Abstract
Background
Like other vaccines, Pfizer BioNTech’s COVID-19 vaccine efficacy against SARS-CoV-2 virus infections begins to decline within a few months after the 2nd dose. On August 12, 2021, the FDA allowed additional Pfizer BioNTch’s COVID-19 vaccine dose (3rd or booster dose) for individuals with weakened immunity. This study aimed to evaluate the short-term adverse reactions (ADRs) of the 2nd and the 3rd doses of the Pfizer BioNTech COVID-19 vaccine.
Methods
Information for this study was collected by Google Form questionnaire (online survey). The results included responses from 442 people, the majority from Saudi Arabia.
Results
The most common local ADRs following the 3rd dose were injection site pain, injection site hypersensitivity, and axillary lymph node swelling. The most common systemic ADRs were fatigue, muscle pain, bone pain, headache, and fever less than 38ºC. Less common systemic ADRs were shivering, fever more than 38ºC, nasal congestion and rhinorrhea, arrhythmia, cough, abdominal pain, chest tightness, nausea, diarrhea, vomiting, and tachypnea. Rare systemic ADRs were constipation, dizziness and vertigo, lack of concentration, sore throat, excessive hair loss, dysmenorrhea and heavy menstruation, and Bell’s palsy. Severe allergic reactions were reported by 2.6% of participants after the 2nd dose, compared with none after the 3rd dose. Nasal congestion and runny nose are more frequent after the 3rd dose. The ADRs of the 2nd and 3rd doses were significantly more prevalent in females. 12% of participants reported ADRs lasting more than one week after the 3rd dose compared to 5% after the 2nd dose. People ≤ 60 years were more affected by the vaccine ADRs.
Conclusion
Most of the ADRs reported after the 3rd vaccine dose were consistent with the Pfizer vaccine information sheet and similar to the 2nd dose ADRs.
Keywords: Pfizer-BioNTech COVID-19 vaccine, adverse drug reactions, booster dose, second dose, injection site pain, fatigue, online questionnaire
Background
The outbreak of the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) in December 2019 caused the pandemic of coronavirus disease 2019 (COVID-19), which killed millions of people worldwide.1 Vaccines designed to target the spike (S) protein of SARS-CoV-2 (Moderna, Pfizer BioNTech, Janssen, and Oxford–AstraZeneca) have been manufactured and given to nearly a billion patients throughout the world.2,3 Two doses of Pfizer BioNTech’s vaccine are given at 21-day intervals. The maximum effect is about 95%, reached one week after the 2nd dose.4,5
Despite the high infection rate of the SARS-CoV-2 virus, the double dose of Pfizer BioNTech’s COVID-19 vaccination program effectively reduced hospitalization and mortality rates.6–8 However, like other vaccines, their efficacy against infections begins to decline within a few months after the 2nd dose.9 Waning immunity also referred to as a secondary vaccine failure, may be reversed through administering another vaccine dosage. The terms “additional dose” or “booster dose” were used to refer to the 3rd dose of the two mRNA vaccines, Pfizer BioNTech and Moderna.
On August 12, 2021, the Food and Drug Administration (FDA) renewed the emergency use authorization (EUA) for the COVID-19 vaccines made by Pfizer BioNTech (for individuals over 12 years old) and Moderna (for individuals over 18 years old) and allowing additional vaccine doses for individuals with weakened immunity. With the flood of new SARS-CoV-2 mutants, countries have begun to provide high-risk groups, and more recently, all adult populations, with a COVID-19 vaccine booster dose.10
The preliminary evidence suggested a robust humoral response after the 3rd Pfizer BioNTech vaccine dose.2,11,12 Booster vaccination with the Pfizer BioNTech vaccine reduced the incidence of infections and the severity of the COVID-19 disease.11,13 In randomized controlled studies, booster vaccination with mRNA-based immunizations were found to be safe and efficacious.14,15
At this time, it is unclear whether the rate and severity of the adverse reactions following the booster dose of the Pfizer BioNTech COVID-19 vaccine are comparable to the 2nd dose. Given that some adverse effects occurred following the 2nd dose but not the first dose of the vaccine, it is crucial to report and follow up on detecting the adverse effects following the booster dose. Moreover, our research group is interested in gathering the side effects of the coronavirus vaccine, we previously published a research article which was the first in its category concluding Pfizer BioNTech COVID-19 vaccine’s second dose side effects,4 and in this manuscript we will shed light on the side effects of the 3rd dose side effects.
The current study aimed to evaluate the short-term side effects after receiving the 2nd and/or the 3rd doses of the Pfizer BioNTech COVID-19 vaccine in a sample of Saudi Arabia residents.
Method
Study Protocol
A cross-sectional study was done retrospectively. The research was carried out for six weeks, from December 15th, 2021, to February 1st, 2022. All the participants in the study received three doses of any of the COVID-19 vaccines. The research ethics committee at the Faculty of Pharmacy, University of Tanta, Egypt, approved the study protocol under Reference No TP/RE/01-22-P-001. The survey was created to meet the guidelines of the Council for International Organizations of Medical Sciences and the local institutional regulations that govern different pharmaceutical research disciplines. A Google form-based questionnaire was prepared in both Arabic and English versions and sent to participants through social media (mainly but not limited to WhatsApp and Facebook). When needed, electronic mail was used to communicate between the investigators and the participants in the study. The questionnaire was validated by our research group and was used in a previously published manuscript.4
There were two types of questions on the questionnaire. The first type covers the subject’s background information, including name (optional), gender, nationality, age category, level of education, and chronic diseases. The second set of questions focused on the information related to COVID-19 infection and vaccination, including the type of vaccine for each of the three doses, the timing of receiving the 3rd dose, previous history of infection and its severity, and timing related to vaccination. The form also included questions about the participants’ symptoms following receiving the 2nd and the 3rd dose and the duration of their persistence. The participants were asked to select from a list of symptoms, including allergic reactions, pain at the administration site, fever, shivering, fatigue, bone or muscle pain, sore throat, headache, tachycardia, limbs’ edema, lymph node swelling, or facial paralysis. The list of symptoms also included abdominal and respiratory-tract associated symptoms. The participants were also instructed to state any other symptoms they experienced and were not listed in the form. At any point during the study, any participant was allowed to opt out of the survey. All precautionary measures were considered to protect the data’s confidentiality.
