Abstract
Background
We became aware through talking with people with asthma that some are using pulse oximeters to monitor their own blood oxygen levels during an asthma attack. Pulse oximeters are marketed by some suppliers as essential equipment for the home medicine cabinet. We wanted to find out if reliable evidence is available on use of pulse oximeters to self monitor asthma exacerbations at home. We decided to include only trials that used pulse oximeters as part of a personalised asthma action plan because it is important that decisions are made on the basis of symptoms as well as oxygen saturation, and that patients have a clear protocol to follow when their asthma worsens.
Objectives
To determine whether pulse oximeters used as part of a personalised asthma action plan for people with asthma are safer and more effective than a personalised asthma action plan alone.
Search methods
We searched the Cochrane Airways Group Specialised Register (CAGR), which includes reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and by handsearching. We also searched ClinicalTrials.gov and the World Health Organization (WHO) trials portal.
Selection criteria
We planned to include randomised controlled trials (RCTs). Participants would have included adults, children or both with a diagnosis of asthma. We planned to include trials in which investigators compared participants who used pulse oximeters to monitor oxygen levels at home during an asthma exacerbation as part of a personalised asthma action plan (PAAP) versus those who used a PAAP without a pulse oximeter. We planned to include studies involving people receiving any treatment regimen provided that no medicine was included as part of the randomisation schedule.
Data collection and analysis
We planned to use standard methods as recommended by The Cochrane Collaboration.
Main results
We found no studies and no evidence to support or refute the use of home pulse oximetry in self management of asthma; therefore, we can make no recommendations about use of a pulse oximeter as part of a PAAP.
Authors' conclusions
We found no reliable data to support or refute patient use of pulse oximeters to monitor oxygen saturation levels when experiencing an asthma attack. People should not use a pulse oximeter without seeking advice from a qualified healthcare professional.
We identified no compelling rationale for home monitoring of oxygen levels in isolation for most people with asthma. Some people have a reduced perception of the severity of their own breathlessness when exposed to hypoxia. If trials on self monitoring of oxygen levels in the blood by pulse oximeter at home by people with asthma are conducted, the pulse oximeter must be given as part of a personalised asthma action plan.
Plain language summary
Pulse oximeters used to self monitor oxygen saturation levels as part of a personalised asthma action plan for people with asthma
We became aware through talking with people with asthma that some are using pulse oximeters to monitor their own blood oxygen levels during an asthma attack. Pulse oximeters are marketed by some suppliers as essential equipment for the home medicine cabinet. We wanted to find out if reliable evidence is available on use of pulse oximeters to self monitor asthma exacerbations at home. We decided to include only trials that used pulse oximeters as part of a personalised asthma action plan because it is important that decisions are made on the basis of symptoms as well as oxygen saturation, and that patients have a clear protocol to follow when their asthma worsens.
Project plan
We planned to include randomised controlled trials (RCTs). Participants would have included adults, children or both with a diagnosis of asthma. We planned to include trials in which investigators compared participants who used pulse oximeters to monitor oxygen levels at home during an asthma exacerbation as part of a personalised asthma action plan (PAAP) versus those who used a PAAP without a pulse oximeter. We planned to include studies involving people taking any treatment regimen provided that no medicine was included as part of the randomisation schedule. We planned to use standard methods as recommended by The Cochrane Collaboration.
Conclusion
We found no evidence to support or refute patient use of home pulse oximetry in self management of asthma; therefore, we can make no recommendations about use of a pulse oximeter as part of a PAAP. People should not use a pulse oximeter without seeking the advice of a qualified healthcare professional.
We identified no compelling rationale for home monitoring of oxygen levels in isolation for most people with asthma. Some people have a reduced perception of the severity of their own breathlessness when exposed to hypoxia (when the whole or part of the body is deprived of adequate oxygen supply). If trials on self monitoring of oxygen levels in the blood by pulse oximeter at home by people with asthma are conducted, the pulse oximeter must be given as part of a personalised asthma action plan.
Background
Description of the condition
Asthma is a common long‐term respiratory condition, and people can have episodes of exacerbation of symptoms, which are known as asthma attacks or flare‐ups (GINA 2014). The trajectory of an exacerbation can be unpredictable, and during an exacerbation an individual's symptoms may worsen rapidly. Asthma exacerbations are usually controlled by the patient who recognises and assesses deterioration in symptoms according to an agreed upon personalised asthma action plan (PAAP, also known as a written action plan (WAP)) and takes medication for relief that was selected from a number of agreed upon actions or thresholds. Patients who do not regain symptom control must seek medical help.
