PURPOSE
To determine the value of tumor cell programmed death-ligand 1 (PD-L1) expression as a predictive biomarker of nivolumab monotherapy efficacy in treatment-naive patients with clear cell renal cell carcinoma (ccRCC) and the efficacy of salvage nivolumab/ipilimumab in patients with tumors unresponsive to nivolumab monotherapy.
METHODS
Eligible patients with treatment-naive ccRCC received nivolumab until progressive disease (PD), toxicity, or completing 96 treatment weeks (part A). Patients with PD before or stable disease at 48 weeks could receive salvage nivolumab/ipilimumab (part B). The primary end point was improvement in 1-year progression-free survival in patients with tumor PD-L1 expression > 20% versus 0%.
RESULTS
One hundred twenty-three patients were enrolled. The objective response rate (ORR) was 34.1% (95% CI, 25.8 to 43.2). ORR by International Metastatic RCC Database Consortium category was favorable-risk 57.1%, intermediate-risk/poor-risk 25.0%, and by sarcomatoid features 36.4%. The ORR was 26.9%, 50.0%, and 75.0% for patients with the tumor PD-L1 expression of 0, 1-20, or > 20%, respectively (trend test P value = .002). The median duration of response was 27.6 (19.3 to not reached) months, with 26 of 42 responders including 17 of 20 with favorable-risk disease remaining progression-free. The 1-year progression-free survival was 34.6% and 75.0% in the PD-L1 = 0% and > 20% categories, respectively (P = .050). Ninety-seven patients with PD or prolonged stable disease were potentially eligible for part B, and 35 were enrolled. The ORR for part B was 11.4%. Grade ≥ 3 treatment-related adverse events occurred in 35% of patients on nivolumab and 43% of those on salvage nivolumab/ipilimumab.
CONCLUSION
Nivolumab monotherapy is active in treatment-naive ccRCC. Although efficacy appears to be less than that of nivolumab/ipilimumab in patients with intermediate-risk/poor-risk disease, favorable-risk patients had notable benefit. Efficacy correlated with tumor PD-L1 status. Salvage nivolumab/ipilimumab was frequently not feasible and of limited benefit.
INTRODUCTION
Nivolumab monotherapy received US Food and Drug Administration approval in 2015 for the treatment of patients with vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor (TKI)–resistant clear cell renal cell carcinoma (ccRCC) on the basis of the results of the CheckMate 025 study.1 In this study, nivolumab demonstrated a statistically significant improvement in overall survival (OS) and objective response rate (ORR). In addition, grade 3 toxicity was significantly less and quality of life significantly improved in patients treated with nivolumab relative to those receiving everolimus. These benefits were sustained at 5 years.2
The combination of nivolumab/ipilimumab received US Food and Drug Administration approval in 2018 for patients with treatment-naive International Metastatic RCC Database Consortium (IMDC)3 intermediate-risk and poor-risk advanced ccRCC on the basis of the results of the CheckMate 214 study comparing the combination with sunitinib.4 In this study, nivolumab/ipilimumab showed improved median progression-free survival (PFS), ORR, and OS relative to sunitinib in patients with IMDC intermediate-risk and poor-risk ccRCC. This benefit relative to sunitinib has been maintained out to a minimum follow-up of 48 months.5 Similar benefits were also seen in the intent-to-treat population.
At the time of study initiation, little information was available on the efficacy and safety profile of nivolumab monotherapy in patients with treatment-naive ccRCC or combination of nivolumab/ipilimumab salvage in patients without response to nivolumab monotherapy. Also, little information was available about biomarkers predictive of either response or resistance to nivolumab monotherapy. CheckMate 009 included a cohort of patients with treatment-naive metastatic ccRCC treated with nivolumab monotherapy at a dose of 10 mg/kg once every 3 weeks and reported responses in only three of 24 (13%) patients, but 74% of patients were alive at 2 years.6 Although pharmacodynamic changes in tumor biopsies were reported, no information was presented about pretreatment predictive biomarkers of response in the treatment-naive cohort. A multi-institutional retrospective analysis reported an ORR of 20% to salvage nivolumab/ipilimumab in 45 patients who previously had been treated with anti–programmed death-ligand 1 (PD-L1) therapy.7 Given these limited data, the HCRN GU16-260 trial was launched to prospectively and more thoroughly fill these knowledge gaps.
METHODS
HCRN-GU16-260 (ClinicalTrials.gov identifier: NCT03117309) was a single-arm, open-label, nonrandomized, multicenter (12 sites), phase II trial. The study was conducted in accordance with International Conference on Harmonization guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki. The Protocol (online only) was approved by the institutional review board for each participating institution. All patients provided written informed consent.
Treatment
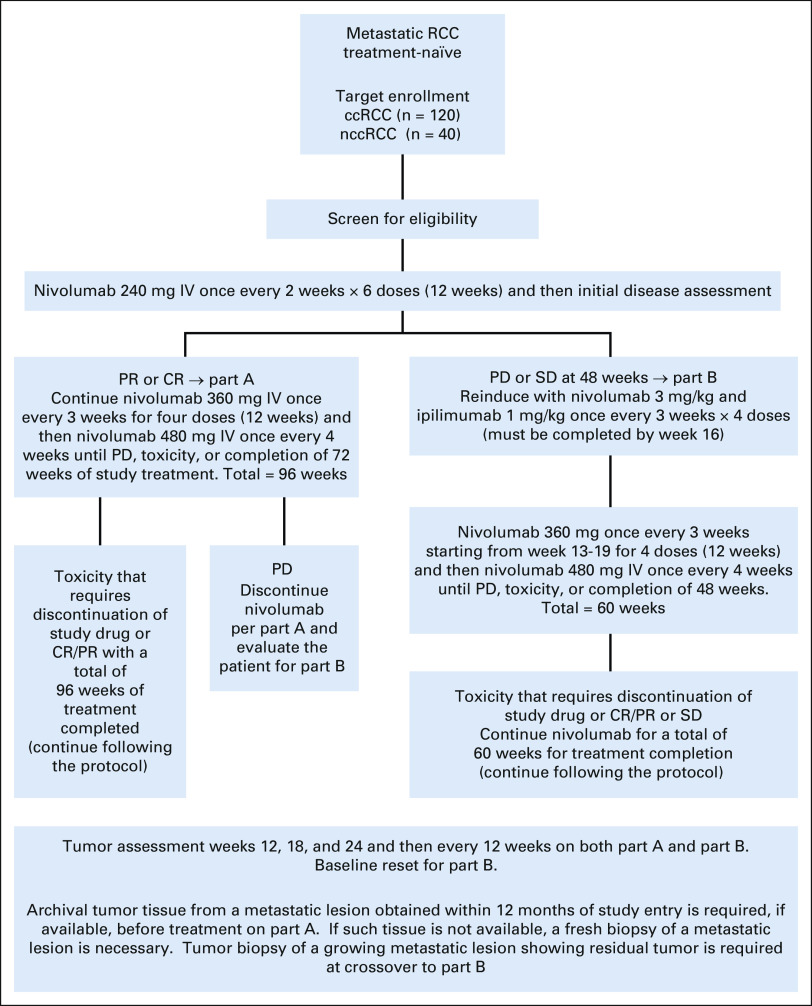
Treatment schema is described in Appendix Figure A1 (online only). In part A, patients received nivolumab monotherapy for up to 96 weeks of total treatment. If patients experienced disease progression (PD) at any time or had a best response of stable disease (SD) at 48 weeks of treatment (prolonged SD), they were potentially eligible for part B, which involved the combination of nivolumab/ipilimumab for 12 weeks followed by nivolumab monotherapy for up to 48 weeks. Imaging was performed at baseline and then every 12 weeks. Patients with asymptomatic disease progression could continue therapy until a repeat scan 6 or more weeks later confirmed disease progression.
