Abstract
The classification of multiple sclerosis (MS) has been established by Lublin in 1996 and revised in 2013. The revision includes clinically isolated syndrome, relapsing-remitting, primary progressive and secondary progressive MS, and has added activity (i.e., formation of white matter lesions or clinical relapses) as a qualifier. This allows for the distinction between active and nonactive progression, which has been shown to be of clinical importance. We propose that a logical extension of this classification is the incorporation of additional key pathological processes, such as chronic perilesional inflammation, neuroaxonal degeneration, and remyelination. This will distinguish MS phenotypes that may present as clinically identical but are driven by different combinations of pathological processes. A more precise description of MS phenotypes will improve prognostication and personalized care as well as clinical trial design. Thus, our proposal provides an expanded framework for conceptualizing MS and for guiding development of biomarkers for monitoring activity along the main pathological axes in MS.
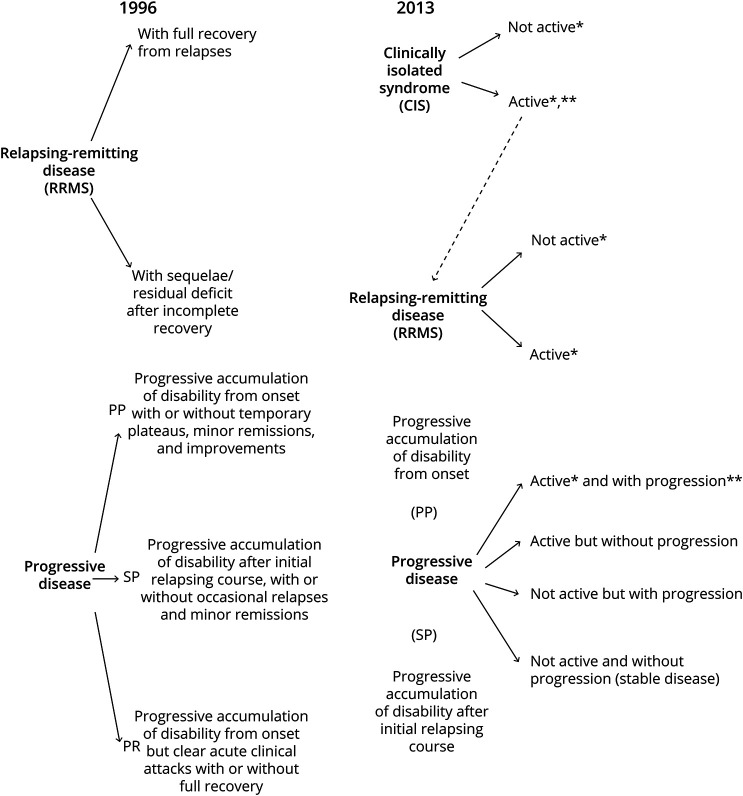
Multiple sclerosis (MS) is an inflammatory demyelinating and neurodegenerative disease with heterogeneous clinical presentations and disease course. To standardize terminology and improve homogeneity in clinical trials, a study1 in 1996 defined 4 distinct MS phenotypes. These phenotypes are based on clinical features consisting of relapsing-remitting, primary progressive, secondary progressive, and progressive-relapsing MS. Because current disease-modifying treatment (DMT) is predominantly effective in reducing relapses, the distinction between a relapsing-remitting and progressive course is useful for identifying patients who do and do not respond to DMTs. The classification was partially revised in 2013.2 It now includes activity, defined as relapses and/or lesion formation on MRI, and disease progression, thereby classifying patients along 2 axes that can be evaluated separately. Patients with MS can thus be active or nonactive and progressing or nonprogressing (Figure 1). The separation of activity from progression has proven to be clinically relevant because the MS treatments, siponimod and rituximab, are effective in active progressive but not in nonactive progressive patients.3,4 Furthermore, the inclusion of lesion formation on MRI reflects the realization that measures other than clinical observation can be used for disease classification.
Figure 1. The 1996 vs 2013 Multiple Sclerosis Phenotype Descriptions for Relapsing and Progressive Disease.
Adapted from Lublin et al. 2014; Neurology. 83:278-286.
