Abstract
Upon osmotic downshock, a few cytoplasmic proteins, including thioredoxin, elongation factor Tu (EF-Tu), and DnaK, are released from Tris-EDTA-treated Escherichia coli cells by an unknown mechanism. We have shown previously that deletion of mscL, the gene coding for the mechanosensitive channel of the plasma membrane with the highest conductance, prevents the release of thioredoxin. We confirm and extend the implication of MscL in this process by showing that the release of EF-Tu and DnaK is severely impaired in MscL-deficient strains. Release of these proteins is not observed in the absence of a Tris-EDTA treatment which disrupts the outer membrane, indicating that, in intact cells, they are transferred to the periplasm upon shock, presumably through the MscL channel.
Mechanosensitive ion channels are channels gated by mechanical forces exerted on cell membranes and are present in a large variety of cells. High-conductance mechanosensitive channels are present in Bacteria (reviewed in reference 25) and Archaea (17). In Escherichia coli, patch-clamp experiments allowed the classification of three families of mechanosensitive channels, with conductances ranging from 100 to 1,500 pS (in 0.1 M KCl): MscM (M for mini), MscS (S for small), and MscL (L for large), which are activated at different thresholds of applied pressure (3). MscL, the channel with the highest conductance and which is activated at the highest membrane tension, has been cloned (24). The mscL gene codes for a 15-kDa protein located in the cytoplasmic membrane (6, 12). Several genes homologous to mscL have been identified in other gram-negative bacteria and in gram-positive bacteria (21). Recently, the pentameric structure of the MscL channel from Mycobacterium tuberculosis, in the closed conformation, was determined by X-ray crystallography (8).
When cultivated in high-osmolarity media, bacteria accumulate high concentrations of osmoprotectants (reviewed in reference 10). Upon a shift to low-osmolarity media, the excretion of these osmoprotectants is required to restore normal turgor and to prevent the cells from bursting. Mechanosensitive channels have been proposed to constitute the pathway for this efflux (5). Osmoprotectants are released at very high rates upon osmotic downshock, but deletion of the mscL gene does not impair the efflux of osmolytes, probably because the different mechanosensitive channels of E. coli constitute a redundant system (1). Indeed, a recent study indicates that E. coli cells lyse upon severe downshock only when both the mscL and yggB genes are deleted (18). The yggB gene is required for MscS activity, but whether it codes for this channel is still unknown. Although MscL alone is not essential for osmolyte efflux, its deletion prevented the release of thioredoxin triggered by osmotic shock (1). Thioredoxin belongs to a class of cytoplasmic proteins which include elongation factor Tu (EF-Tu) and DnaK and which are known to be excreted during osmotic shock from Tris-EDTA-treated E. coli cells (11, 13, 19). Efflux takes place under conditions in which other cytoplasmic proteins are retained and no lysis of the cells occurs (19). Because the release of these proteins is a process which is not yet understood, we have compared the effluxes of EF-Tu and DnaK triggered by osmotic downshock in wild-type and MscL-deficient E. coli strains.
All experiments were performed with the parental E. coli strain AW405 and various derivatives: E. coli AW405 (wild-type strain), AW405 carrying a chromosomal insertion in the mscL gene (the MscL-deficient strain), and an MscL- and RecA-deficient strain carrying the mscL expression plasmid p5-2-2 (restored strain) (24). Cells were grown in M9 minimal medium as previously described (1), and MscL protein synthesis was induced by addition of 2 mM IPTG (isopropyl-β-d-thiogalactopyranoside) to the restored strain. Cells were subjected to an osmotic shock as described in the figure legends and centrifuged. The presence of proteins EF-Tu and DnaK in the supernatant and the pellet was detected by immunoblotting.
Release of EF-Tu and DnaK upon osmotic shock in Tris-EDTA-treated E. coli cells was previously documented (11, 13). We show here that, in untreated cells, no release of EF-Tu and DnaK to the external medium was observed (Fig. 1A and 2A). In contrast, a partial release of both proteins was observed in Tris-EDTA-treated cells. The extent of this release was similar to that previously documented for each of these proteins (11, 13). In Tris-EDTA-treated cells, release of EF-Tu (Fig. 1B) and of DnaK (Fig. 2B) was blocked by gadolinium, an inhibitor of bacterial mechanosensitive channels (5). Since Tris-EDTA treatment disrupts the outer membrane, these results suggest that EF-Tu and DnaK are transferred, as is thioredoxin (1), from the cytoplasm to the periplasm during shock. When intact cells were subjected first to an osmotic downshock and then to a spheroplasting treatment (Tris-EDTA lysozyme treatment) to release periplasmic proteins (11), EF-Tu and DnaK were also partially released to the supernatant, but to a lesser extent (data not shown). Presumably, under these conditions, the loss of potassium, and hence of turgor, resulting from the osmotic shock prevents the spheroplasting procedure from being totally efficient in expelling proteins from the periplasm.
