Abstract
Graves’ disease is an autoimmune disorder that results in hyperthyroidism, caused by autoantibodies to the thyrotropin receptor (TRAbs) stimulating thyroid hormone synthesis, giving rise to a variety of systemic manifestations such as goitre, dermatopathy and orbitopathy.
The authors present the case of a 28-year-old man admitted to hospital for a 3-week history of fatigue, shortness of breath, palpitations and diffuse goitre, after recent mild SARS-CoV-2 infection. Laboratory investigation revealed hyperthyroidism with TRAbs elevation. Thyroid ultrasound confirmed a diffusely heterogeneous and irregular thyroid gland and a nodular image below the sternal notch. Thyroid scintigraphy excluded the nodule and confirmed a Graves’ disease pattern. Following the initiation of methimazole, the patient had complete resolution of symptoms and normalization of thyroid values.
The results suggest a possible association between Graves’ disease and SARS-CoV-2 infection acting as a trigger. Graves’ disease is an important differential diagnosis to keep in mind when patients present with hyperthyroidism after COVID-19 disease.
LEARNING POINTS
Graves’ disease may be induced after SARS-CoV-2 infection by a possible autoimmune pathway.
Graves’ disease induced by SARS-CoV-2 infection responds well to antithyroid medication.
Keywords: Graves’ disease, SARS-CoV-2, hyperthyroidism
INTRODUCTION
Grave’s disease is an autoimmune disorder that results in hyperthyroidism, usually accompanied by an enlarged thyroid gland and occasionally ocular and dermatological manifestations. It is caused by autoantibodies to the thyrotropin receptor (TRAbs) that activate the receptor, thereby stimulating thyroid hormone synthesis and secretion causing a diffuse goitre, resulting in clinical thyrotoxicosis. Thyroglobulin antibodies and thyroid peroxidase antibodies may also be elevated.
A variety of immune mechanisms may be involved in the pathogenesis of Graves’ disease, with predisposing factors such as genetic susceptibility, and environmental factors such as viral infection, stress, smoking and pregnancy, also contributing.
CASE DESCRIPTION
A 28-year-old Caucasian man was admitted to the emergency department with a 3-week history of fatigue on medium exertion, shortness of breath and palpitations. He also reported a significant unexplained weight loss of 8 kg during this time, despite a normal appetite. The symptoms had started nearly 3 weeks after a mild SARS-CoV-2 infection. He did not have a history of other diseases or medication intake.
Physical examination showed non-tender, symmetrical, enlargement of the thyroid gland, but no exophthalmia. His heart rate was 130–150 bpm, sinus rhythm, and blood pressure was 130/65 mmHg. Laboratory investigation showed a thyroid-stimulating hormone (TSH) level of <0.001 mU/ml, free T4 of 7.11 ng/dl, and total T3 of 486 ng/dl. Autoimmunity work-up revealed elevated TRAbs at 8.1 IU/l (positive >1.5 IU/l), anti-peroxidase antibody at 478 IU/ml (<34 IU/ml) and anti-thyroglobulin antibodies at 352 IU/ml (<115 IU/ml). The thyroid values from the previous year were normal (Table 1).
Table 1.
Laboratory values
| May 2020 | January 2021 | February 2021 | July 2021 | Normal values | |
|---|---|---|---|---|---|
| TSH | 0.90 | <0.01 | <0.01 | 3.97 | 0.27–4.20 mU/l |
| Free T3 | 3.2 | 25.80 | 5.81 | 2.09 | 2.00–4.40 pg/ml |
| Free T4 | 10 | 17.8 | 9.3 | 0.95 | 0.93–1.70 ng/dl |
| TSH receptor antibody | |||||
| (TRAbs)7777 | 8.1 | Positive >1.5 IU/ml | |||
| Antithyroid peroxidase antibody | 478 | <34 IU/ml | |||
| Anti-thyroglobulin antibody | 352 | <115 IU/ml |
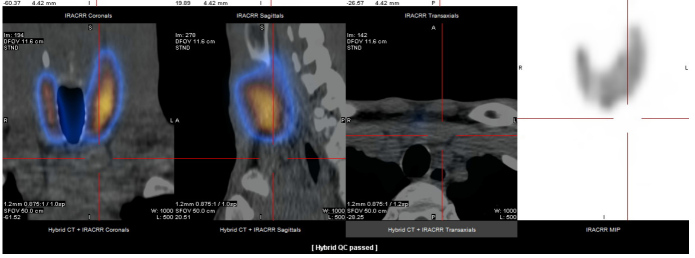
Thyroid ultrasound was performed and showed a diffusely heterogeneous and irregular thyroid, and a nodular image below the sternal notch. To confirm this nodular image, thyroid scintigraphy was performed, which excluded the nodule and confirmed a Graves’ disease pattern (Fig. 1).
Figure 1.
Thyroid scintigraphy excluded the nodule and confirmed a Graves’ disease pattern
Based on the above results and considering the temporal relationship between symptom onset and SARS-CoV-2 infection, the diagnosis of Graves’ disease caused by SARS-CoV-2 virus was established. The patient was discharged with methimazole 20 mg and propranolol 10 mg (for symptom control). After 1 month of therapy, there was symptomatic improvement and a laboratory pattern of subclinical hyperthyroidism was observed (Table 1), followed by complete resolution of symptoms and normalization of thyroid values (Table 1). Six months later, methimazole was discontinued, with no relapse of symptoms.