For part A, patients were enrolled into either cohort A (ccRCC) or cohort B (non-ccRCC). This article describes the results of cohort A. Patients had to have advanced renal cell carcinoma (RCC) with a clear cell component and at least one RECIST version 1.1–defined measurable site of disease. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0-2, age ≥ 18 years, adequate organ and bone marrow function, controlled brain metastases, and a tumor biopsy (core or excisional) of a metastatic lesion obtained within 1 year before study registration.
Patients were excluded if they had active autoimmune disease, a concurrent medical condition requiring use of systemic corticosteroids equivalent to prednisone > 10 mg in a day, or prior systemic therapy for metastatic RCC.
For enrollment into part B, patients must not have experienced a grade ≥ 3 immune-related adverse event (irAE) on nivolumab, excepting an endocrine irAE managed with hormone replacement therapy, and were required to have a repeat tumor biopsy confirming the existence of residual disease. Patients with symptomatic disease progression on nivolumab for whom the investigator, in consultation with the patient, felt that an alternative therapy was more appropriate were also not enrolled.
Tissue Collection
Analysis of PD-L1 expression was performed on formalin-fixed paraffin-embedded tumor tissue sections from metastatic lesions or primary tumors (if sufficient metastatic tissue was unavailable). The tumor sections were stained with an anti–PD-L1 antibody (1:100; E1L3N rabbit monoclonal antibody; Cell Signaling Technology, Danvers, MA) according to a standard protocol.8,9 The percentage of PD-L1–positive tumor cells was independently scored by three pathologists who were blinded to clinical outcomes. Discrepancies in scores were resolved by consensus review. For patients with PD-L1 measures from both metastatic and primary lesions, preference was given to the metastatic lesion score. For statistical analysis, PD-L1 scores were categorized as 0%, 1%-5%, > 5%-20%, or > 20% to study for association with clinical outcomes. The baseline PD-L1 score was used for analysis of efficacy in part B.
Statistical Analysis
The planned number of eligible and treated patients to be enrolled in cohort A was 120.
Part A.
The primary objective of this trial was to use tumor PD-L1 status to predict clinical outcomes (1-year landmark PFS). For the primary outcome, we anticipated that about 45% (or 54) of patients' tumors would have 0% PD-L1 expression and roughly 15% (or 18) would express PD-L1 > 20%. A sample size of 120 patients with ccRCC provided a 90% power using a 0.05-level two-sided test (Fisher's exact) to test the hypothesis that PD-L1 > 20% was associated with a significantly improved 1-year PFS rate (55% v 13% taken as a binomial quantity). Given an expected patient allocation within the four PD-L1 groups of 45%, 25%, 15%, and 15%, the Cochran-Armitage test gives at least 90% power to detect a trend in 1-year PFS of 13%, 19%, 35%, and 55% using a two-sided 0.05-level test for trend. The trend test served as sensitivity analysis of the primary study outcome.
Secondary end points were to use RECIST and immune-related RECIST measurements to determine the ORR, duration of response (DOR), and median PFS for frontline nivolumab monotherapy for all enrolled patients and as a factor of IMDC risk score, tumor PD-L1 expression, and sarcomatoid histology. In addition, we sought to determine the safety of nivolumab monotherapy in the frontline setting.
Part B.
Patients who exhibited a best response of SD at 48 weeks or who experienced progression were considered for part B. The primary end point for part B was ORR using tumor measurements at the start of part B as baseline. It was expected that roughly half of the part A patients would be enrolled in part B. A two-stage design was used. Observing two or more responses among the first 29 part B patients would provide sufficient evidence to continue part B enrollment. With a true uninteresting response rate of 5%, there was a 57% chance of declaring the regimen uninteresting; however, with an interesting response rate of 20%, there was a > 95% chance of continuing. If after enrollment of 60 patients, there were at least 11 total responses, the 95% CI would range from 10.6% to 28.5%, adjusted for the two-stage design.
RESULTS
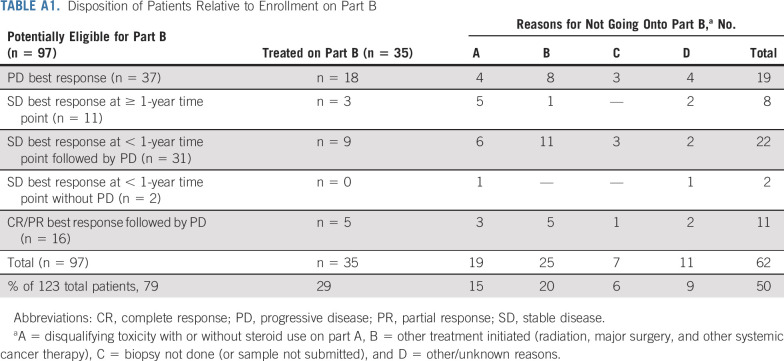
The disposition of patients is shown in the flow diagram (Fig 1). One hundred twenty-three patients with ccRCC were enrolled from May 2017 until December 2019. At the time of data lock (April 7, 2021) and at a median [Q1, Q3] follow-up of 26.9 [20.5, 33.2] months, 35 patients had gone onto part B. Of the remaining 88 patients, 26 were still responding to nivolumab monotherapy and 62 did not enroll in part B because of not meeting eligibility criteria (grade ≥ 3 irAE on part A [19] or inability to obtain a subsequent tumor biopsy or document residual tumor in the biopsy specimen [7]), symptomatic PD prompting the physician/patient to choose an alternative treatment (25), or various other reasons (11; Fig 1, Appendix Table A1 [online only]).
FIG 1.
Flow diagram. aReported separately. ccRCC, clear cell renal cell carcinoma; CR, complete response; irAE, immune-related adverse event; nccRCC, nonclear cell renal cell carcinoma; PD, progressive disease; PR, partial response; SD, stable disease.
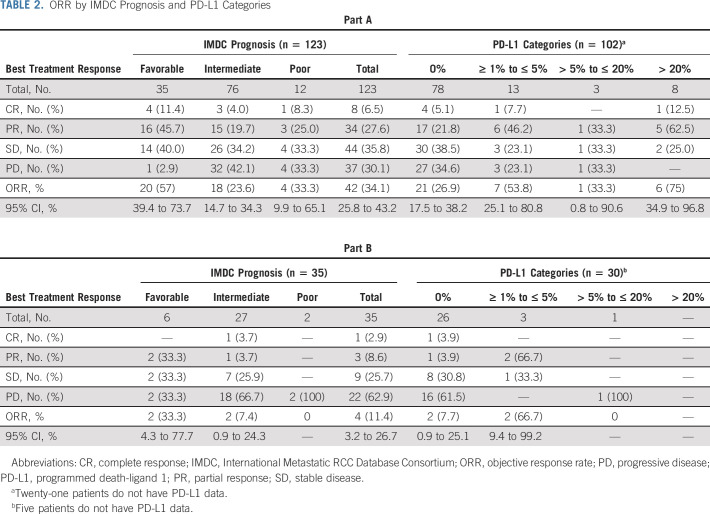
Demographics are displayed in Table 1. Tumor cell PD-L1 expression was 0% in 78 (63.4%) and > 20% in 8 (6.5%) patients. PD-L1 data were not available for 21 patients (17.1%).
TABLE 1.