Authors of the Global Asthma Report have estimated that as many as 334 million people have asthma worldwide (Table 1; Global Asthma Report 2014). The causes of asthma may be genetic, environmental (e.g. inhaling smoke, dust or allergens such as pollen) or both (NICE 2015). Diagnosis is made by an experienced clinician who takes a medical history and relies upon symptoms of asthma and the personal and family history of allergy and asthma. Clinicians may also use tests to help diagnose asthma (e.g. lung function tests), but no definitive test is currently available to diagnose the condition (NICE 2015). There is no agreed internationally accepted definition of asthma, but asthma is a heterogenous inflammatory illness characterised by respiratory symptoms that come and go and by the presence of expiratory airflow obstruction (forced expiratory volume in one second/forced vital capacity (FEV1/FVC) < 0.70), which is variable, whether spontaneous or resulting from treatment.
1. Global burden of asthma.
| THE GLOBAL BURDEN OF ASTHMA: ESTIMATES 2014a |
| 334 million people have asthma |
| 14% of the world's children experience asthma symptoms |
| 8.6% of young adults (aged 18 to 45) experience asthma symptoms |
| 4.5% of young adults have been diagnosed with asthma and/or are taking treatment for asthma |
| The burden of asthma is greatest for children aged 10 to 14 and for the elderly aged 75 to 79 |
| Asthma is the 14th most important disorder in the world in terms of extent and duration of disability |
aReproduced from http://www.globalasthmareport.org/burden/burden.php (accessed 3 October 2015).
Asthma needs to be managed by the individual or by his or her caregiver; management skills can be gained through education, personal experience or professional guidance, but an individualised PAAP is most helpful for management of specific exacerbations/attacks (BTS/SIGN 2014).
Pulse oximetry: accepted clinical usage
Pulse oximetry is a simple, non‐invasive, widely available medical tool that measures oxygen saturation (SpO2) through a device attached to a finger, a toe or an earlobe (Sinha 2014). Pulse oximeters are used by paramedics, by hospital personnel and by some general practitioners (GP) to assess oxygen levels in people who are acutely unwell. They are used to help the practitioner decide whether a patient should be treated with oxygen; however, they also are used to inform decisions about onward referral to secondary care. Measurement of oxygen status by arterial blood gas (ABG) analysis is more accurate and may require that arterial blood samples be sent to a laboratory, depending on local availability (Carruthers 1995).
In acute asthma, oxygen saturation is used as part of a comprehensive assessment to guide selection of appropriate treatment (BTS/SIGN 2014). For management of acute severe asthma in adults, general practitioners use oxygen saturation as a key measure in differentiating moderate asthma (peak expiratory flow rate (PEFR) > 50% to 75% best, SpO2 ≥ 92%) from acute severe asthma (PEFR > 33% to 50% best, SpO2 ≥ 92%) and life‐threatening asthma (PEFR < 33% best, SpO2 < 92%) (BTS/SIGN 2014). Management thereafter consists of SpO2 used to titrate the oxygen given to return SpO2 to levels of 94% to 98% and to maintain saturation at this level. Acute severe or life‐threatening attacks are considered for hospital admission or result in immediate admission, respectively. The Global Initiative for Asthma (GINA) makes reference to the use of pulse oximetry by healthcare practitioners, but not by patients, in adjusting the treatment approach (GINA 2014).
Several risks are associated with use of pulse oximeters.
During an asthma exacerbation, symptoms sometimes can worsen rapidly and deterioration is related to a non‐linear oxygen dissociation curve. SpO2 > 95% can be viewed as normal but < 92% is the shoulder of the curve with a precipitous fall in oxygen delivery.
Limitations of the pulse oximeter device can lead to incorrect readings; these limitations include "motion artefacts, poor perfusion at the site of measurement, irregular rhythms, ambient light or electromagnetic interference, skin pigmentation, nail polish, calibration assumptions, probe positioning…" (Fouzas 2011).
A pulse oximeter designed for adults and used for a child can lead to incorrect readings.
The margin of error for commercially available devices is accuracy to ± 4% in the SpO2 range of 70% to 100%.
Pulse oximetry interpretation in children is complicated by ventilation/perfusion mismatch, which can be worsened following short‐acting beta2‐agonist (SABA) treatment.
Advice at the point of purchase about use of the pulse oximeter device cannot be tailored to an individual unless it comes from an appropriate clinician as part of a PAAP.
Pulse oximetry in children or adults needs to be interpreted in the context of the whole clinical scenario, not in isolation (Sinha 2014).
Extrapolation from the more established use of pulse oximetry in chronic obstructive pulmonary disease (COPD) to its use in asthma must be avoided. Unfortunately, confusion has been reported amongst patients and clinicians regarding diagnosis, terminology and treatment, particularly in cases of overlap syndrome or fixed airflow obstruction in asthma from airway remodelling (Jones 2008). It is noteworthy that in COPD, pulse oximetry may be a useful tool for people with severe disease, and its use can be written into their management plan for early interventions involved in acute exacerbations of COPD (COPD Coalition 2010). In the COPD stable state, SpO2 readings persistently < 92% should trigger an oxygen assessment (Holmes 2013).