FIG. 1.
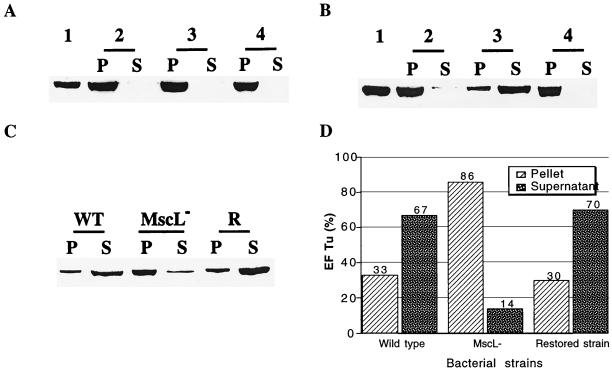
Shock-induced release of EF-Tu. Cells were resuspended at A650 = 5 in 10 mM Tris (pH 7.6) and 20% (wt/vol) sucrose, without (A) or with (B and C) 2.5 mM EDTA for 10 min at room temperature. Isosmotic dilution and hyposmotic shock were performed by diluting 200 μl of the suspension fivefold with the same buffer or distilled water. The suspension was centrifuged, and the presence of EF-Tu in the pellet and in the supernatant was revealed by immunoblotting as previously described (1), with rabbit antibody against EF-Tu. The pellet was suspended and boiled 4 min in 100 μl of electrophoresis buffer (50 mM Tris-HCl [pH 6.8], 2% sodium dodecyl sulfate, 0.2% bromophenol blue, 10% glycerol, 1% β-mercaptoethanol) and a 4-μl aliquot was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. A 200-μl aliquot of the supernatant was incubated overnight with 20% trichloroacetic acid and centrifuged. The precipitated proteins were suspended in 20 μl of electrophoresis buffer and boiled for 4 min, and 4 μl was subjected to electrophoresis. For determination of the total EF-Tu cell content, 1 ml of cells (A650 = 1) was centrifuged, the pellet was suspended in 100 μl of electrophoresis buffer, and a 4-μl sample was subjected to electrophoresis. (A) EF-Tu is not released by cells that are not treated with EDTA. Wild-type cells were suspended in Tris buffer and diluted fivefold with the same buffer or distilled water. Lane 1, total EF-Tu cell content before shock. The remaining lanes show EF-Tu in the pellet (P) and supernatant (S) after isosmotic dilution (lane 2), after osmotic shock (lane 3), and after osmotic shock in the presence of 1 mM gadolinium (lane 4). (B) Release of EF-Tu after EDTA treatment. Wild-type cells were suspended in Tris buffer containing 2.5 mM EDTA and diluted fivefold with the same buffer or distilled water. Lanes 1 to 4 are as above. (C) Release of EF-Tu is impaired in MscL-deficient cells. Wild-type (WT), MscL-deficient (MscL−), and restored (R) cells were EDTA treated and subjected to an osmotic shock. EF-Tu in the pellet and supernatant was revealed by immunoblotting. (D) Quantification by densitometry of the protein bands shown in C.
FIG. 2.
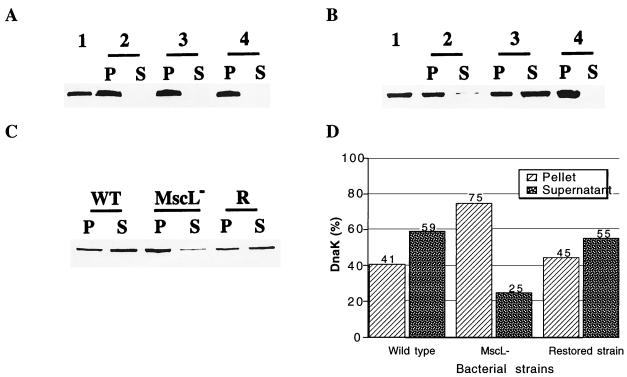
Shock-induced release of DnaK. Experiments were performed as described for Fig. 1, and the presence of DnaK was detected by immunoblotting as previously described (1), with rabbit antibody against DnaK. (A) DnaK is not released by cells that are not treated with EDTA. Wild-type cells were suspended in Tris buffer and diluted fivefold with the same buffer or distilled water. Lane 1, total EF-Tu cell content before shock. The remaining lanes show EF-Tu in the pellet (P) and supernatant (S) after isosmotic dilution (lane 2), after osmotic shock (lane 3), and after osmotic shock in the presence of 1 mM gadolinium (lane 4). (B) Release of DnaK after EDTA treatment. Wild-type cells were suspended in Tris buffer containing 2.5 mM EDTA and diluted fivefold with the same buffer or distilled water. Lanes 1 to 4 are as above. (C) Release of DnaK is impaired in MscL-deficient cells. Wild-type (WT), MscL-deficient (MscL−), and restored (R) cells were EDTA treated and subjected to an osmotic shock. DnaK in the pellet and supernatant was revealed by immunoblotting. (D) Quantification by densitometry of the protein bands shown in C.