Inclusion Criteria
Inclusion included the administration of three doses of Pfizer BioNTech’s COVID-19 vaccines.
Exclusion Criteria
Participants who have not been vaccinated against COVID-19, received less than three doses, or received a vaccine other than Pfizer BioNTech’s COVID-19 vaccine.
Sample Size
The minimal sample size for this questionnaire was 384, with a margin of error of 5% and a confidence level of 95%. The prevalence of adverse events in the population was assumed to be 50% ± 5 (FDA reported adverse events ranging from 14.2% to 84.1%). In this survey, the questionnaire was completed by 674 (643 Arabic forms and 31 English forms) individuals. The total number of participants who met the inclusion and exclusion criteria was 442.
Study Variables
The independent variables in this study included background characteristics such as gender and age group and vaccination timing. In contrast, information related to symptoms experienced after receiving the 2nd and 3rd doses was considered responses.
Statistical Analysis
Background information was analyzed using descriptive statistics. Counts and/or percentages were used to display the responses. To statistically analyze the responses, a Chi-squared test was employed. The statistical analysis was conducted using Prism® (version 8.4.0, GraphPad Software Inc., La Jolla, CA, USA) at a 95% significance level.
Results
Demographic Data, Health Status, Type of COVID-19 Vaccine, the Time Lag Between 2ndand 3rd Vaccine Dose, Previous COVID-19 Infection, Seriousness, and Infection Time
This study involved 442 people, all of Arab heritage. The majority were Saudis (395, 89.4%), with the others hailing from various countries (47, 10.6%). Males made up a small percent of the participants (27.8%), while females made up the bulk (72.2%). Eighty-three percent of the surveyed population were ≤ 60 years old, and 17% were ˃ 60 years old. University education accounted for 50.2% of the participants, while post-university education accounted for 31.7%, and pre-university education accounted for 18.1%. 63.4% of the participants reported no chronic diseases, while 36.4% were chronically diseased patients (58.4% hypertension, 49.7% diabetes, 6.8% immune diseases, 26.7% other diseases). All study populations (442, 100%) received three doses of the Pfizer BioNTech COVID-19 vaccine. The time lag between the 2nd and 3rd vaccine doses was ≤ 6 months for 48.4% of the participants and ˃ six months for 51.6%. Only 13.6% of the study population reported having SARS-CoV-2 (all were not severely ill), while 86.4% reported not being infected. 10% of the SARS-CoV-2 affected persons were infected after their 1st vaccine dose, 28.3% after the 2nd dose, 15% after the 3rd dose, and 46.7% before vaccination, Table 1.
Table 1.
Demographic Data, Health Status, Type of COVID-19 Vaccine, the Time Lag Between 2nd and 3rd Vaccine Dose, Previous COVID-19 Infection, Seriousness, and Infection Time
| Characteristic | Frequency (n and %) |
|---|---|
| Nationality | (n=442) |
| Saudi | 395 (89.4%) |
| Non-Saudi | 47 (10.6%) |
| Sex | (n=442) |
| Male | 123 (27.8%) |
| Female | 319 (72.2%) |
| Age (year) | (n=442) |
| ≤ 60 | 367 (83.0%) |
| > 60 | 75 (17.0%) |
| Education level | (n=442) |
| Pre-university education | 80 (18.1%) |
| University education | 222 (50.2%) |
| Post-university education | 140 (31.7%) |
| Chronic diseases | (n=442) |
| No | 281 (63.6%) |
| Yes | 161 (36.4% |
| Hypertension | 94 (58.4%) |
| Diabetes | 80 (49.7%) |
| Immune diseases | 11 (6.8%) |
| Others | 43 (26.7%) |
| Type of COVID-19 vaccine (1st, 2nd, and 3rd doses) | (n=442) |
| Pfizer BioNTech vaccine | 442 (100%) |
| Time lag between 2nd and 3rd vaccine dose | (n=442) |
| ≤ 6 months | 214 (48.4%) |
| > 6 months | 228 (51.6%) |
| COVID-19 infection | (n=442) |
| No | 382 (86.4%) |
| Yes | 60 (13.6%) |
| Seriousness of infection | (n=60) |
| No | 60 (100%) |
| Yes | 0.0 (0.0%) |
| Time of infection | (n=60) |
| After 1st vaccine dose | 6 (10.0%) |
| After 2nd vaccine dose | 17 (28.3%) |
| After 3rd vaccine dose | 9 (15.0%) |
| Before vaccination | 28 (46.7%) |
Note: Data were presented as frequency; number (n) and percentage (%).
Adverse Reactions Reported After the 3rd Pfizer BioNTech COVID-19 Vaccine
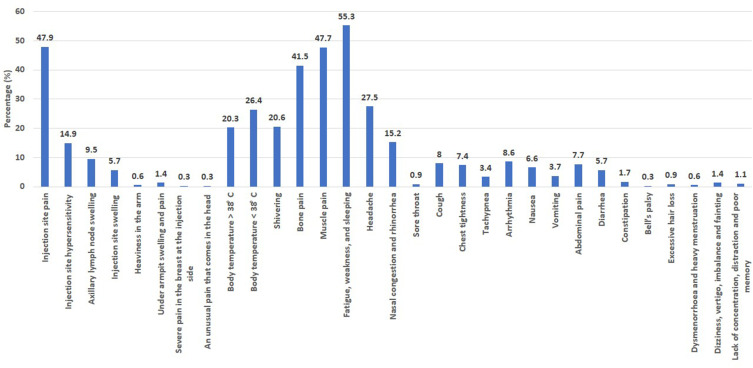
A long list of local and systemic adverse reactions was reported by the 442 individuals who participated in this study, Table 2 and Figure 1. The most common symptoms were fatigue, weakness, and sleeping (55.3%), injection site pain (47.9%), muscle pain (47.7%), bone pain (41.5%), headache (27.5%), mild increase in body temperature ˂ 38ºC (26.4%), severe increase in body temperature ˃ 38ºC (20.4%), shivering 20.6%), nasal congestion and rhinorrhea (15.2%), injection site hypersensitivity (14.9%), axillary lymph node swelling (9.5%), arrhythmia (8.6%), cough (8%), abdominal pain (7.7%), and chest tightness (7.4%).