DISCUSSION
An association between SARS-CoV-2 infection and autoimmune disease is increasingly described, with many reports of patients developing autoantibodies after COVID-19 [1]. Data on SARS-CoV-2 thyroid involvement is scarce, but there are reports of Graves' disease, Hashimoto's disease and postpartum thyroiditis subsequent to SARS-CoV-2 infection [2, 3]. Reported cases of Graves’ disease induced by SARS-CoV-2 may present as a new diagnosis or recurrence in patients with a history of the condition. In the majority of cases, symptoms presented within 2 months of infection [1].
Viral infections are considered a major factor in the pathogenesis of autoimmune thyroid diseases [4, 5] and, although the mechanisms are not completely understood, several have been proposed to explain the link between SARS-CoV-2 infection and Graves' disease. SARS-CoV-2 enters host cells by binding to angiotensin-converting enzyme-2 (ACE2) followed by the subsequent recruitment of transmembrane protease serine 2 (TMPRSS2) to facilitate cytoplasmic entry. The thyroid gland expresses both ACE2 and TMPTSS2 in high quantities, allowing SARS-CoV-2 to enter thyroid cells and directly induce thyroid disease [6].
SARS-CoV-2 may also induce an exaggerated inflammatory response that could trigger a ‘cytokine storm’ that activates or reactivates Graves’ disease. This inflammatory process seems to be mediated by Th1/Th17 as well as interleukin-6, and may affect the hypothalamic-pituitary-thyroid axis, circulating thyroid hormone binding proteins and the peripheral metabolism of thyroid hormones [7–9]. Stress could also have a role in the pathogenesis of Graves’ relapse after COVID-19 [10].
Most patients respond very well to antithyroid medication [1], but it is not known how many relapse.
In our case, the absence of a history of thyroid disease suggested a new diagnosis of Graves’ disease. Additionally, the temporal sequence between SARS-CoV-2 infection and the onset of symptoms strongly suggested that Graves’ disease was triggered by SARS-CoV-2 infection. As in other reported cases, the symptoms began within 2 months of viral infection and responded well to antithyroid medication.
The present case emphasizes the need to consider SARS-CoV-2 infection as a trigger for autoimmune disease, and suggests Graves’ disease should be kept in mind as a differential diagnosis when patients present with hyperthyroidism after COVID-19.
Footnotes
Conflicts of Interests: The authors declare there are no competing interests.
REFERENCES
- 1.Mohammed SR, Tripathi M, Mack N, Teelucksingh S. Relapse of Graves' disease following SARS-CoV-2 infection: a case report and brief literature review. Med Int. 2021;1:25. doi: 10.3892/mi.2021.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feghali K, Atallah J, Norman C. Manifestations of thyroid disease post COVID-19 illness: report of Hashimoto thyroiditis, Graves' disease, and subacute thyroiditis. J Clin Transl Endocrinol Case Rep. 2021 Dec;22:100094. doi: 10.1016/j.jecr.2021.100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiménez-Blanco S, Pla-Peris B, Marazuela M. COVID-19: a cause of recurrent Graves’ hyperthyroidism? J Endocrinol Invest. 2021;44:387–388. doi: 10.1007/s40618-020-01440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prummel MF, Strieder T, Wiersinga WM. The environment and autoimmune thyroid diseases. Eur J Endocrinol. 2004;150(5):605–618. doi: 10.1530/eje.0.1500605. [DOI] [PubMed] [Google Scholar]
- 5.Marinò M, Latrofa F, Menconi F, Chiovato L, Vitti P. Role of genetic and non-genetic factors in the etiology of Graves' disease. J Endocrinol Invest. 2015;38:283–294. doi: 10.1007/s40618-014-0214-2. [DOI] [PubMed] [Google Scholar]
- 6.Duntas LH, Jonklaas J. COVID-19 and thyroid diseases: a bidirectional impact. J Endocr Soc. 2021;5(8):bvab076. doi: 10.1210/jendso/bvab076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costela-Ruiz VJ, Illescas-Montes R, Puerta-Puerta JM, Ruiz C, Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salvi M, Girasole G, Pedrazzoni M, Passeri M, Giuliani N, Minelli R, et al. Increased serum concentrations of interleukin-6 (IL-6) and soluble IL-6 receptor in patients with Graves' disease. J Clin Endocrinol Metab. 1996;81:2976–2979. doi: 10.1210/jcem.81.8.8768861. [DOI] [PubMed] [Google Scholar]
- 9.Croce L, Gangemi D, Ancona G, Liboà F, Bendotti G, Minelli L, et al. The cytokine storm and thyroid hormone changes in COVID-19. J Endocrinol Invest. 2021;44:891–904. doi: 10.1007/s40618-021-01506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marinò M, Latrofa F, Menconi F, Chiovato L, Vitti P. Role of genetic and non-genetic factors in the etiology of Graves' disease. J Endocrinol Invest. 2015;38:283–294. doi: 10.1007/s40618-014-0214-2. [DOI] [PubMed] [Google Scholar]