Further information about how pulse oximeters work can be found in Appendix 1.
How pulse oximetry may be being used at home to self manage asthma
During a workshop for people with asthma, we became aware that some people are using a pulse oximeter at home to monitor their own oxygen levels (Airways Workshop report 2014). Furthermore, pulse oximeters are used by some patients during exacerbations to help them decide when they should seek medical attention from a GP or at a hospital.
Why it is important to do this review
All people with asthma must self manage exacerbations, either in conjunction with a PAAP agreed upon with their clinician that indicates when seeking medical help is appropriate, or without such a plan. It is estimated that around a quarter of people with asthma in the United Kingdom have a PAAP (Asthma UK 2013; Wiener‐Ogilvie 2007). If steps taken by the patient fail to bring symptoms under control, it is important that sufficient time remains for transport to hospital, assessment and intervention, before the onset of complications such as respiratory arrest. As part of a PAAP, once an agreed upon threshold for contacting medical care is met, sufficient remaining time is essential.
Use of a pulse oximeter by people at home who wish to monitor their own (or their child's) oxygen levels during an asthma attack, as a guide for decision making about taking treatment or seeking help, is a new practice and is not recommended by guidelines.
Pulse oximeters are now readily available and can be purchased through websites that promote them as important equipment for people with respiratory conditions such as asthma. Anecdotal evidence from our workshop and from Internet forums stating that healthcare professionals have advised patients to purchase a pulse oximeter to self monitor asthma has raised this as an important question for consideration by the Cochrane Airways Review Group.
Patient access to pulse oximeters has never been easier, and local services and mechanisms to assist with asthma vary among individual countries. Particularly when health care is not free at the point of access, an inexpensive device may be seen as a suitable alternative to visiting a clinician to obtain a comprehensive assessment of an asthma attack. This potential cultural difference should be considered when evidence is interpreted within specific health economies.
This new use of pulse oximeters offers potential benefits. Oximetry could help patients identify and assess loss of control (the first task in a PAAP). In the UK, the National Review of Asthma Deaths (NRAD) highlighted that 45% of people who had died from asthma did not seek or receive medical care (NRAD 2014). By providing an absolute measurement, pulse oximetry might act as an impetus to change health‐seeking behaviour and encourage earlier presentation for some. Furthermore, oximetry is an objective measure that potentially overcomes the likelihood of underestimation of severity.
Potential risks are associated with use of a pulse oximeter at home outside a PAAP and without adequate training. These risks (as listed above) include inaccurate readings for technical reasons and false reassurance if an adequate SpO2 is allowed to override other clinical signs of life‐threatening asthma. Respiratory failure often occurs late in an asthma exacerbation, when it may be too late to seek appropriate help in a hospital setting. In other words, readings of oxygen saturation obtained with a pulse oximeter at home may give false reassurance about the severity of an attack.
For these reasons, we believe that a systematic review of controlled clinical trials is warranted.
Objectives
To determine whether pulse oximeters used as part of a personalised asthma action plan for people with asthma are safer and more effective than a personalised asthma action plan alone.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised controlled trials (RCTs) reported as full‐text articles, published as abstracts only, and provided as unpublished data.
Types of participants
We planned to include adults or children or both with a diagnosis of asthma. We planned to exclude trials including participants with COPD or lung diseases other than asthma. However, we planned to note these trials and consider how to deal with them separately.
Types of interventions
We planned to include trials in which researchers compared participants who used pulse oximeters to monitor oxygen levels at home during an asthma exacerbation as part of a personalised asthma action plan (PAAP) versus those who used a PAAP without a pulse oximeter. We planned to include studies involving people taking any treatment regimen, provided that no medicine was part of the randomisation schedule.
Types of outcome measures
Primary outcomes
Hospital admissions (rate and number of people experiencing one or more events).
Admissions to intensive care unit (ICU) (rate and number of people experiencing one or more events).
Death.
Secondary outcomes
Call to an ambulance/emergency doctor.
Course of oral steroids.
Emergency department (ED) visits.
Length of stay in hospital.
Participant satisfaction.
Asthma control (as measured by a validated questionnaire).
Reporting by investigators of one or more of the outcomes listed here is not an inclusion criterion for the review.
We have chosen harms outcomes to reflect the concern that pulse oximeters used by people having an asthma exacerbation might delay hospitalisation to the point at which harm can occur. We have included participant satisfaction and quality of life outcomes because if these increase while harms increase, pulse oximeters may be giving people false reassurance.