Patient Demographic and Baseline Characteristics (clear cell renal cell carcinoma patients, n = 123)
Efficacy Results: Part A
Response data for part A are shown in Table 2. The ORR (95% CI) for the entire study population was 34.1% (25.8 to 43.2) with a 6.5% complete response (CR). The ORR by immune-related RECIST was 39.0% (30.4 to 48.2). The intermediate-risk and poor-risk populations had response rates of 23.7% (14.7 to 34.8) and 33.3% (9.9 to 65.1), with three and one CR, respectively. The ORR in patients with favorable-risk disease was 57.1% (39.4 to 73.7) with 4 (11.4%) CRs. Of note, only one favorable-risk patient experienced PD at their 12-week computed tomography scan. Eight of 22 (36.4%) patients with sarcomatoid histology responded with no CRs. The ORR by tumor PD-L1 status was 21 of 78 (26.9%), 8 of 16 (50.0%), and 6 of 8 (75.0%) for patients with tumor PD-L1 of 0, 1-20, or > 20%, respectively (trend test P value = .002). Five of 7 (71.4%) favorable-risk patients with PD-L1 ≥ 1 exhibited a tumor response including one CR. However, 14 of the 23 (60.9%) patients with favorable-risk disease and a tumor PD-L1 score of 0% also responded (including three CRs) and this group represented 74% of the responders with favorable-risk disease.
TABLE 2.
ORR by IMDC Prognosis and PD-L1 Categories
Figure 2A displays a waterfall plot of maximum depth of response in target lesions separated by IMDC-risk category for the patients in part A. Some tumor shrinkage was seen in the majority of patients in each category. Figure 2B displays a waterfall plot of maximum depth of response separated by tumor PD-L1 status and demonstrates the above-noted correlation between treatment response and PD-L1 expression.
FIG 2.
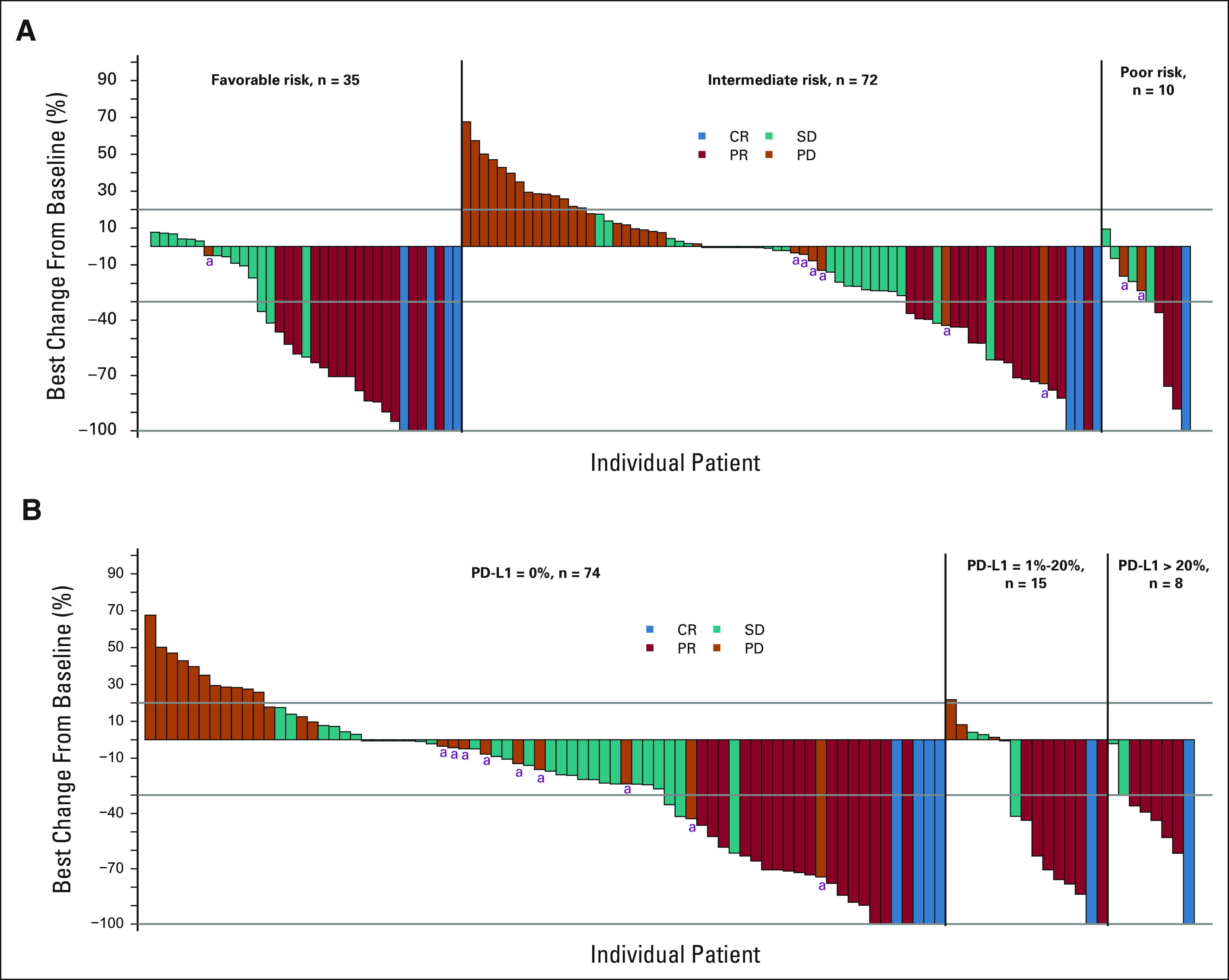
Waterfall plots of maximum tumor shrinkage by (A) IMDC category and (B) PD-L1 status in ccRCC part A. Tumors with PD-L1 status 1-5 and > 5 to 20 are combined combined as there were only three tumors in the latter group. aNew lesions. ccRCC, clear cell renal cell carcinoma; CR, complete response; IMDC, International Metastatic RCC Database Consortium; PD, progressive disease; PD-L1, programmed death-ligand 1; PR, partial response; SD, stable disease.
The median DOR for the whole part A responder population was estimated to be 27.6 (19.3 to not applicable [NA]) months (Appendix Fig A2A, online only). Twenty-six of 42 responders remain progression-free. Of note, only three of 20 responders with favorable-risk disease and one of four with poor-risk disease have exhibited disease progression, whereas the median DOR for the intermediate-risk population was 11.3 (8.3 to NA) months (Appendix Fig A2B).
The median PFS was 8.3 (5.5 to 10.9) months for the whole study population (Fig 3A), 32.5 months for those with IMDC favorable-risk disease, and 5.4 and 5.2 months for patients with intermediate-risk and poor-risk diseases, respectively. The PFS at 12 months was 65.1% (46.8 to 78.5) for those with favorable-risk disease, 28.3% (18.7 to 38.8) for those with intermediate-risk disease, and 33.3% (10.3 to 58.8) for those with poor-risk disease. In total, 32 patients remain progression-free (Fig 3B). The median PFS was 7.7 (4.1 to 10.8) months in the PD-L1 0% expression group and 20.6 (4.2 to NA) months in patients with PD-L1 expression > 20% (Fig 3C). The 1-year PFS rates in the PD-L1 0% and > 20% categories were 34.6% (27 of 78) and 75.0% (6 of 8), respectively (Fisher's exact 2-sided P value = .050). Thus, the study primary end point was met.
FIG 3.