By unlinking activity and progression, the new Lublin classification has taken an important step. However, it considers only white matter lesion formation (“activity”) and remains agnostic to other key pathologic processes such as cortical demyelination,5 chronic perilesional WM inflammation,6 neuroaxonal degeneration, and remyelination. As a result, patients are grouped based on similar clinical phenotypes although they might have different underlying disease-driving pathologic processes. As a logical extension to the current classification, we propose that additional pathologic axes are required to more precisely capture the range of phenotypes observed across patients with MS and within individual patients over time. Thus, we are suggesting a framework that extends beyond the clinical MS classification providing a new conceptual view of MS. This framework will guide biomarker development and may ultimately improve personalized treatment and drug discovery.
The Pathologic Axes of MS and Their Modifiers
The pathologic processes associated with MS have been discussed in detail in several recent reviews.7-9 In this study, we are briefly reviewing the main pathologic axes of MS, which are (1) acute inflammation, (2) chronic parenchymal and interstitial inflammation, (3) white and gray matter demyelination, (4) axonal degeneration, (5) neuronal loss, and (6) remyelination. We hypothesize that the clinical heterogeneity in MS is caused by differing activity along these key pathologic axes, both across patients with MS and within individual patients over time.
(1) Inflammation is the key driver of MS pathology and can be separated into acute and chronic. Acute inflammation consists of bulk invasion of monocytes and lymphocytes into white matter (WM) and to a lesser degree in deep gray matter, with concomitant activation of local microglia and astrocytes. The ensuing demyelination leads to the formation of acute focal WM lesions, characterized by dense infiltration with myelin-laden macrophages, and in lower numbers, lymphocytes, as well as substantial axonal damage.8 (2) Chronic inflammation manifests as diffuse glial activation at the rim of chronic active lesions, which can reach considerable distances into normal-appearing white matter (NAWM) and as predominantly lymphocytic inflammation in the meninges and perivascular spaces. Meningeal inflammation is typically diffuse but may also form follicle-like structures.10 Chronic inflammation is considered compartmentalized; however, persistent blood-brain barrier abnormalities suggest that chronic inflammation is not completely independent of systemic influence. (3) The main inflammatory assault in MS is directed against myelin, which leads to focal demyelination in white and gray matter. A low-grade form of demyelination can occur at the rim of chronic active lesions, which leads to slow expansion and eventually lesion confluency.11 Although MS has been previously believed to affect WM only, the area of cortical and deep gray matter demyelination in secondary progressive MS (SPMS) can exceed that of WM lesions.12 With the exception of loss of myelin, cortical lesions lack the pathologic signatures of WM lesions such as blood-brain barrier breakdown, immune cell infiltration, perivascular cuffs, astrogliosis, loss of oligodendrocyte progenitor cells, and complement activation.13 (4) Axonal damage is abundant in acute MS lesions and becomes less frequent in chronic active and chronic silent lesions.14 One pathologic study has suggested that axonal injury is invariably associated with inflammation and that ongoing axonal damage is absent in patients in whom inflammation has declined to baseline levels.15 Several other studies posit that demyelinated axons degenerate independent of inflammation because of loss of trophic support by the myelin sheath16 and enhanced energy demand with eventual mitochondrial decompensation.17 (5) Neuronal loss in the neocortex is widespread in MS, ranging between 25% and 40%, although less prominent than in classical neurodegenerative diseases. It is unclear how much cortical demyelination contributes to neuronal loss, with some studies reporting enhanced neuronal loss in demyelinated cortical lesions compared with normal-appearing gray matter,13,18 while others describing significant but equivalent levels of neuronal loss in lesional and nonlesional neocortex.19 Synaptic density in the remaining neurons is substantially decreased,20 as is neuronal activity measured by functional MRI. Neuroaxonal damage is believed to be the major determinant of disability. (6) Finally, remyelination is a major repair process in the CNS, which is believed to protect damaged axons, restores axonal conduction, and prevents long-term axonal damage21; remyelination is sparse in most (67%) and extensive in a minority (20%) of patients with MS. It is more pronounced in early MS,22 although robust active remyelination can be observed in patients with MS of all ages.23 It differs between anatomic locations, with the most extensive remyelination in cortical lesions, a lower degree of subcortical and periventricular lesions and near absence of remyelination in cerebellar lesions.24 In this context, a recent single nucleus RNA-sequencing study of brain tissue from patients with MS and controls has uncovered 3 distinct oligodendrocyte response patterns.25 These patterns were determined by individual patient effects only and were not associated with patient metadata such as age, disease duration, and disease category.
