Abstract
In this retrospective analysis, we describe weekly croup and corresponding viral prevalence patterns in a pediatric quaternary care system in metropolitan Atlanta. We characterize a series of 24 patients with croup associated with SARS-CoV-2 infection and show that this clinical presentation increased substantially in frequency during the period of high Omicron vs Delta transmission.
Keywords: children, COVID-19, croup, SARS-CoV-2, tissue adaptation, tropism
Croup represents inflammation of the subglottic upper airway mucosa in children associated with respiratory distress due to epithelial-level edema and airflow obstruction. The majority of croup presentations to the emergency department (ED) are attributable to acute viral infection. Similar to other viral-associated respiratory illnesses, such as bronchiolitis and asthma exacerbations, croup presentations tend to fluctuate in frequency, depending on the local prevalence of specific viral pathogens. Approximately 75% of cases of croup are caused by 1 of 4 parainfluenza virus (PIV) subtypes, with the remaining 25% caused by common seasonal respiratory “cold” viruses that are included in standard multiplex respiratory viral panel (RVP) testing, including endemic coronaviruses [1–3]. Before widespread circulation of the SARS-CoV-2 Omicron variant, less than 10 cases of croup associated with SARS-CoV-2 infection had been published in which multiplex RVP testing was used to confirm the presence of SARS-CoV-2 RNA while excluding other viral etiologies [4–8].
Between December 13, 2021 and January 2, 2022, as metropolitan Atlanta had a sudden spike in the prevalence of SARS-CoV-2 cases, clinicians within our system noted an unexpected increase in ED presentations of croup. We therefore sought to (1) characterize the clinical features of croup associated with SARS-CoV-2 in a series of children presenting during the period of high Omicron variant transmission and (2) identify ED visits for acute SARS-CoV-2 infection and compare the frequency of croup among these children during this Omicron-dominant 3-week study period and a preceding 2-month period during which the Delta variant was dominant in summer 2021.
METHODS
To ascertain the potential novelty of SARS-CoV-2 strain-related clinical presentation, we analyzed patterns of ED visits for COVID-19 diagnosis (ICD-10, U07.1) during 2 time periods with proportional caseloads and where SARS-CoV-2 strain dominance was known: period 1 – Omicron-dominant outbreak (December 13, 2021 to January 2, 2022); period 2 – Delta-dominant outbreak (July 1, 2021 to August 31, 2021) [9]. Among these visits with COVID-19 diagnosis, we then identified those with concurrent croup diagnosis (ICD-10, J05.0) and compared the frequency of this co-association between the 2 time periods. Additionally, we sought to characterize the differential impact on younger children during these 2 outbreaks, by comparing proportional differences in 0- to 4-year-old children diagnosed with COVID-19 during the 2 time periods. Chi-square test for independence was used to confirm the significance of the proportional difference during the 2 time periods.
For the case series, we described patients with croup presenting to the ED during the Omicron-dominant outbreak (December 13, 2021 to January 2, 2022) who had positive RVP testing for SARS-CoV-2 and negative for coinfections. Patients were excluded for the following reasons: no testing was done; or testing was done but could not exclude PIV coinfection. Therefore, those whose testing was done as only a single-plex for SARS-CoV-2 or a 4-plex test (SARS-CoV-2, respiratory syncytial virus [RSV], influenza A and B) were excluded. Additionally, those who had full multiplex RVP testing completed were excluded from the case series for the following reasons: if they were positive for more than 1 pathogen; if they were positive for a pathogen other than SARS-CoV-2; or the RVP results were negative for all pathogens, including SARS-CoV-2 (Supplementary Figure). The multiplex RVP tests include polymerase chain reaction (PCR) testing of the following pathogens: SARS-CoV-2; PIV subtypes 1-4; rhinovirus/enterovirus (RV/EV); RSV; human metapneumovirus (HMPV); influenza A + B, adenovirus; seasonal coronaviruses (229E, HKU1, NL63, OC43); Chlamydophila pneumoniae; Bordetella pertussis; Bordetella parapertussis; and Mycoplasma pneumoniae (BioFire 2.1, bioMerieux, Durham, NC, USA). In general, routine use of RVP testing is discouraged in our system and is more likely to be ordered for ill-appearing patients at the discretion of the clinician. Policies for RVP utilization in our system and availability during the reflected time periods did not differ. This study was approved by the institutional review board at Children’s Healthcare of Atlanta.