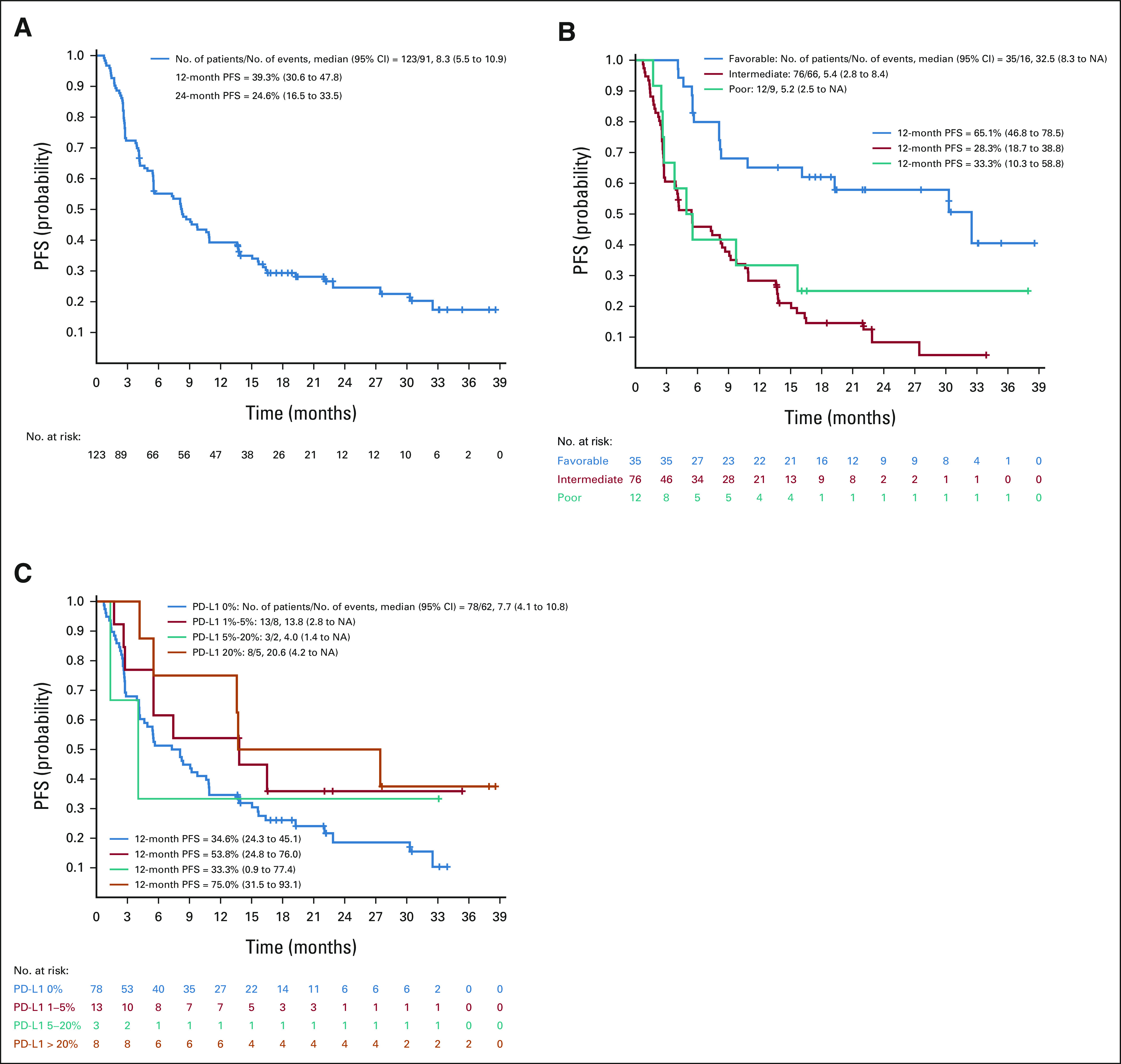
PFS for ccRCC part A: (A) overall (n = 123) and (B) by IMDC and (C) PD-L1 categories. ccRCC, clear cell renal cell carcinoma; IMDC, International Metastatic RCC Database Consortium; NA, not applicable; PD-L1, programmed death-ligand 1; PFS, progression-free survival.
Efficacy Results: Part B
Four of the first 29 patients treated in part B exhibited an objective response, enabling part B to proceed to full accrual, which turned out to be 35 rather than the anticipated 60 patients (Fig 1). The ORR to nivolumab/ipilimumab salvage was 4 of 35 (11.4%) with 1 CR (Table 2). Of note, two of 6 (33.3%) favorable-risk patients but only two of 29 (6.9%) intermediate-risk/poor-risk patients exhibited a response in part B. The immune-related ORR was 6 of 35 (17.1%), with the two additional responses being observed in the intermediate-risk population. Thirty patients on part B had tumor assessable for PD-L1, with 26 (86.7%) being PD-L1 0%. The ORR to salvage nivolumab/ipilimumab was 7.7% (2 of 26) for patients with tumor PD-L1 0% and 50.0% (2 of 4) for patients with PD-L1 ≥ 1%.
Overall Survival
The median OS for the entire population has not been reached, with only 32 patients having died as of the data lock. Specifically, 77.7% of patients are alive at 24 months.
Safety Data
The distribution of toxicities for part A and part B is shown in Table 3. Treatment-emergent grade ≥ 3 toxicity was seen in 35.0% (43 of 123) of patients on nivolumab monotherapy (part A). Eighteen of the 43 patients with grade ≥ 3 toxicities (14.6% of the total) had elevated lipase or amylase, which were almost exclusively asymptomatic. One patient died of respiratory failure. Treatment-emergent grade ≥ 3 toxicities for part B were seen in 42.9% (15 of 35) of patients, with 5 (14.3%) having asymptomatic elevated amylase or lipase. Of note, 40.0% (12 of 30) of patients without grade 3 toxicity on part A experienced grade ≥ 3 toxicity on arm B. One patient died of treatment-related myositis.
TABLE 3.
Incidence of Treatment-Related Adverse Events
DISCUSSION
This study shows that nivolumab monotherapy has efficacy in treatment-naive patients with ccRCC with an ORR (95% CI) of 34.1% (25.8 to 43.2), a CR of 6.5%, a median DOR of 27.6 months, and a median PFS of 8.3 months. Efficacy was seen across all IMDC risk categories with encouraging activity (ORR 57%) in patients with favorable-risk disease. Toxicities were typical of those seen with nivolumab monotherapy in other disease settings, with a substantial minority of grade ≥ 3 toxicity being related to asymptomatic elevations in pancreatic enzymes of uncertain clinical significance.
These results are consistent with several other recently reported frontline studies of single-agent programmed cell death protein 1 (PD-1) pathway blockade in patients with ccRCC.10-12 Although OMNIVORE13 reported a lower ORR for nivolumab monotherapy, this trial included a mix of patients with ccRCC and nonclear cell renal cell carcinoma receiving either frontline or second-line treatment.
Salvage nivolumab/ipilimumab showed modest efficacy with an ORR of only 11.4% with 1 CR. The activity of salvage nivolumab/ipilimumab is also consistent with previous reports, including the TITAN-RCC study where an ORR of 11% was observed in patients with PD in the first 16 weeks and 19% in patients with PD after 16 weeks.10
Because of the study design and patient/investigator decisions, < 40% of patients with PD/prolonged SD were both eligible and consented to receive salvage nivolumab/ipilimumab therapy. Adverse events for nivolumab/ipilimumab were less than that previously reported in the first-line setting, with only 10 patients (29%) experiencing grade ≥ 3 toxicities that were unrelated to elevated pancreatic enzymes. This is perhaps to be expected given that patients who experienced grade ≥ 3 nonendocrine toxicity on nivolumab monotherapy were excluded from enrollment on part B and many endocrine toxicities (eg, thyroiditis and hypophysitis) are permanent and, therefore, cannot recur.
