Abstract
Gradenigo syndrome (GS) was described primarily in the paediatric population, especially in the pre-antibiotic era. GS is rarely reported in the elderly population, especially in the post-antibiotic era. We present the rare case of a 67-year-old man who presented with an incomplete triad of symptoms (without abducens nerve palsy) of GS that failed medical therapy and was successfully treated with surgical intervention (mastoidectomy and petrous apicectomy). Physicians should be familiar with atypical presenting symptoms of GS as it can lead to life-threatening complications, especially in the elderly. GS cases resistant to medical therapy may require prompt appropriate imaging studies and surgical intervention.
LEARNING POINTS
Gradenigo syndrome may present with an incomplete triad (without abducens nerve palsy), especially in the post-antibiotic era.
Gradenigo syndrome can rarely affect immunocompromised elderly patients.
Gradenigo syndrome cases resistant to medical therapy may require prompt appropriate imaging studies and surgical intervention (mastoidectomy and petrous apicectomy).
Keywords: Gradenigo syndrome, elderly, abducens nerve palsy
INTRODUCTION
Gradenigo syndrome (GS) was first described by Giuseppe Gradenigo, one of the pioneers of otology in the late 19th century [1]. In 1904, Dr. Gradenigo published some cases of lateral gaze paralysis, all of which followed an otitis media episode [1]. In 1907, he introduced a triad of symptoms, consisting of deep facial pain resulting from trigeminal nerve involvement, diplopia secondary to abducens nerve palsy, and acute suppurative otitis media [1].
GS is a rare complication of chronic suppurative otitis media that occurs due to the involvement of the petrous apex of the temporal bone (petrous apicitis) [1]. GS was first described primarily in the paediatric population, especially in the pre-antibiotic era [2]. Although GS became rarer in the post-antibiotic era, this syndrome can still occur and cause serious complications, including cranial nerve palsies, intracranial abscess, meningitis, venous sinus thrombosis, subdural empyema and death [3]. Multiple cases of GS affecting adults have been reported in the last two decades as a rare complication of chronic otitis media [1, 4–6]. A systematic review performed by Gore [1] reported that the average age of GS patients was 22.4 years, while Gadre et al. [4] reported a mean age of 39.2 years. GS is rarely reported in the elderly population (age ≥65 years). Herein, we present a rare case of GS in a elderly man that presented with an atypical presentation in an immunocompetent elderly patient, failed medical therapy, and was successfully treated with surgical intervention (mastoidectomy and petrous apicectomy).
CASE DESCRIPTION
A 67-year-old man presented with throbbing left ear pain for 5 months that worsened over 2 weeks. It was associated with left-sided conductive hearing loss and left facial pain that worsened with chewing. He denied ear discharge, diplopia, blurry vision or facial weakness. Before hospitalization, the patient had received multiple courses of antibiotics and underwent myringotomy with no relief of these symptoms. Physical examination showed point tenderness of the left mastoid, left conductive hearing loss, and intact extra-ocular movement. The patient did not have leucocytosis (white blood cell count of 7.9) on presentation. His C-reactive protein was elevated at 2.1 mg/dl, and his erythrocyte sedimentation rate was elevated at 46 mm/hour. A CT scan of the facial bones showed complete opacification of the left mastoid air cells with extensive fluid in the left middle ear (Fig. 1). MRI of the brain showed left mastoid effusion and petrous apicitis involving the paths of the trigeminal, facial, vestibulocochlear and abducens nerves, consistent with Gradenigo syndrome (Fig. 2).
Figure 1.
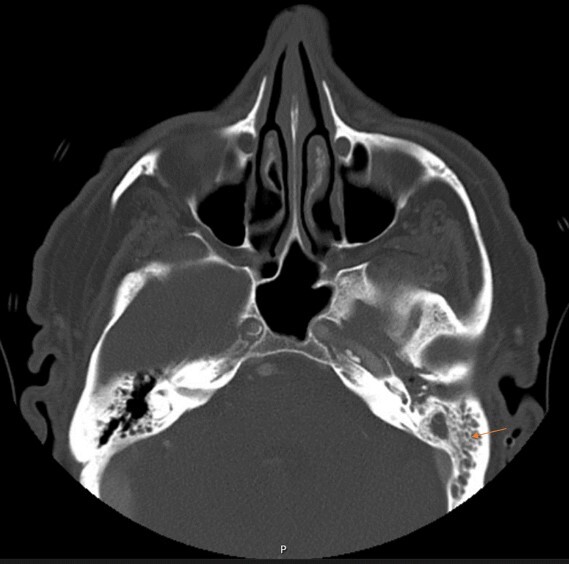
CT scan of facial bones showing complete opacification of the left mastoid air cells with extensive fluid in the left middle ear
Figure 2.
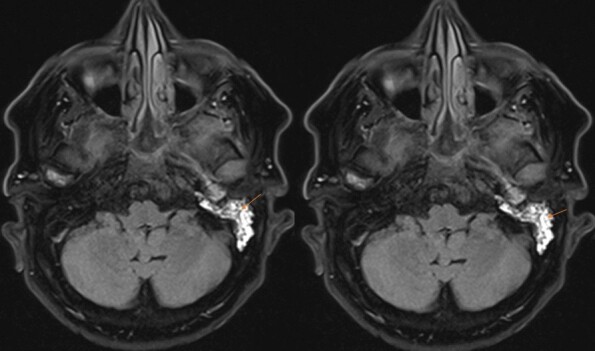
MRI of the brain showing left mastoid effusion and petrous apicitis involving the paths of the trigeminal, facial, vestibulocochlear and abducens nerves, consistent with Gradenigo syndrome
The patient was started on broad-spectrum IV antibiotics with vancomycin, cefepime and metronidazole. Left tympanoplasty with mastoidectomy and petrous apicectomy was performed. Intraoperative tissue cultures came back negative. Extensive laboratory work-up to rule out underlying immunodeficiency, including human immunodeficiency virus, serum immunoglobulins, and a CT scan of the neck and chest, was unremarkable. The patient’s facial and ear pain resolved within 2 days of surgery, and he was eventually discharged on doxycycline and amoxicillin/clavulanate for 4 weeks.