In the MscL-deficient strain, the release of EF-Tu and DnaK was severely impaired and the majority of each protein was found in the cells after the osmotic shock (Fig. 1C and D and 2C and D). Significantly, in the restored strain, effluxes were similar to those found in the wild type (Fig. 1C and D and 2C and D). The data shown are representative of three separate experiments for each protein.
Previously, it has been proposed that cytoplasmic proteins that are released by osmotic downshock belong to an ill-defined osmotically sensitive compartment and that efflux occurs at membrane adhesion sites where the inner and outer membranes are contiguous (19). Some evidence for a partial association of thioredoxin with Bayer adhesion sites has been reported (2). The data presented here are not in favor of this hypothesis. Indeed, EF-Tu and DnaK, as well as thioredoxin (1), are not excreted to the external medium but are transferred to the periplasm, which makes the involvement of contact sites in this process unlikely. Rather, our experiments suggest that the excreted proteins are delivered to the periplasm mostly via the mechanosensitive channel MscL. Effluxes of EF-Tu and DnaK were completely blocked by gadolinium, but not completely inhibited in the MscL-deficient mutant, suggesting that the other mechanosensitive channels could be also implicated, but to a lesser extent. Interestingly, in electrophysiology experiments designed to study the localization of mechanosensitive channels, no significant enrichment of contact zones in these channels could be observed (4).
Efflux of proteins through a channel is certainly unusual, but it has to be kept in mind that the pore of MscL is extremely large. Its structure, as deduced from X-ray diffraction studies, is known only for the closed conformation (8). However, based on its extremely high conductance (1,500 pS in 0.1 M KCl) and based on permeation studies of large organic molecules, the diameter of the open pore was recently estimated to be around 40 Å (9). EF-Tu is composed of a polar head (45 Å by 40 Å by 40 Å) and a tail with a length of 55 Å and a diameter of 25 Å (14). Its size is therefore not incompatible with that of the MscL pore. The exact size of the full DnaK protein is not known. Moreover, although the 1,500-pS MscL channel is certainly the most stable and abundant species, higher degrees of oligomerization of the MscL subunit may exist and account for stretch-activated conductance higher than 1,500 pS, which is observed in patch-clamp experiments (3–5). These channels would have even wider pores.
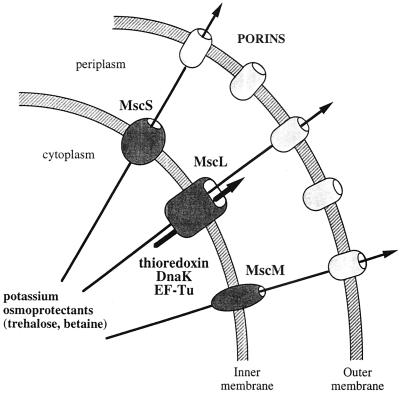
Figure 3 summarizes the possible sequence of events following an osmotic downshock. Upon shock, the membrane tension increases, inducing the opening of the different mechanosensitive channels. Osmolytes are released through these channels at the level of the plasma membrane and then through porins located in the outer membrane. The efflux of osmolytes results in a decrease in the cytoplasmic membrane tension and therefore triggers the closure of the channels. While the channels are open, cytoplasmic proteins, such as thioredoxin, DnaK, and EF-Tu, are released through MscL, the mechanosensitive channel with the highest conductance. They remain trapped in the periplasm unless the outer membrane is disrupted by a Tris-EDTA treatment. It is noteworthy that EF-Tu and DnaK are only partially released during shock while thioredoxin, which is a smaller protein, is totally excreted (1, 19). It therefore appears that, given the brief time during which the channels are open, the size of the excreted proteins is a limiting factor in the process.