Nivolumab monotherapy represents an alternative frontline approach that might be applicable for patients where the addition of ipilimumab or VEGFR TKI therapy poses a concern. However, the nivolumab/ipilimumab combination is likely preferred over nivolumab monotherapy when possible. This is particularly true for intermediate-risk/poor-risk patients and those with sarcomatoid RCC, because of what appears to be a lower ORR, fewer CRs, and shorter PFS and DOR than what was observed in the CheckMate 214 study5,14 as well as the limited ability to salvage patients with later use of the combination. Although these data support this conclusion and further suggest that providing the most active immunotherapy regimen perceived to be tolerable as initial therapy should be the optimal approach in both clinical trials and clinical practice, the CheckMate 8Y8 study, which randomly assigns patients with treatment-naive metastatic ccRCC and IMDC intermediate-risk/poor-risk disease to either nivolumab monotherapy or combination of nivolumab/ipilimumab, will formally address this question.
The data from the current study for patients with IMDC favorable-risk disease are intriguing. Although the high ORR and long DOR relative to the intermediate-risk/poor-risk population appear to be distinct from other studies of anti–PD-(L)1-based monotherapy for ccRCC, these data are in line with previous studies with cytokine-based immunotherapy, which suggested that this population was equally responsive to immunotherapy.15 In addition, data from CheckMate 214 suggest that favorable-risk patients receiving nivolumab/ipilimumab do well in terms of ORR, CR rate, and DOR as patients with intermediate-risk/poor-risk disease. Furthermore, although median PFS for patients with favorable-risk disease was worse for those treated with nivolumab/ipilimumab than those treated with sunitinib, there were more CRs and 5-year survival was greater (58% v 48%) for those on the nivolumab/ipilimumab arm.16 Moreover, for favorable-risk patients, treatment-free survival was three times longer with nivolumab/ipilimumab than sunitinib.17 At the very least, these data suggest that the current algorithm for frontline RCC therapy selection, which excludes patients with favorable-risk disease from receiving immune checkpoint inhibitor (ICI)–only therapy, needs to be re-examined, and perhaps an ICI-specific prediction model needs to be created.
Tumor PD-L1 expression has been generally thought to be an unreliable biomarker for predicting the efficacy of the PD-1 pathway blockade in patients with metastatic ccRCC. For example, in each of the six reported phase III studies comparing the combination PD-1/PD-L1 pathway blockade with sunitinib, the hazard ratio for PFS for the PD-L1+ group overlapped that for the population as a whole4,5,18-22 and only CheckMate 214 showed a greater OS benefit for the combination in the population with PD-L1+ tumors. However, many of these studies were confounded by several factors such as (1) PD-L1 analysis on primary rather than metastatic lesions, (2) using samples collected > 12 months before treatment initiation and/or before an intervening treatment, (3) looking at end points such as median PFS or OS that may be poor measures of immunotherapy efficacy rather than ORR and landmark survival, (4) using mixed combination regimens that do not isolate the impact of the anti–PD-(L)1 component (especially with PD-L1 being a negative predictive biomarker for VEGFR TKIs23), and (5) comparing the results with a control arm rather than measuring within the ICI treatment arm.
In this study, 1-year PFS (the primary study end point) and ORR were directly correlated with tumor PD-L1 expression status, despite the paucity of patients with PD-L1 expression > 20%. However, the ORR in the 78 patients whose tumors lacked PD-L1 expression was 26.9%, encompassing 60% of the total responders. Thus, PD-L1 expression by itself is of limited clinical utility in selecting patients for nivolumab monotherapy, but could serve as an important component of a multifactorial predictive biomarker model. Tissue collected from the patients on this study will be used to test previous gene expression and immunohistochemistry-based profiles of ICI efficacy24,25 and hopefully contributes to such a model.
In summary, this study establishes that nivolumab monotherapy is active in patients with treatment-naive ccRCC and shows encouraging efficacy in patients with favorable-risk disease and high PD-L1–expressing tumors. Although overall efficacy appears to be less than that of the combination of nivolumab/ipilimumab in patients with intermediate-risk/poor-risk disease, these study results may encourage anti–PD-1 monotherapy use in patients who are poor candidates for ipilimumab and inform ICI biomarker development in both the frontline and adjuvant settings.26
ACKNOWLEDGMENT
We acknowledge the contributions to this effort of the patients and their families and the hard work of the team at the HCRN including Robyn Lillie, the Study Coordinator, and the research coordinators and data managers at the investigational sites.
APPENDIX
TABLE A1.
Disposition of Patients Relative to Enrollment on Part B
FIG A1.
Study schema. ccRCC, clear cell renal cell carcinoma; CR, complete response; IV, intravenous; nccRCC, nonclear cell renal cell carcinoma; PD, progressive disease; PR, partial response; RCC, renal cell carcinoma; SD, stable disease.
FIG A2.
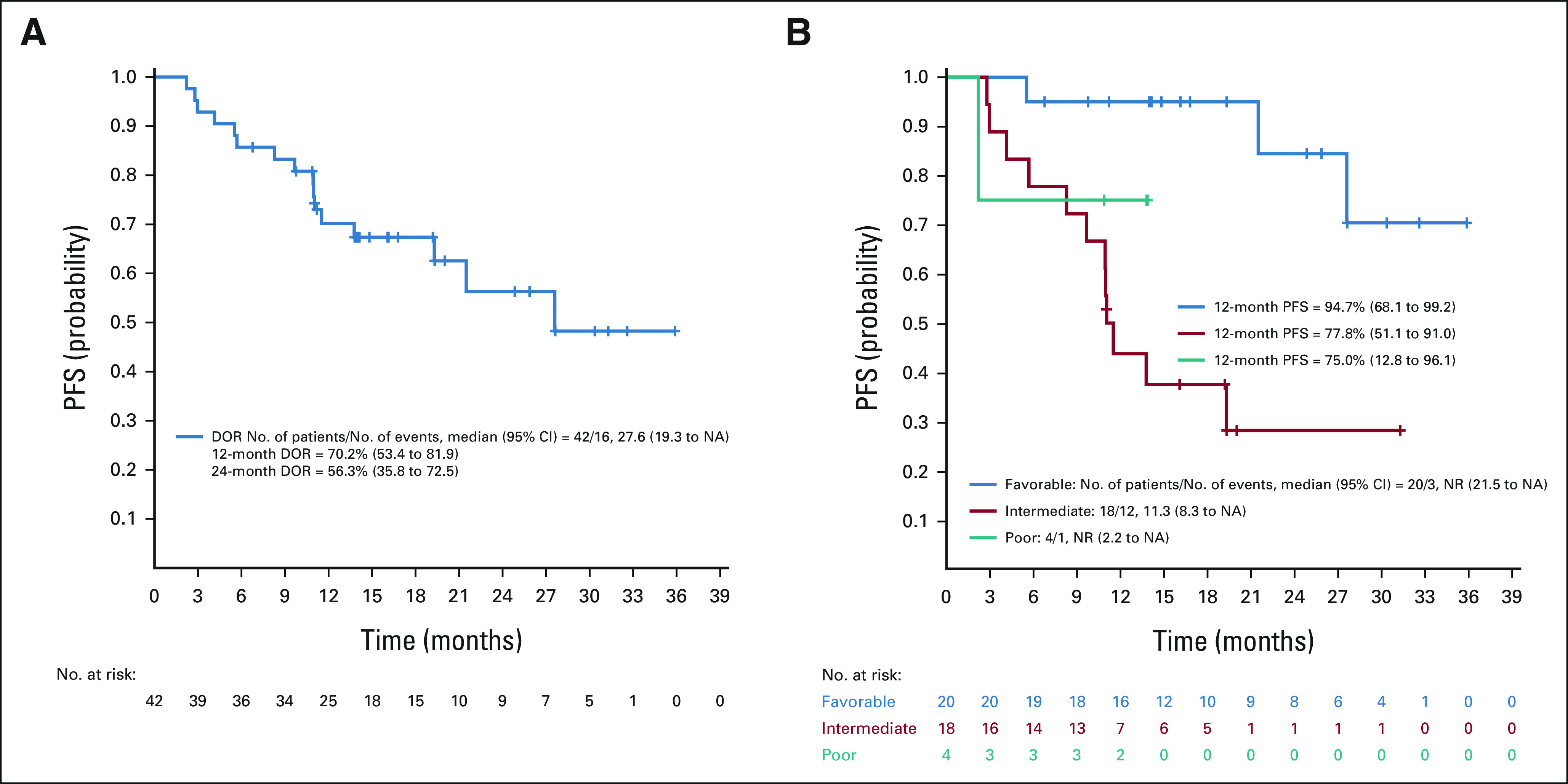
(A) DOR plot, ccRCC part A and (B) DOR by IMDC risk group. ccRCC, clear cell renal cell carcinoma; DOR, duration of response; IMDC, International Metastatic RCC Database Consortium; NA, not applicable; NR, no response; PFS, progression-free survival.