Table 2.
Adverse Reactions Reported After the 2nd and 3rd Pfizer BioNTech COVID-19 Vaccine
| Frequency (n and %) | |||
|---|---|---|---|
| Dose 2 (n = 442) | Dose 3 (n = 442) | Chi-Square | |
| p value | |||
| Adverse reactions | |||
| With AR | 340 (76.9%) | 349 (79.0%) | 0.5165 |
| Without AR | 102 (23.1%) | 93 (21.0%) | |
| Local adverse reactions | |||
| Injection site pain | 251 (73.8%) | 167 (47.9%) | ˂0.0001*** |
| Injection site hypersensitivity | 34 (10.0%) | 52 (14.9%) | 0.0647 |
| Axillary lymph node swelling | 17 (5.0%) | 33 (9.5%) | 0.0274* |
| Injection site swelling | 9 (2.6%) | 20 (5.7%) | 0.0567 |
| Heaviness in the arm | 2 (0.6%) | 2 (0.6%) | 1.0000 |
| Under armpit swelling and pain | 2 (0.6%) | 5 (1.4%) | 0.4509 |
| Neck spasm | 1 (0.3%) | 0 (0.0%) | 0.4935 |
| Severe pain in the breast area on the side of the injection | 0 (0.0%) | 1 (0.3%) | 1.0000 |
| An unusual pain that comes in the head from the left side if the head is touched behind the ear like a pin, only when touched | 1 (0.3%) | 1 (0.3%) | 1.0000 |
| Systemic adverse reactions | |||
| Body temperature ˃ 38ºC | 58 (17.1%) | 71 (20.3%) | 0.2837 |
| Body temperature ˂ 38ºC | 104 (30.6%) | 92 (26.4%) | 0.1117 |
| Shivering | 69 (20.3%) | 72 (20.6%) | 0.9250 |
| Bone pain | 121 (35.6%) | 145 (41.5%) | 0.1177 |
| Muscle pain | 137 (39.0%) | 164 (47.7%) | 0.0779 |
| Fatigue, weakness, and sleeping | 173 (50.9%) | 193 (55.3%) | 0.2529 |
| Headache | 86 (25.3%) | 96 (27.5%) | 0.5455 |
| Nasal congestion and rhinorrhea | 32 (9.4%) | 53 (15.2%) | 0.0273* |
| Sore throat | 13 (3.8%) | 3 (0.9%) | 0.0108* |
| Cough | 18 (5.3%) | 28 (8.0%) | 0.1708 |
| Chest tightness | 29 (8.5%) | 26 (7.4%) | 0.6738 |
| Tachypnea | 7 (2.1%) | 12 (3.4%) | 0.3533 |
| Arrhythmia | 25 (7.4%) | 30 (8.6%) | 0.5762 |
| Nausea | 19 (5.6%) | 23 (6.6%) | 0.6346 |
| Vomiting | 11 (3.2%) | 13 (3.7%) | 0.8363 |
| Abdominal pain | 20 (5.9%) | 27 (7.7%) | 0.3668 |
| Diarrhea | 20 (5.9%) | 20 (5.7%) | 1.0000 |
| Constipation | 2 (0.6%) | 6 (1.7%) | 0.2865 |
| Severe allergy (angioedema) | 9 (2.6%) | 0 (0.0%) | 0.0016** |
| Bell’s palsy | 0 (0.0%) | 1 (0.3%) | 1.0000 |
| Excessive hair loss | 4 (1.2%) | 3 (0.9%) | 0.7219 |
| Loss of appetite | 1 (0.3%) | 0 (0.0%) | 0.4935 |
| Dysmenorrhoea and heavy menstruation | 2 (0.6%) | 2 (0.6%) | 1.0000 |
| Dizziness, vertigo, imbalance and fainting | 8 (2.4%) | 5 (1.4%) | 0.4140 |
| Lack of concentration, distraction and poor memory | 6 (1.8%) | 4 (1.1%) | 0.5410 |
Notes: Data were presented as frequency; number (n) and percentage (%). Correlation between variables was assessed using Fisher’s exact test. *p˂0.05, **p˂0.01, and ***p˂0.001.
Figure 1.
A column presentation showed the adverse reactions reported after the 3rd Pfizer-BioNTech vaccine doses. Data were presented as the percentage of individuals who have experienced a particular adverse reaction relative to the total number of individuals who have experienced the adverse reactions (n=442).
Comparison Between the Adverse Reactions Reported After the 2nd and 3rd Pfizer BioNTech COVID-19 Vaccine
Table 2 showed all the local and systemic adverse reactions reported after the 2nd and 3rd doses of the Pfizer BioNTech COVID-19 vaccine. 76.9% and 79% of the study population suffered adverse reactions after receiving their 2nd and 3rd vaccine doses, respectively, without significant differences (p=5165), Table 2 and Figure 2.
Figure 2.
Comparison between the percentage of individuals with and without adverse reactions after their 2nd and 3rd COVID-19 Pfizer-BioNTech vaccine doses. p value derived from Fisher’s exact test.
