were balanced between groups (Table 1).
TABLE 1.
Baseline Demographics and Disease Characteristics in the ITT Population
Efficacy
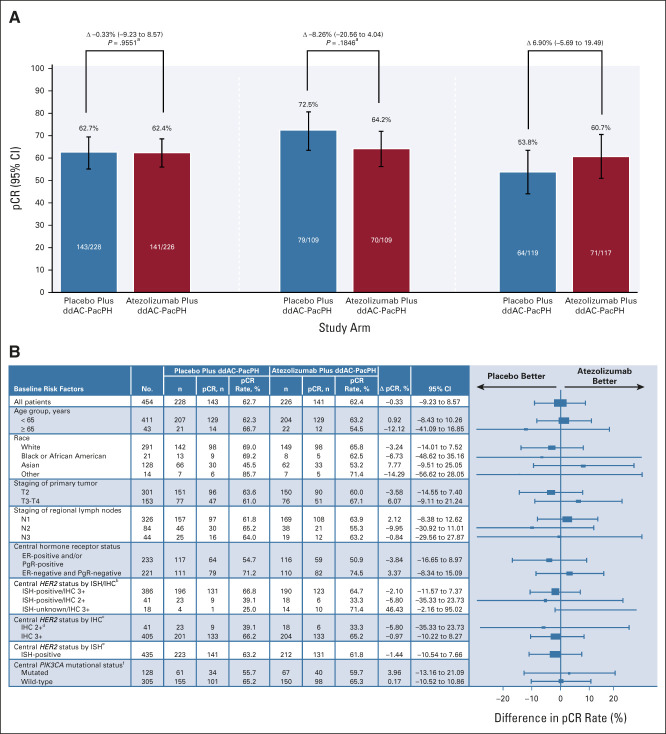
pCR rates in the ITT population were 62.7% (n = 143/228) with placebo and 62.4% (n = 141/226) with atezolizumab (difference –0.33%; 95% CI, –9.23 to 8.57; P = .9551; Fig 2A). pCR rates in the PD-L1–positive population were 72.5% (n = 79/109) and 64.2% (n = 70/109) with placebo and atezolizumab, respectively (difference –8.26%; 95% CI, –20.56 to 4.04; P = .1846; Fig 2A). The results were consistent across subgroups both in the ITT (Fig 2B) and PD-L1–positive (Data Supplement) populations, on the basis of age, race, tumor and nodal staging, and biomarkers, including central HER2, hormone receptor, and PIK3CA mutational status. In patients with PD-L1–negative tumors (secondary end point), pCR rates were 53.8% (n = 64/119) with placebo and 60.7% (n = 71/117) with atezolizumab (difference 6.90%; 95% CI, –5.69 to 19.49; Fig 2A).
FIG 2.
(A) pCR in the ITT (primary end point), PD-L1–positive (primary end point), and PD-L1–negative (secondary end point) populations, and (B) pCR in subgroups of the ITT population. aStratified (Cochran-Mantel-Haenszel test). bISH-positive/IHC 0/1+: number of pCR events in placebo/atezolizumab: n = 0/2 versus n = 0/2. ISH-positive/IHC unknown: n = 1/2 versus n = 2/2. ISH-negative/IHC 3+: n = 1/1 versus n = 0/0. cIHC 0/1+: number of pCR events in placebo/atezolizumab: n = 0/2 versus n = 0/2. IHC unknown: n = 1/2 versus n = 2/2. dPatients whose tumor test results were IHC 2+ had ISH-positive status. eISH-negative: number of pCR events in placebo/atezolizumab: n = 1/1 versus n = 0/0. ISH-unknown: n = 1/4 versus n = 10/14. fPIK3CA missing: number of pCR events in placebo/atezolizumab: n = 14/21 versus n = 11/20. ddAC, dose-dense doxorubicin and cyclophosphamide; ER, estrogen receptor; H, trastuzumab; IHC, immunohistochemistry; ISH, in situ hybridization; ITT, intention-to-treat; N, nodal stage; NE, not estimable; P, pertuzumab; Pac, paclitaxel; pCR, pathologic complete response (ypT0/is ypN0); PD-L1, programmed cell death-ligand 1; PgR, progesterone receptor; T, tumor stage.
Seven patients (3.1%) in the placebo group had an EFS event compared with 12 (5.3%) in the atezolizumab group (Data Supplement; P = .2084); median EFS was not estimable in either group. Overall, 13/19 (68.4%) events were disease recurrences, five were fatal AEs, and one was death due to gastric cancer. There was no disease progression during neoadjuvant treatment.