Search methods for identification of studies
Electronic searches
We searched for trials in the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Trials Search Co‐ordinator for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (see Appendix 2 for further details). We searched all records in the CAGR using the search strategy provided in Appendix 3.
We conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/) using the search terms presented in Appendix 4. We searched all databases from their inception to March 2015, with no restriction on language or type of publication.
Searching other resources
We checked reference lists of all relevant primary studies and review articles for additional references. We searched relevant manufacturers' websites for trial information.
We planned to search for errata or retractions from included studies published in full text on PubMed (www.ncbi.nlm.nih.gov/pubmed).
Data collection and analysis
Selection of studies
Two review authors (EJW, RGC) independently screened titles and abstracts for inclusion of all potential studies identified as a result of the search and to code them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved full‐text study reports/publications, for independent screening to identify studies for inclusion and to identify and record reasons for exclusion of ineligible studies. We resolved disagreements through discussion. It was not necessary to consult with a third person. We planned to identify and exclude duplicates and to collate multiple reports of the same study, so that each study rather than each report is the unit of interest in the review, but did not identify duplicates. We recorded the selection process in sufficient detail to complete a Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram and a Characteristics of excluded studies table (Moher 2009).
Data extraction and management
We planned to use a data collection form that had been piloted on at least one study in the review to document study characteristics and outcome data. One review author (EJW) planned to extract the following study characteristics from included studies.
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and locations, study settings, withdrawals and date of study.
Participants: number, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant medications and excluded medications.
Outcomes: primary and secondary outcomes specified and collected and time points reported.
Notes: funding for trial and notable conflicts of interest for trial authors.
Two review authors (EJW, RGC) planned to independently extract outcome data from included studies. We planned to note in the Characteristics of included studies table if outcome data were not reported in a useable way. We planned to resolve disagreements by reaching consensus or by involving a third person. One review author (EJW) would have transferred data into the Review Manager (RevMan 2014) file. A second review author (RGC) would have spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two review authors (EJW, RGC) planned to independently assess risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We planned to resolve disagreements by discussion or by consultation with another person. We planned to assess risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We planned to grade each potential source of bias as having high, low or unclear risk, and to provide a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We planned to summarise 'Risk of bias' judgements across different studies for each of the domains listed. We planned to consider blinding separately for different key outcomes when necessary (e.g. for unblinded outcome assessment, risk of bias for all‐cause mortality may be very different than for a patient‐reported pain scale). When information on risk of bias was related to unpublished data or correspondence with a trialist, we planned to note this in the 'Risk of bias' table.
When considering treatment effects, we planned to take into account the risk of bias for studies that contributed to those outcomes.
Assesment of bias in conducting the systematic review
We planned to conduct the review according to this published protocol and to report deviations from it in the Differences between protocol and review section of the systematic review.
Measures of treatment effect
We planned to analyse dichotomous data as odds ratios (ORs) and continuous data as mean differences (MDs) or standardised mean differences (SMDs). We planned to enter data presented as a scale with a consistent direction of effect.
We planned to undertake meta‐analyses only when this was meaningful, that is, when treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
We planned to narratively describe skewed data reported as medians and interquartile ranges.
When multiple trial arms were reported in a single trial, we planned to include only the relevant arms. If two comparisons (e.g. drug A vs placebo and drug B vs placebo) were combined in the same meta‐analysis, we planned to halve the control group to avoid double‐counting.
Unit of analysis issues
The unit of analysis was planned to be the participant, with the exception of rates of admission, which we planned to analyse as rate ratios.
Dealing with missing data
We planned to contact investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only). When this was not possible, and missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by performing a sensitivity analysis.
Assessment of heterogeneity
We planned to use the I² statistic to measure heterogeneity among the trials in each analysis. If we had identified substantial heterogeneity, we planned to report this and to explore possible causes by performing prespecified subgroup analyses.
Assessment of reporting biases
If we had been able to pool more than 10 trials, we planned to create and examine a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We planned to use a random‐effects model and to perform a sensitivity analysis by using a fixed‐effect model.
'Summary of findings' table
We planned to create a 'Summary of findings' table using the following outcomes: hospital admissions; admissions to intensive care unit (ICU); death; calls to an ambulance/emergency doctor; courses of oral steroids; emergency department visits; length of stay in hospital; and asthma control. We planned to use GRADEpro software (GRADEpro 2008) and to apply the five GRADE (Grades of Recommendation, Assessment, Development and Evaluation) considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data to the meta‐analyses for prespecified outcomes. We planned to include the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) by using GRADEpro software. We planned to justify all decisions to downgrade or upgrade the quality of studies by using footnotes and to make comments to aid readers' understanding of the review when necessary.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Adults aged 18 and over versus children from birth to two years of age, children aged two to 13 and adolescents aged 13 to 18 (or as reported).