DISCUSSION
Petrous apicitis, an inflammation of the petrous apex of the temporal bone, is believed to be the hallmark of GS [1]. The abducens nerve and the trigeminal ganglion are vulnerable to extradural inflammation in petrous apicitis due to their close proximity to the petrous apex of the temporal bone [4]. Although GS is known for its triad of symptoms (deep facial pain, diplopia and otitis media), only 42% of patients presented with the full triad [4]. Our patient presented with only two symptoms without abducens nerve palsy. He did not complain of diplopia, and extra-ocular movement was normal on presentation, despite the involvement of the abducens nerve on MRI. It has been suggested that the wide use of antibiotics might mask symptoms or affect how patients present, but even old studies in the pre-antibiotic era suggested the triad alone is not a reliable diagnostic indicator.
CT scanning is considered a first-line diagnostic method for any petrous apex lesions, as it is widely available, sensitive and quick [7]. CT scans effectively detect bone erosions and air cell opacification [7]. MRI can provide further details including the aetiology of the lesion if not established, nerve involvement, possible underlying osteomyelitis, neoplastic lesions, and meningeal involvement [6, 7]. However, the CT scan did not show involvement of the apex of the petrous bone in our case, and an MRI was needed to confirm the involvement of trigeminal, facial, vestibulocochlear and abducens nerves. Therefore, physicians should maintain a high level of suspicion of GS in elderly patients with unresolving otitis media/mastoiditis. These patients should undergo MRI for prompt diagnosis and treatment to avoid life-threatening complications. Isolating the causative organism is essential to guide treatment but is not possible for all patients, sometimes secondary to prior antibiotic use. In our case, intra-operative cultures came back negative with no growth. In the retrospective cohort study performed by Gadre et al., cultures were positive in only 34% of patients [4].
Conservative treatment with intravenous antibiotics with or without tympanoplasty or myringotomy is now widely adopted, as opposed to aggressive surgical interventions, which are used if medical therapy fails or in case of intracranial abscess [1, 4]. Most patients with GS usually recover with intravenous antibiotics and those who require surgery recover with tympanostomy tube insertion or mastoidectomy[1,4]. Our patient required aggressive surgical management with mastoidectomy and petrous apicectomy, due to the lack of improvement with IV antibiotics and myringotomy. Only two paediatric cases of GS reported in the literature required aggressive surgical intervention with petrous apicectomy in addition to mastoidectomy [1]. To our knowledge, our case is the first atypical case of Gradenigo syndrome in an elderly man that required mastoidectomy and petrous apicectomy. We believe that our case adds to the published literature given that our case of GS in an elderly man presented with an atypical presentation without diplopia and was successfully treated with aggressive surgical treatment (mastoidectomy and petrous apicectomy).
In conclusion, we describe a rare case of Gradenigo syndrome in an immunocompetent elderly patient who presented without abducens nerve palsy and required surgical intervention with mastoidectomy and petrous apicectomy. It is essential for physicians to be familiar with presenting symptoms for this syndrome as it can lead to life-threatening complications, especially in the elderly population. As outlined above, GS cases resistant to medical therapy may require prompt appropriate imaging studies and surgical intervention.
Footnotes
Conflicts of Interests: The Authors declare that there are no competing interest
REFERENCES
- 1.Gore M. Gradenigo’s syndrome: a review. Ann Med Health Sci Res. 2018;8:220–224. [Google Scholar]
- 2.McLaren J, Cohen MS, El Saleeby CM. How well do we know Gradenigo? A comprehensive literature review and proposal for novel diagnostic categories of Gradenigo's syndrome. Int J Pediatr Otorhinolaryngol. 2020;132:109942. doi: 10.1016/j.ijporl.2020.109942. [DOI] [PubMed] [Google Scholar]
- 3.Burston BJ, Pretorius PM, Ramsden JD. Gradenigo's syndrome: successful conservative treatment in adult and paediatric patients. J Laryngol Otol. 2005;119(4):325–329. doi: 10.1258/0022215054020313. [DOI] [PubMed] [Google Scholar]
- 4.Gadre AK, Chole RA. The changing face of petrous apicitis-a 40-year experience. Laryngoscope. 2018;128(1):195–201. doi: 10.1002/lary.26571. [DOI] [PubMed] [Google Scholar]
- 5.Taklalsingh N, Falcone F, Velayudhan V. Gradenigo's syndrome in a patient with chronic suppurative otitis media, petrous apicitis, and meningitis. Am J Case Rep. 2017;18:1039–1043. doi: 10.12659/AJCR.904648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bano S, Nawaz A, Asmar A, Aemaz Ur Rehman M, Farooq H, Ali H. Gradenigo's syndrome presenting as IX and X cranial nerve palsy without clinically apparent ear infection: a case report and review of literature. eNeurologicalSci. 2022;27:100397. doi: 10.1016/j.ensci.2022.100397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackler RK, Parker DA. Radiographic differential diagnosis of petrous apex lesions. Am J Otol. 1992;13(6):561–574. [PubMed] [Google Scholar]