Safety
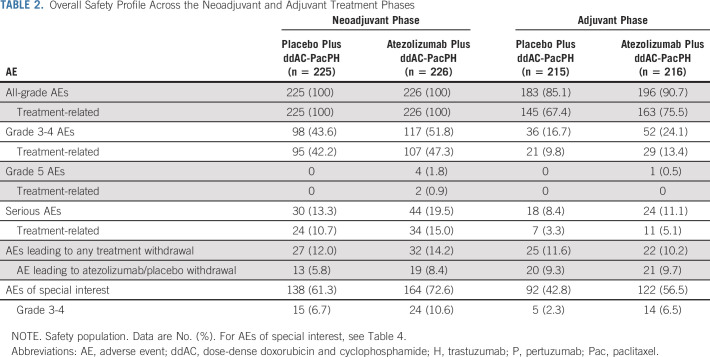
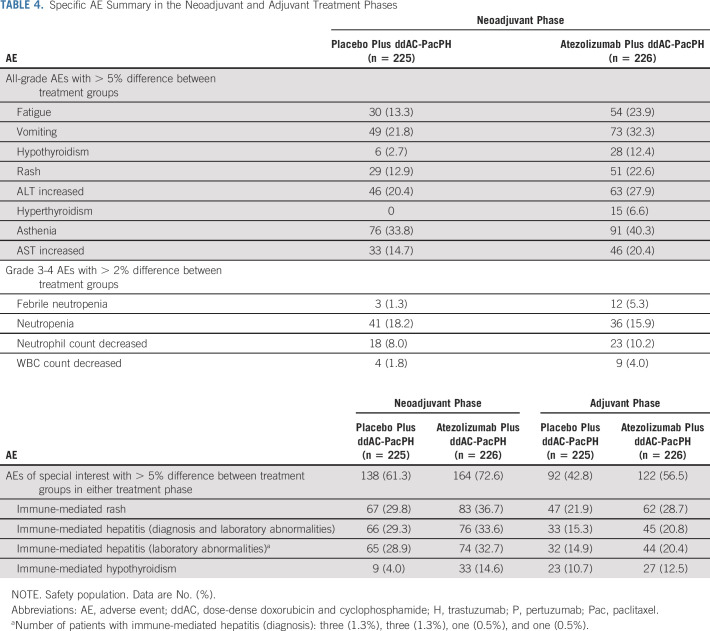
In the neoadjuvant phase, exposure to study drugs did not differ between groups (Data Supplement). The overall incidence of serious AEs, grade 3-4 AEs, and AEs of special interest (AESIs) for atezolizumab was increased in the atezolizumab compared with the placebo group (Table 2). There were similar rates of AEs leading to any study treatment withdrawal between groups (Table 2). Grade 5 AEs were imbalanced in the neoadjuvant phase, with four in the atezolizumab group (alveolitis, septic shock, sepsis, and COVID-19) versus none in the placebo group; two of these fatal AEs (alveolitis and septic shock) were attributed by the investigator to study treatment (Table 3). The grade 5 alveolitis, related to ddAC and atezolizumab, occurred in an 81-year-old patient admitted to hospital because of a traumatic vertebral fracture complicated by pneumonia with suspected pulmonary metastasis. The grade 5 septic shock, related to paclitaxel, PH, and atezolizumab, occurred in a 61-year-old patient with type 2 diabetes and urinary tract infection, aggravated by severe neutropenia. Sepsis also occurred in a 69-year-old patient, caused by relapsed anal fistula leading to perineal ulceration and vulvar infection (in the absence of severe neutropenia). The most common AEs with > 5% difference between treatment groups were fatigue and vomiting (Table 4). The most common grade 3-4 AEs with > 2% difference between treatment groups were febrile neutropenia and neutropenia (Table 4). The most common AESIs with > 5% difference between treatment groups were immune-mediated rash and hepatitis (Table 4). The most common treatment-related AEs with > 10% incidence in either treatment group were diarrhea and nausea (Data Supplement).
TABLE 2.
Overall Safety Profile Across the Neoadjuvant and Adjuvant Treatment Phases
TABLE 3.