Different thresholds or protocols for using pulse oximeters (as defined by trials).
Severity (according to British Thoracic Society (BTS) guideline step 1 vs step 2 vs step 3 vs step 4 vs step 5).
We planned to use the primary outcomes (see Primary outcomes) in subgroup analyses.
We planned to use the formal test for subgroup interactions included in Review Manager (RevMan 2014).
Sensitivity analysis
We planned to carry out a sensitivity analysis by excluding from the primary outcomes studies with a critical risk of bias. We defined this as one or more domains judged to be at high risk of bias when we think that bias could have altered the treatment effect in a way that might change the conclusions of the review.
Results
Description of studies
Results of the search
A search of the CAG Specialised Register yielded 429 references. The two review authors screened the search using Rayyan (Elmagarmid 2014). We selected 18 references for which we obtained the full text. Two review authors screened the full text independently and excluded all 18 references with reasons. The search of ClinicalTrials.gov and the World Health Organization (WHO) Trials Portal yielded 127 records, none of which were relevant. See Figure 1 for our PRISMA study flow diagram.
1.
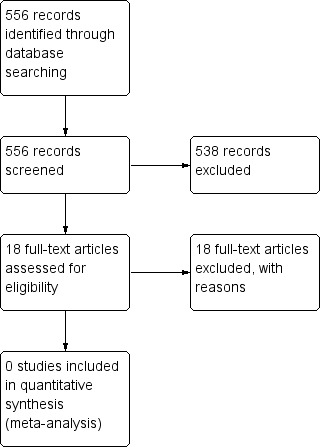
Study flow diagram.
Included studies
We included no studies.
Excluded studies
We excluded 18 studies with reasons. For reasons, see Characteristics of excluded studies.
Risk of bias in included studies
Not applicable.
Effects of interventions
Not applicable.
Discussion
Summary of main results
We identified no included studies.
Overall completeness and applicability of evidence
We identified no included studies.
Quality of the evidence
We identified no included studies.
Potential biases in the review process
Two areas are open to bias in this review process: searching and drawing conclusions. The search was screened independently to keep review authors from influencing each other.
Agreements and disagreements with other studies or reviews
We found no evidence to support or refute the use of home pulse oximetry in self management of asthma; therefore, we cannot make recommendations for or against use of a pulse oximeter as part of a PAAP. In the absence of evidence, healthcare professionals need to weigh existing background information about pulse oximetry when advising individual patients who are considering purchasing a pulse oximeter.
As outlined in the Background, pulse oximetry is subject to error and inaccuracy. Furthermore, the oxygen dissociation curve is not linear during an asthma attack and drops off quickly to below 92% saturation. This creates the risk that, for people with asthma, waiting until SpO2 drops significantly may be waiting too long to seek medical help.
Pulse oximeters are used by doctors, including GPs and physicians at the emergency department/hospital, among a range of indicators in deciding further treatment needs. The sensitivity and specificity of pulse oximeters are not reliable enough for them to be used in isolation for decision making regarding appropriate care. Clinical findings such as peak flow and symptoms are considered together with circumstances in decision making. For example, when clinical findings are equivocal, individuals who live far from the hospital or on their own are more likely to be admitted than those who have more support and live closer to medical help. Use of pulse oximetry as a single indicator of when individuals should seek medical help oversimplifies the situation; we were concerned enough about this to write this review.
Delay among people seeking appropriate medical help when their asthma is getting worse has contributed to fatal and near fatal adverse events (NRAD 2014). Pulse oximetry used incorrectly could add to that delay and worsen outcomes. Pulse oximetry might be beneficial for those who have a lowered perception of the severity of their own symptoms with hypoxia, but this has not been studied.
Authors' conclusions
Implications for practice.
We found no reliable data to support the use of pulse oximeters by patients to monitor their own oxygen saturation levels when they are experiencing an asthma attack. People should not use a pulse oximeter without seeking the advice of a qualified healthcare professional.
We found no compelling rationale for home monitoring of oxygen levels in isolation for most people with asthma. Some people have a reduced perception of the severity of their own breathlessness when exposed to hypoxia. If trials are conducted to study self monitoring of oxygen levels in the blood by pulse oximeter at home, the pulse oximeter must be given as part of a personalised asthma action plan.
Implications for research.
We are reluctant to suggest that research is needed in this area because we found no compelling rationale for home monitoring of blood oxygen levels for most people with asthma, and because peak flow and symptom monitoring are recommended in international guidelines. However, some people have a reduced perception of the severity of their own breathlessness, and this might contribute to delayed presentation; therefore, use of pulse oximeters as part of a personalised asthma action plan could pose an interesting research question.