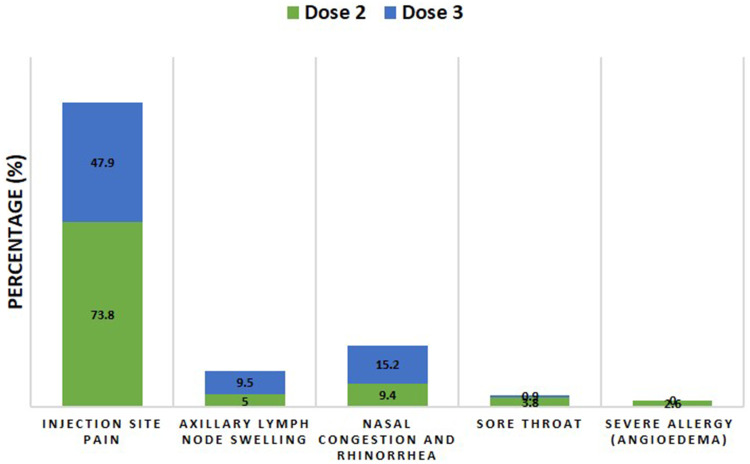
Concerning the post-vaccination local adverse reactions, there was a significant difference between the injection site pain post 2nd and 3rd vaccine doses (p˂0.0001); a higher percentage of the participants (73.8%) were impacted after the 2nd compared to 47.9% after 3rd dose. On the other hand, axillary lymph node swelling was significantly present after the 3rd vaccine dose (9.5%) compared to after the 2nd dose (5%) (p=0.0274), Table 2 and Figure 3.
Figure 3.
A stacked column presentation compared adverse reactions with significant differences (p˂0.05, Fisher’s exact test) reported after 2nd and 3rd Pfizer-BioNTech vaccine doses. Data were presented as the percentage of individuals who have reported a particular adverse reaction relative to the total number of individuals who have reported any adverse reaction (n=340 for the 2nd dose and 349 for 3rd dose).
Regarding the post-vaccination systemic adverse reactions, there were significant differences between the sore throat (p=0.0108) and severe allergy (angioedema) (p=0.0016) post 2nd and 3rd vaccine doses. A higher percentage of the participants (3.8%, sore throat and 2.6% severe allergy) were impacted after the 2nd doses compared to after the 3rd dose (0.9% sore throat and 0% severe allergy). Conversely, nasal congestion and rhinorrhea were significantly present after the 3rd vaccine dose (15.2%) compared to after the 2nd dose (9.4%) (p=0.0273), Table 2 and Figure 3.
For all other adverse reactions reported by the study population, there were no significant differences between those reported after the 2nd and 3rd doses of the vaccine, Table 2.
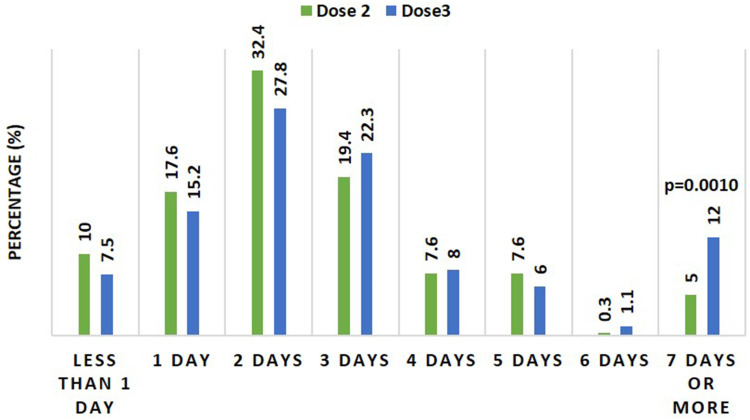
The durations of adverse reactions reported after the 2nd and 3rd doses of the vaccine were about the same, with most participants complaining for two days after vaccination. On the other hand, there was a significant difference (p=0.0010) between the percentage of participants who had their adverse reactions for seven days post-vaccination post 2nd and 3rd vaccine doses; a higher percentage of the participants (12%) were impacted after the 3rd compared to after 2nd dose (5%), Figure 4.
Figure 4.
A column presentation compared duration of adverse reactions reported after 2nd and 3rd Pfizer-BioNTech vaccine doses. Data were presented as the percentage of individuals who have reported a particular duration relative to the total number of individuals who have experienced the adverse reactions (n=340 for the 2nd dose and 349 for 3rd dose). p value derived from Fisher’s exact test.
Effect of Gender Difference on the Adverse Reactions Reported After the 2nd and 3rd Pfizer BioNTech COVID-19 Vaccine
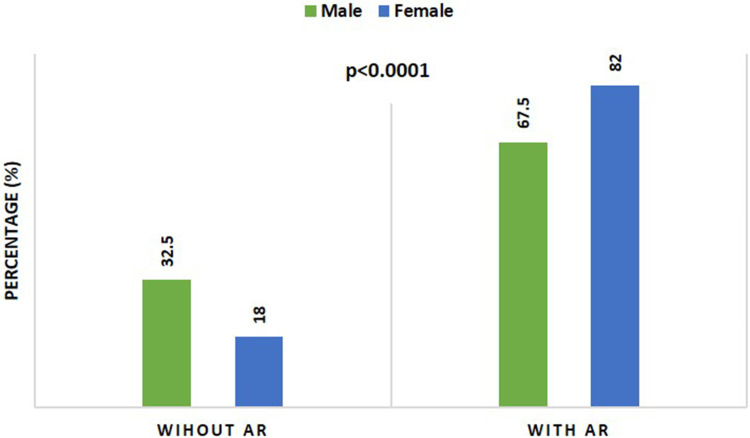
There was a significant increase (p ˂0.0001) in the number of females (523, 82%) who reported exposure to the vaccine adverse reactions compared to the number of males (166, 67.5%), Table 3 and Figure 5.
Table 3.