Deaths Reported Across the Neoadjuvant and Adjuvant Treatment Phases
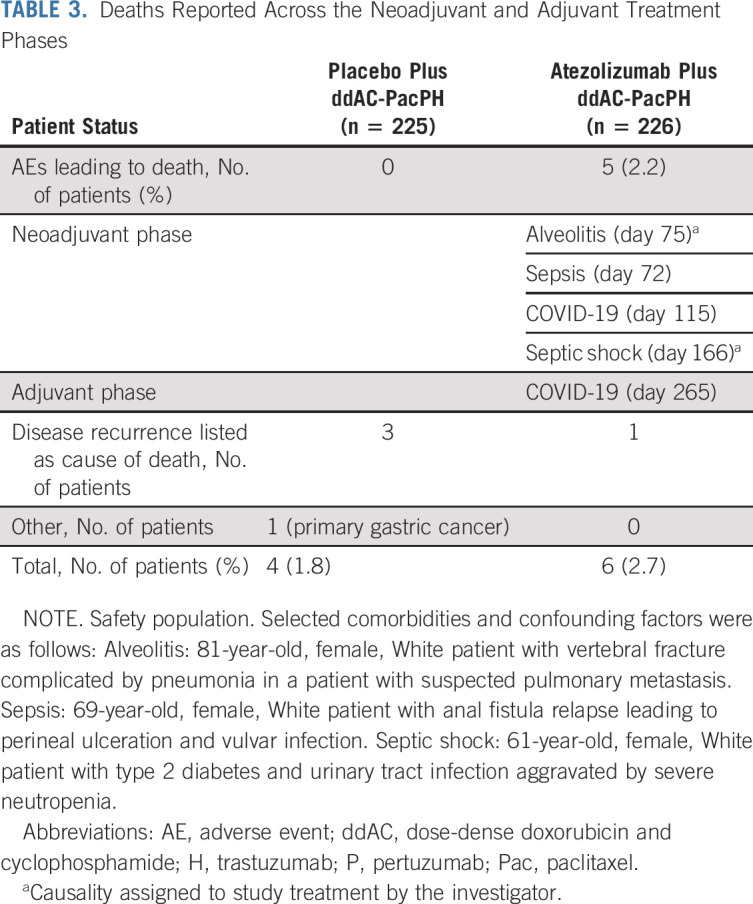
TABLE 4.
Specific AE Summary in the Neoadjuvant and Adjuvant Treatment Phases
Fifty-five and 58 patients with residual disease in the placebo and atezolizumab groups, respectively, switched adjuvant treatment to ado-trastuzumab emtansine with atezolizumab/placebo (Data Supplement). In the adjuvant phase, the mean dose intensity for ado-trastuzumab emtansine was similar between the placebo and atezolizumab groups (96.5% and 96.0%, respectively; Data Supplement). The atezolizumab and ado-trastuzumab emtansine combination had a similar incidence of serious AEs, grade 3-4 AEs, and AEs leading to any treatment withdrawal, compared with atezolizumab and PH or placebo and ado-trastuzumab emtansine (Data Supplement). AESIs were increased in patients who received atezolizumab and ado-trastuzumab emtansine, compared with other groups, although grade 3-4 AESIs occurred with a similar incidence (Data Supplement). There was one grade 5 AE in the adjuvant phase (COVID-19 in the atezolizumab and PH group, not attributed by the investigator to study treatment; Tables 2 and 3). Therefore, across both treatment phases, five grade 5 AEs occurred in the atezolizumab group versus zero in the placebo group.
Both fatal COVID-19 events occurred in the atezolizumab group; any-grade and grade 3-4 COVID-19 infections occurred with a similar incidence in the placebo and atezolizumab groups, in the neoadjuvant (any-grade: n = 5/225 [2.2%] v 2/226 [0.9%]; grade 3-4: 0 v 0) and adjuvant (6/215 [2.8%] v 5/216 [2.3%]; 0 v 0) phases.
DISCUSSION
To our knowledge, IMpassion050 is the first phase III study to report data on cancer immunotherapy in HER2-positive EBC. pCR rates with PH and chemotherapy were high and in accordance with study expectations; however, addition of atezolizumab did not increase pCR rates versus placebo in the ITT or PD-L1–positive populations. When the iDMC recommended stopping randomized atezolizumab/placebo treatment because of an unfavorable benefit-risk profile, only three patients were yet to undergo surgery at clinical cutoff (February 5, 2021). Thus, the assessment of the coprimary end point of pCR in the ITT and PD-L1–positive populations was not affected by the iDMC recommendation.
Preclinical data provide a strong rationale for combining cancer immunotherapy with HER2-targeted therapy in HER2-positive BC.17-19 The lack of pCR improvement with atezolizumab in IMpassion050 is surprising, given the expected greater benefit of cancer immunotherapy in EBC compared with the advanced setting, because of a lower tumor burden, reduced immune escape mechanisms, and a more efficient immune system in patients with EBC.25 Treatment exposure is unlikely to explain the lack of pCR benefit seen with atezolizumab, as exposure to chemotherapy/HER2-targeted therapy was not compromised by adding atezolizumab. Although achieving a pCR has been associated with significantly improved long-term outcomes (eg, EFS, overall survival [OS]) in patients with HER2-positive EBC receiving neoadjuvant anti–HER2-based therapy,10,11 there may be a long-term impact of cancer immunotherapy even with no pCR improvement, given the time required for cancer immunotherapy to exert an antitumor immune response.26 This was seen in early triple-negative BC (TNBC) in the GeparNuevo study, where the addition of durvalumab to chemotherapy significantly improved long-term outcomes despite a nonsignificant numerical pCR increase.27 Furthermore, KEYNOTE-522 showed a significant improvement in pCR and EFS with neoadjuvant pembrolizumab and chemotherapy, followed by adjuvant pembrolizumab, versus placebo (pCR rates: 64.8% with pembrolizumab v 51.2% with placebo; estimated treatment difference: 13.6%; P = .00055; EFS: hazard ratio = 0.63; P = .00031). At the 36-month time point, this EFS benefit with the addition of pembrolizumab was particularly observed in patients without a pCR (67.4% v 56.8%) versus those who did achieve a pCR (94.4% v 92.5%).28 These results may challenge the surrogacy of pCR as an end point when evaluating the long-term benefit of cancer immunotherapy in EBC and argue for powering studies for long-term survival end points. Ongoing studies in HER2-positive EBC, such as the adjuvant ASTEFANIA (ClinicalTrials.gov identifier: NCT04873362) and neoadjuvant/adjuvant APTneo (ClinicalTrials.gov identifier: NCT03595592) trials, may clarify the role of immunotherapy combined with different HER2-targeted therapies as they are powered for long-term efficacy end points.