History
Protocol first published: Issue 3, 2015 Review first published: Issue 9, 2015
| Date | Event | Description |
|---|---|---|
| 13 April 2015 | Amended | Amended typo |
Acknowledgements
We thank Liz Stovold, the information specialist at Cochrane Airways who ran the literature searches. We thank the participants at the Cochrane Airways Asthma Care and Self Care Workshop who identified this question as an important topic for review. We thank the Cochrane editorial base staff and editorial board for providing critical feedback on this review, for arranging peer review and for publishing the review. We thank the patient and clinical referees for providing excellent critical feedback on this review, which improved the clarity of the question.
Chris Cates was the Editor for this review and commented critically on the review.
Cochrane Review Group Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Airways Group.
Disclaimer: The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, the National Health Service or the Department of Health.
The Methods section of this review is based on a standard template used by the Cochrane Airways Group.
Appendices
Appendix 1. Additional information about pulse oximetry
The pulse oximeter shines light of two different wavelengths through the finger, and the device detects absorption of that light by blood and uses that information to provide an estimate of the oxygen saturation (COPD Coalition 2010; Fouzas 2011). Reduction in both price and size of devices has led oxygen saturation (SpO2) to be viewed as an accessible "fifth vital sign" in assessment of acutely unwell patients of any cause in clinical settings including primary care (Schermer 2009).
Oxygen saturation has a non‐linear relationship with partial pressure of oxygen in the blood, which means that once the SpO2 decreases to below 92%, the decline in arterial blood oxygen tension (PaO2) is more rapid.
Appendix 2. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 3. Search strategy to identify relevant trials from the CAGR
#1 AST:MISC1
#2 MeSH DESCRIPTOR Asthma Explode All
#3 asthma*:ti,ab
#4 #1 or #2 or #3
#5 MeSH DESCRIPTOR Oximetry Explode All
#6 oximet*
#7 (blood* NEAR (gas or oxygen)) AND monitor*
#8 oxygen* NEXT saturat*
#9 oxygen* NEXT sats
#10 SpO2
#11 MeSH DESCRIPTOR Self Care
#12 self* NEXT monitor*
#13 home* NEAR2 monitor*
#14 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13
#15 #4 and #14
[Note: in search line #1, MISC1 denotes the field in the record where the reference has ben coded for condition, in this case, asthma]
Appendix 4. Search terms used in trials registries
ClinicalTrials.gov
Intervention: "pulse oximeter"
WHO Trials Portal
"pulse oximeter AND asthma"
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alario 1995 | No oximetry. Randomly assigned to receive medication in the ED |
| Bateman 2000 | No oximetry. Self management vs usual care |
| Berg 1997 | No oximetry. Effects of self management programme on medication compliance, MDI chronology used, peak flow measurements and education |
| Burkhart 2007 | No oximetry. Self management programme including education and peak flow meter |
| der Palen 2001 | No oximetry. Self management programme including education and peak flow meter aimed at behavioural change |
| Ducharme 2011 | No oximetry. Intervention consisting of WAP with dose counter and no pulse oximeter |
| Ekcert 2004 | Not an RCT; a within‐participant study. Physiological experiment on well participants with asthma showing altered perception of symptoms with hypoxia |
| Ferraria 2000 | Wrong intervention. Short‐term hospital monitoring of oximetry during airway clearance |
| Guendelman 2004 | No oximetry. Self management programme including education and peak flow meter |
| Kaya 2007 | Pulse oximetry performed in the ED as part of a clinical scoring correlation |
| Kotses 2007 | No oximetry. Home monitoring with peak flow |
| NCT00282516 | No oximetry. Self management programme including education and peak flow meter |
| Pieters 1990 | Not an RCT. Continuous oximetry |
| Rakos 1985 | Intervention consisting of a simple education pack to give information about asthma. No oximetry |
| Schermer 2002 | No oximetry. Self management programme including education and peak flow meter |
| Silverberg 2012 | Wrong population; participants admitted to the ED |
| Wang 1997 | Randomly assigned to receive medication with oximetry measured for those on salmeterol |
| Yoo 1996 | Randomly assigned to receive nebulised treatments. Children with bronchiolitis and asthma admitted as inpatients and treated with salbutamol nebulisers with or without oxygen |
Differences between protocol and review
We deleted mention of excluding pregnant women, as conversations with specialists have suggested that this group need not be excluded.
Contributions of authors
EJW: screened the search and drafted the review.
RC: screened the search and drafted the review.
Sources of support
Internal sources
-
St George's, University of London, UK.
EJW
External sources
-
National Institute of Health Research, UK.
Core funding of Cochrane Airways Group (EJW)
Declarations of interest
EJW, RGC: none known..