Numerous histologic studies have analyzed the pathologies associated with different MS courses and have concluded that differences are largely quantitative rather than qualitative.26-28 Early relapsing and chronic progressive MS can be seen as opposing ends of the inflammatory spectrum in MS, where intense focal inflammation directed at myelin is replaced by diffuse, glia-cell driven inflammation in perilesional and NAWM. However, relapsing-remitting MS (RRMS)/SPMS does not differ from primary progressive MS (PPMS) regarding overall WM lesion load; presence of active, chronic active, and chronic silent WM lesions (albeit with different proportions); extent of axonal damage14,29; and meningeal inflammation.30 As in RRMS, the correlation between WM lesion load and clinical disability in PPMS is poor.31 These studies suggest that clinical phenotypes do not align well with specific pathomechanisms outside the extremes of the clinical spectrum, early relapsing MS, and late-stage progression, that is, in most patients.
The differing activities of each pathologic axis are caused by a substantial number of modifiers. Important modifiers include human lymphocyte antigens and genetic risk variants (susceptibility)32,33; biological factors such as obesity,34 sex hormones,35 immune senescence36 (predictive of relapse frequency and progression), gut dysbiosis37; and environmental factors such as smoking,38 vitamin D levels,39 and Epstein-Barr virus infection,40 among others. These factors have been shown to be associated with MS susceptibility, clinical outcomes, and/or MS-relevant pathologic processes with varying levels of evidence. Most modifiers exert quantitative rather than qualitative (on-off) effects and may vary throughout the life of the patient, such as pregnancy, smoking, and immune senescence. Furthermore, certain modifiers seem to exert their strongest influence during development, such as low vitamin D and obesity, and confer most MS risk in early life. The multitude of different modifiers that might come into play at different time points suggests that each individual patient carries a unique or near-unique burden of genetic, biological, and environmental factors at a given time point. These massive number of modifier combinations are likely to produce a spectrum of pathway and cellular activation states along the MS-relevant pathologic axes. These may lead to a continuum of possible responses or alternatively to convergence into several major pathways as suggested by the recently described major regenerative (remyelination) patterns in MS.25 This neuropathologic variability in turn predicts high variability of clinical presentations, which is the clinical experience with MS. It also provides new context for the clinical categories, suggesting that these are fluid rather than distinct entities and can result from different pathologic activity profiles. This further argues that the clinical categories do not correspond to unique pathologic processes.
Defining MS Phenotypes Through Key Pathologic Processes
Given that the clinical categories provide poor mechanistic separation, especially in patients outside of early relapsing MS or late-stage progression, we propose an expanded framework for MS classification that is based on pathologic processes rather than clinical presentation. We argue that MS can be described as a combination of multiple, interconnected pathologic processes that present with different degrees of activity at different time points. This approach provides a ground truth about the disease state that clinical observations cannot provide.
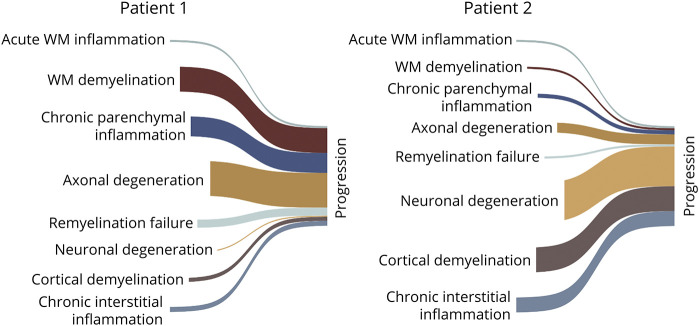
By quantifying the extent and type of activity along each pathologic axis, our model would be able to assign specific profiles for individual patients with MS. While the clinical classification into relapsing-remitting and secondary progressive MS is intuitive, it may obscure individual-level pathologic profiles. For example, MS progression may be driven by chronic perilesional inflammation, meningeal inflammation, or inflammation-independent degeneration of denuded axons, which the description “progression” is not able to distinguish (Figure 2). This suggests that additional pathologic axes might provide improved resolution of MS phenotypes.
Figure 2. Multiple Sclerosis Clinical Phenotypes May Result From Different Axes Activity Profiles.
Examples of the same clinical presentation (nonactive progression) caused by different pathologic axes activity profiles. Progression can be driven by chronic parenchymal inflammation (chronic smoldering inflammation in chronic active lesions) and axonal degeneration (patient 1) or by interstitial (meningeal) inflammation resulting in cortical demyelination and neuronal degeneration (patient 2). Indentation of axes indicates that activity occurs at later stage relative to others.