Michael B. Atkins
Stock and Other Ownership Interests: Werewolf Pharma, Pyxis
Consulting or Advisory Role: Genentech, Novartis, Bristol Myers Squibb, Merck, Exelixis, Eisai, Agenus, Werewolf Pharma, Surface Oncology, Iovance Biotherapeutics, Pyxis, Pneuma Respiratory, Leads Biolabs, Fathom Biotechnology, Aveo, Cota Healthcare, Adagene, Idera, Ellipses Pharma, AstraZeneca, PACT Pharma, Seattle Genetics, Pfizer, Scholar Rock, Asher Biotherapeutics, Calithera Biosciences, Takeda, Sanofi, Simcha Therapeutics, GlaxoSmithKline
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst)
Naomi B. Haas
Consulting or Advisory Role: Pfizer, Merck Sharp & Dohme, Calithera Biosciences, Eisai, Exelixis, AVEO, Roche/Genentech
Expert Testimony: Lilly (I)
David F. McDermott
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Genentech/Roche, Pfizer, Exelixis, Novartis, Array BioPharma, Peloton Therapeutics, EMD Serono, Jounce Therapeutics, Alkermes, Lilly, Eisai, Calithera Biosciences, Iovance Biotherapeutics, Werewolf Therapeutics, Synthekine, AVEO
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), Genentech (Inst), Novartis (Inst), Alkermes (Inst)
Other Relationship: Beth Israel Deaconess Medical Center
Uncompensated Relationships: X4 Pharma, AVEO
Mehmet A. Bilen
Consulting or Advisory Role: Exelixis, Sanofi, Nektar, EMD Serono, Eisai, Janssen, Genomic Health, Pfizer, Bristol Myers Squibb, Bayer, Calithera Biosciences, AstraZeneca, Seattle Genetics
Research Funding: Bayer (Inst), Bristol Myers Squibb (Inst), Genentech/Roche (Inst), Incyte (Inst), Nektar (Inst), AstraZeneca (Inst), Tricon Pharmaceuticals (Inst), Pfizer (Inst), Seattle Genetics (Inst), Xencor (Inst), Exelixis (Inst), Advanced Accelerator Applications (Inst), Genome & Company (Inst), Peloton Therapeutics (Inst), Merck (Inst)
Mark Stein
Consulting or Advisory Role: Merck Sharp & Dohme, Exelixis, Xencor, Janssen Oncology, Vaccitech, Bristol Myers Squibb/Medarex
Research Funding: Oncoceutics (Inst), Merck Sharp & Dohme (Inst), Janssen Oncology (Inst), Medivation/Astellas (Inst), Advaxis (Inst), Suzhou Kintor Pharmaceuticals (Inst), Harpoon (Inst), Bristol Myers Squibb (Inst), Genocea Biosciences (Inst), Lilly (Inst), Nektar (Inst), Seattle Genetics (Inst), Xencor (Inst), Tmunity Therapeutics Inc (Inst), Exelixis (Inst), Bellicum Pharmaceuticals
Jeffrey A. Sosman
Honoraria: Bristol Myers Squibb, Array BioPharma, Jazz Pharmaceuticals, Apexian Pharmaceuticals, Iovance Biotherapeutics
Consulting or Advisory Role: Bristol Myers Squibb, Array BioPharma, Apexigen, Jazz Pharmaceuticals, Iovance Biotherapeutics
Elizabeth R. Plimack
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Merck, Seattle Genetics, Pfizer, Infinity Pharmaceuticals, MEI Pharma, AstraZeneca/MedImmune, Astellas Pharma, Aveo, Bristol Myers Squibb/Medarex, Calithera Biosciences, Genentech, Janssen, Regeneron
Research Funding: Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Astellas Pharma (Inst), Genentech/Roche (Inst)
Patents, Royalties, Other Intellectual Property: US Patent No.: 14/588,503, Filed January 2, 2015 (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/66377
Moshe Ornstein
Consulting or Advisory Role: Pfizer, Eisai, Exelixis, Merck, AVEO, Bristol Myers Squibb Foundation
Speakers' Bureau: Bristol Myers Squibb
Research Funding: Bristol Myers Squibb (Inst), Pfizer (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst), Astellas Medivation (Inst), Aravive (Inst), Surface Oncology (Inst)
Travel, Accommodations, Expenses: Exelixis, Bristol Myers Squibb, Pfizer
Michael Hurwitz
Employment: Pfizer (I), Gamida Cell (I), Arvinas (I)
Consulting or Advisory Role: Nektar, Janssen, Crispr Therapeutics, Bristol Myers Squibb/Celgene, Exelixis
Research Funding: Apexigen, Astellas Pharma, AstraZeneca/MedImmune, Bayer, Bristol Myers Squibb, Corvus Pharmaceuticals, Lilly, Endocyte, Genentech, Genmab, Innocrin Pharma, Iovance Biotherapeutics, Merck, Nektar, Novartis, Pfizer, Progenics, Sanofi/Aventis, Seattle Genetics, Torque, Unum Therapeutics, Achilles Therapeutics
David J. Peace
Stock and Other Ownership Interests: Merck, Abbott Laboratories
Patents, Royalties, Other Intellectual Property: US Patent No. 8,557,777 B2: Methods of Treating Prostate Cancer Using Prostate Specific Antigen and Tumor Endothelial Marker Peptides. October 15, 2013 (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/345598
Sabina Signoretti
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca/MedImmune, Merck, Crispr Therapeutics
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca (Inst), Exelixis (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Receives royalties from BioGenex
Other Relationship: AACR, NCI
Alessia Cimadamore
Honoraria: AstraZeneca/Merck
Catherine J. Wu
Stock and Other Ownership Interests: BioNTech (I)
Research Funding: Pharmacyclics/Janssen
David Braun
Honoraria: LM Education/Exchange Service
Consulting or Advisory Role: Bristol Myers Squibb, Octane Co, Defined Health, Dedham Group, Adept Field Solutions, Slingshot Insights, Blueprint Partnership, Charles River Associates, Trinity Group, Insight Strategy, Schlesinger Associates, Exelixis, AVEO, Catenion
Travel, Accommodations, Expenses: Bristol Myers Squibb
David Einstein
Honoraria: OncLive
Research Funding: Bristol Myers Squibb (Inst), Cardiff Oncology (Inst), Foundation Medicine (Inst), Puma Biotechnology (Inst)
Paul J. Catalano
Research Funding: Regeneron (Inst), Astellas Pharma (Inst), AstraZeneca (Inst)
Hans Hammers
Honoraria: Bristol Myers Squibb
Consulting or Advisory Role: Bristol Myers Squibb, Pfizer, Exelixis, Bayer, Novartis, Merck, ARMO BioSciences, Corvus Pharmaceuticals, Surface Oncology, Lilly
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), Aravive (Inst), Surface Oncology (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Merck, Pfizer, Lilly, Novartis
No other potential conflicts of interest were reported.