Consistent with reports suggesting a prognostic role for PD-L1 expression in BC,28-31 patients with PD-L1–positive tumors in IMpassion050 demonstrated higher pCR rates than those with PD-L1–negative tumors. Conversely, no pCR benefit of adding atezolizumab to PH and chemotherapy was observed in patients with PD-L1–positive tumors. This was surprising given that, in PANACEA, pembrolizumab and trastuzumab showed durable clinical benefit in patients with PD-L1–positive, trastuzumab-resistant, HER2-positive advanced BC; with significantly greater TIL levels in objective responders and those with disease control (despite small sample sizes).32 Furthermore, KATE2 showed a possible survival advantage by increasing PFS and OS with the addition of atezolizumab to ado-trastuzumab emtansine for patients with PD-L1–positive tumors previously treated with a taxane and trastuzumab, although the exploratory nature of this subgroup analysis means confirmation of the results is required.21 Nonetheless, there is increasing evidence from neoadjuvant trials of early TNBC that PD-L1 status may not be a predictor of benefit from checkpoint inhibitor therapy in the early setting, and other additional factors, such as induction of an immune response or enrichment of TILs, may be more important.29,33 In the preliminary results from the NeoTRIP trial of neoadjuvant atezolizumab plus carboplatin/nab-paclitaxel in TNBC, atezolizumab did not significantly increase pCR rate versus placebo in the overall population; atezolizumab increased pCR by > 10% only in immune-rich groups (PD-L1–positive and TIL-high disease).30
Similar to the results observed with the ITT and PD-L1–positive populations in IMpassion050, no other biomarker showing an increased treatment benefit from atezolizumab versus placebo was identified. As observed previously,34-42 patients with tumors either hormone receptor–positive, HER2 IHC 2+, or PIK3CA-mutated tended to have lower pCR rates in IMpassion050, compared with those with hormone receptor-negative, HER2 IHC 3+, or PIK3CA-wild-type tumors, respectively, potentially reflecting lower addiction to the HER2 pathway and/or intrinsic resistance to anti-HER2 therapies. However, it is important to note the small sample size of some of these subgroups, limiting interpretation of the results.
In IMpassion050, the overall safety profile was consistent with the known profile for atezolizumab in combination studies.21,29,43,44 Nonetheless, five fatal AEs occurred (all in the atezolizumab arm), two of which were considered treatment-related by the investigator (alveolitis and septic shock), and two were attributed to COVID-19. This imbalance in deaths with atezolizumab seems to emerge upon combination with an intense cytotoxic regimen, unlike what has been observed in combination with single-agent chemotherapy.43,45,46 This emphasizes the need for careful patient monitoring and AE management. The added toxicity of cancer immunotherapies makes the demonstration of a clear survival advantage paramount, particularly in a curative setting such as EBC. Most AESIs were grade 1-2; however, some, such as immune-related endocrine dysfunctions (eg, hypothyroidism and adrenal insufficiency), are chronic and warrant careful patient selection in a curative setting.
IMpassion050, as a large clinical data set with ongoing correlative analyses with tumor- and immune-related features, may help to identify patients most likely to benefit from cancer immunotherapy in HER2-positive EBC. However, EFS and OS were secondary end points only, and IMpassion050 was not powered for long-term outcomes, which limits full understanding of the long-term impact of atezolizumab in this indication. Follow-up is ongoing and may be hypothesis-generating with regards to the long-term benefit of atezolizumab in EBC.
In conclusion, in the IMpassion050 primary analysis, atezolizumab and neoadjuvant ddAC-PacPH for high-risk, HER2-positive EBC did not increase pCR rates versus placebo in either the ITT or the PD-L1–positive population. Current neoadjuvant SOC for HER2-positive EBC (PH and chemotherapy) remains valid; further data are needed to clarify the role of cancer immunotherapy in this setting.