RESULTS
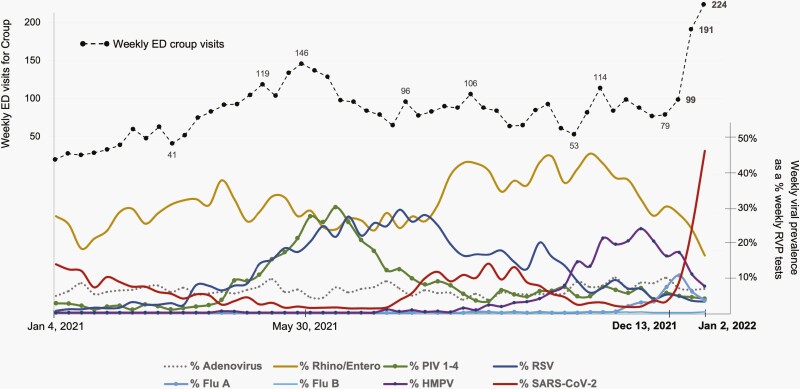
Between January 4, 2021 and January 2, 2022, our system encountered nearly 218 387 ED visits, with 34 670 (15.9%) of those visits leading to inpatient admission. During this 52-week period, a total of 19 865 (median of 382 RVPs per week), multiplex RVP specimens were collected between ED and inpatient units on two of the medical campuses within our system. Results of these fluctuating weekly RVP positivity data are displayed in the lower half of Figure 1 on a pathogen-specific basis.
Figure 1.
Patterns of weekly ED visits with croup diagnosis and corresponding weekly virus prevalence in a pediatric quaternary care system. January 4, 2021 to January 2, 2022. Abbreviation: ED, emergency department.
During the 3-week period ending on January 2, 2022, weekly ED croup visits for our system nearly tripled, in parallel to increasing SARS-CoV-2 prevalence (Figure 1). The overall admission rate for croup presentations during this 3-week study period fluctuated from 12.1% to 15.6%. In comparison, during a peak in PIV activity (May-June 2021) associated with a substantial increase in croup presentations to the ED, hospital admission rates for croup fluctuated from 11.6% to 14.4%. Remarkably, during this 3-week Omicron period, the prevalence of all other respiratory viruses decreased substantially. In contrast, the ongoing activity of RSV and RV/EV during the previous SARS-CoV-2 Delta surge (also shown in Figure 1) contributed to unprecedented hospital volumes and strain for our system during August 2021.
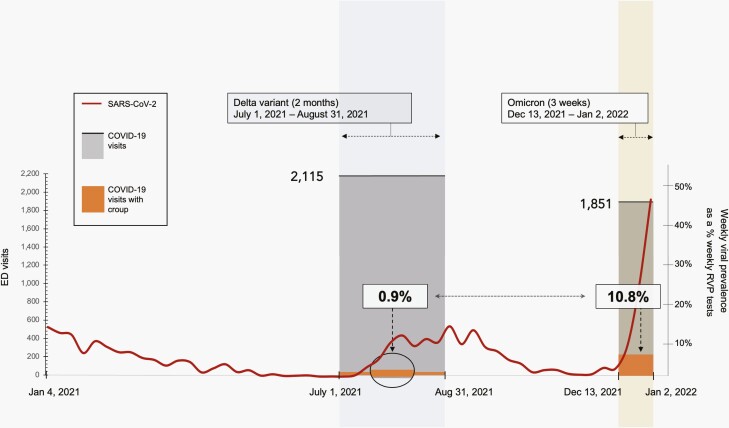
During the 2-month Delta-dominant period, there were 44 940 total ED visits. Of those visits, 2115 (4.7%) were associated with a COVID-19 diagnosis. Of these COVID-19 visits, 609 (28.8%) were 0-4 years of age, and 107 (17.6%) of those 0- to 4-year-old visits were associated with hospital admission. Twenty (0.9%) of the 2115 COVID-19 visits also included a diagnosis of croup. In comparison, during the 3-week Omicron-dominant period, there were 15 423 total ED visits, 1851 (12%) of which were associated with COVID-19 diagnosis. Of those COVID-19 visits, 951 (51.2%) were 0-4 years of age, a substantial 77.8% increase in the proportion of this age group during Omicron vs Delta outbreaks (P < .001). Of these 951 0- to 4-year-old visits for COVID-19, 155 (16.1%) were associated with hospital admission. Two hundred (10.8%) of the 1851 COVID-19 visits also included a diagnosis of croup, representing a 12-fold increase in the frequency of croup associated with COVID-19 (P < .001) (Figure 2).
Figure 2.