A recent study has demonstrated that MRI biomarkers assessing WM lesions, WM integrity, and atrophy can provide remarkable insights into MS heterogeneity by identifying subcategories in which clinical phenotypes have little to no organizing value. In this study, 3 MS subtypes were identified based on changes in MRI patterns over time.41 Patients exhibited early cortical atrophy (cortical-led phenotype), early reduction in the T1/T2 ratio across various NAWM areas (NAWM-led phenotype), or early and extensive accrual of T2 lesions followed by severe deep gray matter atrophy. These 3 subgroups exhibited substantial differences regarding risk of progression, relapse rate, and treatment responses. This example illustrates that MRI pattern–based MS subtypes, which correlate with different pathologic processes (neuronal damage, WM tissue damage, and inflammation/demyelination), predict disease activity, disability progression, and treatment response better than conventional clinical phenotypes. Similarly, the incorporation of different oligodendrocyte response patterns25 into the classification system and its quantification with biomarkers might be highly informative about potential responses to pre-remyelinating drugs.
Monitoring Pathologic Activities in MS
Precision phenotyping requires a panel of quantitative biomarkers. These biomarkers reflect aspects of different axes pathologies but do not have to be specific for MS because they are not used for diagnosis. Creating and validating this panel remains a major unmet challenge in clinical MS research. Despite substantial effort over the past few decades, the only biomarkers currently in clinical use are radiologic, namely gadolinium enhancement, T2 lesion formation, and to a lesser degree, brain atrophy on serial MRIs.
Recent developments in MRI methods have made advances in visualizing previously inaccessible pathologies. These include chronic glial cell activation with quantitative susceptibility mapping,42 meningeal inflammation and cortical demyelination,43,44 and remyelination with myelin water fraction imaging.45 While these nonconventional MRI techniques are currently limited to academic research centers, they are likely to eventually translate into clinical trials and clinical practice. In addition, a growing number of serum-derived and CSF-derived markers are under investigation. The most promising biomarker is light-chain neurofilaments (NfL), which reflects neuroaxonal damage. NfL levels in serum and CSF derived from the same patients are highly associated and have shown predictive value for new or enlarging T2 lesions, brain volume loss, and risk of disability worsening.46 As a caveat, these observations have been made at a group level,47 whereas NfL varies considerably between individuals and overlap substantially, e.g., between MS patients with and without enhancing lesions. This can be overcome through longitudinal assessment of NfL, where individual patients serve as their own control. Other serum and CSF markers include the intermediate filament protein, GFAP, associated with astrocyte activation48; the glycoprotein chitinase 3-like 1 (CHI3L1),49 expressed by astrocytes and microglia/macrophages; sCD163, a marker of activated microglia and macrophages50; the chemoattractant, CXCL13, required for the development of B-cell follicles and secondary lymphoid structures51; and osteopontin,52,53 an early activation marker of T cells. In addition, with a growing number of MS biomarker studies, specific patterns of the disease process are coming to light. For example, NfL levels in CSF correlated with brain atrophy, whereas CHI3L1 levels correlated with spinal cord atrophy.54 Similarly, elevated CXCL12 and osteopontin levels in the CSF were associated with PPMS and elevated IL-10 with RRMS.55
Overall, their association with disease activity is still tenuous and their utility for clinical use is unconfirmed. Biomarkers that consist of several parameters might be better suited to capture the activation state of specific cells or pathways. This can be exploited, e.g., with cell-type specific exosomes, which contain hundreds of RNAs, and may thus reflect the state of their parent cell with high grain resolution,56 whereas individual markers such as GFAP and CHI3L1 are crude measures of glial activity. Finally, genetic markers may eventually prove useful for prognostication because preliminary studies suggest genetic influence over the relapse rate.57
In summary, a pipeline exists for potential biomarkers of different MS-associated pathologic processes, which are in different stages of development. So far, their validation has been a slow process. It might require a more concerted effort to accelerate this process.
Summary and Future Considerations
The classification scheme for MS is based on clinical presentation. Recently, the categories of “active” and “nonactive” progressive MS have been introduced. This has been clinically useful because only active progressive, but not nonactive progressive patients respond to MS treatments.