ACKNOWLEDGMENT
The authors thank the patients and their families, participating study investigators, and clinical sites. Research support in the form of third-party medical writing assistance, including the initial draft, was provided by Stephen Salem, BSc (Health Interactions, Manchester, UK; funded by F. Hoffmann-La Roche Ltd).
Jens Huober
Honoraria: Roche, Lilly, Pfizer, Celgene, MSD Oncology, AstraZeneca, AbbVie, Novartis, Seagen, Daiichi Sankyo/AstraZeneca, Gilead Sciences, Eisai
Consulting or Advisory Role: Novartis, Roche, Pfizer, Lilly, Celgene, AstraZeneca, AbbVie, Eisai, MSD Oncology, Hexal, Daiichi Sankyo/Astra Zeneca
Research Funding: Novartis (Inst), Celgene (Inst), Hexal, Lilly (Inst), Roche
Travel, Accommodations, Expenses: Novartis, Roche, Pfizer, Celgene, Daiichi Sankyo
Uncompensated Relationships: German Breast Group
Carlos H. Barrios
Stock and Other Ownership Interests: MedSIR, Tummi
Honoraria: Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, Eisai, MSD, Lilly, Bayer, AstraZeneca, Zodiac Pharma
Consulting or Advisory Role: Boehringer Ingelheim, Roche/Genentech, Novartis, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Libbs, MSD Oncology, United Medical, Lilly
Research Funding: Pfizer (Inst), Novartis (Inst), Amgen (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Roche/Genentech (Inst), Lilly (Inst), Sanofi (Inst), Taiho Pharmaceutical (Inst), Mylan (Inst), Merrimack (Inst), Merck (Inst), AbbVie (Inst), Astellas Pharma (Inst), Biomarin (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Abraxis BioScience (Inst), AB Science (Inst), Asana Biosciences (Inst), Medivation (Inst), Exelixis (Inst), ImClone Systems (Inst), LEO Pharma (Inst), Millennium (Inst), Janssen (Inst), Clinica Atlantis (Inst), INC Research (Inst), Halozyme (Inst), Covance (Inst), Celgene (Inst), inVentiv Health (Inst), Shanghai Henlius Biotech (Inst), Polyphor (Inst), PharmaMar (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Novartis, Pfizer, BMS Brazil, AstraZeneca, MSD Oncology, Lilly
Naoki Niikura
Honoraria: Chugai Pharma, AstraZeneca, Eisai, Novartis, Daiichi Sankyo/UCB Japan, Nippon Kayaku, Lilly Japan, MSD, Pfizer, Nihon Medi-Physics, Taiho Pharmaceutical, Takeda, Kyowa Kirin
Consulting or Advisory Role: Roche, Pfizer, AstraZeneca, MSD, Daiichi Sankyo
Research Funding: Chugai Pharma, Nihon Medi-Physics, Daiichi Sankyo, Eisai, Pfizer (Inst), Nippon Kayaku (Inst), Kyowa Kirin, Takeda, Lilly Japan (Inst), Roche
Michał Jarząb
Honoraria: Roche, Novartis, Pfizer
Consulting or Advisory Role: Pfizer, Novartis, Lilly, Roche
Speakers' Bureau: Exact Sciences, Roche, Novartis, Pfizer
Research Funding: Roche
Travel, Accommodations, Expenses: Pfizer, Lilly, Roche, Novartis
Yuan-Ching Chang
Research Funding: Roche
Shannon L. Huggins-Puhalla
Consulting or Advisory Role: AbbVie, Roche/Genentech
Research Funding: AbbVie (Inst), Pfizer (Inst), AstraZeneca (Inst)
José Pedrini
Research Funding: Roche
Lyudmila Zhukova
Honoraria: AstraZeneca, Novartis, Lilly
Consulting or Advisory Role: Novartis
Research Funding: Roche
Vilma Graupner
Employment: Roche
Stock and Other Ownership Interests: Roche
Daniel Eiger
Employment: Roche
Stock and Other Ownership Interests: Roche
Research Funding: Novartis
Volkmar Henschel
Employment: Roche
Stock and Other Ownership Interests: Roche
Nino Gochitashvili
Employment: Roche Products Limited
Stock or Other Ownership: Roche
Chiara Lambertini
Employment: Roche
Eleonora Restuccia
Employment: Roche
Stock and Other Ownership Interests: Roche
Hong Zhang
Consulting or Advisory Role: Roche/Genentech
Research Funding: Roche
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the European Society for Medical Oncology Virtual Plenary 2021, June 17-18, 2021.
SUPPORT
The study was sponsored by F. Hoffmann-La Roche Ltd.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked because of a potential increase in risk of patient reidentification.