See accompanying editorial on page 2867
PRIOR PRESENTATION
Presented in part at the 2020 ASCO virtual annual meeting, May 29-31, 2020, and the 2022 ASCO GU meeting, San Francisco, CA, February 17-19, 2022.
SUPPORT
Supported by Bristol Myers Squibb (CA209-669) via a contract with the Hoosier Cancer Research Network. Funds for correlative work were provided by a Department of Defense Translational Team Science Grant (KC170216) to M.B.A. and C.J.W. and the Dana Farber Harvard Kidney Cancer SPORE Grant (NCI P50CA101942) to D.F.M. and (NCI P30 CA051008) to the Georgetown Lombardi Comprehensive Cancer Center.
CLINICAL TRIAL INFORMATION
DATA SHARING STATEMENT
Upon request, and subject to review, HCRN and Georgetown University will provide the data that support the clinical and biomarker findings of this study to interested investigators or regulatory authorities. Subject to certain criteria, conditions, and exceptions, HCRN and Georgetown University may also provide access to individual deidentified participant data to such entities. Data can be requested by e-mailing Dr Michael B. Atkins at mba41@georgetown.edu. Potential access to data will be available from the date of manuscript publication through at least December 31, 2027.
AUTHOR CONTRIBUTIONS
Conception and design: Michael B. Atkins, David F. McDermott, David Einstein, Hans Hammers
Administrative support: Michael B. Atkins
Provision of study materials or patients: Michael B. Atkins, Naomi B. Haas, David F. McDermott, Mark Stein, Robert Alter, Elizabeth R. Plimack, Michael Hurwitz, David J. Peace, David Einstein, Hans Hammers
Collection and assembly of data: Michael B. Atkins, Opeyemi A. Jegede, Naomi B. Haas, Mehmet A. Bilen, Mark Stein, Jeffrey A. Sosman, Robert Alter, Elizabeth R. Plimack, Moshe Ornstein, David J. Peace, Sabina Signoretti, Thomas Denize, Alessia Cimadamore, David Einstein, Hans Hammers
Data analysis and interpretation: Michael B. Atkins, Opeyemi A. Jegede, Naomi B. Haas, David F. McDermott, Mehmet A. Bilen, Mark Stein, Elizabeth R. Plimack, Moshe Ornstein, Michael Hurwitz, Alessia Cimadamore, Catherine J. Wu, David Braun, David Einstein, Paul J. Catalano, Hans Hammers
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Phase II Study of Nivolumab and Salvage Nivolumab/Ipilimumab in Treatment-Naive Patients With Advanced Clear Cell Renal Cell Carcinoma (HCRN GU16-260-Cohort A)
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Michael B. Atkins
Stock and Other Ownership Interests: Werewolf Pharma, Pyxis
Consulting or Advisory Role: Genentech, Novartis, Bristol Myers Squibb, Merck, Exelixis, Eisai, Agenus, Werewolf Pharma, Surface Oncology, Iovance Biotherapeutics, Pyxis, Pneuma Respiratory, Leads Biolabs, Fathom Biotechnology, Aveo, Cota Healthcare, Adagene, Idera, Ellipses Pharma, AstraZeneca, PACT Pharma, Seattle Genetics, Pfizer, Scholar Rock, Asher Biotherapeutics, Calithera Biosciences, Takeda, Sanofi, Simcha Therapeutics, GlaxoSmithKline
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst)
Naomi B. Haas
Consulting or Advisory Role: Pfizer, Merck Sharp & Dohme, Calithera Biosciences, Eisai, Exelixis, AVEO, Roche/Genentech
Expert Testimony: Lilly (I)
David F. McDermott
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Genentech/Roche, Pfizer, Exelixis, Novartis, Array BioPharma, Peloton Therapeutics, EMD Serono, Jounce Therapeutics, Alkermes, Lilly, Eisai, Calithera Biosciences, Iovance Biotherapeutics, Werewolf Therapeutics, Synthekine, AVEO
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), Genentech (Inst), Novartis (Inst), Alkermes (Inst)
Other Relationship: Beth Israel Deaconess Medical Center
Uncompensated Relationships: X4 Pharma, AVEO
Mehmet A. Bilen
Consulting or Advisory Role: Exelixis, Sanofi, Nektar, EMD Serono, Eisai, Janssen, Genomic Health, Pfizer, Bristol Myers Squibb, Bayer, Calithera Biosciences, AstraZeneca, Seattle Genetics
Research Funding: Bayer (Inst), Bristol Myers Squibb (Inst), Genentech/Roche (Inst), Incyte (Inst), Nektar (Inst), AstraZeneca (Inst), Tricon Pharmaceuticals (Inst), Pfizer (Inst), Seattle Genetics (Inst), Xencor (Inst), Exelixis (Inst), Advanced Accelerator Applications (Inst), Genome & Company (Inst), Peloton Therapeutics (Inst), Merck (Inst)
Mark Stein
Consulting or Advisory Role: Merck Sharp & Dohme, Exelixis, Xencor, Janssen Oncology, Vaccitech, Bristol Myers Squibb/Medarex
Research Funding: Oncoceutics (Inst), Merck Sharp & Dohme (Inst), Janssen Oncology (Inst), Medivation/Astellas (Inst), Advaxis (Inst), Suzhou Kintor Pharmaceuticals (Inst), Harpoon (Inst), Bristol Myers Squibb (Inst), Genocea Biosciences (Inst), Lilly (Inst), Nektar (Inst), Seattle Genetics (Inst), Xencor (Inst), Tmunity Therapeutics Inc (Inst), Exelixis (Inst), Bellicum Pharmaceuticals
Jeffrey A. Sosman
Honoraria: Bristol Myers Squibb, Array BioPharma, Jazz Pharmaceuticals, Apexian Pharmaceuticals, Iovance Biotherapeutics
Consulting or Advisory Role: Bristol Myers Squibb, Array BioPharma, Apexigen, Jazz Pharmaceuticals, Iovance Biotherapeutics
Elizabeth R. Plimack
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Merck, Seattle Genetics, Pfizer, Infinity Pharmaceuticals, MEI Pharma, AstraZeneca/MedImmune, Astellas Pharma, Aveo, Bristol Myers Squibb/Medarex, Calithera Biosciences, Genentech, Janssen, Regeneron
Research Funding: Bristol Myers Squibb (Inst), Merck Sharp & Dohme (Inst), Astellas Pharma (Inst), Genentech/Roche (Inst)
Patents, Royalties, Other Intellectual Property: US Patent No.: 14/588,503, Filed January 2, 2015 (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/66377
Moshe Ornstein
Consulting or Advisory Role: Pfizer, Eisai, Exelixis, Merck, AVEO, Bristol Myers Squibb Foundation
Speakers' Bureau: Bristol Myers Squibb
Research Funding: Bristol Myers Squibb (Inst), Pfizer (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst), Astellas Medivation (Inst), Aravive (Inst), Surface Oncology (Inst)
Travel, Accommodations, Expenses: Exelixis, Bristol Myers Squibb, Pfizer
Michael Hurwitz
Employment: Pfizer (I), Gamida Cell (I), Arvinas (I)
Consulting or Advisory Role: Nektar, Janssen, Crispr Therapeutics, Bristol Myers Squibb/Celgene, Exelixis
Research Funding: Apexigen, Astellas Pharma, AstraZeneca/MedImmune, Bayer, Bristol Myers Squibb, Corvus Pharmaceuticals, Lilly, Endocyte, Genentech, Genmab, Innocrin Pharma, Iovance Biotherapeutics, Merck, Nektar, Novartis, Pfizer, Progenics, Sanofi/Aventis, Seattle Genetics, Torque, Unum Therapeutics, Achilles Therapeutics
David J. Peace
Stock and Other Ownership Interests: Merck, Abbott Laboratories
Patents, Royalties, Other Intellectual Property: US Patent No. 8,557,777 B2: Methods of Treating Prostate Cancer Using Prostate Specific Antigen and Tumor Endothelial Marker Peptides. October 15, 2013 (Inst)
Open Payments Link: https://openpaymentsdata.cms.gov/physician/345598
Sabina Signoretti
Consulting or Advisory Role: Bristol Myers Squibb, AstraZeneca/MedImmune, Merck, Crispr Therapeutics
Research Funding: Bristol Myers Squibb (Inst), AstraZeneca (Inst), Exelixis (Inst), Novartis (Inst)
Patents, Royalties, Other Intellectual Property: Receives royalties from BioGenex
Other Relationship: AACR, NCI
Alessia Cimadamore
Honoraria: AstraZeneca/Merck
Catherine J. Wu
Stock and Other Ownership Interests: BioNTech (I)
Research Funding: Pharmacyclics/Janssen
David Braun
Honoraria: LM Education/Exchange Service