Adverse Reactions Reported After the 2nd and 3rd Pfizer-BioNTech COVID-19 Vaccine According to the Participant’s Gender
| Frequency n (%) | |||
|---|---|---|---|
| Male | Female | Chi-Square | |
| (n=246) | (n=638) | p value | |
| With adverse reactions (AR) | 166 (67.5%) | 523 (82.0%) | ˂0.0001*** |
| Without adverse reactions (AR) | 80 (32.5%) | 115 (18.0%) | |
| Local adverse reactions (LAR) | |||
|
97 (58.4%) | 321 (61.4%) | 0.5585 |
|
4 (2.4%) | 82 (15.7%) | ˂0.0001*** |
|
11 (6.6%) | 39 (7.5%) | 0.8512 |
|
0 (0.0%) | 29 (5.5%) | 0.0040** |
|
0 (0.0%) | 4 (0.8%) | 0.5866 |
|
1 (0.6%) | 6 (1.1%) | 0.8684 |
|
0 (0.0%) | 1 (0.2%) | 0.5444 |
|
0 (0.0%) | 1 (0.2%) | 0.5444 |
| An unusual pain that comes in the head from the left side if the head is touched behind the ear like a pin, only when touched | 0 (0.0%) | 2 (0.4%) | 0.9760 |
| Systemic adverse reactions (SAR) | |||
|
29 (17.5%) | 100 (19.1%) | 0.7183 |
|
41 (24.7%) | 155 (29.6%) | 0.2585 |
|
28 (16.9%) | 113 (21.6%) | 0.2270 |
|
40 (26.1%) | 226 (43.2%) | ˂0.0001*** |
|
56 (33.7%) | 245 (46.8%) | 0.0040** |
|
74 (44.6%) | 292 (55.8%) | 0.0146* |
|
26 (15.7%) | 156 (29.8%) | 0.0005*** |
|
17 (10.2%) | 68 (13.0%) | 0.4197 |
|
3 (1.8%) | 13 (2.5%) | 0.8337 |
|
9 (5.4%) | 37 (7.1%) | 0.5722 |
|
9 (5.4%) | 46 (8.8%) | 0.2176 |
|
6 (3.6%) | 13 (2.5%) | 0.6158 |
|
8 (4.8%) | 47 (9.0%) | 0.1184 |
|
3 (1.8%) | 39 (7.5%) | 0.0137* |
|
1 (0.6%) | 23 (4.4%) | 0.0375* |
|
2 (1.2%) | 45 (8.6%) | 0.0018** |
|
4 (2.4%) | 36 (6.9%) | 0.0503 |
|
0 (0.0%) | 8 (1.5%) | 0.2352 |
|
1 (0.6%) | 8 (1.5%) | 0.6000 |
|
1 (0.6%) | 0 (0.0%) | 0.5444 |
|
0 (0.0%) | 7 (1.3%) | 0.2919 |
|
0 (0.0%) | 1 (0.2%) | 0.5444 |
|
0 (0.0%) | 4 (0.8%) | 0.5866 |
|
0 (0.0%) | 13 (2.5%) | 0.0848 |
|
0 (0.0%) | 10 (1.9%) | 0.1550 |
Notes: Data were presented as frequency; number (n) and percentage (%). Correlation between variables was assessed using Fisher’s exact test. *p˂0.05, **p˂0.01, and ***p˂0.001.
Figure 5.
Comparison between the percentage of individuals with and without adverse reactions after their 2nd and 3rd COVID-19 Pfizer-BioNTech vaccine doses according to their gender. p value derived from Fisher’s exact test.
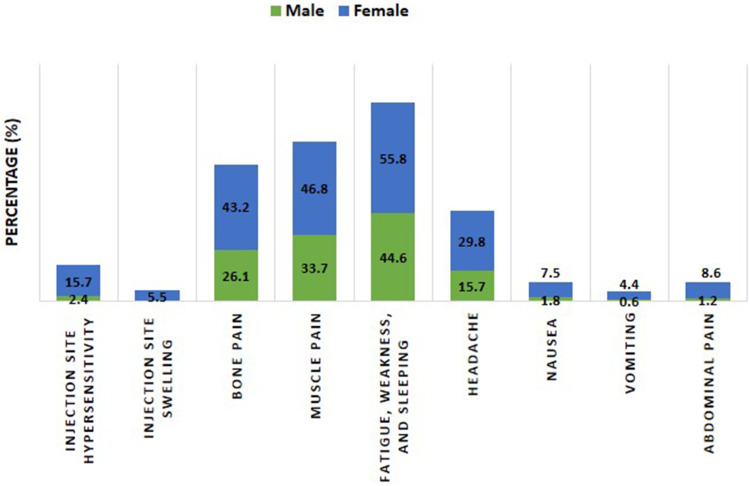
There were significant increases in females’ number who reported injection site hypersensitivity, injection site swelling, bone pain, muscle pain, fatigue, headache, nausea, vomiting, and abdominal pain compared to males’ number (p˂0.0001, p=0.0040, p˂0.0001, p=0.0040, p=0.0146, p=0.0005, p=0.0137, p=0.0375, p=0.0018, respectively), Table 3 and Figure 6.
Figure 6.
A stacked column presentation compared adverse reactions with significant differences (p˂0.05, Fisher’s exact test) reported after 2nd and 3rd Pfizer-BioNTech vaccine doses according to their gender. Data were presented as the percentage of individuals who have reported a particular adverse reaction relative to the total number of individuals who have reported any adverse reaction (n=166 for the males and 523 for females).
Effect of Age Difference on the Adverse Reactions Reported After the 2nd and 3rd Pfizer BioNTech COVID-19 Vaccine
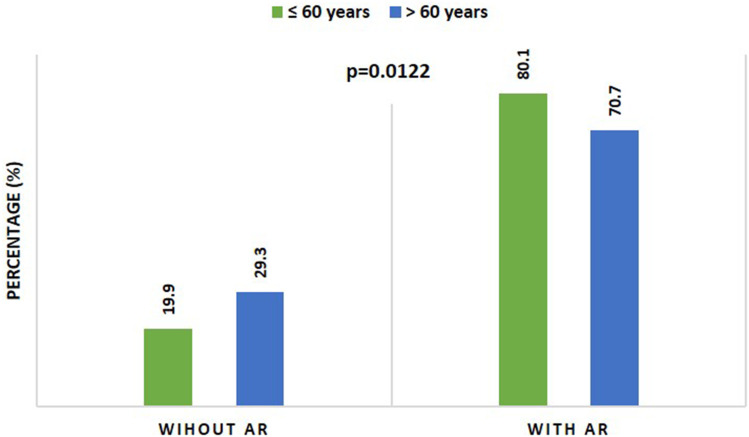
There was a significant increase (p=0.0137) in the number of participants aged ≤ 60 years (584, 80.1%) who reported exposure to the vaccine adverse reactions compared to the number of participants aged ˃ 60 years (105, 70.7%), Table 4 and Figure 7.