Frequency of laryngotracheitis (croup) among ED visits with COVID-19 diagnosis. Delta vs Omicron SARS-Cov-2 outbreaks within a pediatric quaternary care system. Abbreviation: ED, emergency department.
To further characterize the clinical features of croup associated with SARS-CoV-2 infection, we then identified 36 patients with croup who underwent testing by multiplex RVP. Twenty-four (66.7%) of those patients were exclusively SARS-CoV-2-positive. Among these 24 patients, the median age was 12 months (interquartile range [IQR], 5, 21.5). Eighteen (75%) of these patients were male and 6 (25%) were female. In terms of ethnicity, 10 (41.7%) were Caucasian, 7 (29.2%) were African American, 5 (20.8%) were Hispanic, and 2 (8.3%) were Asian. Thirteen (54.2%) were discharged home from the ED, while 11 (45.8%) were admitted to the hospital, 2 of whom (18.2%) were admitted to the intensive care unit (ICU). One patient required supplemental oxygen with heliox. The median length of stay (LOS) for those admitted to the hospital was 24 hours (IQR, 24, 36). Patient management was according to the standard clinical pathway for croup in our system (Supplementary Table and Supplementary Figure).
DISCUSSION
During a 3-week period from December 13, 2021 through January 2, 2022, we found a substantial increase in croup presentations during a decline in the prevalence of all other respiratory viral pathogens known to cause croup. These patients presented during a surge of the SARS-CoV-2 Omicron variant, which was first identified in South Africa in November 2021 and became the dominant circulating strain in metropolitan Atlanta by mid-December 2021 [9]. While our case series of 24 patients with croup associated with SARS-CoV-2 showed frequent hospitalization (45.8%) and ICU admission (18.2%), interpretation of clinical severity is limited by the fact that RVP testing was done in only a fraction of those with croup and was more likely to have been done in more ill-appearing patients needing hospital admission.
The Omicron variant has been associated with increased transmissibility and attenuated lower respiratory tract disease in comparison to SARS-CoV-2 Delta variant [10–12]. Although previous reports indicate that croup associated with SARS-CoV-2 presentation likely existed prior to emergence of the Omicron variant [4–8], the frequency increased substantially during the Omicron outbreak. Additionally, the proportion of 0- to 4-year-old children diagnosed with COVID-19 nearly doubled during Omicron, in comparison to the Delta-dominant period. These data underscore the ongoing need for vaccination efforts in this susceptible population. As with any observational data, bias could have played a role in the identification of croup and COVID-19 during the Omicron study period. However, the magnitude of change we detail between the 2 study periods makes this less likely.
Altogether, our observational clinical findings support recent animal and ex vivo models suggesting that in comparison to Delta, the Omicron variant displays upper respiratory tissue tropism, a process by which a pathogen adapts to new tissue as an infectious target, as a means of improving fitness for survival and transmissibility. As a subset of upper respiratory tract infection, croup in children has an “adult” equivalent: laryngitis. Interestingly, a recent study in Sweden described a series of young adult patients presenting with odynophagia and laryngitis in the setting of Omicron infection, adding further support for evolving tissue tropism [13].
CONCLUSIONS
Our data provide further evidence of a croup clinical infectious syndrome associated with SARS-CoV-2 that appears to resemble clinical features of croup caused by other respiratory viruses and increased substantially in frequency during the Omicron variant surge. However, our data also indicate a dramatic increase in frequency of younger children being diagnosed with COVID-19 during ED visits in association with the Omicron variant, when compared with a recent Delta variant surge. Overall, this supports ongoing efforts to immunize younger children, who continue to be adversely affected by COVID-19.
Supplementary Material
Notes
Acknowledgments. We would like to thank Dr. Daniel Hirsh and Aziza Mustafa for their assistance with this project, as well as Children’s Healthcare of Atlanta for clinical and technology support that helped make this project possible.
Financial support. There was no funding for this project to report.
Potential conflicts of interest. S.K.’s institution has received funding from NIH to conduct clinical trials of Moderna and Janssen COVID-19 vaccines. His institution has received funding from Pfizer to conduct clinical trials of Pfizer COVID-19 vaccine. C.A.R.’s institution has received funding to conduct clinical research unrelated to this manuscript from BioFire Inc., GSK, MedImmune, Micron, Merck, Novavax, PaxVax, Regeneron, Pfizer, and Sanofi-Pasteur. She is co-inventor of patented RSV vaccine technology unrelated to this manuscript, which has been licensed to Meissa Vaccines, Inc. Her institution has received funding from NIH to conduct clinical trials of Moderna and Janssen COVID-19 vaccines. All authors have submitted the ICMJE Form for Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
Sujit Sharma, Pediatric Emergency Medicine Associates (PEMA), LLC, Atlanta, Georgia, USA; Children’s Healthcare of Atlanta, Division of Emergency Medicine, Atlanta, Georgia, USA; PhenoPhys, LLC, Atlanta, Georgia, USA.