AUTHOR CONTRIBUTIONS
Conception and design: Jens Huober, Carlos H. Barrios, Naoki Niikura, Shannon L. Huggins-Puhalla, Chiara Lambertini, Hong Zhang
Provision of study materials or patients: Jens Huober, Michał Jarząb, Yuan-Ching Chang, José Pedrini, Lyudmila Zhukova,
Collection and assembly of data: Carlos H. Barrios, Naoki Niikura, Michał Jarząb, Yuan-Ching Chang, José Pedrini, Lyudmila Zhukova, Vilma Graupner, Volkmar Henschel, Nino Gochitashvili, Chiara Lambertini, Hong Zhang
Data analysis and interpretation: Jens Huober, Carlos H. Barrios, Naoki Niikura, Michał Jarząb, Shannon L. Huggins-Puhalla, Vilma Graupner, Daniel Eiger, Volkmar Henschel, Nino Gochitashvili, Chiara Lambertini, Eleonora Restuccia, Hong Zhang
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Atezolizumab With Neoadjuvant Anti–Human Epidermal Growth Factor Receptor 2 Therapy and Chemotherapy in Human Epidermal Growth Factor Receptor 2–Positive Early Breast Cancer: Primary Results of the Randomized Phase III IMpassion050 Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jens Huober
Honoraria: Roche, Lilly, Pfizer, Celgene, MSD Oncology, AstraZeneca, AbbVie, Novartis, Seagen, Daiichi Sankyo/AstraZeneca, Gilead Sciences, Eisai
Consulting or Advisory Role: Novartis, Roche, Pfizer, Lilly, Celgene, AstraZeneca, AbbVie, Eisai, MSD Oncology, Hexal, Daiichi Sankyo/Astra Zeneca
Research Funding: Novartis (Inst), Celgene (Inst), Hexal, Lilly (Inst), Roche
Travel, Accommodations, Expenses: Novartis, Roche, Pfizer, Celgene, Daiichi Sankyo
Uncompensated Relationships: German Breast Group
Carlos H. Barrios
Stock and Other Ownership Interests: MedSIR, Tummi
Honoraria: Novartis, Roche/Genentech, Pfizer, GlaxoSmithKline, Sanofi, Boehringer Ingelheim, Eisai, MSD, Lilly, Bayer, AstraZeneca, Zodiac Pharma
Consulting or Advisory Role: Boehringer Ingelheim, Roche/Genentech, Novartis, GlaxoSmithKline, Eisai, Pfizer, AstraZeneca, Libbs, MSD Oncology, United Medical, Lilly
Research Funding: Pfizer (Inst), Novartis (Inst), Amgen (Inst), AstraZeneca (Inst), Boehringer Ingelheim (Inst), GlaxoSmithKline (Inst), Roche/Genentech (Inst), Lilly (Inst), Sanofi (Inst), Taiho Pharmaceutical (Inst), Mylan (Inst), Merrimack (Inst), Merck (Inst), AbbVie (Inst), Astellas Pharma (Inst), Biomarin (Inst), Bristol Myers Squibb (Inst), Daiichi Sankyo (Inst), Abraxis BioScience (Inst), AB Science (Inst), Asana Biosciences (Inst), Medivation (Inst), Exelixis (Inst), ImClone Systems (Inst), LEO Pharma (Inst), Millennium (Inst), Janssen (Inst), Clinica Atlantis (Inst), INC Research (Inst), Halozyme (Inst), Covance (Inst), Celgene (Inst), inVentiv Health (Inst), Shanghai Henlius Biotech (Inst), Polyphor (Inst), PharmaMar (Inst)
Travel, Accommodations, Expenses: Roche/Genentech, Novartis, Pfizer, BMS Brazil, AstraZeneca, MSD Oncology, Lilly
Naoki Niikura
Honoraria: Chugai Pharma, AstraZeneca, Eisai, Novartis, Daiichi Sankyo/UCB Japan, Nippon Kayaku, Lilly Japan, MSD, Pfizer, Nihon Medi-Physics, Taiho Pharmaceutical, Takeda, Kyowa Kirin
Consulting or Advisory Role: Roche, Pfizer, AstraZeneca, MSD, Daiichi Sankyo
Research Funding: Chugai Pharma, Nihon Medi-Physics, Daiichi Sankyo, Eisai, Pfizer (Inst), Nippon Kayaku (Inst), Kyowa Kirin, Takeda, Lilly Japan (Inst), Roche
Michał Jarząb
Honoraria: Roche, Novartis, Pfizer
Consulting or Advisory Role: Pfizer, Novartis, Lilly, Roche
Speakers' Bureau: Exact Sciences, Roche, Novartis, Pfizer
Research Funding: Roche
Travel, Accommodations, Expenses: Pfizer, Lilly, Roche, Novartis
Yuan-Ching Chang
Research Funding: Roche
Shannon L. Huggins-Puhalla
Consulting or Advisory Role: AbbVie, Roche/Genentech
Research Funding: AbbVie (Inst), Pfizer (Inst), AstraZeneca (Inst)
José Pedrini
Research Funding: Roche
Lyudmila Zhukova
Honoraria: AstraZeneca, Novartis, Lilly
Consulting or Advisory Role: Novartis
Research Funding: Roche
Vilma Graupner
Employment: Roche
Stock and Other Ownership Interests: Roche
Daniel Eiger
Employment: Roche
Stock and Other Ownership Interests: Roche
Research Funding: Novartis
Volkmar Henschel
Employment: Roche
Stock and Other Ownership Interests: Roche
Nino Gochitashvili
Employment: Roche Products Limited
Stock or Other Ownership: Roche
Chiara Lambertini
Employment: Roche
Eleonora Restuccia
Employment: Roche
Stock and Other Ownership Interests: Roche
Hong Zhang
Consulting or Advisory Role: Roche/Genentech
Research Funding: Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Slamon DJ, Clark GM, Wong SG, et al. : Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177-182, 1987 [DOI] [PubMed] [Google Scholar]