We posit that this classification system can be further improved by incorporating additional key pathologic processes. This will provide a framework to view MS as a combination of interdependent pathobiological axes that vary in expression between different patients. An advantage of this framework is that it provides a ground truth and overcomes the paradox that clinical categories do not align with specific pathologies. Moreover, by quantifying activity patterns across multiple pathologic axes, patients with MS can be phenotyped according to their activity profiles. This profiling will ultimately allow clinicians to improve prognostication and to administer personalized care, e.g., by determining whether a patient will respond to a specific DMT based on his or her activity profile. Moreover, clinical trials can be better powered by enriching for patients with specific pathobiological features that are predicted to respond to the trial drug.
The main limitation of our proposal is that the biomarker panels which cover the principal pathologic axes are not available and that this scheme can currently not be actualized in clinical practice. For now, our proposal may serve to conceptualize how underlying pathologic processes may be incorporated into the MS classification scheme and to highlight the need for the coordinated development of biomarkers.
A second limitation is that we do not know which axes activities provide the most meaningful phenotypic separation. This can only be ascertained as biomarkers become available. However, our framework can be easily adapted to accommodate new pathobiological insights. This may include monitoring the newly postulated specific oligodendrocyte response patterns,25 the presence of neurotoxic and neuroprotective glial subpopulations,58 and determinants of neuroaxonal damage, such as mitochondrial dysfunction.59 Because major efforts in MS research are currently directed at identifying biomarkers, we are hopeful that several new biomarkers will become available for clinical use in the foreseeable future that cover at least a portion of the pathologic axes activity.
GLOSSARY
- DMT
disease-modifying treatment
- MS
multiple sclerosis
- NAWM
normal-appearing white matter
- NfL
neurofilaments
- PPMS
primary progressive MS
- RRMS
relapsing-remitting MS
- WM
white matter
Appendix. Authors
Acknowledgment
The authors acknowledge helpful discussion with Dr. Stephen Krieger.
Study Funding
Supported in part by NIH grants R01 NS102267 and R01 NS112907 (D.P.) and a career transition fellowship from the Consortium of Multiple Sclerosis Centers and the National Multiple Sclerosis Society (M.R.L.).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National multiple sclerosis society (USA) advisory committee on clinical trials of new agents in multiple sclerosis. Neurology. 1996;46(4):907-911. [DOI] [PubMed] [Google Scholar]
- 2.Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kappos L, Bar-Or A, Cree BAC, et al. , EXPAND Clinical Investigators. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263-1273. [DOI] [PubMed] [Google Scholar]
- 4.Hawker K, O'Connor P, Freedman MS, et al. , OLYMPUS Trial Group. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460-471. [DOI] [PubMed] [Google Scholar]
- 5.Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain a J Neurol. 2005;128(pt 11):2705-2712. [DOI] [PubMed] [Google Scholar]
- 6.Sucksdorff M, Matilainen M, Tuisku J, et al. Brain TSPO-PET predicts later disease progression independent of relapses in multiple sclerosis. Brain. 2020;143(11):3318-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lassmann H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front Immunol. 2018;9:3116-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuhlmann T, Ludwin S, Prat A, Antel J, Bruck W, Lassmann H. An updated histological classification system for multiple sclerosis lesions. Acta Neuropathologica. 2017;133(1):13-24. [DOI] [PubMed] [Google Scholar]
- 9.Dutta R, Trapp BD. Relapsing and progressive forms of multiple sclerosis: insights from pathology. Curr Opin Neurol. 2014;27(3):271-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130(pt 4):1089-1104. [DOI] [PubMed] [Google Scholar]
- 11.Dal-Bianco A, Grabner G, Kronnerwetter C, et al. Slow expansion of multiple sclerosis iron rim lesions: pathology and 7 T magnetic resonance imaging. Acta Neuropathologica. 2017;133(1):25-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calabrese M, Magliozzi R, Ciccarelli O, Geurts JJG, Reynolds R, Martin R. Exploring the origins of grey matter damage in multiple sclerosis. Nat Rev Neurosci. 2015;16(3):147-158. [DOI] [PubMed] [Google Scholar]