New
References
References to studies excluded from this review
Alario 1995 {published data only}
- Alario AJ, Lewander WJ, Dennehy P, Seifer R, Mansell AL. The relationship between oxygen saturation and the clinical assessment of acutely wheezing infants and children. Pediatric Emergency Care 1995;11(6):331‐9. [DOI] [PubMed] [Google Scholar]
Bateman 2000 {published data only}
- Bateman ED, Kruger MJ. A computer‐based home‐monitoring disease management programme, Pulmassist Plus(r) (PAP), achieves significant improvement in quality of life, and healthcare costs in moderate and severe asthma [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161:A457. [Google Scholar]
Berg 1997 {published data only}
- Berg J, Dunbar‐Jacob J, Sereika SM. An evaluation of a self‐management program for adults with asthma. Clinical Nursing Research 1997;6(3):225‐8. [DOI] [PubMed] [Google Scholar]
Burkhart 2007 {published data only}
- Burkhart PV, Rayens MK, Oakley MG, Abshire DA, Zhang M. Testing an intervention to promote children's adherence to asthma self‐management. Journal of Nursing Scholarship 2007;39(2):133‐40. [DOI] [PubMed] [Google Scholar]
der Palen 2001 {published data only}
- Palen J, Klein JJ, Zielhuis GA, Herwaarden CL, Seydel GR. Behavioural effect of self‐treatment guidelines in a self‐management program for adults with asthma. Patient Education and Counselling 2001;43(2):161‐9. [DOI] [PubMed] [Google Scholar]
Ducharme 2011 {published data only}
- Ducharme FM, Zemek RI, Chalut D, McGillivray D, Noya FJD, Resendes S, et al. Written action plan in pediatric emergency room improves asthma prescribing, adherence, and control. American Journal of Respiratory and Critical Care Medicine 2011;183(2):195‐203. [DOI] [PubMed] [Google Scholar]
Ekcert 2004 {published data only}
- Eckert DJ, Catcheside PG, Smith JH, Frith PA, McEvoy RD. Hypoxia suppresses symptom perception in asthma. American Journal of Respiratory and Critical Care Medicine 2004;169(11):1224‐30. [DOI] [PubMed] [Google Scholar]
Ferraria 2000 {published data only}
- Ferreira SM, Gusmao AL, Andrade MA, Amorim D, Krebsky PB, Andrade FMD, et al. SpO2 in asthmatic patients submitted to the bronchial clearance maneuvers with active cycle (AC) of the breathing or vibrocompression (VC). European Respiratory Journal 2000;16:320s. [Google Scholar]
Guendelman 2004 {published data only}
- Guendelman S, Meade K, Chen YQ, Benson M. Asthma control and hospitalizations among inner‐city children: results of a randomized trial. Telemedicine Journal and e‐health 2004;10(Suppl 2):S6‐14. [PubMed] [Google Scholar]
Kaya 2007 {published data only}
- Kaya Z, Turktas I. Correlation of clinical score to pulmonary function and oxygen saturation in children with asthma attack. Allergologia et Immunopathologia 2007;35(5):169‐73. [DOI] [PubMed] [Google Scholar]
Kotses 2007 {published data only}
- Kotses H, Thurber L, Mosby A, McLacklan A, Harver A. Effects of home monitoring on subjective and objective indicators of asthma in pediatric patients: caregivers' perceived symptoms and child peak flow [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco. 2007:Poster #904.
NCT00282516 {published data only}
- Callahan CW. Asthma in‐home monitoring (AIM) trial. https://clinicaltrials.gov/ct2/show/NCT00282516 (accessed 16 June 2015).
Pieters 1990 {published data only}
- Pieters W, Houben L, Terpstra G, Kreukniet J, Raaijmakers J. Pulse‐oximetry and nocturnal bronchial obstruction [abstract]. European Respiratory Journal Supplement 1990;3:215S. [Google Scholar]
Rakos 1985 {published data only}
- Rakos RF, Grodek MV, Mack KK. The impact of a self‐administered behavioral intervention program on pediatric asthma. Journal of Psychosomatic Research 1985;29(1):101‐8. [DOI] [PubMed] [Google Scholar]
Schermer 2002 {published data only}
- Schermer TR, Thoonen BP, Boom G, Akkermans RP, Grol RP, Folgering HT, et al. Randomized controlled economic evaluation of asthma self‐management in primary health care. American Journal of Respiratory and Critical Care Medicine 2002;166(8):1062‐72. [DOI] [PubMed] [Google Scholar]
Silverberg 2012 {published data only}
- Silverberg JI, Rodenas M, Sinert R, Joks R. Emergency department treatment of adults with acute asthma exacerbations: effect on exhaled nitric oxide levels. Allergy and Asthma Proceedings 2012;33(6):514‐8. [DOI] [PubMed] [Google Scholar]
Wang 1997 {published data only}
- Wang TH, Woodcock AJ, Sullivan CE. Improvement of overnight oxygen saturation in asthmatics with salmeterol treatment [abstract]. European Respiratory Journal Supplement 1997;10:3s. [Google Scholar]
Yoo 1996 {published data only}
- Yoo ES, Seo JW, Lee SJ. Arterial oxygen desaturation after salbutamol nebulization in wheezy infants and children. Journal of the Korean Pediatric Society 1996;39(7):953‐61. [Google Scholar]
Additional references
Airways Workshop report 2014
- Cochrane Airways. Identifying and overcoming challenges in asthma care and self‐care. http://airways.cochrane.org/sites/airways.cochrane.org/files/uploads/Identifying%20and%20overcoming%20challenges%20in%20asthma%20care%20and%20self%20care.pdf 2014 (accessed 18 February 2015).