Table 4.
Adverse Reactions Reported After the 2nd and 3rd Pfizer-BioNTech COVID-19 Vaccine According to the Participant’s Age
| Frequency n (%) | |||
|---|---|---|---|
| ≤ 60 Years | ˃ 60 Years | Chi-Square | |
| (n=734) | (n=150) | p value | |
| With adverse reactions (AR) | 584 (80.1%) | 105 (70.7%) | 0.0137* |
| Without adverse reactions (AR) | 150 (19.9%) | 45 (29.3%) | |
| Local adverse reactions (LAR) | |||
|
346 (59.2%) | 72 (68.6%) | 0.0127* |
|
77 (13.2%) | 9 (8.6%) | 0.2475 |
|
45 (7.7%) | 5 (4.8%) | 0.3864 |
|
27 (4.6%) | 2 (1.9%) | 0.3109 |
|
3 (0.5%) | 1 (1.0%) | 0.8785 |
|
7 (1.2%) | 0 (0.0%) | 0.5491 |
|
1 (0.2%) | 0 (0.0%) | 0.3331 |
|
1 (0.2%) | 0 (0.0%) | 0.3331 |
|
2 (0.3%) | 0 (0.0%) | 0.7005 |
| Systemic adverse reactions (SAR) | |||
|
119 (13.9%) | 10 (9.5%) | 0.0128* |
|
161 (27.6%) | 35 (33.3%) | 0.2766 |
|
127 (21.7%) | 14 (13.3%) | 0.0664 |
|
240 (41.1%) | 26 (24.8%) | 0.0022** |
|
265 (45.4%) | 36 (34.3%) | 0.0452* |
|
311 (53.3%) | 55 (52.4%) | 0.9532 |
|
163 (27.9%) | 19 (18.1%) | 0.0477* |
|
72 (12.3%) | 13 (12.4%) | 0.8838 |
|
15 (2.6%) | 1 (1.0%) | 0.5090 |
|
40 (6.8%) | 6 (5.7%) | 0.8285 |
|
55 (9.4%) | 0 (0.0%) | 0.0021** |
|
19 (3.3%) | 0 (0.0%) | 0.1210 |
|
54 (9.2%) | 1 (1.0%) | 0.0074** |
|
39 (6.7%) | 3 (2.9%) | 0.1988 |
|
23 (3.9%) | 1 (1.0%) | 0.2123 |
|
44 (7.5%) | 3 (2.9%) | 0.1236 |
|
37 (6.3%) | 3 (2.9%) | 0.2393 |
|
8 (1.4%) | 0 (0.0%) | 0.4767 |
|
8 (1.4%) | 1 (1.0%) | 0.9045 |
|
1 (0.2%) | 0 (0.0%) | 0.3331 |
|
5 (0.9%) | 2 (1.9%) | 0.6470 |
|
1 (0.2%) | 0 (0.0%) | 0.3331 |
|
4 (0.7%) | 0 (0.0%) | 0.8785 |
|
10 (1.7%) | 3 (2.9%) | 0.6860 |
|
9 (1.5%) | 1 (1.0%) | 0.9831 |
Notes: Data were presented as frequency; number (n) and percentage (%). Correlation between variables was assessed using Fisher’s exact test. *p˂0.05 and **p˂0.01.
Figure 7.
Comparison between the percentage of individuals with and without adverse reactions after their 2nd and 3rd COVID-19 Pfizer-BioNTech vaccine doses according to their age categories. p value derived from Fisher’s exact test.
There was a significant increase in the number of participants aged ˃ 60 years who reported injection site pain (72, 68.6%) compared to the number of participants aged ≤ 60 years (346, 59.2%) (p=0.0127), Table 4 and Figure 8.
Figure 8.
A stacked column presentation compared adverse reactions with significant differences (p˂0.05, Fisher’s exact test) reported after 2nd and 3rd Pfizer-BioNTech vaccine doses according to their age categories. Data were presented as the percentage of individuals who have reported a particular adverse reaction relative to the total number of individuals who have reported any adverse reaction (n=584 for age ≤ 60 and 105 for age ˃ 60 years).
Conversely, there were significant increases in the number of participants aged ≤ 60 years who reported body temperature ˃ 38ºC, bone pain, muscle pain, headache, chest tightness, and arrhythmia compared to the number of participants aged ˃ 60 years (p=0.0128, p=0.0022, p=0.0452, p=0.0477, p=0.0021, p=0.0074, respectively), Table 4 and Figure 8.
Discussion
To date, there are few data on the safety of the 3rd dose (booster) of the Pfizer BioNTech COVID-19 vaccine. Therefore, the present study aimed to evaluate the short-term side effects after receiving the 2nd and/or the 3rd doses of the Pfizer BioNTech COVID-19 vaccine in a sample of Saudi Arabia residents. In the current study, 79% of the participants reported local and/or systemic adverse reactions after the 3rd dose of the Pfizer BioNTech COVID-19 vaccine. The most common local adverse reactions among participants following their booster dose were injection site pain (47.9%), injection site hypersensitivity (14.9%), and axillary lymph node swelling (9.5%). In comparison, the most common systemic adverse effects were fatigue (55.3%), muscle pain (47.7%), bone pain (41.5%), headache (27.5%), and fever less than 38ºC (26.4%). Less common systemic adverse reactions were shivering (20.6%), fever more than 38ºC (20.3%), nasal congestion and rhinorrhea (15.2%), arrhythmia (8.6%), cough (8%), abdominal pain (7.7%), chest tightness (7.4%), nausea (6.6%), diarrhea (5.7%), vomiting (3.7%), and tachypnea (3.4%). Rare adverse reactions were constipation (1.7%), dizziness and vertigo (1.4%), lack of concentration (1.1%), sore throat (0.9%), excessive hair loss (0.9%), dysmenorrhea and heavy menstruation (0.6%), and Bell’s palsy (0.3%). Most of the reported adverse reactions were mild to moderate, and no serious adverse events occurred among the participants. Around 7.5% of the participants reported adverse reactions that lasted for less than a day following the vaccination. The adverse reactions lasted two to three days in 27.8% and 22.3% of the participants, respectively. Only 12% of the participants reported adverse reactions that lasted seven days or more post-vaccination.