- 13.Peterson JW, Bö L, Mörk S, Chang A, Trapp BD. Transected neurites, apoptotic neurons, and reduced inflammation in cortical multiple sclerosis lesions. Ann Neurol. 2001;50(3):389-400. [DOI] [PubMed] [Google Scholar]
- 14.Kuhlmann T, Lingfeld G, Bitsch A, Schuchardt J, Brück W. Acute axonal damage in multiple sclerosis is most extensive in early disease stages and decreases over time. Brain. 2002;125(pt 10):2202-2212. [DOI] [PubMed] [Google Scholar]
- 15.Frischer JM, Bramow S, Dal-Bianco A, et al. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(pt 5):1175-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Griffiths I, Klugmann M, Anderson T, et al. Axonal swellings and degeneration in mice lacking the major proteolipid of myelin. Science. 1998;280(5369):1610-1613. [DOI] [PubMed] [Google Scholar]
- 17.Mahad DJ, Ziabreva I, Campbell G, et al. Mitochondrial changes within axons in multiple sclerosis. Brain. 2009;132(pt 5):1161-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magliozzi R, Howell OW, Reeves C, et al. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010;68(4):477-493. [DOI] [PubMed] [Google Scholar]
- 19.Carassiti D, Altmann DR, Petrova N, Pakkenberg B, Scaravilli F, Schmierer K. Neuronal loss, demyelination and volume change in the multiple sclerosis neocortex. Neuropathol Appl Neurobiol. 2018;44(4):377-390. [DOI] [PubMed] [Google Scholar]
- 20.Jürgens T, Jafari M, Kreutzfeldt M, et al. Reconstruction of single cortical projection neurons reveals primary spine loss in multiple sclerosis. Brain. 2016;139(pt 1):39-46. [DOI] [PubMed] [Google Scholar]
- 21.Duncan ID, Brower A, Kondo Y, Curlee JF, Schultz RD. Extensive remyelination of the CNS leads to functional recovery. Proc Natl Acad Sci U S A. 2009;106(16):6832-6836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patrikios P, Stadelmann C, Kutzelnigg A, et al. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129(pt 12):3165-3172. [DOI] [PubMed] [Google Scholar]
- 23.Chang A, Staugaitis SM, Dutta R, et al. Cortical remyelination: a new target for repair therapies in multiple sclerosis. Ann Neurol. 2012;72(6):918-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldschmidt T, Antel J, König FB, Brück W, Kuhlmann T. Remyelination capacity of the MS brain decreases with disease chronicity. Neurology .2009;72(22):1914-1921. [DOI] [PubMed] [Google Scholar]
- 25.Macnair W, Calini D, Agirre E, et al. Single nuclei RNAseq stratifies multiple sclerosis patients into three distinct white matter glia responses. BioRxiv. 2022:487263. [Google Scholar]
- 26.Kuhlmann T. Relapsing-remitting and primary progressive MS have the same cause(s): the neuropathologist's view: 2. Mult Scler. 2013;19(3):268-269. [DOI] [PubMed] [Google Scholar]
- 27.Lassmann H. Relapsing-remitting and primary progressive MS have the same cause(s): the neuropathologist's view: 1. Mult Scler. 2013;19(3):266-267. [DOI] [PubMed] [Google Scholar]
- 28.Luchetti S, Fransen NL, van Eden CG, Ramaglia V, Mason M, Huitinga I. Progressive multiple sclerosis patients show substantial lesion activity that correlates with clinical disease severity and sex: a retrospective autopsy cohort analysis. Acta Neuropathologica. 2018;135(4):511-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tallantyre EC, Bo L, Al-Rawashdeh O, et al. Greater loss of axons in primary progressive multiple sclerosis plaques compared to secondary progressive disease. Brain. 2009;132(pt 5):1190-1199. [DOI] [PubMed] [Google Scholar]
- 30.Choi SR, Howell OW, Carassiti D, et al. Meningeal inflammation plays a role in the pathology of primary progressive multiple sclerosis. Brain. 2012;135(pt 10):2925-2937. [DOI] [PubMed] [Google Scholar]
- 31.Stevenson VL, Miller DH, Rovaris M, et al. Primary and transitional progressive MS: a clinical and MRI cross-sectional study. Neurology. 1999;52(4):839-845. [DOI] [PubMed] [Google Scholar]
- 32.Dyment DA, Herrera BM, Cader MZ, et al. Complex interactions among MHC haplotypes in multiple sclerosis: susceptibility and resistance. Hum Mol Genet. 2005;14(14):2019-2026. [DOI] [PubMed] [Google Scholar]
- 33.International Multiple Sclerosis Genetics C. Multiple sclerosis genomic map implicates peripheral immune cells and microglia in susceptibility. Science. 2019;365(6460):eaav7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gianfrancesco MA, Acuna B, Shen L, et al. Obesity during childhood and adolescence increases susceptibility to multiple sclerosis after accounting for established genetic and environmental risk factors. Obes Res Clin Pract. 2014;8(5):e435-e447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. New Engl J Med. 1998;339(5):285-291. [DOI] [PubMed] [Google Scholar]