- 2.Costa RLB, Czerniecki BJ: Clinical development of immunotherapies for HER2+ breast cancer: A review of HER2-directed monoclonal antibodies and beyond. NPJ Breast Cancer 6:10, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gianni L, Pienkowski T, Im YH, et al. : 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol 17:791-800, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Swain SM, Miles D, Kim SB, et al. : Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): End-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol 21:519-530, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Cardoso F, Kyriakides S, Ohno S, et al. : Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 30:1194-1220, 2019 [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network (NCCN) : NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Breast Cancer. Version 4. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf [Google Scholar]
- 7.Korde LA, Somerfield MR, Carey LA, et al. : Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol 39:1485-1505, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burstein HJ, Curigliano G, Loibl S, et al. : Estimating the benefits of therapy for early stage breast cancer the St Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann Oncol 30:1541-1557, 2019 [DOI] [PubMed] [Google Scholar]
- 9.von Minckwitz G, Huang CS, Mano MS, et al. : Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 380:617-628, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Cortazar P, Zhang L, Untch M, et al. : Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384:164-172, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Spring LM, Fell G, Arfe A, et al. : Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin Cancer Res 26:2838-2848, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldman AD, Fritz JM, Lenardo MJ: A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat Rev Immunol 20:651-668, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denkert C, von Minckwitz G, Darb-Esfahani S, et al. : Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: A pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol 19:40-50, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Ignatiadis M, Van den Eynden G, Roberto S, et al. : Tumor-infiltrating lymphocytes in patients receiving trastuzumab/pertuzumab-based chemotherapy: A TRYPHAENA substudy. J Natl Cancer Inst 111:69-77, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salgado R, Denkert C, Campbell C, et al. : Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: A secondary analysis of the NeoALTTO trial. JAMA Oncol 1:448-454, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccart M, Procter M, Fumagalli D, et al. : Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer in the APHINITY trial: 6 years' follow-up. J Clin Oncol 39:1448-1457, 2021 [DOI] [PubMed] [Google Scholar]
- 17.Stagg J, Loi S, Divisekera U, et al. : Anti–ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti–PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A 108:7142-7147, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagai Y, Tsuchiya H, Runkle EA, et al. : Disabling of the erbB pathway followed by IFN-γ modifies phenotype and enhances genotoxic eradication of breast tumors. Cell Rep 12:2049-2059, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller P, Kreuzaler M, Khan T, et al. : Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med 7:315ra188, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Herbst RS, Soria J-C, Kowanetz M, et al. : Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515:563-567, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emens LA, Esteva FJ, Beresford M, et al. : Trastuzumab emtansine plus atezolizumab versus trastuzumab emtansine plus placebo in previously treated, HER2-positive advanced breast cancer (KATE2): A phase 2, multicentre, randomised, double-blind trial. Lancet Oncol 21:1283-1295, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Schmid P, Adams S, Rugo HS, et al. ,: Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med 379:2108-2121, 2018 [DOI] [PubMed] [Google Scholar]
- 23.Baselga J, Lewis Phillips GD, Verma S, et al. : Relationship between tumor biomarkers and efficacy in EMILIA, a phase III study of trastuzumab emtansine in HER2-positive metastatic breast cancer. Clin Cancer Res 22:3755-3763, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institutes of Health (NIH); National Cancer Institute; Division of Cancer Treatment and Diagnosis : Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0, 2017. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf [Google Scholar]
- 25.Szekely B, Bossuyt V, Li X, et al. : Immunological differences between primary and metastatic breast cancer. Ann Oncol 29:2232-2239, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Gutzmer R, Stroyakovskiy D, Gogas H, et al. : Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): Primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 395:1835-1844, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Loibl S, Schneeweiss A, Huober JB, et al. : Durvalumab improves long-term outcome in TNBC: Results from the phase II randomized GeparNUEVO study investigating neodjuvant durvalumab in addition to an anthracycline/taxane based neoadjuvant chemotherapy in early triple-negative breast cancer (TNBC). J Clin Oncol 39, 2021 (abstr 506) [Google Scholar]