Our results are consistent with the ZOE-COVID study conducted in the United Kingdom. The most common systemic symptom reported was fatigue, and the most common local symptom was injection site pain. Our data conversely showed a higher prevalence of systemic side effects among the Saudi compared to the British population (55.3% vs 15.9%).10 Furthermore, our findings closely mirror the adverse effects documented previously in the Pfizer vaccine trial. In phase three clinical trial conducted by Pfizer, which included 300 participants, around 94.4% of the participants reported local and systemic adverse reactions after receiving the booster dose. Injection site pain (83%) was the most comment adverse reaction, followed by fatigue (63.7%), headache (48.4%), muscle pain (39%), and chills (29.1%). These adverse reactions were observed within seven days following the vaccination.16 The adverse effects recorded by Pfizer after the booster dose were similar to those reported after the two doses of the vaccine.
In this study, the reported local and systemic adverse reactions following the 3rd dose of the Pfizer BioNTech COVID-19 vaccine (booster) were consistent with those documented after the 2nd dose of the vaccine. However, pain around the injection site was less frequent after the booster dose (47.9%) than the pain after the 2nd dose (73.8%). This finding is consistent with the previous study reported by Menni et al.10 On the other hand, participants reported that swelling of axillary lymph nodes, a sign of the immune system’s response, was more common following the 3rd dose (9.5%) than after the 2nd dose (5%). Similarly, in a phase three clinical trial conducted by Pfizer, swelling of the lymph nodes was reported at a higher rate (5%) among participants who received the booster dose than those following the 2nd dose (0.4%), which lasted from two to eight days.16
Concerning systemic adverse reactions, severe allergy (angioedema) was reported by 2.6% of the participants following the 2nd dose; however, none of the participants have reported severe allergies following the booster dose. Similarly, in a phase three clinical trial conducted by Pfizer, no cases of anaphylaxis or Bell’s Palsy were reported following the 3rd dose.16 Severe allergic reactions following the 2nd dose of the vaccine are rare but have been documented by several groups.4,17 In contrast, respiratory symptoms, including nasal congestion and rhinorrhea, were more common following the 3rd dose of the vaccine.
The findings of the current study show that the adverse effects of the 2nd and 3rd Pfizer BioNTech COVID-19 vaccines were significantly more prevalent in females (82.0%) than males (67.5%). Specifically, of the 638 female participants (319 effectively receiving the 2nd and 3rd doses), 55.8% reported fatigue, weakness, and sleeping; 46.8% muscle pain; 43.2% bone pain; 29.8% headache; 15.7% injection site hypersensitivity; 8.6% abdominal pain; 7.5% nausea; 5.5% injection site swelling; and 4.4% vomiting, all significantly higher than male volunteers. This observation is very similar to previous studies on COVID-19 vaccines, which reported a significant association between the adverse effects and female sex.4,18,19 A possible explanation could be hormonal and genetic differences between genders.20,21 For instance, male sex hormones (androgens) have been shown to influence cytokine levels and immune responses resulting in immunosuppression.22
The durations of adverse reactions reported following the 2nd and 3rd doses were similar and lasted two to three days after vaccination. However, a higher percentage of individuals (12%) reported adverse effects that lasted over a week after the 3rd dose of the vaccine compared to after the 2nd dose (5%). Overall, local and systemic adverse reactions reported after the Pfizer BioNTech COVID19 vaccine booster dose lasted 1–2 days median.16 Adverse reactions that lasted over a week following the booster Pfizer BioNTech COVID-19 vaccine have not been reported yet.
Our study results showed significant differences in the adverse effects between the two age groups after vaccination. The younger age group (≤ 60 years old) was more affected by muscle pain (45.4%), bone pain (41.1%), headache (27.9%), body temperature above 38°C (13.9%), chest tightness (9.4%), and arrhythmia (9.2%) than the older age group (≥ 60 years old). The study by19 regarding the Pfizer BioNTech COVID-19 vaccine side effects among healthcare workers in the Czech Republic reported that injection site pain (89.8%), fatigue (62.2%), headache (45.6%), muscle pain (37.1%), and chills (33.9%) were more prevalent in the younger age group. This observation is similar to our study regarding muscle pain, headache, and chills. However, the injection site pain was more frequent in the older age group of our sample (68.6%) than in the younger age group (59.2%). Our previously published paper also showed that influenza-like symptoms were more common among those under 60 years of age, while pain at the injection site was more common among those above 60 years.4 While the elderly tend to display injection site reactions after vaccination, they are less prone to experience systemic adverse effects than younger people.23 Although it remains to be tested, this variation might be due to the decreased vaccine reactogenicity in the elderly.24
Limitations and Strengths
Few or no studies have documented the adverse reactions of the COVID-19 vaccine booster doses, especially post-marketing vaccine follow-up. The current study is one of the leading studies investigating the adverse reactions of Pfizer BioNTech COVID-19 vaccine booster dose. Furthermore, this study used a sample of the same person to compare the side effects that occurred after the 2nd and 3rd doses of the vaccine. Our study has several limitations. First, our observations included a limited number of participants, mainly from Saudi Arabia. Second, the observed adverse reactions were based on the patient’s self-reported data, which might result in misclassification or an information bias. Third, the survey was distributed via social media, and therefore the sample may not reflect the whole vaccinated population, leading to selection bias. Finally, the current study reported short-term adverse reactions following the booster dose. Future studies are urgently needed to evaluate the long-term adverse effects of the booster dose.