Beesan Agha, Pediatric Emergency Medicine Associates (PEMA), LLC, Atlanta, Georgia, USA; Children’s Healthcare of Atlanta, Division of Emergency Medicine, Atlanta, Georgia, USA.
Carlos Delgado, Pediatric Emergency Medicine Associates (PEMA), LLC, Atlanta, Georgia, USA; Children’s Healthcare of Atlanta, Division of Emergency Medicine, Atlanta, Georgia, USA.
Karen Walson, Children’s Healthcare of Atlanta, Division of Critical Care Medicine, Atlanta, Georgia, USA.
Charles Woods, Department of Pediatrics, University of Tennessee College of Medicine, Chattanooga, Tennessee, USA.
Mark D Gonzalez, Children’s Healthcare of Atlanta, Division of Pathology, Atlanta, Georgia, USA.
Robert Jerris, Children’s Healthcare of Atlanta, Division of Pathology, Atlanta, Georgia, USA.
Gregory Sysyn, Children’s Healthcare of Atlanta, Division of Critical Care Medicine, Atlanta, Georgia, USA.
James Beiter, Pediatric Emergency Medicine Associates (PEMA), LLC, Atlanta, Georgia, USA; Children’s Healthcare of Atlanta, Division of Emergency Medicine, Atlanta, Georgia, USA.
Satoshi Kamidani, Children’s Healthcare of Atlanta, Division of Infectious Disease, Atlanta, Georgia, USA; Department of Pediatrics, Division of Pediatric Infectious Disease, Emory University School of Medicine, Atlanta, Georgia, USA.
Christina A Rostad, Children’s Healthcare of Atlanta, Division of Infectious Disease, Atlanta, Georgia, USA; Department of Pediatrics, Division of Pediatric Infectious Disease, Emory University School of Medicine, Atlanta, Georgia, USA.
References
- 1. Rihkanen H, Rönkkö E, Nieminen T, et al. Respiratory viruses in laryngeal croup of young children. J Pediatr 2008; 152:661–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams JV, Harris PA, Tollefson SJ, et al. Human metapneumovirus and lower respiratory tract disease in otherwise healthy infants and children. N Engl J Med 2004; 350:443–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bjornson CL, Johnson DW.. Croup in children. CMAJ 2013; 185:1317–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pitstick CE, Rodriguez KM, Smith AC, Herman HK, Hays JF, Nash CB.. A curious case of croup: laryngotracheitis caused by COVID-19. Pediatrics 2021; 14:7. [DOI] [PubMed] [Google Scholar]
- 5. Tsoi K, Chan KC, Chan L, Mok G, Li AM, Lam HS.. A child with SARS-CoV2-induced croup. Pediatr Pulmonol 2021; 56:2377–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Venn AMR, Schmidt JM, Mullan PC.. Pediatric croup with COVID-19. Am J Emerg Med 2021; 43:287.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cassedy EA, Kim S, Silver AH.. An unexpected cause of croup in a pediatric patient. Clin Pediatr 2021; 60:574–6. [DOI] [PubMed] [Google Scholar]
- 8. Brewster RCL, Parsons C, Laird-Gion J, et al. COVID-19-associated croup in children. Pediatrics 2022. doi: 10.1542/peds.2022-056492 [DOI] [PubMed] [Google Scholar]
- 9. CDC. COVID Data Tracker. Accessed January 25, 2022. https://covid.cdc.gov/covid-data-tracker/#variant-proportions
- 10. Halfmann PJ, Iida S, Iwatsuki-Horimoto K, et al. SARS-CoV-2 Omicron virus causes attenuated disease in mice and hamsters. Nature 2022; 603:687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lundberg AL, Lorenzo-Redondo R, Ozer EA, et al. Has Omicron changed the evolution of the pandemic? JMIR Public Health Surveill 2022; 8:e35763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hui KPY, Ho JCW, Cheung M-C, et al. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 2022;603:715–20. [DOI] [PubMed] [Google Scholar]
- 13. Piersiala K, Kakabas L, Bruckova A, Starkhammar M, Cardell LO.. Acute odynophagia: a new symptom of COVID-19 during the SARS-CoV-2 Omicron variant wave in Sweden. J Intern Med 2022. :1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.