- 28.Schmid P, Cortes J, Dent R, et al. : KEYNOTE-522: Phase III study of neoadjuvant pembrolizumab + chemotherapy versus placebo + chemotherapy, followed by adjuvant pembrolizumab versus placebo for early-stage triple-negative breast cancer. Ann Oncol 32, 2021. (abstr VP7-2021) [Google Scholar]
- 29.Mittendorf EA, Zhang H, Barrios CH, et al. : Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 396:1090-1100, 2020 [DOI] [PubMed] [Google Scholar]
- 30.Bianchini G, Huang C, Egle D, et al. : Tumour infiltrating lymphocytes (TILs), PD-L1 expression and their dynamics in the NeoTRIPaPDL1 trial. Ann Oncol 31, 2020. (abstr LBA13) [Google Scholar]
- 31.Loibl S, Untch M, Burchardi N, et al. : A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: Clinical results and biomarker analysis of GeparNuevo study. Ann Oncol 30:1279-1288, 2019 [DOI] [PubMed] [Google Scholar]
- 32.Loi S, Giobbie-Hurder A, Gombos A, et al. : Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): A single-arm, multicentre, phase 1b-2 trial. Lancet Oncol 20:371-382, 2019 [DOI] [PubMed] [Google Scholar]
- 33.Schmid P, Cortes J, Pusztai L, et al. : Pembrolizumab for early triple-negative breast cancer. N Engl J Med 382:810-821, 2020 [DOI] [PubMed] [Google Scholar]
- 34.Loibl S, Jackisch C, Schneeweiss A, et al. : Dual HER2-blockade with pertuzumab and trastuzumab in HER2-positive early breast cancer: A subanalysis of data from the randomized phase III GeparSepto trial. Ann Oncol 28:497-504, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Meisel JL, Zhao J, Suo A, et al. : Clinicopathologic factors associated with response to neoadjuvant anti-HER2-directed chemotherapy in HER2-positive breast cancer. Clin Breast Cancer 20:19-24, 2020 [DOI] [PubMed] [Google Scholar]
- 36.Zhao J, Krishnamurti U, Zhang C, et al. : HER2 immunohistochemistry staining positivity is strongly predictive of tumor response to neoadjuvant chemotherapy in HER2 positive breast cancer. Pathol Res Pract 216:153155, 2020 [DOI] [PubMed] [Google Scholar]
- 37.Lillemoe TJ, Rendi M, Tsai ML, et al. : HER2 testing characteristics can predict residual cancer burden following neoadjuvant chemotherapy in HER2-positive breast cancer. Int J Breast Cancer 2021:6684629, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneeweiss A, Chia S, Hegg R, et al. : Evaluating the predictive value of biomarkers for efficacy outcomes in response to pertuzumab- and trastuzumab-based therapy: An exploratory analysis of the TRYPHAENA study. Breast Cancer Res 16:R73, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.de Haas SL, Hurvitz SA, Martin M, et al. : Biomarker analysis from the neoadjuvant KRISTINE study in HER2-positive early breast cancer (EBC). Cancer Res 77, 2017. (abstr P6-07-09) [Google Scholar]
- 40.Berns K, Horlings HM, Hennessy BT, et al. : A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell 12:395-402, 2007 [DOI] [PubMed] [Google Scholar]
- 41.Loibl S, Majewski I, Guarneri V, et al. : PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: Pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann Oncol 27:1519-1525, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loibl S, von Minckwitz G, Schneeweiss A, et al. : PIK3CA mutations are associated with lower rates of pathologic complete response to anti–human epidermal growth factor receptor 2 (HER2) therapy in primary HER2-overexpressing breast cancer. J Clin Oncol 32:3212-3220, 2014 [DOI] [PubMed] [Google Scholar]
- 43.Schmid P, Rugo HS, Adams S, et al. : Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21:44-59, 2020 [DOI] [PubMed] [Google Scholar]
- 44.Hamilton EP, Kaklamani V, Falkson C, et al. : Atezolizumab in combination with trastuzumab emtansine or with trastuzumab and pertuzumab in patients with HER2-positive breast cancer and atezolizumab with doxorubicin and cyclophosphamide in HER2-negative breast cancer: Safety and biomarker outcomes from a multi-cohort Phase Ib study. Cancer Res 80, 2020. (abstr PD1-05) [Google Scholar]
- 45.Cortes J, Cescon DW, Rugo HS, et al. : Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 396:1817-1828, 2020 [DOI] [PubMed] [Google Scholar]
- 46.Miles D, Gligorov J, André F, et al. : Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol 32:994-1004, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli (https://vivli.org/ourmember/roche/). For up-to-date details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than one data source external to Roche cannot, and should not, be linked because of a potential increase in risk of patient reidentification.