Conclusion
Our study is one of the first to investigate the adverse reactions of the Pfizer BioNTech COVID-19 vaccine booster dose in the Middle East. The observed adverse effects following the Pfizer BioNTech COVID-19 vaccine booster dose were mild to moderate and are similar to those observed following the first two doses. Our findings agree with previous studies and with those reported by the FDA Fact Sheet and manufacturing company.
Acknowledgments
This research work was funded by the Institutional Fund Projects under grant no. (IFPDP-20-22). Therefore, the authors gratefully acknowledge technical and financial support from the Ministry of Education and King Abdulaziz University, Deanship of Scientific Research, Jeddah, Saudi Arabia.
Data Sharing Statement
The current manuscript contains all the necessary data. The corresponding author can provide the data used in this study upon request.
Ethics Approval and Consent to Participate
The research ethics committee at the Faculty of Pharmacy, University of Tanta, Egypt, approved the study protocol under Reference No TP/RE/01-22-P-001.
In terms of the informed consent statement, study participants provided a form that they accepted the study once they completed the survey. This research was carried out in line with the Helsinki Declaration.
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Kumar A, Singh R, Kaur J, et al. Wuhan to world: the COVID-19 pandemic. Front Cell Infect Microbiol. 2021;11:242. doi: 10.3389/FCIMB.2021.596201/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu K, Choi A, Koch M, et al. Preliminary analysis of safety and immunogenicity of a SARS-CoV-2 variant vaccine booster. medrxiv.org. 2021. doi: 10.1101/2021.05.05.21256716 [DOI] [Google Scholar]
- 3.World Health Orgainisation. WHO news updates. WwwWhoInt; 2021. Available from: https://www.who.int/news-room/news-updates. Accessed April 14, 2022.
- 4.El-Shitany N, Harakeh S, Badr-Eldin S, et al. Minor to moderate side effects of Pfizer-BioNTech COVID-19 vaccine among Saudi residents: a retrospective cross-sectional study. Int J Gen Med. 2021;14:1389–1401. doi: 10.2147/IJGM.S310497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack F, Thomas S, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMOA2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haas E, McLaughlin J, Khan F, et al. Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer–BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Lancet Infect Dis. 2022;22(3):357–366. doi: 10.1016/S1473-3099(21)00566-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernal JL, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in England: test negative case-control study. BMJ. 2021;373:n1088. doi: 10.1136/bmj.n1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabezas C, Coma E, Mora-Fernandez N, et al. Associations of BNT162b2 vaccination with SARS-CoV-2 infection and hospital admission and death with covid-19 in nursing homes and healthcare workers in Catalonia: prospective cohort study. BMJ. 2021;374:n1868. doi: 10.1136/BMJ.N1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levin E, Lustig Y, Cohen C, et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/nejmoa2114583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menni C, May A, Polidori L, et al. COVID-19 vaccine waning and effectiveness and side effects of boosters: a prospective community study from the ZOE COVID study. Lancet Infect Dis. 2022;22:1002–1010. doi: 10.2139/ssrn.3980542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barda N, Dagan N, Cohen C, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–2100. doi: 10.1016/S0140-6736(21)02249-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ducloux D, Colladant M, Chabannes M, et al. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021;100(3):702–704. doi: 10.1016/J.KINT.2021.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bar-On Y, Goldberg Y, Mandel M, et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385(15):1393–1400. doi: 10.1056/NEJMOA2114255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu X, Shaw R, Stuart ASV, et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): a single-blind, randomised, non-inferiority trial. Lancet. 2021;398(10303):856–869. doi: 10.1016/S0140-6736(21)01694-9/ATTACHMENT/FB270FA3-26F7-4D43-AAE1-B475113E0263/MMC1.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munro APS, Janani L, Cornelius V, et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, Phase 2 trial. Lancet. 2021;398(10318):2258–2276. doi: 10.1016/S0140-6736(21)02717-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfizer V and RBPACMBD-S. BNT162b2 [COMIRNATY (COVID-19 vaccine, mRNA)] evaluation of a booster dose (Third Dose). Vaccines and Related Biological Products Advisory Committee September 17, 2021 Meeting Briefing Document- Sponsor. 2021:1–53.
- 17.Alhazmi A, Alamer E, Daws D, et al. Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Vaccines. 2021;9(6):674. doi: 10.3390/VACCINES9060674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayadevan R, Shenoy R. Survey of symptoms following COVID-19 vaccination in India. Public Glob Health. 2021. doi: 10.1101/2021.02.08.21251366 [DOI] [Google Scholar]
- 19.Riad A, Pokorná A, Attia S, et al. Prevalence of COVID-19 vaccine side effects among healthcare workers in the Czech Republic. J Clin Med. 2021;10(7):1428. doi: 10.3390/JCM10071428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anker M. Addressing sex and gender in epidemic-prone infectious diseases. World Health Organization; 2007:1–46. Available from: https://www.who.int/csr/resources/publications/SexGenderInfectDis.pdf. Accessed April 23, 2022. [Google Scholar]
- 21.Jacobsen H, Klein S. Sex differences in immunity to viral infections. Front Immunol. 2021;12:3483. doi: 10.3389/FIMMU.2021.720952/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Trigunaite A, Dimo J, Jørgensen T. Suppressive effects of androgens on the immune system. Cell Immunol. 2015;294(2):87–94. doi: 10.1016/J.CELLIMM.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 23.Hervé C, Laupèze B, Del Giudice G, et al. The how’s and what’s of vaccine reactogenicity. Npj Vaccines. 2019;4(1):39. doi: 10.1038/s41541-019-0132-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Yousfi M, Mercier S, Breuillé D, et al. The inflammatory response to vaccination is altered in the elderly. Mech Ageing Dev. 2005;126(8):874–881. doi: 10.1016/J.MAD.2005.03.008 [DOI] [PubMed] [Google Scholar]