Consulting or Advisory Role: Bristol Myers Squibb, Octane Co, Defined Health, Dedham Group, Adept Field Solutions, Slingshot Insights, Blueprint Partnership, Charles River Associates, Trinity Group, Insight Strategy, Schlesinger Associates, Exelixis, AVEO, Catenion
Travel, Accommodations, Expenses: Bristol Myers Squibb
David Einstein
Honoraria: OncLive
Research Funding: Bristol Myers Squibb (Inst), Cardiff Oncology (Inst), Foundation Medicine (Inst), Puma Biotechnology (Inst)
Paul J. Catalano
Research Funding: Regeneron (Inst), Astellas Pharma (Inst), AstraZeneca (Inst)
Hans Hammers
Honoraria: Bristol Myers Squibb
Consulting or Advisory Role: Bristol Myers Squibb, Pfizer, Exelixis, Bayer, Novartis, Merck, ARMO BioSciences, Corvus Pharmaceuticals, Surface Oncology, Lilly
Research Funding: Bristol Myers Squibb (Inst), Merck (Inst), Aravive (Inst), Surface Oncology (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, Merck, Pfizer, Lilly, Novartis
No other potential conflicts of interest were reported.
REFERENCES
- 1.Motzer RJ, Escudier B, McDermott DF, et al. : Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 373:1803-1813, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Escudier B, George S, et al. : Nivolumab versus everolimus in patients with advanced renal cell carcinoma: Updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer 126:4156-4167, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heng DY, Xie W, Regan MM, et al. : External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: A population-based study. Lancet Oncol 14:141-148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Tannir NM, McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 378:1277-1290, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albiges L, Tannir NM, Burotto M, et al. : Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 5:e001079, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choueiri TK, Fishman MN, Escudier B, et al. : Immunomodulatory activity of nivolumab in metastatic renal cell carcinoma. Clin Cancer Res 22:5461-5471, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gul A, Stewart TF, Mantia CM, et al. : Salvage ipilimumab and nivolumab in patients with metastatic renal cell carcinoma after prior immune checkpoint inhibitors. J Clin Oncol 38:3088-3094, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cogswell J, Inzunza HD, Wu Q, et al. : An analytical comparison of Dako 28-8 PharmDx assay and an E1L3N laboratory-developed test in the immunohistochemical detection of programmed death-ligand 1. Mol Diagn Ther 21:85-93, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rimm DL, Han G, Taube JM, et al. : A prospective, multi-institutional, pathologist-based assessment of 4 immunohistochemistry assays for PD-L1 expression in non-small cell lung cancer. JAMA Oncol 3:1051-1058, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimm M-O, Esteban E, Barthélémy P, et al. : Efficacy of nivolumab/ipilimumab in patients with initial or late progression with nivolumab: Updated analysis of a tailored approach in advanced renal cell carcinoma (TITAN-RCC). J Clin Oncol 39, 2021. (abstr 4576) [Google Scholar]
- 11.McDermott DF, Huseni MA, Atkins MB, et al. : Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med 24:749-757, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDermott DF, Lee JL, Bjarnason GA, et al. : Open-label, single-arm phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced clear cell renal cell carcinoma. J Clin Oncol 39:1020-1028, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKay RR, McGregor BA, Xie W, et al. : Optimized management of nivolumab and ipilimumab in advanced renal cell carcinoma: A response-based phase II study (OMNIVORE). J Clin Oncol 38:4240-4248, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tannir NM, Signoretti S, Choueiri TK, et al. : Efficacy and safety of nivolumab plus ipilimumab versus sunitinib in first-line treatment of patients with advanced sarcomatoid renal cell carcinoma. Clin Cancer Res 27:78-86, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott DF, Cheng SC, Signoretti S, et al. : The high-dose aldesleukin “select” trial: A trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clin Cancer Res 21:561-568, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tannir NM, Motzer RJ, McDermott DF, et al. : First-line nivolumab plus ipilimumab (NIVO+IPI) versus sunitinib (SUN) in patients with long-term survival of ≥5 years in the CheckMate 214 trial. Int Kidney Cancer Soc 2021, 2021. (abstr CTR11) [Google Scholar]
- 17.Regan MM, Jegede OA, Mantia CM, et al. : Treatment-free survival after immune checkpoint inhibitor therapy versus targeted therapy for advanced renal cell carcinoma: 42-Month results of the CheckMate 214 trial. Clin Cancer Res 27:6687-6695, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rini BI, Motzer RJ, Powles T, et al. : Atezolizumab plus bevacizumab versus sunitinib for patients with untreated metastatic renal cell carcinoma and sarcomatoid features: A prespecified subgroup analysis of the IMmotion151 clinical trial. Eur Urol 79:659-662, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powles T, Plimack ER, Soulieres D, et al. : Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial. Lancet Oncol 21:1563-1573, 2020 [DOI] [PubMed] [Google Scholar]
- 20.Choueiri TK, Powles T, Burotto M, et al. : Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 384:829-841, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motzer R, Alekseev B, Rha SY, et al. : Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 384:1289-1300, 2021 [DOI] [PubMed] [Google Scholar]
- 22.Choueiri TK, Motzer RJ, Rini BI, et al. : Updated efficacy results from the JAVELIN renal 101 trial: First-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma. Ann Oncol 31:1030-1039, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choueiri TK, Figueroa DJ, Fay AP, et al. : Correlation of PD-L1 tumor expression and treatment outcomes in patients with renal cell carcinoma receiving sunitinib or pazopanib: Results from COMPARZ, a randomized controlled trial. Clin Cancer Res 21:1071-1077, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, Banchereau R, Hamidi H, et al. : Molecular subsets in renal cancer determine outcome to checkpoint and angiogenesis blockade. Cancer Cell 38:803-817.e4, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ficial M, Jegede OA, Sant'Angelo M, et al. : Expression of T-cell exhaustion molecules and human endogenous retroviruses as predictive biomarkers for response to nivolumab in metastatic clear cell renal cell carcinoma. Clin Cancer Res 27:1371-1380, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choueiri TK, Tomczak P, Park SH, et al. : Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med 385:683-694, 2021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Upon request, and subject to review, HCRN and Georgetown University will provide the data that support the clinical and biomarker findings of this study to interested investigators or regulatory authorities. Subject to certain criteria, conditions, and exceptions, HCRN and Georgetown University may also provide access to individual deidentified participant data to such entities. Data can be requested by e-mailing Dr Michael B. Atkins at mba41@georgetown.edu. Potential access to data will be available from the date of manuscript publication through at least December 31, 2027.