FIG. 3.
Release of osmolytes and proteins during osmotic downshock. Osmotic downshock triggers the opening mechanosensitive channels in the plasma membrane. Osmolytes are released through MscL, MscS, and possibly MscM to the periplasm. Osmolytes can then diffuse out of the cell through porins. Cytoplasmic proteins such as thioredoxin, EF-Tu, and DnaK, are excreted mostly through MscL, the channel with the largest pore, to the periplasm, where they remain trapped because their size does not allow them to cross the outer membrane.
Does the transfer of thioredoxin, EF-Tu, and DnaK from the cytoplasm to the periplasm during osmotic shock have any physiological significance? It could be only a side effect resulting from the opening of MscL. However, if the only role of MscL is to catalyze the release of osmoprotectants such as glycine betaine or trehalose, why is its pore so large? Despite their apparent differences, the three proteins released by MscL have several common properties and their functions somewhat overlap. EF-Tu, in addition to its usual function in translation, was recently shown to catalyze disulfide formation and reduction, like thioredoxin (22). EF-Tu was also shown to have chaperone properties (7, 16) like those of DnaK and osmoprotectants (10). Moreover, the cellular level of both DnaK (15, 20) and thioredoxin (23) has been shown to increase upon an osmotic upshock, as does that of osmoprotectants. It is possible that after a shift back to low-osmolarity conditions, high concentrations of these proteins in the cytoplasm are deleterious to the cell or their presence is needed in the periplasm.
Acknowledgments
The strains used in this study were a gift from P. Blount. Rabbit antibody against EF-TU was a gift of A. Parmeggiani. We thank G. Moeck for correction of the manuscript and A. Boutan for technical assistance.
REFERENCES
- 1.Ajouz B, Berrier C, Garrigues A, Besnard M, Ghazi A. Release of thioredoxin via the mechanosensitive ion channel MscL during osmotic downshock of Escherichia coli cells. J Biol Chem. 1998;273:26670–26674. doi: 10.1074/jbc.273.41.26670. [DOI] [PubMed] [Google Scholar]
- 2.Bayer M E, Bayer M H, Lunn C A, Pigiet V. Association of thioredoxin with inner membrane and adhesion sites in Escherichia coli. J Bacteriol. 1987;149:2659–2666. doi: 10.1128/jb.169.6.2659-2666.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berrier C, Besnard M, Ajouz B, Coulombe A, Ghazi A. Multiple mechanosensitive ion channels from Escherichia coli, activated at different thresholds of applied pressure. J Membr Biol. 1996;151:175–187. doi: 10.1007/s002329900068. [DOI] [PubMed] [Google Scholar]
- 4.Berrier C, Coulombe A, Houssin C, Ghazi A. A patch-clamp study of ion channels of inner and outer membranes and of contact zones of Escherichia coli, fused into giant liposomes. Pressure-activated channels are localized in the inner membrane. FEBS Lett. 1989;259:27–32. doi: 10.1016/0014-5793(89)81486-3. [DOI] [PubMed] [Google Scholar]
- 5.Berrier C, Coulombe A, Szabó I, Zoratti M, Ghazi A. Gadolinium ion inhibits loss of metabolites induced by osmotic shock and large stretch-activated channels in bacteria. Eur J Biochem. 1992;206:559–565. doi: 10.1111/j.1432-1033.1992.tb16960.x. [DOI] [PubMed] [Google Scholar]
- 6.Blount P, Sukharev S I, Moe P C, Shroeder M J, Guy H R, Kung C. Membrane topology and multimeric structure of a mechanosensitive channel protein of Escherichia coli. EMBO J. 1996;15:4798–4805. [PMC free article] [PubMed] [Google Scholar]
- 7.Caldas T D, El Yaagoubi A, Richarme G. Chaperone properties of bacterial elongation factor EF-Tu. J Biol Chem. 1998;273:11478–11482. doi: 10.1074/jbc.273.19.11478. [DOI] [PubMed] [Google Scholar]
- 8.Chang G, Spencer R H, Lee A T, Barclay M T, Rees D C. Structure of the MscL homolog from Mycobacterium tuberculosis: a gated mechanosensitive ion channel. Science. 1998;282:2220–2225. doi: 10.1126/science.282.5397.2220. [DOI] [PubMed] [Google Scholar]