Asthma UK 2013
- Asthma UK. Compare your care. How asthma care in the UK matches up to standards. http://www.asthma.org.uk/compareyourcare 2013 (accessed 25 February 2015).
BTS/SIGN 2014
- British Thoracic Society/Scottish Intercollegiate Guidelines Network (BTS/SIGN). SIGN 141 British guideline on the management of asthma. A national clinical guideline. October 2014. https://www.brit‐thoracic.org.uk/document‐library/clinical‐information/asthma/btssign‐asthma‐guideline‐2014/ (accessed 2 February 2015).
Carruthers 1995
- Carruthers DM, Harrison BDW. Arterial blood gas analysis or oxygen saturation in the assessment of acute asthma?. Thorax 1995;50(2):186‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
COPD Coalition 2010
- International COPD Coalition. Clinical use of pulse oximetry, pocket reference 2010. https://www.theipcrg.org/download/attachments/689660/oximetry_pocket_guide.pdf?version=1&modificationDate=1347261955000 (accessed 15 January 2015).
Elmagarmid 2014
- Elmagarmid A, Fedorowicz Z, Hammady H, Ilyas I, Khabsa M, Ouzzani M. Rayyan: a systematic reviews web app for exploring and filtering searches for eligible studies for Cochrane Reviews. Evidence‐Informed Public Health: Opportunities and Challenges. Abstracts of the 22nd Cochrane Colloquium; 2014 21‐26 Sep; Hyderabad, India. John Wiley & Sons, 2014.
Fouzas 2011
- Fouzas S, Priftis KN, Anthracopoulos MB. Pulse oximetry in pediatric practice. Pediatrics 2011;128(4):740‐52. [DOI] [PubMed] [Google Scholar]
GINA 2014
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention. http://www.ginasthma.org/local/uploads/files/GINA_Report_2014_Jun11.pdf (accessed 9 December 2014).
Global Asthma Report 2014
- Marks G, Pearce N, Strachan D, Asher I. The Global Asthma Report 2014. http://www.globalasthmareport.org/ (accessed 18 February 2014).
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. Ontario: McMaster University, 2008.
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holmes 2013
- Holmes S, Peffers SJ. Pulse oximetry in primary care opinion sheet. https://www.pcrs‐uk.org/system/files/Resources/Opinion‐sheets/os28_pulse_oximetry.pdf (accessed 10 December 2014).
Jones 2008
- Jones R, Dickson‐Spillman M, Mather M, Marks D, Shackell B. Accuracy of diagnostic registers and management of chronic obstructive pulmonary disease: the Devon primary care audit. Respiratory Research 2008;9(1):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed.1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2015
- National Clinical Guideline Centre. Asthma: diagnosis and monitoring of asthma in adults, children and young people. http://www.nice.org.uk/guidance/dg12/resources/asthma‐diagnosis‐and‐monitoring‐draft‐guideline2 (accessed 23 January 2015).
NRAD 2014
- Royal College of Physicians. Why asthma still kills. The National Review of Asthma Deaths (NRAD) Confidential Enquiry report. https://www.rcplondon.ac.uk/sites/default/files/why‐asthma‐still‐kills‐full‐report.pdf. London: RCP, 2014, (accessed 22 January 2015).
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schermer 2009
- Schermer T, Leenders J, in 't Veen H, Bosch W, Wissink A, Smeele I, et al. Pulse oximetry in family practice: indications and clinical observations in patients with COPD. Family Practice 2009;26(6):524‐31. [DOI] [PubMed] [Google Scholar]
Sinha 2014
- Sinha IP, Mayell SJ, Halfhide C. Pulse oximetry in children. Archives of Disease in Childhood 2014;99(3):117‐8. [DOI] [PubMed] [Google Scholar]
Wiener‐Ogilvie 2007
- Wiener‐Ogilvie S, Pinnock H, Huby G, Sheikh A, Partridge MR, Gillies J. Do practices comply with key recommendations of the British Asthma Guideline? If not, why not?. Primary Care Respiratory Journal 2007;16(6):369‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]