- 36.von Wyl V, Decard BF, Benkert P, et al. Influence of age at disease onset on future relapses and disability progression in patients with multiple sclerosis on immunomodulatory treatment. Eur J Neurol. 2020;27(6):1066-1075. [DOI] [PubMed] [Google Scholar]
- 37.Jangi S, Gandhi R, Cox LM, et al. Alterations of the human gut microbiome in multiple sclerosis. Nat Commun. 2016;7:12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sundström P, Nyström L. Smoking worsens the prognosis in multiple sclerosis. Mult Scler. 2008;14(8):1031-1035. [DOI] [PubMed] [Google Scholar]
- 39.Rhead B, Baarnhielm M, Gianfrancesco M, et al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol Genet. 2016;2(5):e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296-301. [DOI] [PubMed] [Google Scholar]
- 41.Eshaghi A, Young AL, Wijeratne PA, et al. Identifying multiple sclerosis subtypes using unsupervised machine learning and MRI data. Nat Commun. 2021;12(1):2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wisnieff C, Ramanan S, Olesik J, Gauthier S, Wang Y, Pitt D. Quantitative susceptibility mapping (QSM) of white matter multiple sclerosis lesions: interpreting positive susceptibility and the presence of iron. Magn Reson Med. 2015;74(2):564-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Absinta M, Vuolo L, Rao A, et al. Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis. Neurology. 2015;85(1):18-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beck ES, Gai N, Filippini S, Maranzano J, Nair G, Reich DS. Inversion recovery susceptibility weighted imaging with enhanced T2 weighting at 3 T improves visualization of subpial cortical multiple sclerosis lesions. Invest Radiol. 2020;55(11):727-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laule C, Moore GRW. Myelin water imaging to detect demyelination and remyelination and its validation in pathology. Brain Pathol. 2018;28(5):750-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology. 2019;92(10):e1007-e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berger T, Stüve O. Neurofilament light chain: an important step toward a disease biomarker in multiple sclerosis. Neurology. 2019;92(10):451-452. [DOI] [PubMed] [Google Scholar]
- 48.Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep. 2018;8(1):14798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martínez MAM, Olsson B, Bau L, et al. Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult Scler. 2015;21(5):550-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Housley WJ, Pitt D, Hafler DA. Biomarkers in multiple sclerosis. Clin Immunol. 2015;161(1):51-58. [DOI] [PubMed] [Google Scholar]
- 51.Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187(4):655-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Comabella M, Pericot I, Goertsches R, et al. Plasma osteopontin levels in multiple sclerosis. J Neuroimmunol. 2005;158(1-2):231-239. [DOI] [PubMed] [Google Scholar]
- 53.Vogt MHJ, Lopatinskaya L, Smits M, Polman CH, Nagelkerken L. Elevated osteopontin levels in active relapsing-remitting multiple sclerosis. Ann Neurol. 2003;53(6):819-822. [DOI] [PubMed] [Google Scholar]
- 54.Schneider R, Bellenberg B, Gisevius B, et al. Chitinase 3–like 1 and neurofilament light chain in CSF and CNS atrophy in MS. Neurol Neuroimmunol Neuroinflamm. 2020;8(1):e906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marastoni D, Magliozzi R, Bolzan A, et al. CSF levels of CXCL12 and osteopontin as early markers of primary progressive multiple sclerosis. Neurol Neuroimmunol Neuroinflamm. 2021;8(6):e1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang L, Zhang L. Circulating exosomal miRNA as diagnostic biomarkers of neurodegenerative diseases. Front Mol Neurosci. 2020;13:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vandebergh M, Andlauer TFM, Zhou Y, et al. Genetic variation in WNT9B increases relapse hazard in multiple sclerosis. Ann Neurol. 2021;89(5):884-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liddelow SA, Guttenplan KA, Clarke LE, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541(7638):481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Licht-Mayer S, Campbell GR, Canizares M, et al. Enhanced axonal response of mitochondria to demyelination offers neuroprotection: implications for multiple sclerosis. Acta Neuropathol. 2020;140(2):143-167. [DOI] [PMC free article] [PubMed] [Google Scholar]