- 9.Cruickshank C C, Minchin R F, Le Dain A C, Martinac B. Estimation of the pore size of the large conductance mechano-sensitive ion channel of Escherichia coli. Biophys J. 1997;73:1925–1931. doi: 10.1016/S0006-3495(97)78223-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Csonka L N, Epstein W. Osmoregulation. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1210–1223. [Google Scholar]
- 11.El Yaacoubi A, Kohiyama M, Richarme G. Localization of DnaK (chaperone 70) from Escherichia coli in an osmotic-shock-sensitive compartment of the cytoplasm. J Bacteriol. 1994;176:7074–7078. doi: 10.1128/jb.176.22.7074-7078.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Häse C C, Minchin R F, Kloda A, Martinac B. Cross-linking studies and membrane localization and assembly of radiolabelled large mechanosensitive ion channel (MscL) of Escherichia coli. Biochem Biophys Res Commun. 1997;232:777–782. doi: 10.1006/bbrc.1997.6370. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson G R, Rosenbusch J P. Abundance and membrane association of elongation factor EF-Tu in E. coli. Nature. 1976;261:23–26. doi: 10.1038/261023a0. [DOI] [PubMed] [Google Scholar]
- 14.Kabsch W, Gast W H, Schulz G E, Leberman R. Low resolution structure of partially trypsin-degraded polypeptide elongation factor, EF-Tu, from Escherichia coli. J Mol Biol. 1977;117:999–1012. doi: 10.1016/s0022-2836(77)80009-0. [DOI] [PubMed] [Google Scholar]
- 15.Kilstrup M, Jacobsen S, Hammer K, Vogensen F K. Induction of heat shock proteins DnaK, GroEL, and GroES by salt stress in Lactococcus lactis. Appl Environ Microbiol. 1997;63:1826–1837. doi: 10.1128/aem.63.5.1826-1837.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kudlicki W, Coffman A, Kramer G, Hardesty B. Renaturation of rhodanese by translational elongation factor (EF) Tu. Protein refolding by EF-Tu flexing. J Biol Chem. 1997;272:32206–32210. doi: 10.1074/jbc.272.51.32206. [DOI] [PubMed] [Google Scholar]
- 17.Le Dain A C, Saint N, Kloda A, Ghazi A, Martinac B. Mechanosensitive ion channels of the archaeon Haloferax volcanii. J Biol Chem. 1998;273:12116–12119. doi: 10.1074/jbc.273.20.12116. [DOI] [PubMed] [Google Scholar]
- 18.Levina N, Totemeyer S, Stokes N R, Louis P, Jones M A, Booth I R. Protection of Escherichia coli cells against extreme turgor by activation of MscS and MscL mechanosensitive channels: identification of genes required for MscS activity. EMBO J. 1999;18:1730–1737. doi: 10.1093/emboj/18.7.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lunn C A, Pigiet V P. Localization of thioredoxin from Escherichia coli in an osmotically sensitive compartment. J Biol Chem. 1982;257:11424–11430. [PubMed] [Google Scholar]
- 20.Meury J, Kohiyama M. Role of heat shock protein DnaK in osmotic adaptation of Escherichia coli. J Bacteriol. 1991;173:4404–4410. doi: 10.1128/jb.173.14.4404-4410.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moe P C, Blount P, Kung C. Functional and structural conservation in the mechanosensitive channel implicates elements crucial for mechanosensation. Mol Microbiol. 1998;28:583–592. doi: 10.1046/j.1365-2958.1998.00821.x. [DOI] [PubMed] [Google Scholar]
- 22.Richarme G. Protein-disulfide isomerase activity of elongation factor EF-Tu. Biochem Biophys Res Commun. 1998;252:156–161. doi: 10.1006/bbrc.1998.9591. [DOI] [PubMed] [Google Scholar]
- 23.Scharf C, Reithdorf S, Ernst H, Engelmann H, Völker U, Hecker M. Thioredoxin is an essential protein induced by multiple stresses in Bacillus subtilis. J Bacteriol. 1998;180:1869–1877. doi: 10.1128/jb.180.7.1869-1877.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sukharev S I, Blount P, Martinac B, Blattner F R, Kung C. A large conductance mechanosensitive channel in E. coli encoded by mscl alone. Nature. 1994;368:265–268. doi: 10.1038/368265a0. [DOI] [PubMed] [Google Scholar]
- 25.Sukharev S I, Blount P, Martinac B, Kung C. Mechanosensitive channels of Escherichia coli: the MscL gene, protein, and activities. Annu Rev Physiol. 1997;59:633–657. doi: 10.1146/annurev.physiol.59.1.633. [DOI] [PubMed] [Google Scholar]